Abstract
Background
Toxoplasmosis is one of the most important cosmopolitan life-threatening diseases in immune-compromised patients. It is caused by an intracellular protozoon: Toxoplasma gondii (T. gondii). The parasite can cause pneumonia, encephalitis or disseminated disease in immune-deficient patients and dangerous congenital anomalies in infants born to mothers infected during early pregnancies. The present study aims to evaluate the prevalence of toxoplasmosis in Egyptian cancer patients and to correlate the prevalence with type of malignancy and the different cancer treatment modalities.
Materials and Methods
Blood samples from 150 cancer patients and 50 control subjects have been examined for presence of anti-toxoplasma antibodies using a lateral flow chromatographic immunoassay.
Results
Among cancer patients included in this study, the prevalence of anti- T.gondii antibodies was 20% for IgG and 4% for IgM, while in the control group it was 8% and 2% in the same order. This difference was statistically significant for IgG (P =0.003) but not for IgM (P = 0.44). Patients with solid organ tumors treated with chemotherapy had the highest prevalence rate of toxoplasmosis (28%). It was also found higher in males (26%) than females (10%) and higher among urban (18%) than rural dwellers (16%).
Conclusion
Cancer patients showed a significantly higher rate of infection with T. gondii than their cross-matched control. For that reason, we recommend the inclusion of a screening test for toxoplasmosis in their routine workup.
Keywords: Cancer, chemotherapy, prevalence, radiation, Toxoplasma gondii
Introduction
The Apicomplexan, coccidian, protozoan parasite T. gondii can infect almost all animals and humans causing the parasitic disease toxoplasmosis which can cause severe ophthalmic, neurological and systemic diseases in the newborns and persons with debilitated immune system (Robert-Gangneux, 2012; Liu et al., 2017).
T. gondii has a worldwide prevalence and it is anticipated to infect 30% of the world’s population (Montoya and Liesenfeld, 2004). This is attributed to the association of infection with several risk factors as contact with cats, nutritional habits and the environmental conditions (El Deeb et al., 2012). Man can contract the infection from many sources but primarily from eating raw or undercooked meat containing tissue cysts or water contaminated with oocysts from the stool of infected cats (Baldursson and Karanis, 2011). In immunocompetent individuals, the infection with T. gondii is generally controlled by the immune system and often pass unnoticed, however, it is life threatening in the immunocompromised individuals (Da Cunha et al., 1994; Pott and Castelo, 2013; Agrawal et al., 2014; Lu et al., 2015).
Reactivation of a latent toxoplasmosis infection usually occurs if the immune system is jeopardized. In this case, the patient represents with neurological signs and symptoms that varies from headache up to convulsions and hemiparesis (Barratt et al., 2010). Acute acquired infection is not uncommon in the immunocompromised individuals and when occurs, multiple organs will be involved. Encephalitis is by far the most common presentation in these patients but also retinochoroiditis and pneumonia are common (Machala et al., 2015). Cancer patients are at high risk of developing severe disease, from one hand being immunocompromised from the cancer itself and from the other hand reactivation of a latent T. gondii infection can occur due to cancer therapy (Frenkel et al., 1978).
Several studies showed a link between reactivation of a latent infection and a diversity of cancers as ocular tumors, meningiomas and hematological malignancies (Maciel et al., 2000; Khurana et al., 2005; Kojima et al., 2010).
Moreover, T. gondii is incriminated to be responsible for the progression of malignant diseases by inhibiting apoptosis and increasing dendritic cells and macrophages motility (Carmen and Sinai, 2007; Baumgartner, 2011).
The prevalence of toxoplasmosis in Egyptian cancer patients has not been adequately studied. So, in our work we aimed to evaluate its current status in Egypt and to correlate the prevalence with type of malignancy and different cancer treatment modalities.
Materials and Methods
This cross-sectional study was performed over the period from April 2014 to July 2015 on blood samples collected from a total of 200 subjects, including study groups (number: n=150) and control group (n=50).
The patients were recruited from the Clinical Oncology Department- Kasr Al Ainy Faculty of Medicine- Cairo University after approval of Cairo University ethical committee and with their consent in participating in the study. Participants were divided into four groups:
- Group A: includes 50 patients having hematological origin tumors (leukemias, lymphomas or multiple myelomas) and treated with chemotherapy.
- Group B: includes 50 patients having solid organ tumors and treated with chemotherapy.
- Group C: includes 50 patients having solid organ tumors and treated by radiotherapy.
- Group D: includes 50 apparently healthy individuals not known to have malignancy (referred to as control group).
One blood sample from each participant was used for detection of Toxoplasma gondii antibodies using the Onsite Toxo IgG/IgM Rapid Test, CTK Biotech, California, USA.
This is a lateral flow chromatographic immunoassay for the simultaneous detection and differentiation of IgG and IgM anti- T.gondii in human serum or plasma.
Statistical Methods:
Descriptive analysis was presented as frequency with percentage for categorical data and mean with standard deviation for continuous variables. Different groups were compared using Chi square (χ2) test and p-value of less than 0.05 was considered significant. SPSS version 17 was used for all statistical analysis.
Results
In the present study, we detected 34 positive cases of toxoplasmosis among 200 participants (17%). A higher seroprevalence of toxoplasmosis was detected in cancer patients (20%) than in controls (4%) (Table 1).
Table 1.
Positive and Negative Cases of T.gondii in Different Groups
| Positive | Negative | OR* | |||
|---|---|---|---|---|---|
| Number | percentage | Number | Percentage | ||
| Group A (total=50) | 6 | 12% | 44 | 88% | 1.5 |
| Group B (total=50) | 14 | 28% | 36 | 72% | 4.4 |
| Group C (total=50) | 10 | 20% | 40 | 80% | 2.9 |
| Group D (total=50) | 4 | 8% | 46 | 92% | control |
| Total (200) | 34 | 17% | 166 | 83% | |
OR, Odds Ratio
In cancer patients, the prevalence of T.gondii was 20% for IgG and 4% for IgM, while in the control it was 8% and 2% in the same order. This difference was statistically significant for IgG (P =0.003) but not for IgM (P = 0.44) (Figure 1).
Figure 1.
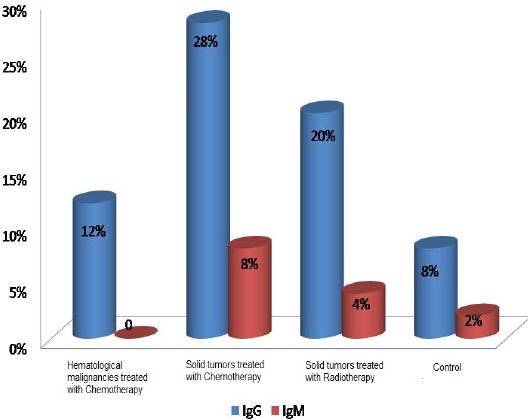
Distribution of Positive Cases of T.gondii According to the Immunoglobulin Detected in Different Groups
Patients with solid organ tumors had higher prevalence rate of toxoplasmosis (24%) than patients with hematological malignancies (12%) but this difference was statistically insignificant (p=0.06) (Table 2).
Table 2.
Prevalence of T.gondii in Cancer Patients According to the Origin of Neoplasm and Type of Treatment
| Positive | Negative | OR* | |||
|---|---|---|---|---|---|
| Number | % | Number | % | ||
| Solid tumors (total=100) | 24 | 24% | 76 | 76% | 3.6 |
| Haematological malignancies (total=50) | 6 | 12% | 44 | 88% | 1.5 |
| Radiotherapy (total=50) | 10 | 20% | 40 | 80% | 2.9 |
| Chemotherapy (total=100) | 20 | 20% | 80 | 80% | 2.8 |
OR, Odds Ratio
Moreover, the prevalence of T.gondii was not affected by the type of treatment. Patients treated with radiation and those treated with chemotherapy had same positivity rates (20% both) (Table 2).
A higher prevalence of T.gondii was detected in males (18%) than in females (16%) (Table 3) and it was also higher in urban dwellers (18%) than rural dwellers (15%) (Table 4) Both differences were not statistically significant (p value = 0.74, 0.64 respectively).
Table 3.
Gender Distribution of Positive Cases of T.gondii in Different Groups of the Study
| Male | female | P value | |
|---|---|---|---|
| Group A | 3/24 (12.5%) | 3/26 (11.5%) | 0.91 |
| Group B | 4/12 (33.3%) | 10/38 (26.3%) | 0.32 |
| Group C | 7/26(26.9%) | 3/24(12.5%) | 0.48 |
| Group D | 2/27 (7.4%) | 2/23 (8.7%) | 0.86 |
| total | 16/89 (17.9%) | 18/111 (16.2%) | 0.74 |
Table 4.
Residency Distribution of Positive Cases of T.gondii in Different Groups of the Study
| rural | urban | P value | |
|---|---|---|---|
| Group A | 4/27(14.8%) | 2/23(8.6%) | 0.54 |
| Group B | 5/18(27.7%) | 9/32(28.1%) | 0.87 |
| Group C | 3/16(18.7%) | 7/34(20.5%) | 0.87 |
| Group D | 1/25 (4%) | 3/25 (12%) | 0.29 |
| total | 13/86 (15.1%) | 21/114 (18.4%) | 0.64 |
Discussion
The immune system plays an integral role in controlling and clearing parasitic infections (Sanad et al., 2014). Some of these infections in the immunocompromised hosts could be more hostile and life threatening (Mohammadi Manesh et al., 2014)
Although opportunistic parasites have a serious impact on the health of immunosuppressed patients, routine diagnosis of these parasites may be ignored during therapy and thus their prevalence among Egyptian cancer patients have not yet been adequately studied.
The present study focused on evaluating the current status of toxoplasmosis in adult Egyptian cancer patients, in correlation with the type of malignancy and the type of treatment they receive.
In the present study, among cancer patients, the prevalence of anti- T.gondii antibodies was 20% for IgG and 4% for IgM, while in the control it was 8% and 2% in the same order. This difference was statistically significant for IgG (P =0.003) but not for IgM (P = 0.44). This could be credited to the reactivation of a latent disease when the host immunity is weakened, while the risk of acquiring new infection is seemingly similar in all individuals.
Similar to our results, in a recent meta-analysis study about the prevalence of toxoplasmosis in immunocompromised patients, the authors reported presence of anti- T.gondii IgG antibodies in 26% of cancer patients compared to 12% in their controls (p<0.001) and IgM in 11.4% in cancer patients and 2.7% in the control groups (p<0.01) (Wang et al., 2017).
Another meta-analysis study about the prevalence of toxoplasmosis in Chinese cancer patients that incorporated nineteen studies including 4,493 cases and 6,797 controls, the overall seroprevalence of T. gondii was higher in the population with cancer compared with those without (20.59% versus 6.31%, P < 0.001) (Jiang et al., 2015).
In Egypt, studies of the prevalence of toxoplasmosis in Egyptian cancer patients are scarce and not recent. Khalil et al., (1991) reported the presence of anti- toxoplasma antibodies in 36% of cancer patients and in 20% of control while, El Shazly et al., (1996) examined the sera of 25 cancer patients for anti-Toxoplasma IgG and IgM antibodies before and after chemotherapy treatment and found seropositive IgG in 22 (88%) of patients before treatment and in 23 (92%) after treatment and the IgM was positive in three (12%) patients before treatment and in six (24%) after treatment.
Other studies were conducted in different countries to estimate the prevalence of T.gondii in cancer patients. Rai et al., (2003) investigated the presence of T. gondii in Nepalese patients with ocular malignancies versus patients with other ocular diseases and reported that patients with ocular malignancies had higher positive rates. Also, Yazar et al., (2004) detected anti-T.gondii IgG in 63% Turkish cancer patients compared to 19% in the controls. Shin et al., (2009) also noted high titers of IgG antibodies against T.gondii in patients with neoplasms in Korea.
The prevalence of T.gondii in the present study was higher in patients having solid organ tumors (24%) than in patients with hematological malignancies (12%) but this difference was not statistically significant (p=0.06). Moreover, the type of treatment doesn’t seem to affect the prevalence of T.gondii. The prevalence in patients treated with chemotherapy was equal to the prevalence in those treated with irradiation (20% both). This shows that the type of malignancy and the type of treatment has no effect on the prevalence of the disease.
Yuan et al., (2007) revealed higher positivity rates of toxoplasmosis in Chinese cancer patients compared to control. He also found that patients having rectal and nasopharyngeal tumors had significantly higher rates of positive anti- Toxoplasma IgG than the other cancer groups.
In the present study, the prevalence of T.gondii was higher in males (18%) than females (16%) with no significant statistical difference. This may be due to the increased risk of exposure of males due to more outdoor activities and the habit of eating fast foods.
This goes in agreement with the work of Wang et al., (2015) who also found a higher prevalence of toxoplasmosis in males with no significant differences in gender (male, 8.96%; female, 7.45%, P < 0.4).
In contrast, some studies reported statistically significant higher infection rate of T. gondii in males than in females (Shimelis et al., 2009; Jones et al., 2014) While in Ethiopia, females were more affected than males HIV infected patients (Walle et al., 2013).
The prevalence of T.gondii was also higher in urban dwellers (18%) than rural dwellers (15%) with no significant statistical difference. This could be attributed to eating habits like consuming undercooked meat and petting of cats in the houses that are more in urban communities.
This goes in agreement with Walle et al., (2013) who reported higher rates of toxoplasmosis in urban than rural dwellers and attributed this to environmental variation, eating behaviors as well as pet- keeping.
One of the limitations of this study is that all the patients were recruited from only one hospital, although the largest University Hospital in the Middle-East with around 3,000 newly diagnosed case/year however, further studies including centers from different parts of the country are needed. Also, the small number of study population was another limitation.; this was due to the fact that the study was self-funded; nevertheless, due to the promising results the authors believe that it will open the door for further studies.
Another limitation is that diagnosis of toxoplasmosis relies mainly on serology which may be underestimating the true prevalence in cancer patients due to insufficient antibodies production.
Moreover, correlation between the length of affection with cancer and length of treatment that may affect the immuno-competent state of the patients and rising antibodies titer is recommended to be done in further studies.
Cancer patients showed a significantly higher rate of infection with T. gondii. For that reason, we recommend the inclusion of a screening test for toxoplasmosis in their routine workup. This is a very important, cost effective and life saving measurement wherever parasitic infections are common and neoplasms are at rise as in under-developed countries. As seropositive patients are vulnerable to reactivation of the infection, while seronegative patients are vulnerable to primary infection. By screening and raising awareness to avoid contracting the infection we can reduce infection related fatality rates.
Source of support
None.
Disclosure
The authors report no conflicts of interest.
References
- 1.Agrawal SR, Singh V, Ingale S, et al. Toxoplasmosis of spinal cord in acquired immunodeficiency syndrome patient presenting as paraparesis: a rare entity. J Glob Infect Dis. 2014;6:178–81. doi: 10.4103/0974-777X.145248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baldursson S, Karanis P. Waterborne transmission of protozoan parasites: review of worldwide outbreaks-an update 2004-2010. Water Res. 2011;45:6603–14. doi: 10.1016/j.watres.2011.10.013. [DOI] [PubMed] [Google Scholar]
- 3.Barratt JL, Harkness J, Marriott D, et al. Importance of non-enteric protozoan infections in immunocompromised people. Clin Microbiol Rev. 2010;23:795–836. doi: 10.1128/CMR.00001-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baumgartner M. Enforcing host cell polarity: an apicomplexan parasite strategy towards dissemination. Curr Opin Microbiol. 2011;14:436–44. doi: 10.1016/j.mib.2011.07.003. [DOI] [PubMed] [Google Scholar]
- 5.Carmen JC, Sinai AP. Suicide prevention: disruption of apoptotic pathways by protozoan parasites. Mol Microbiol. 2007;64:904–16. doi: 10.1111/j.1365-2958.2007.05714.x. [DOI] [PubMed] [Google Scholar]
- 6.Da Cunha S, Ferreira E, Ramos I, et al. Cerebral toxoplasmosis after renal transplantation. Case report and review. Acta Med Port. 1994;7:61–6. [PubMed] [Google Scholar]
- 7.El Deeb HK, Salah Eldin H, Khodeer S, Allah AA. Prevalence of Toxoplasma gondii infection in antenatal population in Menoufia, governorate Egypt. Acta Trop. 2012;124:185–91. doi: 10.1016/j.actatropica.2012.08.005. [DOI] [PubMed] [Google Scholar]
- 8.El Shazly AM, Afify EM, Morsy TA. Antibodies against toxoplasma in children and adults with malignancy. J Egypt Soc Parasitol. 1996;26:781–7. [PubMed] [Google Scholar]
- 9.Frenkel JK, Amare M, Larsen W. Immune competence in a patient with Hodgkin's disease and relapsing toxoplasmosis. Infection. 1987;6:84–91. doi: 10.1007/BF01642165. [DOI] [PubMed] [Google Scholar]
- 10.Jiang C, Li Z, Chen P, Chen L. The seroprevalence of Toxoplasma gondii in Chinese population with cancer: A systematic review and meta-analysis. Medicine (Baltimore) 2015;94:e2274. doi: 10.1097/MD.0000000000002274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jones JL, Kruszon-Moran D, Rivera HN, et al. Toxoplasma gondii seroprevalence in the United States 2009–2010 and Comparison with the Past Two Decades. Am J Trop Med Hyg. 2014;90:1135–9. doi: 10.4269/ajtmh.14-0013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jones JL, Kruszon-Moran D, Wilson M, et al. Toxoplasma gondii infection in the United States: seroprevalence and risk factors. Am J Epidemiol. 2001;154:357–65. doi: 10.1093/aje/154.4.357. [DOI] [PubMed] [Google Scholar]
- 13.Khalil HM, Makled MK, Azab ME, et al. Opportunistic parasitic infections in immunocompromised hosts. J Egypt Soc Parasitol. 1991;21:657–8. [PubMed] [Google Scholar]
- 14.Khurana S, Dubey ML, Malla N. Association of parasitic infections and cancers. Indian J Med Microbiol. 2005;23:74–9. doi: 10.4103/0255-0857.16044. [DOI] [PubMed] [Google Scholar]
- 15.Kojima M, Nakamura N, Murayama K, et al. Reactive lymphoid hyperplasia with giant follicles associated with a posttherapeutic state of hematological malignancies. A report of eight cases. Tumori. 2010;96:143–8. doi: 10.1177/030089161009600123. [DOI] [PubMed] [Google Scholar]
- 16.Liu XC, He Y, Han DG, et al. Detection of Toxoplasma gondii in chicken and soil of chicken farms in Nanjing region, China. Infect Dis Poverty. 2017;6:62. doi: 10.1186/s40249-017-0277-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lu N, Liu C, Wang J, et al. Toxoplasmosis complicating lung cancer: a case report. Int Med Case Rep J. 2015;8:37–40. doi: 10.2147/IMCRJ.S76488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Machala L, Kodym P, Maly M, et al. Toxoplasmosis in immunocompromised patients. Epidemiol Mikrobiol Imunol. 2015;64:59–65. [PubMed] [Google Scholar]
- 19.Maciel E, Siqueira I, Queiroz AC, et al. Toxoplasma gondii myelitis in a patient with adult T-cell leukemia-lymphoma. Arq Neuropsiquiatr. 2000;58:1107–9. doi: 10.1590/s0004-282x2000000600019. [DOI] [PubMed] [Google Scholar]
- 20.Mohammadi Manesh R, Hosseini Safa A, Sharafi SM, et al. Parasites and chronic renal failure. J Renal Inj Prev. 2014;3:87–90. doi: 10.12861/jrip.2014.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Montoya JG, Liesenfeld O. Toxoplasmosis. Lancet. 2004;363:1965–76. doi: 10.1016/S0140-6736(04)16412-X. [DOI] [PubMed] [Google Scholar]
- 22.Pott H, Jr, Castelo A. Isolated cerebellar toxoplasmosis as a complication of HIV infection. Int J STD AIDS. 2013;24:70–2. doi: 10.1258/ijsa.2012.012189. [DOI] [PubMed] [Google Scholar]
- 23.Rai SK, Upadhyay MP, Shrestha HG. Toxoplasma infection in selected patients in Kathmandu, Nepal. Nepal Med Coll J. 2003;5:89–91. [PubMed] [Google Scholar]
- 24.Robert-Gangneux F, Darde ML. Epidemiology of and diagnostic strategies for toxoplasmosis. Clin Microbiol Rev. 2012;25:264–96. doi: 10.1128/CMR.05013-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sanad MM, Thagfan FA, Al Olayan EM, et al. Opportunistic coccidian parasites among Saudi cancer patients presenting with diarrhea: prevalence and immune status. J Parasitol Res. 2014;9:55–63. [Google Scholar]
- 26.Shimelis T, Tebeje M, Tadesse E, et al. Sero-prevalence of latent Toxoplasma gondii infection among HIV-infected and HIV-uninfected people in Addis Ababa Ethiopia: a comparative cross-sectional study. BMC Res Notes. 2009;2:213. doi: 10.1186/1756-0500-2-213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shin D-W, Cha D-Y, Hua QJ, et al. Seroprevalence of Toxoplasma gondii infection and characteristics of seropositive patients in general hospitals in Daejeon, Korea. Korean J Parasitol. 2009;47:125–30. doi: 10.3347/kjp.2009.47.2.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Walle F, Kebede N, Tsegaye A, et al. Seroprevalence and risk factors for toxoplasmosis in HIV infected and non-infected individuals in Bahir Dar, Northwest Ethiopia. Parasit Vectors. 2013;6:15. doi: 10.1186/1756-3305-6-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang L, He LY, Meng DD, et al. Seroprevalence and genetic characterization of Toxoplasma gondii in cancer patients in Anhui Province, Eastern China. Parasit Vectors. 2015;15:162. doi: 10.1186/s13071-015-0778-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang ZD, Liu HH, Ma ZX, et al. Toxoplasma gondii infection in immunocompromised patients: A Systematic Review and Meta-Analysis. Front Microbiol. 2017;8:389. doi: 10.3389/fmicb.2017.00389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yazar S, Yaman O, Eser B, et al. Investigation of anti- toxoplasma gondii antibodies in patients with neoplasia. J Med Microbiol. 2004;53:1183–6. doi: 10.1099/jmm.0.45587-0. [DOI] [PubMed] [Google Scholar]
- 32.Yuan Z, Gao S, Liu Q, et al. Toxoplasma gondii antibodies in cancer patients. Cancer Lett. 2007;254:71–4. doi: 10.1016/j.canlet.2007.02.011. [DOI] [PubMed] [Google Scholar]


