Abstract
Objectives
To evaluate immunohistochemically the expression of GLUT-3 and GLUT-4 in oral epithelial dysplasia (OED) and the oral squamous cell carcinoma (OSCC) and assess possible involvement in the malignant transformation of oral lesions.
Methods
Tissue samples of 15 cases of OSCC and 15 of OED were subjected to immunohistochemistry with anti-GLUT-3 and anti-GLUT-4 antibodies. Five fields of each case were analyzed, to provide percentages of positive cells at 400X magnification.
Result
GLUT-3 and GLUT-4 were positive in 100% of the analyzed samples, the percentage immunolabeling for GLUT-3 ranging from 19% to 73% in the OED group and 10% to 89% in the OSCC group. Positive immunolabeling for GLUT-4 ranged from 15.2% to 79.9% in the OSCC group and 27.1% to 92.6% in the OED group. Statistical analysis with the Mann-Whitney test revealed that there was a higher expression of GLUT-4 in the OED group than in the OSCC group (p=0.04) without any significant difference in the GLUT-3 expression (p=0.852).
Conclusion
GLUT-4 expression may indicate some role in oncogenic mechanisms which can determine a malignant phenotype. Thus, it is suggested that further studies on the role of GLUT-3 in oral carcinogenesis be conducted.
Keywords: Oral epithelial dysplasia, oral squamous cell carcinoma, GLUT proteins, immunohistochemistry
Introduction
Oral cancer has the sixth highest mortality rate among all other cancers (Liu et al., 2012) and the oral squamous cell carcinoma (OSCC) is the most common histological subtype (Liu et al., 2012; Dantas et al., 2016). Potentially malignant lesions (PMLs) in the oral cavity is a risk factor for the development of oral squamous cell carcinoma. Histopathology test should be administered to detect oral epitelial dysplasia (OED) as an indicator for malignant transformation and progression (Warnalulasuriya et al., 2008). However, the progression to the malignant phenotype is not observed in most PMLs (Angadi and Angadi, 2015).
The histopathological examination is still the gold standard to evaluate dysplasia (Silveira et al., 2009; Fonseca-Silva et al., 2016); however, the accuracy of histopathological examination is insufficient in predicting lessions with high probability of malignant transformation (Chaves et al., 2017). In oral carcinogenesis, recent studies have shown that proteins involved in the cellular metabolism have received much attention (Demasi et al., 2010; Pereira et al., 2013). Thus, the identification of the molecular markers which may signalize the behavior and the malignant transformation is very important.
Tumoral cells can replace the oxidative phosphorylation with glycolysis as an energy source. The generated adenosine triphosphate (ATP) is the driving force for mitosis once it is the main source of energy for the nuclei (Demasi et al., 2010). Glycolysis is not essential for neoplasia to occur but it is a favorable and strategical opportunity for survival and proliferation when oxygen and nutrients are lacking as it occurs during oral carcinogenesis (Pereira et al., 2013).
The transport of glucose into the cells is mediated by active and passive means. Passive transport is performed by a family of glucose transporters known as the GLUT family. Many isoforms of GLUT have been described and their expression are specific to cell types and controlled by the extracellular environment. The GLUT family is comprised of fourteen members: GLUT-1 to GLUT-14 (Macheda et al., 2005, Rao et al., 2012).
GLUT-3 is the main glucose transporter in neuronal cells and its expression levels in brain regions are related to the use of glucose in this organ. This protein can also be found in intracellular vesicles of lymphocytes, monocytes/macrophages, and platelets being translocated to the cell membrane when metabolic energy demand is high (Simpsom et al., 2008). GLUT-3 was also detected with high expression in mouse spermatozoa, controlling the rate of glucose uptake and the metabolism required for the motility and maturation of these cells (Thorens and Mueckler, 2010).
Among the GLUT isoforms, GLUT-4 is one of the most studied, because it plays an important role in glucose homeostasis throughout the body. GLUT-4 is primarily expressed in adipose tissue and skeletal muscle (Thorens and Mueckler, 2010). In physiologic conditions when glucose levels are high, as in the post prandial state for exemple, the subsequent increase in circulating insulin activates intracellular signaling cascades that translocate GLUT-4 to the cell membrane (Assefa et al., 2017). Several cells of oral tissues such as the periodontium, or sensitive parts of the tongue are glucose dependent for their metabolic activities (Toyono et al., 2011).
It is known that tumoral cells utilize this metabolic pathway as the main source of energy, explaining why tumoral cells have a high proliferation rate even in the presence of low tension of oxygen (Voelxen et al., 2017). Besides, the expression of such proteins has been correlated with the prognosis of tumors (Kunkel et al., 2003; Macheda et al., 2005; Moreno-Sanchez et al., 2007; Ayala et al., 2010; Ohba et al., 2010; Azad et al., 2016). The high rate of GLUT expression is considered as an important early event in the development of head and neck cancers (Reisser et al., 1999).
The main aim of this study was to verify whether the changes in the glucose transport mechanism are strongly associated with malign phenotypes and whether these changes occur in early stages of the oral carcinogenesis, still in the OED stage. Thus, this study aimed to analyze the immunohistochemical expression of GLUT-3 and GLUT-4 in OED and OSCC lesions to better elucidate their biological behavior.
Materials and Methods
This study consisted of an observational, analytical and cross-sectional study, through the diagnosis and immunoexpression analysis of malignant and premalignant lesions. The present study analysed 15 formalin-fixed and paraffin-embedded cases (FFPE) of OEDs and 15 FFPE cases of OSCCs, obtained from incisional biopsies in patients of the outpatient Stomatology Clinic of the Federal University of Ceará - Sobral Campus between 2012 and 2013. The Research Ethics Committee of the Ceará Dental Academy,Continual Education Center (ACO/CEC), approved this clinical-laboratory study.
Histomorphometric analysis
The specimens were fixed in 10% formol with reference to the selected cases. Afterwards, they were embedded in paraffin, submitted to 5 μm (micrometres) thick cuts, stained with hematoxylin-eosin, and mounted on glass slides for histopathological analysis. Samples were classified utility according to the WHO classification (Barnes et al, 2005; Westra, Lewis, 2017). The obtained data were reviewed and analysed.
Immunohistochemical Reaction
For immunohistochemistry, 3-mm-thick sections were cut from paraffin-embedded material. The tissue samples were treated with anti-GLUT-3 (GTX15311, GeneTex®, San Antonio, TX, USA) and anti-GLUT-4 (GTX61787, GeneTex®, San Antonio, TX, USA) antibodies by performing the streptavidin-biotin immunohistochemical technique (Labeled Streptavidin-Biotin – LSAB).
All tissue samples were processed using standard methods, and serial sections were used for IHC. After deparaffinization and rehydration, slides were subjected to heat-induced epitope retrieval in 10 mmol/L citrate buffer (pH=6.0, 30 min, 99 ºC). Endogenous peroxidase activity was blocked for 10 minutes in 0.3% hydrogen peroxide. After blocking with 1% goat serum for 10 minutes at room temperature, the sections were incubated with primary antibodies for at least 60 minutes at 4°C overnight, being the antibody dilutions 1:300 for GLUT-3 and 1:200 for GLUT-4. The samples were incubated with the secondary antibody LSAB Kit (DAKO®, Carpentaria, CA, USA) for 10 minutes at room temperature. Next, incubation was performed in a chromogen solution prepared with DAB (3-3’-diaminobenzidine) for 10 minutes in a dark chamber (DAKO®, Carpentaria, CA, USA) and Harris hematoxylin was used for counterstaining.
Finally, the assembly was carried out on glass slides, which were examined under an optical microscope NIKON® Eclipse E200. The human placenta tissue was used as positive control for GLUT-3 and the striated cardiac muscle was used as positive control for GLUT-4. Erythrocytes were used as internal positive control; and in parallel with the incubated sections, the negative control was performed, excluding the application of the primary antibody.
Evaluation of IHC staining and statistical analysis
Five fields were randomly selected (400x magnification), visualized through the above-mentioned optical microscope system Leica DM2000, and photographed by digital cam Leica DFC290 HD using Las software at maximum resolution. The quantitative analysis of GLUT-3 and GLUT-4 protein expression was performed by a single examiner, which counted the absolute values of marked cells in different intervals by using Image J software (National Institutes of Health, Bethesda, Maryland, USA). The raw data were transferred to SPSS (version 17.0) for statistical analysis. Means and medians were obtained and the Mann-Whitney test was used. The probability value of p<0.05 indicated statistically significant difference.
Results
In the OSCC group, 10 patients were male and 5 were female; whereas, in the OED group, 6 patients were male and 9 were female. The age of patients ranged from 17 to 84 years old, and the patients in the OED group were younger than those in the other one. The mean age of all patients was 61 years old. Most of elderly male patients at the OSCC group were affected by OSCC lesions. Regarding the OED group, a high percentage of young women was affected.
The GLUT-3 immunoexpression was positive in all cases of OED and OSCC (Figure 1A and Figure 1B). However, different percentage values were obtained. The GLUT-4 expression was also evidenced in all cases of OED and OSCC (Figures 1C and 1D). The nuclear staining was not observed for GLUT-4; however, an intense immunostaining in the membrane of cells undergoing dysplasia and neoplasia was found. The intense immunostaining was seen in the positive controls (Figure 1E and Figure 1F).
Figure 1.
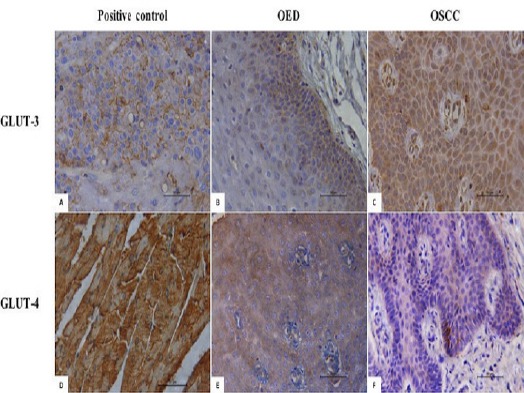
Photomicrographs Evidencing the Immunoexpression of GLUT-3 and GLUT-4 in OED (A) and OSCC (B), and Immunopositivity GLUT-4 in OED (C) and OSCC (D). Positive control (E and F). (LSAB, 400X) (OED; oral epithelial dysplasia, OSCC; oral squamous cell carcinoma).
The means of immune positive cells varied from 10% to 89% in the OSCC group, demonstrating a discreet expression of GLUT-3. No significant association was observed between the immunolabeling of this transporter and age as well as gender. Regarding the GLUT-4 expression and OSCC lesions, the means of immune positive cells ranged from 15.2% to 79.9%, being the last sample the one with the highest number of marked cells. There was no significant association between the GLUT-4 immunolabeling and age as well as gender. However, 80% of the samples with immunolabeling over 80% were obtained from the base of the tongue and floor of the mouth.
In the OED group, the means of immunolabeled cells for GLUT-3 varied approximately from 19% to 73%. There was no significant association between the GLUT-3 expression and age as well as gender. Regarding the GLUT-4 expression, the means of immunolabeled cells ranged from 27.1% to 92.6%. The immune marking was discovered mainly on the epithelial basal layer and on the initial part of the epithelial spinous layer. No significant association was observed between GLUT-4 expression and age, gender, and the location of the lesions. Data were analysed using SPSS (version 17.0) and running Mann-Whitney test. The data are shown in Table 1. No difference was found between the OED and OSCC groups concerning GLUT-3 expression (p=0.852). Considering the expression of GLUT-4 in two types of pathologies, there were statistically significant differences (p=0.04) between the immunolabeling of OED and OSCC lesions, in a way that the expression of GLUT-4 was higher in the OED group (Table 2).
Table 1.
Number of Positive Cells for GlUT-3 in Oral Epithelial Dysplasia and Oral Squamous Cell Carcinoma
| Analyzed Lesion | Quantity of Immunolabeled Cells by the Antibody GLUT-3 | |||
|---|---|---|---|---|
| N | Median | Q25-Q75 | P* | |
| OED | 15 | 37.74 | 21.25 – 47.01 | 0.852 |
| OSCC | 15 | 33.33 | 26.86 – 50.72 | |
, Mann-Whitney Test; OED, oral epithelial dysplasia; OSCC, oral squamous cell carcinoma
Table 2.
Number of Positive Cells for GLUT-4 in Oral Epithelial Dysplasia and Oral Squamous Cell Carcinoma
| Analyzed Lesion | Quantity of Immunolabeled Cells by the Antibody GLUT-4 | |||
|---|---|---|---|---|
| N | Median | Q25-Q75 | P* | |
| Oral epithelial dysplasia | 15 | 58.62 | 46.76 – 68.91 | 0.040 |
| Epidermoid Carcinoma | 15 | 42.84 | 30.10 – 55.84 | |
, Mann-Whitney Test; OED, oral epithelial dysplasia; OSCC, oral squamous cell carcinoma
Discussion
Malignant neoplastic cells exhibit an altered metabolism which is characterized by the increased absorption and utilization of glucose (Ortega et al., 2009; Perez-Sayans et al., 2011). This phenomenon can be explained by several mechanisms, including the adaptation of cells to the hypoxic tumor microenvironment and the maintenance of tumor cell viability, which depends in part on the ability of these cells to perform anaerobic glycolysis (Perez-Sayans et al., 2011). Hypoxia is an important characteristic of solid tumors and advanced head and neck carcinomas and it is associated with a poor prognosis (Eckert et al., 2012).
Few studies have investigated the expression of GLUT-3 and GLUT-4 in OSCC and, to the authors’ knowledge, there is no investigation on the immunoexpression of these glucose in OED. According to the high index of immunolabelling in the OED group, it is believed that the GLUT expression can be a factor that can determine a tumor phenotype. Therefore, it can be a sign of malign phenotype (Reisser et al., 1999; Kunkel et al., 2003; Demeda et al., 2014). In the present study, no difference was discovered between OSCC and OED groups concerning GLUT-3 expression. However, expression of GLUT-4 was higher in the OED group.
GLUT-3 mediate glucose uptake by cancer cells, regulating energy metabolism, and have been related to the aggressive behavior of some tumors (Ogane et al., 2010; Eckert et al., 2012). Szablewski (2013) analyzed GLUT in melanocytic lesions and verified that GLUT-3 was expressed in all lesions under the investigation, while GLUT-1 was expressed by 87.5% of all cases. It is known that that GLUT-1 plays an important role in glucose uptake in cancer cells (Tian et al., 2004; Roh et al., 2009; Pereira et al., 2016). Ayala et al., (2010) studied the GLUT-1 and GLUT-3 immunolabeling in OSCC and detected high expression levels of GLUT-1 in nearly all of the cases under the investigation. Although GLUT-3 appeared in only 21.1% of the studied samples, it was not found in the normal oral epithelium. Contrary to these findings, our study showed 100% immunolabeling of the analyzed samples. Zhou et al., (2008) found no immunoexpression of this glucose transporter in any of 38 head and neck carcinomas. On the other hand, Tian et al., (2004) observed GLUT-3 immunostaining in 16 out of 19 OSCC cases (84.2%). Demeda et al., (2014) studied metastatic and non-metastatic lower lip squamous cell carcinoma and found that immunostaining for GLUT-3 was much lower than that for GLUT-1. In addition, they observed that GLUT-3 staining prevailed in central areas of tumor nests, islands and sheets. In the present study, GLUT-3 immunostaining was discovered in the basal and suprabasal layer, suggesting that the expression of GLUT-3 represents a secondary glucose uptake mechanism in OED and OSCC lesions.
The discreet immunolabeling pattern presented in the cell membrane was like the one found in other studies (Moreno-Sánchez et al., 2009, Krzeslak et al., 2012, and Szablewski et al., 2013), revealing that GLUT-3 is present at a low frequency in oral spinous cell carcinomas. GLUT-3 serves as a prognosis biomarker for many types of malign lesions even though it is not directly related to the oral carcinogenesis process; however, few studies have reported this protein as marker in oral cancer lesions (Azad et al., 2016).
Based on our results, there was higher expression of GLUT-4 in dysplastic lesions than in carcinomatous ones, which differs from Reisser’s et al., (1999) finding that compared the immune expression of GLUT in carcinomas. Higher expression of GLUT-4 in dysplastic lesions can reflect its role in some oncogenic mechanisms, leading to a tumoral phenotype in early stages of carcinogenesis. Therefore, it is can be concluded that the overexpression of this protein is an indicator of a malignancy. In other words, the high levels of GLUT-4 expression are correlated with malign transformation of cells and malign phenotype mainly in colorectal and pancreatic cancers (Harada et al., 2001; Kunkel et al., 2003; Ohba et al., 2010).
A pilot study identified the presence of GLUT-4 and located it in healthy human gingival cells, observing a peri-nuclear marking on connective tissue and gingival epithelium, more evident in the basal layer than in the superficial layers. Based on our findings, it seems that the greater marking found in the basal layer was due to its higher metabolic activity, emphasizing the importance of GLUT-4 in the energy metabolism of oral tissues (Rao et al., 2012). In our study, we also identified a more evident expression of GLUT-4 in the basal layer; however, the nuclear staining was not observed. It seems that higher expression found in the membrane can be justified by the high methabolic rates in OED and OSCC.
In human OSCC cell lines is evidenced atypical mRNA expression of GLUT-4 using RT-PCR (Fuzukumi et al., 2000; Chang et al., 2017), generating differentiated immunohistochemical marking patterns. Chang et al., (2017) revealed that GLUT-4 mediated HNSCC metastasis was independent of glucose concentration and the innate glucose transport function of GLUT-4 and the immunohistochemical staining results showed stronger staining of the GLUT-4 protein in tumor tissues than in the adjacent normal tissues. It seems that the ectopic overexpression of GLUT-4 can lead to a lower threshold for activating its downstream molecules, rendering the ligand (glucose) concentration nonconsequential. They showed that GLUT-4 overexpression promotes tumor metastasis and is significantly associated with poor prognosis in HNSCC patients through a glucose indirect pathway in cancer cells, leading to the activation of the TRIM24 pathway.
There are few studies representing the immunohistochemical evaluation of the GLUT-3 and GLUT-4 expression in OED and OSCC. Thus, there is no evidence that dysplastic lesions display a more intense immunolabeling than carcinomatous ones and no elucidation as to why such difference between the lesions exist. Our study; however, demonstrated a higher immune expression of GLUT-4 in dysplastic than in carcinomatous lesions. In a nutshell, we indicated the involvement of GLUT-4 in the early stages of the oral carcinogenesis, including in OED cases.
Funding
This study was supported by Fundação Cearense de Apoio ao Desenvolvimento Científico e Tecnológico (FUNCAP - # BP1-0031-00088.01.00/10).
References
- 1.Angadi VC, Angadi PV. GLUT-1 immunoexpression in oral epithelial dysplasia, oral squamous cell carcinoma, and verrucous carcinoma. J Oral Sci. 2015;57:115–22. doi: 10.2334/josnusd.57.115. [DOI] [PubMed] [Google Scholar]
- 2.Assefa B, Mahmoud AM, Pfeiffer AFH, et al. Insulin-like growth factor (IGF) binding protein-2, independently of IGF-1, induces GLUT-4 translocation and glucose uptake in 3T3-L1 adipocytes. Oxid Med Cell Longev. 2017;2017 doi: 10.1155/2017/3035184. Article ID 3035184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ayala FR, Rocha RM, Carvalho KC, et al. GLUT1 and GLUT3 as potential prognostic markers for oral squamous cell carcinoma. Molecules. 2010;15:2374–87. doi: 10.3390/molecules15042374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Azad N, Maurya MK, Kar M, et al. Expression of GLUT-1 in oral squamous cell carcinoma in tobacco and non-tobacco users. J Oral Biol Craniofac Res. 2016;6:25–31. doi: 10.1016/j.jobcr.2015.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barnes L, Eveson JW, Reichart P, Sidransky D. Neck tumours. Vol. 2005. Lyon: IARC Press; 2005. World health organization classification of tumours pathology and genetics of head and. [Google Scholar]
- 6.Chang YC, Chi LH, Chang WM, et al. Glucose transporter 4 promotes head and neck squamous cell carcinoma metastasis through the TRIM24-DDX58 axis. J Hematol Oncol. 2017;10:11. doi: 10.1186/s13045-016-0372-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chaves FN, Bezerra T, de Barros Silva PG, et al. Evaluation of the p-AKT, p-JNK and FoxO3a function in oral epithelial dysplasia. Oral Dis. 2017;23:367–78. doi: 10.1111/odi.12623. [DOI] [PubMed] [Google Scholar]
- 8.Dantas TS, de Barros Silva PG, Sousa EF, et al. Influence of educational level, stage, and histological type on survival of oral cancer in a Brazilian population: A retrospective study of 10 years observation. Medicine. 2016;95:e2314. doi: 10.1097/MD.0000000000002314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Demasi AP, Costa AF, Altemani A, et al. Glucose transporter protein 1 expression in mucoepidermoid carcinoma of salivary gland: correlation with grade of malignancy. Int J Exp Pathol. 2010;91:107–13. doi: 10.1111/j.1365-2613.2009.00702.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Demeda CF, Carvalho CHP, Aquino ARL, et al. Expression of Glucose Transporters 1 and 3 in Metastatic and Non-Metastatic Lower Lip Squamous Cell Carcinoma. Braz Dent J. 2014;25:372–8. doi: 10.1590/0103-6440201300054. [DOI] [PubMed] [Google Scholar]
- 11.Eckert AW, Lautner MH, Schütze A, et al. Coexpression of hypoxia-inducible factor-1αand glucose transporter-1 is associated with poor prognosis in oral squamous cell carcinoma patients. Histopathology. 2011;58:1136–47. doi: 10.1111/j.1365-2559.2011.03806.x. [DOI] [PubMed] [Google Scholar]
- 12.Fukuzumi M, Hamakawa H, Onishi A, et al. Gene expression of GLUT isoforms and VHL in oral squamous cell carcinoma. Cancer Lett. 2000;161:133–40. doi: 10.1016/s0304-3835(00)00613-3. [DOI] [PubMed] [Google Scholar]
- 13.Fonseca-Silva T, Diniz MG, de Sousa SF, Gomez RS, Gomes CC. Association between histopathological features of dysplasia in oral leukoplakia and loss of heterozygosity. Histopathology. 2016;68:456–60. doi: 10.1111/his.12746. [DOI] [PubMed] [Google Scholar]
- 14.Harada H, Kobayashi K, Maeda A. Predictive indicators for occult lymph node metastasis in N0 cases of squamous tongue carcinoma. J Oral Tumor. 2001;13:313–7. [Google Scholar]
- 15.Liu W, Shi LJ, Wu L, et al. Oral cancer development in patients with leukoplakia--clinicopathological factors affecting outcome. PLoS One. 2012;7:e34773. doi: 10.1371/journal.pone.0034773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Krześlak A, Forma E, Bernaciak M, Romanowicz H, Bryś M. Gene expression of O-GlcNAc cycling enzymes in human breast cancers. Clin Exp Med. 2012;12:61–5. doi: 10.1007/s10238-011-0138-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kunkel M, Reichert TE, Benz P, et al. Overexpression of Glut-1 and increased glucose metabolism in tumors are associated with a poor prognosis in patients with oral squamous cell carcinoma. Cancer. 2003;97:1015–24. doi: 10.1002/cncr.11159. [DOI] [PubMed] [Google Scholar]
- 18.Macheda ML, Rogers S, Best JD. Molecular and cellular regulation of glucose transporter (GLUT) proteins in cancer. J Cell Physiol. 2005;202:654–62. doi: 10.1002/jcp.20166. [DOI] [PubMed] [Google Scholar]
- 19.Moreno-Sanchez R, Rodriguez-Enriquez S, Marin-Hernandez A, Saavedra E. Energy metabolism in tumor cells. FEBS J. 2007;274:1393–418. doi: 10.1111/j.1742-4658.2007.05686.x. [DOI] [PubMed] [Google Scholar]
- 20.Ogane N, Yasuda M, Shimizu M, et al. Clinicopathological implications of expressions of hypoxia-related molecules in esophageal superficial squamous cell carcinoma. Ann Diagn Pathol. 2010;14:23–9. doi: 10.1016/j.anndiagpath.2009.10.003. [DOI] [PubMed] [Google Scholar]
- 21.Ohba S, Fujii H, Ito S, et al. Overexpression of GLUT-1 in the invasion front is associated with depth of oral squamous cell carcinoma and prognosis. J Oral Pathol Med. 2010;39:74–8. doi: 10.1111/j.1600-0714.2009.00814.x. [DOI] [PubMed] [Google Scholar]
- 22.Ortega AD, Sánchez-Aragó M, Giner-Sánchez D, et al. Glucose avidity of carcinomas. Cancer Lett. 2009;276:125–35. doi: 10.1016/j.canlet.2008.08.007. [DOI] [PubMed] [Google Scholar]
- 23.Pereira KMA, Chaves FN, Viana TSA, et al. Oxygen metabolism in oral cancer: HIF and GLUT's (Review) Oncol Lett. 2013;6:311–6. doi: 10.3892/ol.2013.1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pereira KMA, Feitosa SG, Lima ATT, et al. Immunohistochemical evaluation of glucose transporter type 1 in epithelial dysplasia and oral squamous cell carcinoma. Asian Pac J Cancer Prev. 2016;17:147–51. doi: 10.7314/apjcp.2016.17.1.147. [DOI] [PubMed] [Google Scholar]
- 25.Pérez-Sayáns M, Suárez-Peñaranda JM, Pilar GD, et al. Hypoxia-inducible factors in OSCC. Cancer Lett. 2011;313:1–8. doi: 10.1016/j.canlet.2011.08.017. [DOI] [PubMed] [Google Scholar]
- 26.Rao SR, Sundaram S, Lavu V. Immuno-localization of glucose transporter 4 in healthy human gingiva. Indian J Dent Res. 2012;23:565–7. doi: 10.4103/0970-9290.107327. [DOI] [PubMed] [Google Scholar]
- 27.Reisser C, Eichhorn K, Herold-Mende C, Born AI, Bannasch P. Expression of facilitative glucose transport proteins during development of squamous cell carcinomas of the head and neck. Int J Cancer. 1999;80:194–8. doi: 10.1002/(sici)1097-0215(19990118)80:2<194::aid-ijc6>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 28.Roh JL, Cho KJ, Kwon GY, et al. The prognostic value of hypoxia markers in T2-staged oral tongue cancer. Oral Oncol. 2009;45:63–8. doi: 10.1016/j.oraloncology.2008.03.017. [DOI] [PubMed] [Google Scholar]
- 29.Silveira EJD, Lopes MFF, Silva LMM, et al. Lesões orais com potencial de malignização: análise clínica e morfológica de 205 casos. J Bras Patol Med Lab. 2009;45:233–8. [Google Scholar]
- 30.Simpson IA, Dwyer D, Malide D, et al. The facilitative glucose transporter GLUT3:20 years of distinction. Am J Physiol Endocrinol Metab. 2008;295:242–53. doi: 10.1152/ajpendo.90388.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Szablewski L. Expression of glucose transporters in cancers. Biochim Biophys Acta. 2013;1835:164–9. doi: 10.1016/j.bbcan.2012.12.004. [DOI] [PubMed] [Google Scholar]
- 32.Thorens B, Mueckler M. Glucose transporters in the 21st Century. Am J Physiol Endocrinol Metab. 2010;298:141–5. doi: 10.1152/ajpendo.00712.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tian M, Zhang H, Nakasone Y, Mogi K, Endo K. Expression of Glut-1 and Glut-3 in untreated oral squamous cell carcinoma compared with FDG accumulation in a PET study. Eur J Nucl Med Mol Imaging. 2004;31:5–12. doi: 10.1007/s00259-003-1316-9. [DOI] [PubMed] [Google Scholar]
- 34.Toyono T, Seta Y, Kataoka S, et al. Differential expression of the glucose transporters in mouse gustatory papillae. Cell Tissue Res. 2011;345:243–52. doi: 10.1007/s00441-011-1210-x. [DOI] [PubMed] [Google Scholar]
- 35.Voelxen NF, Blatt S, Knopf P, et al. Comparative metabolic analysis in head and neck cancer and the normal gingiva. Clin Oral Investig. 2018;22:1033–43. doi: 10.1007/s00784-017-2185-0. [DOI] [PubMed] [Google Scholar]
- 36.Warnakulasuriya S, Reibel J, Bouquot J, Dabelsteen E. Oral epithelial dysplasia classification systems: predictive value, utility, weaknesses, and scope for improvement. J Oral Pathol Med. 2008;37:127–33. doi: 10.1111/j.1600-0714.2007.00584.x. [DOI] [PubMed] [Google Scholar]
- 37.Westra WH, Lewis JS., Jr Update from the 4th edition of the world health organization classification of head and neck tumours: oropharynx. Head Neck Pathol. 2017;11:41–7. doi: 10.1007/s12105-017-0793-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhou S, Wang S, Wu Q, Fan J, Wang Q. Expression of glucose transporter-1 and -3 in the head and neck carcinoma –the correlation of the expression with the biological behaviors. ORL J Otorhinolaryngol Relat Spec. 2008;70:189–94. doi: 10.1159/000124293. [DOI] [PubMed] [Google Scholar]


