Abstract
Objective
Breast carcinoma is the most common malignant tumor and the leading cause of carcinoma deaths in women. Its etiology is multifactorial, implicating reproductive factors, hormonal imbalances and genetic predispositions. Studies have shown that Cycloxygenase-2 (COX-2) plays an important role in the carcinogenesis and increased expression has been regarded as a poor prognostic factor. The objective of our study is 1. To study COX-2 expression in normal breast tissue, DCIS and invasive breast cancer. 2. To determine COX-2 expression with clinicopathological prognostic parameters.
Methods
Radical mastectomy specimens were studied for COX-2 expression by immunohistochemistry in 50 patients diagnosed as breast carcinoma. COX-2 expression is quantified as IHS Score and separately calculated for normal breast epithelium near the tumor, DCIS and invasive areas. Relationship between COX-2 expression with various clinicopathological parameters was evaluated.
Result
The results of our study suggest an association of the expression of COX-2 to the factors associated with poor prognosis in breast cancer, such as larger tumor size, positive lymph node status, higher T stage and N stage and lymphovascular invasion. There was a higher COX-2 expression in the DCIS component as compared to the invasive ductal carcinoma component and the adjoining breast epithelium.
Conclusion
Our study established the role of COX-2 in carcinogenesis and its association with adverse prognostic factors.
Keywords: COX-2, IDC, DCIS
Introduction
Breast carcinoma is the most common malignant tumor and the leading cause of carcinoma deaths in women, with more than 16,00,000 new cases being diagnosed worldwide annually. It is the commonest cancer among women in India with an incidence of approximately 1,45,000 cases annually and around 70,000 deaths annually.
The molecular characterization of this malignancy is an indicator for tumor prognosis and aggressiveness and may contribute to routine clinical decision making. Additionally, identifying specific molecular patterns helps to introduce specifically targeted therapies for cancer treatment. The classical molecular prognostic parameters of breast cancer are estrogen receptor (ER), progesterone receptor (PR) expression and Her-2-neu receptor expression (Pakkiri et al., 2009; Ross et al., 2009).
Studies have shown that Cycloxygenase-2 (COX-2) plays an important role in the development of some human cancers, particularly pulmonary, colon and breast carcinoma as well as their pre-invasive lesions. Cyclooxygenase (also known as Prostaglandin endoperoxide synthase) catalyzes the conversion of arachidonic acid to prostaglandin endoperoxide, which is the rate limiting step in prostaglandin and thromboxane biosynthesis. Two isoforms of prostaglandin synthase have been identified and are often referred to as COX-1 and COX-2 (Williams and DuBois, 1996).
COX-1 is constitutively produced by most of the body tissues, while COX-2 is an inducible enzyme and is produced under certain specific conditions like inflammation and tumor microenvironment. COX-2 plays a role in the regulation of estrogen by producing prostaglandin E2, which increases the expression of the cytochrome P450 enzyme complex (also known as aromatase) that catalyzes androgen to produce estrogen (Brueggemeier et al., 2003; Diaz-Cruz et al., 2005; Richards et al., 2002).
During progression of cancer, prostaglandins mediate several mechanisms, including cell proliferation, apoptosis, and angiogenesis.
Therefore, the aim of our present study is to determine the COX-2 expression in infiltrating duct carcinoma, adjacent normal breast epithelium and DCIS (ductal carcinoma in situ).
The study was designed: 1. To determine COX-2 expression by immunohistochemistry in invasive breast cancer, adjacent normal breast tissue and DCIS (wherever possible). 2.To evaluate the COX-2 expression with clinical and histological prognostic parameters including hormone receptor status.
Materials and Methods
Fifty patients of breast carcinoma in whom mastectomy was performed and a diagnosis of invasive duct carcinoma was made on histopathological examination, were recruited in this study after proper written consent from the patients.
All received specimens were properly labelled mastectomy specimens, well preserved in 10% neutral buffered formalin, sent with patient’s history and proper clinical details on the requisition form.
Relevant clinical data with regard to sociodemographic variables, clinical history including family history and radiological findings were obtained.
Following adequate fixation for about 12-24 hours, the representative tissue sections were submitted for routine processing, following which the paraffin embedded serial sections of 3-4 micron thickness, were obtained. These were stained with Haematoxylin and Eosin stain for routine histopathological examination and immunohistochemistry was applied thereafter.
In our study we used COX-2 as the primary immunohistochemical marker. ER, PR and Her2-neu expression of the cases was also recorded, wherever available.
COX-2 expression was objectively evaluated by:
COX-2 Quantity Score
0 = No staining
1 = 1%-10% nuclei staining
2 = 11%-50% nuclei staining
3 = 51%-80% nuclei staining
4 = ≥81% nuclei staining
COX-2 Staining Intensity Score
0 = No staining
1 = Weak staining
2 = Moderate staining
3 = Strong staining
COX-2 IHS Score
COX-2 IHS score is obtained by multiplying the quantity score and staining intensity score.
0 – 3 = Negative or faint staining
4 – 8 = Moderate/ Intermediate staining
9 – 12 = Strong/ High staining
Intermediate and high staining are considered positive.
Statistical analysis
The collected data were transformed into variables, coded and entered in Microsoft Excel. Data were analyzed and statistically evaluated using SPSS-PC-17 version. Quantitative data was expressed in mean, standard deviation and difference between two comparable groups were tested by Student’s t-test (unpaired) or Mann Whitney ‘U’ test while qualitative data were expressed in percentage. Statistical differences between the proportions were tested by chi square test or Fisher’s exact test.
Results
This study was performed on 50 patients who were diagnosed as having invasive breast carcinoma. The age ranged from 25 to 80 years with a median age of 51.49 years. Most of the cases (56%) were in the age group of 35 to 44 years and 55 to 64 years with the lowest number (6%) being in the age group of 25 to 34 years.
The component of DCIS was seen in 18 (36%) out of 50 cases.
Histological grading was done in all the cases using Richardson Blooms scoring. Maximum number of cases were of grade 2 (52%). Grade 1 and grade 3 were seen in 16 (32%) and 8 (16%) cases respectively. Lymphovascular invasion was present in 28 (56%) cases.
The carcinomas were staged according to the TNM AJCC 8th edition. There were 11(22%), 17 (34%) and 22(44%) cases in the T1, T2 and T3 category, respectively.
N1, N2 and N3 stages were seen in 15(30%), 18(36%) and 3(6%) cases respectively. Fourteen (28%) cases did not show any lymph node involvement.
Figure 1.
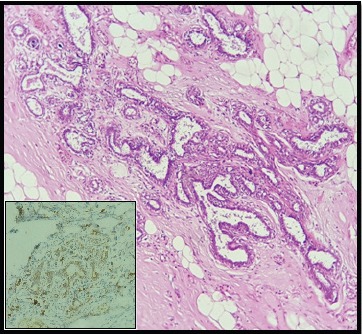
Ducts in the Adjoining Non-Neoplastic Breast Parenchyma Showing Intermediate Positivity for COX-2 (100x)
Figure 2.
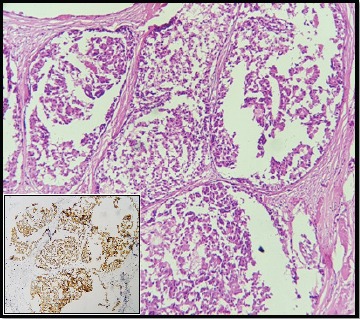
DCIS Component Showing High Positivity for COX-2 (100x)
Figure 3.
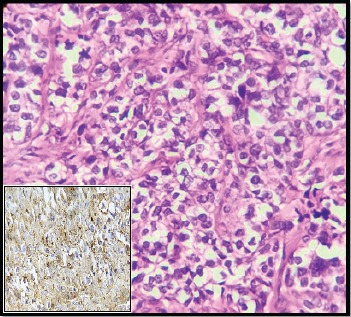
IDC Showing Intermediate Cytoplasmic Positivity for COX-2 (400x)
Figure 4.
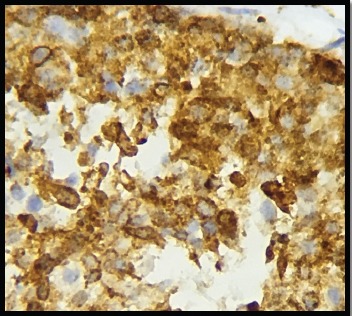
IDC Showing High Cytoplasmic Positivity for COX-2 in a Patient with Lymph Node Involvement (400x)
The immunohistochemical profiles of the patients were also studied in 39 cases. ER and PR positivity was found in 20 (51.3%) and 17 (43.6%) of the cases. Her-2-neu was overexpressed in 15 cases (38.5%). According to the receptor patterns 14 (35.9%) expressed a triple negative pattern.
COX-2 expression was studied in IDC as well as the DCIS component and adjoining non neoplastic breast epithelium. In IDC, high expression of COX-2 was seen in 14 (28%) cases. Twenty one (42%) cases were negative for COX-2 expression, 15 (30%) cases showed intermediate expression. Mean age of the patients with positive COX-2 expression was 48.41 years while in patients with negative COX-2 expression it was 54.57 years.
The DCIS component revealed high COX-2 expression in ten cases. Intermediate expression of COX-2 was seen in four (22.2%) cases whereas four (22.2%) cases did not express COX-2.
The mean tumor size in patients showing COX-2 expression was 6.93 cm while it was 2.81 cm in patients with negative COX-2 expression. There was a positive correlation between COX-2 expression and larger tumor size with a significant P-value (<0.001). Twenty eight (96.6%) cases with positive COX-2 expression were in T2 and T3 stage while all the 21 (100%) cases with negative COX-2 expression were in T1 and T2 stage. There exists a significant correlation between positive COX-2 expression and higher T stage.
The mean number of involved lymph nodes in patients with positive COX-2 expression was 5.36 while in cases with negative COX-2 expression was 5.18. Nodal metastasis was seen in 25 (86.2%) cases while four (13.8%) cases showed no metastasis to lymph nodes in the COX-2 positive group. In the COX-2 negative category, 11 (52.4%) cases showed nodal involvement while ten (47.6%) cases did not show any metastasis to lymph nodes. There was a significantly positive correlation between positive COX-2 expression and lymph node involvement.
In the COX-2 positive group, no lymph node metastasis was seen in four (13.8%) cases, ten (34.5%) were in N1 stage, 14 (48.2%) were in N2 stage and one (3.5%) in N3 stage. In the COX-2 negative group, N0 stage was seen in ten (47.6%) cases, N1 in five (23.8%) cases, N2 stage in four (19%) cases and N3 stage in two (9.6%) cases.
ER, PR and Her-2-neu status were available only in 39 cases.
COX-2 was expressed in 60.9% of non triple negative cases of ductal carcinoma and in 39.1% of triple negative cases. COX-2 negativity was seen in 68.7% of non triple negative cases and 31.3% of triple negative cases.
In the COX-2 positive group, 14 (48.3%) cases belonged to grade 2, nine (31%) cases belonged to grade 1 and six (20.7%) cases belonged to grade 3. In cases with negative COX-2 expression, 12 (57.1%), seven (33.4%) and two (9.5%) cases fell in the category of histological grade 2, 1 and 3, respectively.
Lymphovascular invasion was present in 26 (89.7%) cases and absent in three (10.3%) cases of the COX-2 positive group. In cases with negative COX-2 expression, lymphovascular invasion was seen only in two (9.5%) cases while it was absent in 19 (90.5%) cases. There was a significantly positive correlation between COX-2 expression and lymphovascular invasion.
Discussion
In our study, 58% of the breast carcinoma cases showed COX-2 positivity. This finding is comparable with the findings of some studies (Mosalpuria et al., 2014; Rozenowicz et al., 2012). While other researchers quote either a higher or lower COX-2 expression (Costa et al., 2002; Lee et al., 2010; Davies et al., 2003; Misron et al., 2015). The variation of COX-2 expression in the different studies can be possibly attributed to tissue sampling (paraffin or frozen section), method of analysis, and the positive cut-off point.
We found no significant correlation between COX-2 expression and the patient’s age at presentation. This finding was in accordance with the studies done by Costa et al., (2002) and Singh (2004) which showed no association between the mean age of presentation and the expression of COX-2. Similar results were obtained in various other studies performed by Dannenberg and Howe (2003), Lee et al., (2010) and Nam et al., (2005).
In the present study, there was a highly significant correlation between tumor size and COX-2 expression with a P value of <0.001. Similar findings were observed by Dannenberg and Howe (2003), Ristimaki et al., (2002) and Arun and Goss (2004) with P values of <0.0001, <0.0001 and <0.001, respectively. However, in the studies performed by Lee et al., (2010) and Misron et al., (2015), no significant correlation was found between these variables. According to Muhammad et al., (2013), since tumor size is one of the most powerful predictors of tumor behaviour and as it constitutes the basis of major staging systems, positive COX-2 expression correlates with its poor prognosis.
In the present study, 25 out of 29 COX-2 positive cases showed metastasis to lymph nodes. Of the 21 COX-2 negative cases, 11 cases were node positive and 10 cases were node negative. There was a significant correlation between COX-2 positivity and lymph node involvement as indicated by a P value of 0.01. These findings were in concordance with the studies done by Rozenowicz et al., (2012) and Dannenberg and Howe (2003). However, Costa et al., (2002) and Misron et al., (2015) showed that there was no significant correlation between COX-2 positivity and node status. Correlation between lymph node positivity and higher COX-2 expression suggests its association with tumor spread and a poor prognosis.
In our study, no significant correlation was found between COX-2 status and estrogen receptor status (P value = 0.74), progesterone receptor status (P value = 0.91) or HER-2-neu expression (P value = 0.74). Our finding is in accordance to the study done by Lee (2010). However, studies done by some authors (Rozenowicz et al., 2012; Jana et al., 2014) showed a negative correlation of COX-2 expression with ER and PR expression and positive correlation with HER2neu expression. Ristimaki (2002) suggested that elevated COX-2 expression in ER-positive cancers could be due to the enhancement of microenvironment for cancer cells to grow by inducing estrogen production. Nam (2005) opined that HER-2-neu is known to be a poor prognostic factor in breast cancer and so is the expression of COX-2, thus a positive correlation between the two implies poor prognosis.
We were unable to derive any significant correlation between COX-2 expression and triple negative breast cancer in our study. However, Misron et al., (2015) and Mosalpuria et al., (2014) stated that COX-2 protein is overexpressed in triple negative breast cancer patients. Since the number of triple negative cases was very few in our study they may not be the exact representation of this group and hence there was no correlation.
In the present research a correlation between COX-2 expression and the histological grade of the tumor was not deduced. Our findings are in accordance with the findings of Costa et al., (2002) and Lee et al., (2010). However, they are discordant with the study done by Jana et al., (2014), who found a significantly positive correlation between COX-2 positivity and a higher tumor grade with a P value of <0.01. Tumor grade holds a high prognostic value as a marker of poor prognosis, higher expression of COX-2 in higher histologic grade suggests the same.
We found a highly significant correlation between the COX-2 expression and the presence of lymphovascular invasion (P value <0.001). These findings correlate with the findings of the study done by Muhammad et al., (2013), who stated that this positive correlation indicates aggressive biological behaviour. However, studies done by Misron et al., (2015) and Davies et al., (2003) obtained a non significant P value and no correlation between COX-2 expression and lymphovascular invasion.
The expression of COX-2 in the adjoining normal epithelium in our study was 38%. In the other studies, the range of COX-2 expression in the adjoining normal epithelium varies between 0% and 81%. We demonstrated COX-2 positivity in 77.8% of the DCIS cases and 58% of IDC. These findings are in concordance with many authors Half et al., (2002), Koki et al., (2002) and Boland et al., (2004). The higher frequency of COX-2 in DCIS versus invasive cancer suggests that up-regulation of COX-2 is a relatively early event in mammary carcinogenesis and thus has a role in tumorigenesis.
In this study we could determine the pattern of expression of COX-2 in breast carcinoma in the Indian population for the first time.
The results of our study thus suggest an association of the expression of COX-2 to the factors associated with poor prognosis in breast cancer, such as larger tumor size, positive lymph node status, higher T stage and N stage and lymphovascular invasion. There was a higher COX-2 expression in the DCIS component as compared to the invasive ductal carcinoma component and the adjoining breast epithelium. Further studies are required to ascertain the definite prognostic role of COX-2 in correlation with multiple other factors. Such studies will be helpful to assess the role of selective COX-2 inhibitors in not just the treatment but also in the chemoprevention of human breast cancer.
Funding Statements
Nil.
Acknowledgements
Nil.
References
- 1.Arun B, Goss P. The role of COX-2 inhibition in breast cancer treatment and prevention. Semin Oncol. 2004;31:22–9. doi: 10.1053/j.seminoncol.2004.03.042. [DOI] [PubMed] [Google Scholar]
- 2.Boland G, Butt I, Prasad R, Knox W, Bundred N. COX-2 expression is associated with an aggressive phenotype in ductal carcinoma in situ. Br J Cancer. 2004;90:423–9. doi: 10.1038/sj.bjc.6601534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brueggemeier R, Richards J, Petrel T. Aromatase and cyclooxygenases: enzymes in breast cancer. J Steroid Biochem Mol Biol. 2003;86:501–7. doi: 10.1016/s0960-0760(03)00380-7. [DOI] [PubMed] [Google Scholar]
- 4.Costa C, Soares R, Reis-Filho J, et al. Cyclo-oxygenase 2 expression is associated with angiogenesis and lymph node metastasis in human breast cancer. J Clin Pathol. 2002;55:429–34. doi: 10.1136/jcp.55.6.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dannenberg AJ, Howe LR. The role of cox-2 in breast and cervical cancer. Prog Exp Tum Res. 2003;37:90–106. doi: 10.1159/000071368. [DOI] [PubMed] [Google Scholar]
- 6.Davies G, Salter J, Hills M, et al. Correlation between cyclooxygenase-2 expression and angiogenesis in human breast cancer. Clin Cancer Res. 2003;9:2651–6. [PubMed] [Google Scholar]
- 7.Díaz-Cruz E, Shapiro C, Brueggemeier R. Cyclooxygenase inhibitors suppress aromatase expression and activity in breast cancer cells. J Clin Endocrinol Metab. 2005;90:2563–70. doi: 10.1210/jc.2004-2029. [DOI] [PubMed] [Google Scholar]
- 8.Half E, Tang X, Gwyn K, Sahin A. Cyclooxygenase-2 expression in human breast cancers and adjacent ductal carcinoma in situ. Cancer Res. 2002;62:1676–81. [PubMed] [Google Scholar]
- 9.Jana D, Sarkar D, Ganguly S, et al. Role of cyclooxygenase 2 (COX-2) in prognosis of breast cancer. Indian J Surg Oncol. 2014;5:59–65. doi: 10.1007/s13193-014-0290-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Koki A, Khan N, Woerner B, et al. Characterization of cyclooxygenase-2 (COX-2) during tumorigenesis in human epithelial cancers: evidence for potential clinical utility of COX-2 inhibitors in epithelial cancers. Prostaglandins Leukot Essent Fatty Acids. 2002;66:13–8. doi: 10.1054/plef.2001.0335. [DOI] [PubMed] [Google Scholar]
- 11.Lee J, Bae J, Woo S, Kim H, Kim C. Correlation between COX-2 expression and hormone receptors in invasive ductal breast cancer. J Korean Surg Soc. 2010;78:140. [Google Scholar]
- 12.Misron N, Looi L, Mustapha N. Cyclooxygenase-2 expression in invasive breast carcinomas of no special type and correlation with pathological profiles suggest a role in tumorigenesis rather than cancer progression. Asian Pac J Cancer Prev. 2015;16:1553–8. doi: 10.7314/apjcp.2015.16.4.1553. [DOI] [PubMed] [Google Scholar]
- 13.Mosalpuria K, Hall C, Krishnamurthy S, et al. Cyclooxygenase-2 expression in non-metastatic triple-negative breast cancer patients. Mol Clin Oncol. 2014;2:845–50. doi: 10.3892/mco.2014.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Muhammad E, Edin H, Guirguis M, Osman S. Immunohistochemical cyclooxygenase-2 (COX-2) and P53 expression in breast carcinoma with correlation to clinico-pathological parameters. Med J Cairo Univ. 2013;81:253–66. [Google Scholar]
- 15.Nam E, Lee S, Im S, et al. Expression of cyclooxygenase-2 in human breast cancer: Relationship with HER-2/neu and other clinicopathological prognostic factors. Cancer Res Treat. 2005;37:165. doi: 10.4143/crt.2005.37.3.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pakkiri P, Lakhani S, Smart C. Current and future approach to the pathologist's assessment for targeted therapy in breast cancer. Pathology. 2009;41:89–99. doi: 10.1080/00313020802563551. [DOI] [PubMed] [Google Scholar]
- 17.Richards J, Petrel T, Brueggemeier R. Signaling pathways regulating aromatase and cyclooxygenases in normal and malignant breast cells. J Steroid Biochem Mol Biol. 2002;80:203–12. doi: 10.1016/s0960-0760(01)00187-x. [DOI] [PubMed] [Google Scholar]
- 18.Ristimaki A, Sivula A, Lundin J, Lundin M. Prognostic significance of elevated cyclooxygenase-2 expression in breast cancer. Cancer Res. 2002;62:632–5. [PubMed] [Google Scholar]
- 19.Ross J, Slodkowska E, Symmans W, et al. The HER-2 receptor and breast cancer: Ten years of targeted anti-HER-2 therapy and personalized medicine. Oncologist. 2009;14:320–68. doi: 10.1634/theoncologist.2008-0230. [DOI] [PubMed] [Google Scholar]
- 20.Rozenowicz R, Santos R, Rodrigues F, et al. Cox-2 and its association with prognostic factors and response to neoadjuvant chemotherapy in patients with breast cancer. Eur J Surg Oncol. 2012;38:803. doi: 10.1590/s0100-69912010000500003. [DOI] [PubMed] [Google Scholar]
- 21.Singh Ranger G, Jewell A, Thomas V, Mokbel K. Elevated expression of cyclooxygenase-2 in breast cancer and ductal carcinoma in situ has no correlation with established prognostic markers. J Surg Oncol. 2004;88:100–3. doi: 10.1002/jso.20142. [DOI] [PubMed] [Google Scholar]
- 22.Williams C, DuBois R. Prostaglandin endoperoxide synthase: why two isoforms? Am J Physiol Gastrointest Liver Physiol. 1996;270:393–400. doi: 10.1152/ajpgi.1996.270.3.G393. [DOI] [PubMed] [Google Scholar]


