Abstract
Background
Ovarian cancer has been regarded as most deadly gynaecological cancer in the world. In Oman, ovarian cancer is the third most prevalent gynaecological cancer affecting Omani women. The awareness of risk factors, symptoms and seeking early medical care play a role in the improvement of survival rates. The aim of this study is to explore knowledge, risk factors, symptoms and the time taken to seek early medical help for ovarian cancer among Omani women attended Sultan Qaboos University Hospital.
Methods
The ovarian Cancer Awareness and Measure (CAM) questionnaire (translated into Arabic) was used to collect data.
Results
A total of 499 women participated. The most recognised risk factors were having ovarian cysts (71.3%), smoking (67.5%) and having close relative with ovarian cancer (63.5%); the least recognised were having in vitro fertilization (25.5%), having children (26.3%) and using talcum powder in the genital area (31.5%). The most recognised symptoms were persistent pain in the pelvis (67.7%), persistent pain in the abdomen (60.3%) and extreme fatigue (56.5%); the least recognised were feeling full persistently (22.8%), passing more urine than usual (31.1%) and changes in bowel habits (32.1%). Multinomial logistic regression showed recognition of risk factors and symptoms were associated with a higher level of education, a higher income, increased age, higher number of pregnancies, a longer duration of marriage and having a family history of ovarian cancer. Most of the barriers to seeking medical help were for several emotional, practical and healthcare service reasons.
Conclusion
The overall level of recognition of risk factors and symptoms of ovarian cancer among Omani women were low with several emotional, practical and service barriers preventing them from seeking early medical help. More measures to raise national cancer awareness levels are needed, and support for women to overcome these barriers to minimized delays in the presentation.
Keywords: Ovarian cancer, symptoms, awareness, Barriers, Oman
Introduction
Ovarian cancer has been regarded as the most deadly cancer, a “silent killer”, which is responsible for more deaths than any other gynaecological cancer worldwide (Office for national stastistics, 2012; Permuth-Wey and Sellers, 2009). In 2008, ovarian cancer accounted for 4% of all cancers in women, with over 225,500 new diagnoses each year and 140,200 deaths among the female population (Jemal et al., 2011). The symptoms of ovarian cancer are present in both early and late stages of the disease, with a better prognosis if diagnosed at an earlier stage (Goff etal., 2000). However, only 30% of women are diagnosed in the early stages of the disease (Colombo et al., 2006).
The high mortality rates associated with ovarian cancer are attributed to the non-specific nature of symptoms, such as bloating, lower back pain, pelvic pain and abdominal pain (Olson et al., 2001). Therefore, the potentially serious symptoms may not be recognised by patients or they might be ye or misinterpreted as symptoms of benign diseases, such as irritable bowel syndrome, ageing and stress (Low et al., 2013). A study has been conducted in the UK showed that most women with ovarian cancer presented initially to their general practitioner and half of them were having symptoms for more than a month (Hamilton et al., 2009).
Screening for ovarian cancer has not been proved to decrease the death rate and, therefore, there is no standard routine screening or test even for high risk ovarian cancer patients such as patients with inherited genetic syndrome (Buys et al., 2011). However, women with family history of ovarian cancer or familial ovarian cancer syndromes are at a higher risk of developing ovarian cancer (Permuth-Wey and Sellers, 2009). The other risk factors of ovarian cancer include infertility, endometriosis, postmenopausal hormone-replacement therapy, cigarette smoking and alcohol consumption. On the other hand, increased parity, using oral contraceptives, oophorectomy and breast feeding were found to decrease the risk of ovarian cancer (Hunn and Rodriguez, 2012).
In the developed countries, the incidence of ovarian cancer is high and this increases with age (Siegel et al., 2014). However, the majority of deaths from cancer generally occur in developing countries, mainly due to the late-stage presentation, delay in timely diagnosis, poor availability of tests or screening and limited access to standard cancer treatments (Jassem et al., 2013; Jemal et al., 2011). Poor public knowledge of early symptoms of cancer have been considered to be the predominant reason for late-stage presentation or delay in the diagnosis (Macleod et al., 2009).
In Oman, ovarian cancer was the fourth most commonly diagnosed cancer affecting Omani women after breast cancer, thyroid cancer and leukaemia between 1998 and 2012 with total of 130 cancer diagnoses recorded during this period (Ministry of Health, 2013). In 2013, ovarian cancer became the third most commonly diagnosed gynecological cancer after breast and cervical cancer, with a total of 24 Omani women diagnosed with ovarian cancer (Ministry of Health, 2013). Like other developing countries, women in Oman may present with advanced stages of cancer at the time of diagnosis (Kumar et al., 2011).
Studies conducted in Oman showed that general awareness of risk factors and symptoms of cancer were low among the public (Al-Azri et al., 2014; Al-Azri et al., 2016; Al-Azri et al., 2017). There were also other social and cultural barriers in Oman which contribute to the delay in the diagnosis of cancer, particularly among certain groups of women, such as unmarried, widowed, divorced, or separated (Al-Azri et al., 2016).
Many studies worldwide have shown that public awareness levels of ovarian cancer were poor and women are often diagnosed at late stages, when treatment is difficult (Lockwood-Rayermann et al., 2009). Improved Omani women awareness of risk factors, recognition of symptoms and seeking early medical help could improves the prognosis and decrease death from ovarian cancer. To our knowledge, there were no previous studies conducted in Oman to identify the level of awareness of Omani women for ovarian cancer. The aim of this study is, therefore, to determine the levels of awareness among Omani women for risk factors, symptoms and barriers to seek early medical for ovarian cancer.
Materials and Methods
The ovarian Cancer Awareness and Measure (CAM) questionnaire is a site-specific version of the generic CAM, developed in the UK, which was validated for use as a survey questionnaire, by telephone, face-to-face, online or to be “self-completed” (Simon et al., 2012; Cancer Research UK, 2011). The ovarian CAM includes questions which measure the awareness levels of ovarian cancer for risk factors (12 items), warning signs (11 items), barriers to seek medical help (10 items) and the anticipated time to seek medical help (11 items) (Cancer Research UK, 2011). The internal reliability of the ovarian CAM (Cronbach’s α = 0.88) and test-retest reliability (r = 0.84) were found to be high in a previously validated study conducted in the UK (Simon, et al., 2012). The ovarian CAM questionnaire has been used also in a previous study (Low et al., 2013).
The socio-demographic variables section of the ovarian CAM was modified to be relevant to the Omani population. The ovarian CAM was translated from English to Arabic and back-translated into English by several people proficient in both languages. Based on the standardized items, the Cronbach’s alpha of the Arabic version of the ovarian CAM for the first recruited 50 participants was found to be high (Cronbach’s α = 0.90).
Setting of the study
The Sultan Qaboos University Hospital (SQUH) is tertiary teaching and training hospital for undergraduate medical students and for postgraduate clinicians of various medical, surgical specialities and subspecialties. The hospital is located in Muscat governorate, the capital city of Oman, which receives patients from primary care health centres and also from secondary general hospitals from all over Oman.
SQUH was chosen to collect the data as data could be collected from heterogeneous groups of women, most of them from different regions of Oman.
Recruitment of participants
This cross-sectional study has been conducted from 1st December 2016 to 31st March 2017. Two female medical students were trained on how to use the ovarian CAM questionnaire, in order to distribute and collect data through face to face interview from the study’s participants. All adult women and attendees (age >18 years) attending the out-patients’ clinics of SQUH during the study period were invited to participate in the study. The aim of the study was explained to the women by the medical students. Women who agreed to participate were asked to sign a consent form and the questionnaire was introduced to them by the medical students while waiting to see their doctors for the consultation. Women who were very ill and emergency cases were excluded.
Sample size calculation
There have been no previous studies conducted in Oman to determine the awareness levels of Omani women in terms of risk factors, symptoms and barriers to seeking early medical care, specifically relating to ovarian cancer. For the sample size calculation, we assumed that Omani women had an awareness level of 50%. Thus, with a precision of 5% and a desired confidence level of 95%, we determined that around 400 subjects were needed. However, to overcome missing and poor responses, we decided to involve 550 women in this study.
Data Analysis
Data were analyzed using the Statistical Package for the Social Sciences (SPSS), version 22 (IBM Corp. Chicago, Illinois). For descriptive purposes, categorized variables were presented as numbers and percentages. The Chi-squared test (χ2) was used to find associations between the demographic factors and ovarian cancer symptoms and reported barriers. A binary logistic regression model was used to adjust it for the factors. A P value of <0.05 was considered to be significant. The study was approved by the Medical Research and Ethics Committee of the College of Medicine and Health Sciences, at the Sultan Qaboos University (MREC # 1190).
Results
Socio-demographic characteristics of participants
A total of 499 women participated in this study, from a total of 550 invited (response rate = 90.7%). Their ages ranged from 18-68 years. The overall mean age of the participants was 30.5 ± 8.6 years; the majority (61.9%) of the participants were married, and more than half of them had completed university and postgraduate education (64.9%). A small subset of participants (26.4%) had family history of cancer (Table 1).
Table 1.
Socio-Demographic Variables of Participants
| Variables | n | % |
|---|---|---|
| Age in years (n = 499) | ||
| 18-25 | 155 | 31.1 |
| 26-30 | 137 | 27.5 |
| >30 | 207 | 41.5 |
| Marital status (n = 499) | ||
| Single | 171 | 34.3 |
| Married | 309 | 61.9 |
| Divorced/Widowed | 19 | 3.8 |
| Education (n = 499) | ||
| No formal education | 63 | 12.6 |
| Secondary education | 112 | 22.4 |
| University | 298 | 59.7 |
| Postgraduate | 26 | 5.2 |
| Smoking status (n = 499) | ||
| Was, but stopped | 9 | 1.8 |
| No | 490 | 98.2 |
| Family income in OMR (n = 494) | ||
| <500 | 71 | 14.2 |
| 500–1,000 | 211 | 42.3 |
| 1,000–2,000 | 151 | 30.3 |
| >2,000 | 61 | 12.2 |
| Duration of marriage (n = 326) | ||
| 1-5 years | 116 | 35.6 |
| 6-11 years | 104 | 31.9 |
| 16-20 years | 44 | 13.5 |
| >20 years | 62 | 19 |
| Number of pregnancies (n = 444) | ||
| 0 | 163 | 36.7 |
| 1 | 47 | 10.6 |
| 2–4 | 154 | 34.7 |
| >4 | 80 | 18 |
| Family history of cancer (n = 489) | ||
| Yes | 129 | 26.4 |
| No | 360 | 73.6 |
Recognition of ovarian cancer risk factors
The most recognised risk factors for ovarian cancer were having ovarian cysts (71.3%), followed by smoking (67.5%), having a close relative with ovarian cancer (63.5%), having a past history of breast cancer (57.3%), having endometriosis (50.2%), being over 50-years-old (43.4%), using Hormone Replacement Therapy (HRT) (42.9%), being overweight (a BMI over 25) (37.9%), having gone through the menopause (34.7%), using talcum powder in the genital area (31.5%), not having children (26.3%) and having in vitro fertilization (IVF) (25.5%) (Table 2).
Table 2.
Risk Factors Agreements of Ovarian Cancer
| Statements | Agree n (%) | Not sure n (%) | Disagree n (%) |
|---|---|---|---|
| Having ovarian cysts | 356 (71.3) | 105 (21.0) | 38 (7.6) |
| Smoking | 337 (67.5) | 99 (28) | 63 (12.6) |
| Having a close relative with ovarian cancer | 317(63.5) | 116 (23.2) | 66 (13.2) |
| Having a past history of breast cancer | 286 (57.3) | 140 (28.1) | 73 (14.6) |
| Having endometriosis | 250 (50.2) | 210 (42.2) | 38 (7.6) |
| Being over 50 years old | 216 (43.4) | 168 (33.7) | 114 (22.9) |
| Using HRT (Hormone Replacement Therapy) | 213 (42.9) | 230 (46.4) | 53 (10.7) |
| Being overweight (BMI >25) | 189 (37.9) | 216 (43.3) | 94 (18.8) |
| Having gone through the menopause | 173 (34.7) | 197 (39.6) | 128 (25.7) |
| Using talcum powder in the genital area | 157 (31.5) | 273 (54.7) | 69 (13.8) |
| Not having children | 131 (26.3) | 207 (41.5) | 161 (32.2) |
| Having IVF treatment | 127 (25.5) | 264 (52.9) | 107 (21.5) |
In the multivariate analyses, after adjusting for other variables, women with a secondary level of education were more likely than women who were literate alone to recognise the following as risk factors for ovarian cancer: “being overweight (BMI over 25)” (OR = 3.40; 95% CI: 1.28-8.99), “having endometriosis” (OR = 3.58; 95% CI: 1.35-9.51), “having ovarian cysts” (OR = 2.77; 95% CI: 1.14-6.75) and “not having children” (OR = 3.32; 95% CI:1.01-1097). Also, women with a university education were more likely than literate women to recognise the following as risk factors for ovarian cancer: “being overweight (BMI over 25)” (OR = 3.62; 95% CI:1.34-9.80), “having endometriosis” (OR = 7.12; 95% CI:2.61-19.44) and “having ovarian cysts” (OR = 2.89; 95% CI: 1.16-7.15). Also, women with a postgraduate education were more likely than literate women to recognise “having endometriosis” as a risk factor for ovarian cancer (OR = 3.93; 95% CI: 1.04-14.82) (Table 3).
Table 3.
Recognition of Risk Factors of Ovarian Cancer by Socio-Demographic Variables
| Variables | Odds ratio of recognition of risk factors (95% CI) | |||||
|---|---|---|---|---|---|---|
| Having a close relative with ovarian cancer | Having a past history of breast cancer | Using HRT (Hormone Replacement Therapy) | Being overweight (BMI >25) | Having endometriosis | Having ovarian cysts | |
| Age group | ||||||
| Up to 30 years | 1 | 1 | 1 | 1 | 1 | 1 |
| > 30 years | 1.10 (0.60-2.03) | 0.94 (0.54-1.78) | 1.3 (0.68-2.53) | 0.63 (0.34-1.17) | 1.52 (0.83-2.80) | 1.14 (0.60-2.16) |
| Education | ||||||
| No formal | 1 | 1 | 1 | 1 | 1 | 1 |
| Secondary | 0.76 (0.32-1.78) | 1.99 (0.85-4.66) | 0.79 (0.29-2.18) | 3.40 (1.28-8.99)* | 3.58 (1.35-9.51)* | 2.77 (1.14-6.75)* |
| University | 0.82 (0.35-1.97) | 1.71 (0.72-4.05) | 1.75 (0.65-4.72) | 3.62 (1.34-9.80)* | 7.12 (2.61-19.44)* | 2.89 (1.16-7.15)* |
| PG/Others | 1.06 (0.29-3.84) | 0.94 (0.28-3.18) | 1.41 (0.37-5.44) | 3.11 (0.83-11.61) | 3.93 (1.04-14.82)* | 2.62 (0.73-9.37) |
| Family income (OMR) | ||||||
| <500 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 |
| 500 – 1,000 | 1.93 (0.76-4.89) | 2.37 (0.97-5.81) | 2.0 (0.59-6.79) | 0.53 (0.21-1.33) | 1.45 (0.57-3.67) | 1.94 (0.78-4.82) |
| 1,000 – 2,000 | 3.30 (1.23-8.88)* | 4.37 (1.67-11.45)* | 7.14 (2.04-24.95)* | 0.86 (0.33-2.26) | 0.76 (0.28-2.04) | 1.21 (0.46-3.18) |
| > 2,000 | 3.96 (1.20-13.02)* | 3.59 (1.15-11.23)* | 5.63 (1.37-23.16)* | 0.58 (0.19-1.85) | 0.72 (0.23-2.30) | 0.58 (0.18-1.85) |
| Duration of marriage | ||||||
| 1-5 years | 1 | 1 | 1 | 1 | 1 | 1 |
| 6-11 years | 0.82 (0.42-1.59) | 0.84 (0.44-1.60) | 0.53 (0.26-1.08) | 0.89 (0.46-1.73) | 1.19 (0.62-2.28) | 1.28 (0.64-2.55) |
| 16-20 years | 1.62 (0.59-4.43) | 0.83 (0.32-2.18) | 0.72 (0.24-2.12) | 1.57 (0.59-4.23) | 1.03 (0.38-2.80) | 1.23 (0.44-3.48) |
| >20 years | 0.72 (0.26-2.06) | 0.85 (0.30-2.38) | 0.97 (0.31-3.01) | 2.06 (0.71-5.97) | 2.37 (0.81-6.95) | 2.37 (0.74-7.60) |
| No. of pregnancies | ||||||
| 0 | 1 | 1 | 1 | 1 | 1 | 1 |
| 1 | 0.23 (0.09-0.54)* | 0.67 (0.28-0.64) | 0.61 (0.23-1.61) | 0.52 (0.20-1.36) | 1.30 (0.52-3.26) | 0.29 (0.12-0.71)* |
| 2-4 | 0.74 (0.34-1.61) | 0.62 (0.29-1.33) | 0.96 (0.44-2.09) | 1.76 (0.83-3.74) | 2.20 (1.03-4.70)* | 1.12 (0.51-2.46) |
| >4 | 0.32 (0.11-0.93)* | 0.45 (0.16-1.28) | 0.26 (0.09-0.81) | 1.03 (0.37-2.92) | 1.21 (0.43-3.43) | 0.53 (0.18-1.59) |
| Family history | ||||||
| Yes | 0.94 (0.55-1.61) | 1.32 (0.78-2.22) | 1.20 (0.68-2.11) | 1.23 (0.73-2.09) | 1.49 (0.87-2.53) | 1.23 (0.71-2.14) |
| No | 1 | 1 | 1 | 1 | 1 | 1 |
| Using talcum powder in the genital area | Being over 50 years old | Having IVF treatment | Not having children | Having gone through the menopause | Being a smoker | |
| Age group | ||||||
| Up to 30 years | 1 | 1 | 1 | 1 | 1 | 1 |
| > 30 years | 0.98 (0.51-1.88) | 1.08 (0.58-2.02) | 1.32 (0.65-2.70) | 1.40 (0.72-2.71) | 3.58 (1.86-6.90) | 0.78 (0.43-1.45) |
| Education | ||||||
| No formal | 1 | 1 | 1 | 1 | 1 | 1 |
| Secondary | 2.57 (0.99-6.68) | 1.10 (0.46-2.65) | 0.99 (0.34-2.86) | 3.32 (1.01-1097)* | 0.82 (0.32-2.08) | 1.74 (0.76-3.98) |
| University | 1.43 (0.55-3.72) | 1.01 (0.41-2.48) | 1.63 (0.58-4.54) | 3.10 (0.93-10.31) | 0.90 (0.35-2.36) | 2.47 (1.05-5.80) |
| PG/Others | 0.84 (0.20-3.60) | 0.53 (0.14-1.95) | 1.17 (0.26-5.33) | 1.57 (0.31-8.09) | 0.99 (0.27-3.62) | 2.07 (0.59-7.22) |
| Family income (OMR) | ||||||
| <500 | 1 | 1 | 1 | 1 | 1 | 1 |
| 500 – 1,000 | 3.66 (1.21-11.07)* | 1.17 (0.46-3.01) | 2.30 (0.67-7.89) | 2.10 (0.67-6.57) | 0.75 (0.28-1.97) | 1.62 (0.68-3.86) |
| 1,000 – 2,000 | 4.28 (1.37-13.38)* | 3.76 (1.38-10.23)* | 3.63 (1.01-13.06)* | 2.08 (0.63-6.81) | 0.99 (0.36-2.74) | 2.11 (0.82-5.38) |
| >2,000 | 2.18 (0.56-8.44) | 2.31 (0.71-7.60) | 3.34 (0.77-14.49) | 2.12 (0.54-8.32) | 0.63 (0.19-2.13) | 2.33 (0.74-7.31) |
| Duration of marriage | ||||||
| 1-5 years | 1 | 1 | 1 | 1 | 1 | 1 |
| 6-11 years | 0.90 (0.45-1.78) | 0.56 (0.29-1.09) | 0.73 (0.34-1.58) | 1.07 (0.53-2.18) | 0.59 (0.29-1.18) | 1.40 (0.73-2.71) |
| 16-20 years | 0.63 (0.21-1.93) | 1.60 (0.60-4.26) | 0.32 (0.08-1.18) | 0.87 (0.29-2.60) | 0.65 (0.24-1.80) | 1.89 (0.71-5.00) |
| >20 years | 2.79 (0.93-8.36) | 1.02 (0.35-2.96) | 0.81 (0.24-2.82) | 0.92 (0.29-2.92) | 0.56 (0.19-1.67) | 1.79 (0.62-5.11) |
| No. of pregnancies | ||||||
| 0 | 1 | 1 | 1 | 1 | 1 | 1 |
| 1 | 0.59 (0.22-1.57) | 0.81 (0.33-1.99) | 2.10 (0.80-5.54) | 1.22 (0.44-3.32) | 0.30 (0.11-0.81) | 0.44 (0.17-1.14) |
| 2-4 | 0.73 (0.34-1.59) | 0.83 (0.39-1.75) | 0.61 (0.26-1.43) | 1.03 (0.45-2.35) | 0.93 (0.44-1.97) | 0.39 (0.17-0.92) |
| >4 | 0.54 (0.18-1.61) | 0.32 (0.11-0.93) | 1.08 (0.33-3.57) | 0.77 (0.25-2.39) | 0.28 (0.10-0.81) | 0.28 (0.09-0.85) |
| Family history | ||||||
| Yes | 1.49 (0.86-2.59) | 1.53 (0.90-2.60) | 1.08 (0.59-1.97) | 2.04 (1.17-3.56)* | 1.10 (0.63-1.91) | 0.83 (0.49-1.42) |
| No | 1 | 1 | 1 | 1 | 1 | 1 |
p < 0.05
Women from the higher income group (>1000 Omani riyal [OMR]) were more likely than women from the lower income group (<500 OMR) to recognise the following as risk factors for ovarian cancer: “having a close relative with ovarian cancer”(OR = 3.30; 95% CI:1.23-8.88), “having a past history of breast cancer” (OR = 4.37; 95% CI:1.67-11.45), “using Hormone Replacement Therapy” (OR = 7.14; 95% CI:2.04-24.95), “using talcum powder in the genital area” (OR = 4.28; 95% CI: 1.21-11.07), “being over 50 years old” (OR = 3.76; 95% CI: 1.38-10.23) and “having IVF treatment” (OR = 3.63; 95% CI: 1.01-13.06) (Table 3).
Women who had one and more pregnancies were more likely than women who had never been pregnant to recognise the following as ovarian cancer: “having a close relative with ovarian cancer” (OR = 0.32; 95% CI: 0.11-0.93), “having endometriosis” (OR = 2.20; 95% CI:1.03-4.70), “having ovarian cysts” (OR = 0.29; 95% CI:0.12-0.71). Women with a family history of cancer were more likely than women without a family history of cancer to recognise “not having children” as a risk factor for ovarian cancer (OR = 2.04; 95% CI: 1.17-3.56) (Table 3).
Recognition of ovarian cancer symptoms
The recognition of ovarian cancer symptoms varied from 22.8%-67.7%. The most frequently recognised ovarian cancer symptoms were persistent pain in the pelvis (67.7%), followed by persistent pain in the abdomen (60.3%), extreme fatigue (56.5%), persistent bloating (52.5%), back pain (52.3%), increased abdominal size (47.6%), postmenopausal bleeding (44.8%), difficulty eating most days (37.5%), changes in bowel habits (32.1%), passing more urine than usual (31.1%) and feeling full persistently (22.8%) (Figure 1).
Figure 1.
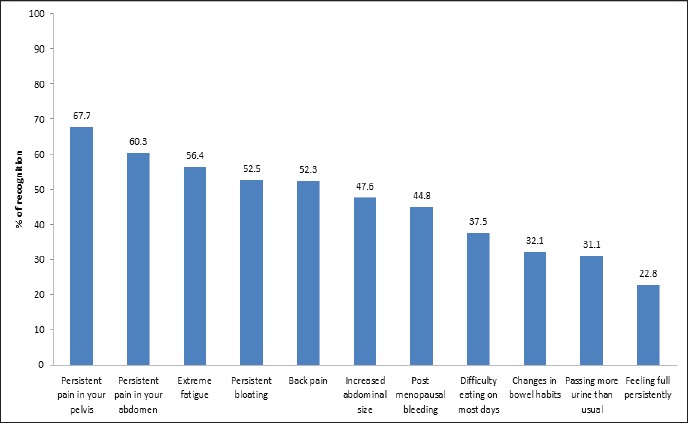
Graph Showing Percentage of Distribution of Recognition of Ovarian Cancer Symptoms Amongst Participants
In the multivariate analyses, after adjusting for other variables, women who were more than 30-years-old were more likely than women who were less than 30-years-old to recognise “postmenopausal bleeding” as a symptom of ovarian cancer (OR = 2.81; 95% CI:1.48-5.32). Women with a higher income (>2000 OMR) were more likely than women with a lower income (<500 OMR) to recognise “passing more urine than usual” as a symptom of ovarian cancer (OR = 5.26; 95% CI: 1.15-24.10) (Table 4)
Table 4.
Recognition of Ovarian Cancer Symptoms by Socio-Demographic Variables
| Variables | Odds ratio of recognition of signs and symptoms (95% CI) | |||||
|---|---|---|---|---|---|---|
| Persistent pain in your abdomen | Persistent pain in your pelvis | Persistent bloating | Increased abdominal size | feeling full persistently | ||
| Age group | ||||||
| Up to 30 years | 1 | 1 | 1 | 1 | 1 | |
| > 30 years | 1.03 (0.57-1.88) | 1.55 (0.80-3.01) | 1.11 (0.59-2.07) | 1.01 (0.56-1.83) | 1.13 (0.56-2.28) | |
| Education | ||||||
| No formal | 1 | 1 | 1 | 1 | 1 | |
| Secondary | 2.13 (0.91-5.0) | 3.03 (1.26-7.30)* | 1.53 (0.63-3.74) | 2.07 (0.88-4.86) | 0.73 (0.23-2.30) | |
| University | 2.91 (1.22-6.96)* | 4.01 (1.61-10.02)* | 1.57 (0.64-3.85) | 1.36 (0.57-3.25) | 1.68 (0.55-5.14) | |
| Postgraduate | 3.37 (1.0-11.70)* | 3.08 (0.80-11.81) | 1.07 (0.30-3.79) | 1.8 (0.54-6.31) | 1.60 (0.36-7.23) | |
| Family income (OMR) | ||||||
| <500 | 1 | 1 | 1 | 1 | 1 | |
| 500–1,000 | 1.11 (0.46-2.67) | 1.42 (0.56-3.61) | 1.49 (0.58-3.80) | 0.94 (0.39-2.26) | 0.62 (0.21-1.86) | |
| 1,000–2,000 | 1.42 (0.56-3.60) | 1.60 (0.59-4.33) | 2.39 (0.89-6.47) | 1.40 (0.55-3.57) | 0.90 (0.28-2.85) | |
| >2,000 | 0.90 (0.30-2.76) | 1.26 (0.37-4.24) | 2.76 (0.84-9.01) | 1.65 (0.54-5.07) | 0.74 (0.19-2.92) | |
| Duration of marriage | ||||||
| 1-5 years | 1 | 1 | 1 | 1 | 1 | |
| 6-11 years | 0.52 (0.28-1.0)* | 0.30 (0.15-0.62)* | 0.25 (0.13-0.51)* | 0.76 (0.40-1.43) | 0.79 (0.38-1.67) | |
| 16-20 years | 0.66 (0.25-1.73) | 0.40 (0.14-1.17) | 0.12 (0.04-0.35)* | 0.37 (0.14-0.98)* | 0.70 (0.22-2.25) | |
| >20 years | 0.37 (0.13-1.06) | 0.39 (0.12-1.26) | 0.31 (0.11-0.91)* | 0.71 (0.25-1.98) | 0.69 (0.19-2.54) | |
| No. of pregnancies | ||||||
| 0 | 1 | 1 | 1 | 1 | 1 | |
| 1 | 0.82 (0.34-1.97) | 0.73 (0.27-1.97) | 1.96 (0.78-4.96) | 1.12 (0.47-2.66) | 1.48 (0.54-4.03) | |
| 2-4 | 1.07 (0.50-2.26) | 1.11 (0.47-2.63) | 2.94 (1.32-6.54) | 1.05 (0.50-2.17) | 1.16 (0.49-2.73) | |
| >4 | 1.92 (0.69-5.39) | 1.53 (0.49-4.79) | 1.74 (0.59-5.09) | 0.54 (0.19-1.49) | 0.73 (0.21-2.60) | |
| Family history | ||||||
| Yes | 0.93 (0.55-1.56) | 0.87 (0.49-1.53) | 0.99 (0.58-1.72) | 1.67 (0.98-2.82) | 1.54 (0.84-2.83) | |
| No | 1 | 1 | 1 | 1 | 1 | |
| Difficulty eating on most days | Passing more urine than usual | Changes in bowl habits | Extreme fatigue | Back pain | Postmenopausal bleeding | |
| Age group | ||||||
| Up to 30 years | 1 | 1 | 1 | 1 | 1 | 1 |
| > 30 years | 1.10 (0.59-2.05) | 1.31 (0.68-2.53) | 0.97 (0.52-1.83) | 1.52 (0.81-2.83) | 1.13 (0.61-2.08) | 2.81(1.48-5.32)* |
| Education | ||||||
| No formal | 1 | 1 | 1 | 1 | 1 | 1 |
| Secondary | 0.99 (0.35-2.82) | 1.34 (0.45-3.94) | 1.82 (0.58-5.68) | 1.05 (0.44-2.49) | 3.30 (1.26-8.48)* | 0.87(0.36-2.09) |
| University | 1.55 (0.55-4.38) | 1.54 (0.53-4.43) | 2.23 (0.72-6.97) | 1.11 (0.46-2.67) | 5.30 (2.03-14.07)* | 1.15(0.47-2.82) |
| PG/Others | 1.82 (0.47-7.13) | 1.07 (0.25-4.55) | 2.06 (0.47-9.04) | 2.58 (0.70-9.51) | 9.14 (2.35-35.60)* | 0.55(0.16-1.94) |
| Family Income (OMR) | ||||||
| <500 | 1 | 1 | 1 | 1 | 1 | 1 |
| 500 – 1,000 | 0.80 (0.30-2.14) | 3.57 (0.92-13.78) | 0.84 (0.31-2.28) | 1.47 (0.60-3.61) | 0.85 (0.34-2.12) | 0.42(0.17-1.06) |
| 1,000 – 2,000 | 0.69 (0.24-1.98) | 2.84 (0.70-11.42) | 0.77 (0.26-2.22) | 1.43 (0.55-3.70) | 1.41 (0.54-3.70) | 0.52(0.20-1.39) |
| > 2,000 | 1.13 (0.34-3.82) | 5.26 (1.15-24.10)* | 2.51 (0.72-8.76) | 1.56 (0.50-4.91) | 1.52 (0.48-4.84) | 0.52(0.16-1.68) |
| Duration of Marriage | ||||||
| 1-5 years | 1 | 1 | 1 | 1 | 1 | 1 |
| 6-11 years | 0.33 (0.17-0.65)* | 0.55 (0.27-1.10) | 0.63 (0.32-1.23) | 0.26 (0.13-0.51)* | 0.69 (0.36-1.33) | 0.27(0.14-0.56) |
| 16-20 years | 0.20 (0.07-5.93)* | 0.18 (0.06-0.59)* | 0.21 (0.06-0.69) | 0.26 (0.09-0.69)* | 0.76 (0.28-2.05) | 0.24(0.09-0.65) |
| >20 years | 0.17 (0.05-0.56)* | 0.28 (0.09-0.89)* | 0.42 (0.13-1.39) | 0.16 (0.56-0.48)* | 0.37 (0.12-1.09) | 0.14(0.05-0.42) |
| No. of pregnancies | ||||||
| None | 1 | 1 | 1 | 1 | 1 | 1 |
| One | 0.89 (0.35-2.25) | 2.32 (0.77-7.0) | 0.67 (0.27-1.69) | 0.41 (0.17-1.01) | 1.41 (0.58-3.46) | 0.92(0.36-2.31) |
| 2-4 | 2.09 (0.96-4.57) | 3.61 (1.42-9.21)* | 0.90 (0.42-1.93) | 0.86 (0.40-1.88) | 0.97 (0.46-2.04) | 4.03(1.82-8.96)* |
| >4 | 1.72 (0.55-5.40) | 4.68 (1.34-16.28)* | 0.44 (0.13-1.45) | 1.17 (0.42-3.27) | 2.31 (0.80-6.6) | 8.35(2.85-24.40)* |
| Family history | ||||||
| Yes | 1.25 (0.71-2.19) | 1.90 (1.09-3.32)* | 2.33 (1.31-4.13)* | 1.10 (0.66-1.86) | 0.94 (0.80-6.64) | 0.79(0.46-1.36) |
| No | 1 | 1 | 1 | 1 | 1 | 1 |
p < 0.05
Women with a secondary education were more likely than literate women to recognise the following as symptoms of ovarian cancer: “persistent pain in the pelvis” (OR = 3.03; 95% CI: 1.26-7.30) and “back pain” (OR = 3.30; 95% CI: 1.26-8.48). Women with a university education were significantly more likely than those who are only literate to recognise the following as symptoms of ovarian cancer: “persistent pain in the abdomen” (OR = 2.91; 95% CI: 1.22-6.96), “persistent pain in the pelvis” (OR = 4.01; 95% CI: 1.61-10.02) and “back pain” (OR = 5.30; 95% CI: 2.03-14.07). Additionally, women with a postgraduate education were significantly more likely than those who are only literate to recognise the following as symptoms of ovarian cancer: “persistent pain in the pelvis” (OR = 3.37; 95% CI: 1.00-11.70) and “back pain” (OR = 9.14; 95% CI: 2.35-35.60) (Table 4).
Women who were married from between 6-11 years were significantly more likely than women who were married less than 6 years to recognise the following as symptoms of ovarian cancer: “persistent pain in the abdomen” (OR = 0.52; 95% CI: 0.28-1.0), “persistent pain in the pelvis” (OR = 0.30; 95% CI: 0.15-0.62), “persistent bloating” (OR = 0.25; 95% CI: 0.13-0.51), “difficulty eating on most days” (OR = 0.33; 95% CI: 0.17-0.65), “extreme fatigue” (OR = 0.26; 95% CI: 0.13-0.51) (Table 4).
Women who were married from between 16-20 years were significantly more likely than women who were married less than 6 years to recognise the following as ovarian cancer symptoms: “persistent bloating” (OR = 0.12; 95% CI: 0.04-0.35), “increased abdominal size” (OR = 0.37; 95% CI: 0.14-0.98), “difficulty eating most days” (OR = 0.20; 95% CI: 0.07-5.93), “passing more urine than usual” (OR = 0.18; 95% CI: 0.06-0.59), “extreme fatigue” (OR = 0.26; 95% CI: 0.09-0.69). Similarly, women who were married for more than 20 years were significantly more likely than women who were married for less than five years to recognise the following ovarian cancer symptoms: “persistent bloating” (OR = 0.31; 95% CI: 0.11-0.91), “difficulty eating most days” (OR = 0.17; 95% CI: 0.05-0.56), “passing more urine than usual” (OR = 0.28; 95% CI: 0.09-0.89) and “extreme fatigue” (OR = 0.16; 95% CI: 0.56-0.48) (Table 4).
Women who had had two to four times pregnancies were more likely than those who had never been pregnant women to recognise the following as ovarian cancer symptoms: “passing more urine than usual” (OR = 3.61; 95% CI: 1.42-9.21) and “postmenopausal bleeding” (OR = 4.03; 95% CI: 1.82-8.96). Also, women who had more than four pregnancies were more likely than those who had never been pregnant to recognise the following as ovarian cancer symptoms: “passing more urine than usual” (OR = 4.68; 95% CI: 1.34-16.28) and “postmenopausal bleeding” (OR = 8.35; 95% CI: 2.85-24.40).
Women with a family history of ovarian cancer were more likely than women without a family history of ovarian cancer to recognise the following symptoms as ovarian cancer symptoms: “passing more urine than usual” (OR = 1.90; 95% CI: 1.09-3.32) and “changes in bowel habits” (OR = 2.33; 95% CI: 1.31-4.13) (Table 4).
Barriers and the anticipated time to seek early medical help
The most common barriers to seeking early medical help were emotional barriers. The most common reasons for emotional barriers were being “too scared”, followed by being “too embarrassed”, and that they “would not feel confident talking with a doctor”. The most common practical barriers were being “too busy”, followed by “too many other things to worry about” and it was ‘‘too difficult to arrange transport”. The most common service barriers were that it was “difficult to make an appointment with doctor” followed by “my doctor was difficult to talk to” and “worry about wasting doctor’s time” (Figure 2).
Figure 2.
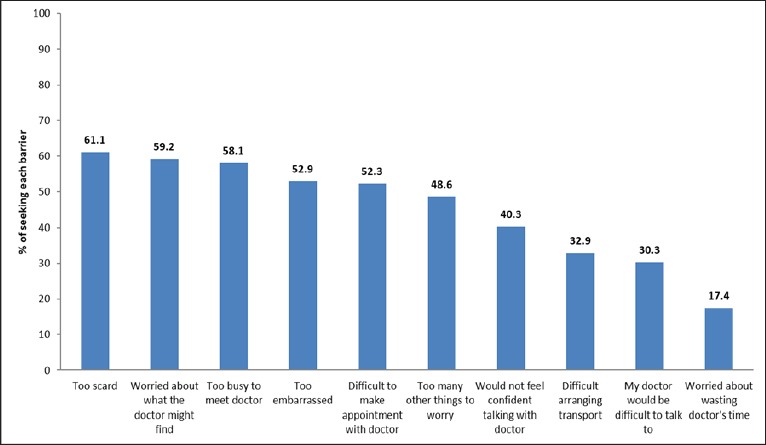
Graph Showing Percentage of Distribution of Reported Barriers to Seek Medical Help Amongst Participants
The anticipated time to seeking early medical help was varied by symptom. The shorter anticipated time of two weeks was noted with symptoms of unexplained vaginal bleeding, post-coital bleeding, postmenopausal bleeding and pain during intercourse. On the other hand, women anticipated a longer time of more than six months for symptoms of excessive or unwanted hair growth, difficulty in eating or feeling full very quickly, unexplained weight loss and loss of appetite (Table 5).
Table 5.
Participants’ Expected Time to Consult a Doctor in Response to Possible Symptoms of Ovarian Cancer
| Variables | Within two weeks n (%) | Within a month n (%) | Within six months n (%) | After six months n (%) | I will not go n (%) |
|---|---|---|---|---|---|
| Unexplained bleeding from the vagina | 462 (92.8) | 19 (3.8) | 10 (2.0) | 3 (0.6) | 4 (0.8) |
| Post-coital bleeding | 385 (77.8) | 47 (9.5) | 23 (4.6) | 8 (1.6) | 32 (6.5) |
| Postmenopausal bleeding | 370 (74.3) | 59 (11.8) | 31 (6.2) | 16 (3.2) | 22 (4.4) |
| Pain during intercourse | 346 (70.2) | 65 (13.2) | 29 (5.9) | 15 (3.0) | 38 (7.7) |
| Abdominal pain | 346 (69.3) | 75 (15.0) | 32 (6.4) | 2 (0.4) | 44 (8.8) |
| Bloating | 335 (67.3) | 78 (15.7) | 29 (5.8) | 11 (2.2) | 45 (9.0) |
| Nausea or vomiting | 330 (66.1) | 89 (17.8) | 36 (7.2) | 14 (2.8) | 30 (6.0) |
| Pelvic pain | 324 (64.9) | 100 (20.0) | 26 (5.2) | 4 (0.8) | 45 (9.0) |
| Lower abdominal heaviness | 323 (64.9) | 87 (17.5) | 45 (9.0) | 15 (3.0) | 28 (5.6) |
| Increased abdominal size | 321 (64.6) | 77 (15.5) | 43 (8.7) | 12 (2.4) | 44 (8.9) |
| Lower back pain | 290 (58.8) | 95 (19.1) | 36 (7.2) | 6 (1.2) | 68 (13.7) |
| Constipation | 297 (58.6) | 100 (20.1) | 40 (8.0) | 24 (4.8) | 42 (8.4) |
| Passing more urine than usual | 280 (56.7) | 98 (19.8) | 52 (10.5) | 20 (4.0) | 44 (8.9) |
| Extreme fatigue | 256 (51.3) | 84 (16.8) | 78 (15.6) | 23 (4.6) | 58 (11.6) |
| Persistent change in bowel habits | 254 (51.2) | 106 (21.4) | 67 (13.5) | 31 (6.3) | 38 (7.7) |
| Loss of appetite | 244 (48.9) | 113 (22.6) | 62 (12.4) | 28 (5.6) | 52 (10.4) |
| Disturbance in menstrual cycle | 231 (46.3) | 106 (21.2) | 114 (22.8) | 35 (7.0) | 13 (2.6) |
| Excessive or unwanted hair growth | 222 (44.7) | 76 (15.3) | 66 (13.3) | 40 (8.0) | 93 (18.7) |
| Difficulty in eating or feeling full very quickly | 212 (42.6) | 101 (20.3) | 85 (17.1) | 28 (5.6) | 72 (14.5) |
| Unexplained weight loss | 205 (41.1) | 113 (22.6) | 84 (16.8) | 24 (4.8) | 73 (14.6) |
Discussion
Ovarian cancer has been regarded as a “silent killer” due to the majority of patients diagnosed at later stages of the disease (60% at stages III/IV) with low 5-year survival rates (6–22%) (Cancer Research UK, 2014). To our knowledge this is the first study conducted in Oman to evaluate the level of awareness among Omani women of ovarian cancer risk factors, symptoms and anticipated time to seek early medical help should they develop suspicious symptoms. The recognition level of ovarian cancer symptoms among Omani women in this study was varied from 22.8-67.7%. Previous studies conducted in several countries, such as USA, Malaysia and Jordon, have shown that knowledge of risk factors of ovarian cancer among women were also low as our study (Freij et al., 2017; Keng et al., 2015; Lockwood-Rayermann et al., 2009).
The most recognised ovarian cancer risks factors among Omani women were having ovarian cysts, smoking and having a close relative with ovarian cancer, and the least recognised were being overweight, getting the menopause and infertility. A previous study conducted in Oman showed that the majority of participants were aware that smoking is a risk factor for cancer though less of the participants identified being overweight as a risk factor (Al-Azri,et al., 2014). In high- and low-income countries, smoking, being overweight and obesity are still among the most common risk factors leading to death due to cancer (Danaei, 2005). The lesser recognised symptoms of being overweight, getting the menopause and infertility as risk factors for ovarian cancer in our study indicates the lack of educational program and awareness in the community to identify the risk factors.
Women in this study with family history of ovarian cancer were more likely to recognise ovarian cancer symptoms, such as “passing more urine than usual” and “changes in bowel habits”, than women without a family history of cancer. Having a family history of cancer has been found to be the single greatest risk factor for ovarian cancer; hence, women who have a family history of breast or ovarian cancer are at the highest risk (Antoniou et al., 2003). Indeed, increasing women’s awareness of risk factors might help in reducing delays in diagnosing ovarian cancer (Lockwood-Rayermann et al., 2009).
The most recognised ovarian cancer symptoms among Omani women were persistent pain in the pelvis and abdomen, extreme fatigue and persistent bloating; the least recognised symptoms were difficulty eating, changes in bowel habits, frequent urination and feeling full persistently. A previous study conducted in Oman to measure the public’s awareness of the most common cancer symptoms showed that the most frequently recognised symptoms were “unexplained lump/swelling” and “persistent unexplained pain” (Al-Azri, et al., 2016).
Symptoms of bloating, increased abdominal size, pelvic or abdominal pain, difficulty eating or feeling full quickly, and frequent or urgent urination have been categorized as ovarian cancer index symptoms that are significantly associated with ovarian cancer (Goff et al., 2007). Nevertheless, despite women recognising these symptoms as potential ovarian cancer symptoms, which could lead to nearly diagnosis, the majority of women are still diagnosed at later stages (Cancer Research UK UK, 2014). The reasons for this might be because ovarian cancer is uncommon, on average a general practitioner may only see one patient with ovarian cancer every five years, reducing the likelihood of considering the diagnosis (Choices, 2012).
It has also been noted that there is no association between symptom awareness and anticipated time for early help-seeking, as some women might worry about seeking early medical help because they might be “scared of cancer” or “worried about what the doctor might find” (Al-Azri et al., 2017). Also, although, some symptoms of ovarian cancer, such as pain, bloating, abnormal bleeding are better recognised by women, a more accurate recognition of vague and non-specific symptoms, such as eating difficulties and changes in bowel or bladder habits, are difficult to recognise and are often mistaken for benign conditions, contributing to the overall late presentation of cancer patients (Smith and Anderson, 1985).
The role of education in increasing awareness among women of risk factors and symptoms of ovarian cancer in this study confirms findings from earlier studies conducted in Oman, which showed that people with higher levels of education recognized cancer symptoms better than those who were literate alone or less educated (Al-Azri et al., 2016; Al-Azri et al., 2017). Indeed, previous studies have shown that a low level of education is associated with a delayed presentation among breast and colon cancer patients, whereas those with a higher level of education showed a greater knowledge of early symptoms (McCaffery et al., 2003, Macleod et al., 2009).
The observed positive association between education level and awareness of ovarian cancer risk factor and symptoms indicate that educated women are in a better position to access and understand information regarding ovarian cancer. Thus, education is an important factor in increasing awareness of cancer symptoms and predisposing cancer risk factors, such as smoking, physical inactivity, obesity and unhealthy diet (Albano et al., 2007).
The association between longer duration of marriage, increased number of pregnancies and ageing with an improved recognition of ovarian cancer symptoms might be due to the more frequent visits of these women to the hospitals or local health cancers, and getting more health education. Indeed, the higher the frequency of pregnancy, the more times that these women attended local health centres or hospitals, and the higher the chance that they will be exposed to cancer-related educational materials or meeting healthcare professionals. There is evidence that tailored print information distributed among the hospitals or local health centres is effective in increasing cancer awareness levels and knowledge of cancer prevention (Austoker et al.,2009).
The most common barriers to seeking timely help among the Omani women in our study were emotional reasons, such as being “too scared”, “too embarrassed” and “would not feel confident talking with doctor”. Previous studies conducted in Oman and the UK showed similar findings, particularly among the females’ participants. (Robb et al., 2009; Hubbard et al., 2014; Al-Azri et al., 2015). Although there are many Omani and expatriate female physicians in the local health centres, Oman is a conservative society (similar to other GCC countries) and some women might be worried about seeing a male doctor and might feel too embarrassed to have a pelvic examination. A study has been conducted in the United Arab Emirates showing that the majority of women refused gynaecological and abdominal examination to be carried out by male medical students (McLean, et al., 2010).
Indeed, emotional barriers, such as the recognition of the seriousness of symptoms and how much match their expectations. Fear of consultation and embarrassment (particular when symptoms affect a sensitive part of the body) might also be factors which contribute to the delay in seeking early medical help in ovarian cancer (Burgess et al., 2001). Furthermore, women also fear a cancer diagnosis because the disease can be incurable and there is a risk of losing some sexual characteristics as a result of surgical or chemotherapy treatments (Smith etal., 2003). A study conducted in the UK showed that the fear of cancer is a contributor to delayed presentation (Macleod, et al., 2009).
Practical and services barriers being identified by women such as “too busy”, “too many other things to worry about”, “difficult to arrange transport”, “difficult to make an appointment with doctor”, “my doctor is difficult to talk to” and “worry of wasting doctor’s time” were being identified by more women than men in a previous study (Al-Azri et al., 2015). Women sometimes prioritise family commitments over their own health, which could cause a delay in seeking medical help (Burgess et al., 2001).
The issues of the difficulty to make an appointment and to talk to the doctor were expected, as contacting doctors in the local health centres is not a common or usual practice in Oman or in other GCC countries, unlike the UK or other European countries. On the other hand, women can be seen by any doctors in the local health centres if they present as walk-ins during working hours. Although women can drive, they still have less access to transport in comparison to men, particularly if they need to travel from rural area to the hospital in urban areas and cities.
This study has some limitations. Firstly, there we no previous studies conducted in Oman to identify the level of awareness among Omani women of risk factors, symptoms and barriers to seek early medical help for ovarian cancer. Although this study has been conducted among women attending a teaching hospital in Muscat, the capital city of Oman, which could affect the generalisability, we believe that this is not a major issue, as most of women are originally from other regions and cities around the country. Nevertheless, a larger national study with recruitment of women from all regions of Oman is needed for better representative sampling.
Secondly, the majority of women who attended the hospital were attending for different medical or surgical reasons, and the possibility that some of them would have a report bias regarding ovarian cancer is therefore considered to be at a minimum, but this cannot be ruled out completely. Thirdly, although some women had a family history of cancer and, similarly, an interference or bias, the questionnaire asked specifically about ovarian cancer symptoms, not about cancer in general. Indeed, the ovarian CAM questionnaire did not recommend to eliminate participants with a family history of cancer from the analysis.
Finally, although the medical students were trained to be neutral when administering the questionnaire, there might have been some disparity in the meaning of some statements when translated to Arabic, which is beyond our control. Nonetheless, the Cronbach’s alpha of the Arabic version of the ovarian CAM from the pilot study was found to be high and the ovarian CAM was designed to be administered as an interview, either face-to-face or over the telephone to yield the best quality data (Cancer Research, 2011).
In conclusion, the findings from this study showed that, the overall level of recognition of risk factors and symptoms of ovarian cancer among Omani women were low with several emotional, practical and service barriers preventing them from seeking early medical help. Thus, strengthening education among women in Oman with regards to risk factors, early symptoms and the need to seek early medical help should help to increase ovarian cancer awareness and reduce the delay in the diagnosis. Leaflets could be distributed in local health centres and hospitals in different governorates and regions in Oman could help to increase ovarian cancer awareness.
Omani women are in need of support to eliminate the emotional barriers to seeking early medical help, particularly for reasons such as scarring, embarrassment and not feeling confident enough to talk to the doctor. Building the trust of women at an interpersonal level with the healthcare professionals is important to attend cancer services and disclose the possible cancer symptoms (Rowe and Calnan, 2006).
The campaigns of the Oman Cancer Association (OCA) that have been conducted in the community, particularly to promote self-breast examination, have shown their effectiveness (Oman Cancer Association, 2017). However, there is also a need to include ovarian cancer awareness in these campaigns particularly for non-specific symptoms such as excessive or unwanted hair growth, difficulty in eating or feeling full very quickly, unexplained weight loss and loss of appetite.
Finally, further research may help to further understand the reasons for these emotional, practical and services barriers preventing Omani women from seeking early medical help should they experience these symptoms.
Compliance with Ethical Standards
The study has been approved by the Medical Research and Ethics Committee of the College of Medicine and Health Sciences at Sultan Qaboos University, Muscat, Oman (MREC#1189).
Conflict of Interest Statement
The authors declare that they have no conflict of interest.
Funding
None.
References
- 1.Al-Azri M, Al-Hamedi I, Al-Awisi H, Al-Hinai M, Davidson R. Public awareness of warning signs and symptoms of cancer in Oman: a community-based survey of adults. Asian Pac J Cancer Prev. 2015;16:2731–37. doi: 10.7314/apjcp.2015.16.7.2731. [DOI] [PubMed] [Google Scholar]
- 2.Al-Azri M, Al-Kindi J, Al-Harthi T, et al. Awareness of stomach and colorectal cancer risk factors, symptoms and time taken to seek medical help among public attending primary care setting in Muscat Governorate, Oman. J Cancer Educ. 2017 doi: 10.1007/s13187-017-1266-8. doi:10.1007/s13187-017-1266-8. [DOI] [PubMed] [Google Scholar]
- 3.Al-Azri M, Al-Maskari A, Al-Matroushi S, et al. Awareness of cancer symptoms and barriers to seeking medical help among adult people attending primary care settings in Oman. Health Serv Res Manag Epidemiol. 2016;3 doi: 10.1177/2333392816673290. doi:10.1177/2333392816673290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Al-Azri M, Al-Rasbi K, Al-Hinai M, Davidson R, Al-Maniri A. Awareness of risk factors for cancer among Omani adults-A community based study. Asian Pac J Canc Prev. 2014;15:5401–6. doi: 10.7314/apjcp.2014.15.13.5401. [DOI] [PubMed] [Google Scholar]
- 5.Albano JD, Ward E, Jemal A, et al. Cancer mortality in the United States by education level and race. J Natl Cancer Inst. 2007;99:1384–94. doi: 10.1093/jnci/djm127. [DOI] [PubMed] [Google Scholar]
- 6.Antoniou A, Pharoah PD, Narod S, et al. Average risks of breast and ovarian cancer associated with BRCA1 or BRCA2 mutations detected in case series unselected for family history: A combined analysis of 22 studies. Am J Hum Genet. 2003;72:1117–30. doi: 10.1086/375033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Austoker J, Bankhead C, Forbes LJL, et al. Interventions to promote cancer awareness and early presentation: systematic review. Br J Cancer. 2009;101:31–9. doi: 10.1038/sj.bjc.6605388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Burgess C, Hunter MS, Ramirez AJ. A qualitative study of delay among women reporting symptoms of breast cancer. Br J Gen Pract. 2001;51:967–71. [PMC free article] [PubMed] [Google Scholar]
- 9.Buys SS, Partridge E, Black A, et al. Effect of screening on ovarian cancer mortality: the prostate, lung, colorectal and ovarian (PLCO) cancer screening randomized controlled trial. JAMA. 2011;305:2295–303. doi: 10.1001/jama.2011.766. [DOI] [PubMed] [Google Scholar]
- 10.Cancer Research UK. Ovarian cancer awareness measure. 2011. [Accessed 23 Novembrt 2017]. https://www.cancerresearchuk.org/sites/default/files/health_professional_ovarian_cancer_awareness_measure_toolkit_version_2_1_09_02_11.pdf.html .
- 11.Cancer Research UK. Ovarian cancer survival statistics. 2014. [Accessed 23 Novembrt 2017]. http://www.cancerresearchuk.org/health-professional/cancer-statistics/statistics-by-cancer-type/ovarian-cancer.html .
- 12.Choices N. Know the symptoms of ovarian cancer –live well 2012. 2012. [Accessed 23 Novembrt 2017]. http://www.nhs.uk/conditions/cancer-of-the-ovary/pages/symptoms.aspx.html .
- 13.Colombo N, Van Gorp T, Parma G, et al. Ovarian cancer. Crit Rev Oncol Hematol. 2006;60:159–79. doi: 10.1016/j.critrevonc.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 14.Danaei G, Vander Hoorn S, Lopez AD, Murray CJ, Ezzati M. Causes of cancer in the world: comparative risk assessment of nine behavioural and environmental risk factors. Lancet. 2005;366:1784–93. doi: 10.1016/S0140-6736(05)67725-2. [DOI] [PubMed] [Google Scholar]
- 15.Freij M, Al Qadire M, Khadra M, et al. Awareness and knowledge of ovarian cancer symptoms and risk factors: A survey of Jordanian women. Clin Nurs Res. 2017 doi: 10.1177/1054773817704749. 1054773817704749. [DOI] [PubMed] [Google Scholar]
- 16.Goff BA, Mandel L, Muntz HG, Melancon CH. Ovarian carcinoma diagnosis. Cancer. 2000;89:2068–75. doi: 10.1002/1097-0142(20001115)89:10<2068::aid-cncr6>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 17.Goff BA, Mandel LS, Drescher CW, et al. Development of an ovarian cancer symptom index: possibilities for earlier detection. Cancer. 2007;109:221–7. doi: 10.1002/cncr.22371. [DOI] [PubMed] [Google Scholar]
- 18.Hamilton W, Peters TJ, Bankhead C, Sharp D. Risk of ovarian cancer in women with symptoms in primary care: population based case-control study. BMJ. 2009;339 doi: 10.1136/bmj.b2998. doi:10.1136/bmj.b2998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hubbard G, Macmillan I, Canny A, et al. Cancer symptom awareness and barriers to medical help-seeking in Scottish adolescents: a cross-sectional study. BMC Pub Health. 2014;14:1117. doi: 10.1186/1471-2458-14-1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hunn J, Rodriguez GC. Ovarian cancer: Etiology, risk factors, and epidemiology. Clin Obstet Gynecol. 2012;55:3–23. doi: 10.1097/GRF.0b013e31824b4611. [DOI] [PubMed] [Google Scholar]
- 21.Jassem J, Ozmen V, Bacanu F, et al. Delays in diagnosis and treatment of breast cancer: a multinational analysis. Eur J Public Health. 2013;24:761–7. doi: 10.1093/eurpub/ckt131. [DOI] [PubMed] [Google Scholar]
- 22.Jemal A, Bray F, Center MM, et al. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 23.Keng SL, Abdul Wahab SB, Chiu LB, Yusuf A. Awareness of ovarian cancer risk factors among women in Malaysia: a preliminary study. Asian Pac J Cancer Prev. 2015;16:537–40. doi: 10.7314/apjcp.2015.16.2.537. [DOI] [PubMed] [Google Scholar]
- 24.Kumar S, Burney I, Al-Ajmi A, Al-Moundhri MS. Changing trends of breast cancer survival in Sultanate of Oman. J Oncol. 2011;2011:316243. doi: 10.1155/2011/316243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lockwood-Rayermann S, Donovan HS, Rambo D, Kuo CW. Women's awareness of ovarian cancer risks and symptoms. Am J Nurs. 2009;109:36–45. doi: 10.1097/01.NAJ.0000360309.08701.73. [DOI] [PubMed] [Google Scholar]
- 26.Low EL, Waller J, Menon U, et al. Ovarian cancer symptom awareness and anticipated time to help-seeking for symptoms among UK women. J Fam Plann Reprod Health Care. 2013;39:163–71. doi: 10.1136/jfprhc-2012-100473. [DOI] [PubMed] [Google Scholar]
- 27.Macleod U, Mitchell ED, Burgess C, MacDonald S, Ramirez AJ. Risk factors for delayed presentation and referral of symptomatic cancer: evidence for common cancers. Br J Cancer. 2009;101:92–101. doi: 10.1038/sj.bjc.6605398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McCaffery K, Wardle J, Waller J. Knowledge, attitudes, and behavioral intentions in relation to the early detection of colorectal cancer in the United Kingdom. Prev Med. 2003;36:525–35. doi: 10.1016/s0091-7435(03)00016-1. [DOI] [PubMed] [Google Scholar]
- 29.McLean M, Al Ahbabi S, Al Ameri M, et al. Muslim women and medical students in the clinical encounter. Med Educ. 2010;44:306–15. doi: 10.1111/j.1365-2923.2009.03599.x. [DOI] [PubMed] [Google Scholar]
- 30.Ministry of Health. Cancer incidence in Oman. Department of non-communicable disease. Directorate general of primary health care. Ministry of health, Sultanat of Oman. 2013 [Google Scholar]
- 31.Office for national stastistics. Cancer incidence and mortality in the United Kingdom 2008-10. 2012 [Google Scholar]
- 32.Olson SH, Mignone L, Nakraseive C, et al. Symptoms of ovarian cancer. Obstet Gynecol. 2001;98:212–7. doi: 10.1016/s0029-7844(01)01457-0. [DOI] [PubMed] [Google Scholar]
- 33.Oman Cancer Association. Clinical and self breast examination. 2017. [Accessed 23 Novembrt 2017]. http://www.oca.om/index.php/corporate-3/art2-8.html .
- 34.Permuth-Wey J, Sellers T. Epidemiology of ovarian cancer. In: Verma M, editor. Cancer epidemiology. 472 ed. Humana Press; 2009. pp. 413–37. [DOI] [PubMed] [Google Scholar]
- 35.Robb K, Stubbings S, Ramirez A, et al. Public awareness of cancer in Britain: a population-based survey of adults. Br J Cancer. 2009;101:18–23. doi: 10.1038/sj.bjc.6605386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rowe R, Calnan M. Trust relations in health care-the new agenda. Eur J Public Health. 2006;16:4–6. doi: 10.1093/eurpub/ckl004. [DOI] [PubMed] [Google Scholar]
- 37.Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics 2014. CA Cancer J Clin. 2014;64:9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 38.Simon AE, Wardle J, Grimmett C, et al. Ovarian and cervical cancer awareness: development of two validated measurement tools. J Fam Plann Reprod Health Care. 2012;38:167–74. doi: 10.1136/jfprhc-2011-100118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Smith EM, Anderson B. The effects of symptoms and delay in seeking diagnosis on stage of disease at diagnosis among women with cancers of the ovary. Cancer. 1985;56:2727–32. doi: 10.1002/1097-0142(19851201)56:11<2727::aid-cncr2820561138>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 40.Smith LK, Pope C, Botha JL. Patients'help-seeking experiences and delay in cancer presentation: a qualitative synthesis. Lancet. 2003;366:825–31. doi: 10.1016/S0140-6736(05)67030-4. [DOI] [PubMed] [Google Scholar]


