Abstract
Background
No approved pharmacological agents are available for the treatment and prevention of acute kidney injury (AKI). The nervous system has been reported to play an important role, directly or indirectly via the immune system, in the pathophysiology of AKI. Neuromodulation, such as vagus nerve stimulation and pulsed ultrasound, is emerging as an innovative therapeutic treatment for various diseases including AKI. However, a lack of effective methods to selectively stimulate or inhibit neurons has hampered the complete understanding of the roles of the nervous system in AKI because electrical stimulation is nonspecific for cell types.
Summary
A novel technique called optogenetics optically controls cells in living tissues, typically neurons, which have been genetically modified to express light-sensitive opsins. For example, channelrhodopsin-2 (ChR2), an opsin, is a nonselective cation channel residing in a cell membrane, which rapidly opens its gate after exposing to monochromatic light in the “blue” wavelength. Unlike electrodes, blue light can selectively depolarize ChR2-expressing neurons, mainly via the Na+ entry, evoking an action potential. Optogenetics that use ChR2 and several variants to modulate kinetic properties and inhibitory opsins help in understanding the roles of the nervous system in AKI, thus leading to a clinical application of neuromodulation to AKI treatment.
Keywords: optogenetics, acute kidney injury, vagus nerve stimulation, pulsed ultrasound
Introduction
Acute kidney injury (AKI) is associated with high mortality and morbidity, and AKI episodes can lead to chronic kidney disease and end stage renal disease. Despite therapeutic interventions, no approved pharmacological agents are available for the treatment and prevention of AKI [1]. The unsuccessful development of a therapeutic strategy for AKI can be attributed to the inadequate understanding of the pathophysiology of AKI and adverse effects of pharmacological agents. Thus, sustained efforts to grasp the complex AKI pathophysiology and innovative therapies are clearly needed. Recently, the nervous system has been reported to play an important role, directly or via the immune system, in the pathophysiology of various kidney diseases including AKI [2]. Optogenetics is a groundbreaking technique in the field of neuroscience that helps in stimulating or inhibiting specific neurons by light illumination. Here we elaborate optogenetics including its history and discuss its usefulness to reveal important roles of the nervous system in the pathophysiology and therapeutic strategy of AKI.
Optogenetics: a groundbreaking intervention tool in neuroscience
Since Luigi Galvani observed muscle contraction evoked by electrical stimulation in the end of the 18th century, electrical stimulation has been broadly used to manipulate neuronal cells. However, it is nonspecific for cell types because it simultaneously excites all neurons located close to the electrode tip. In the 1970s, Francis Crick, who found the double helix of DNA with James Watson in 1953, stated the need for a new tool to control neurons with cellular and temporal precision [3]. Thirty years later, optogenetics finally achieved his aim to further elucidate the complexities of nervous system [4].
Optogenetics refers to the combined use of optics and genetics to manipulate cells or optically control cells in living tissues, typically neurons, which have been genetically modified to express light-sensitive opsins. Its history began with the discovery of channelrhodopsin (ChR)-1 and ChR2 from the green alga Chlamydomonas reinhardtii in 2002 and 2003 [5–7]. ChR2 was reported as a nonselective cation channel, which rapidly opens its gate after blue light application (maximum activation at 470 nm) [8]. ChR1 and ChR2 are expressed in the eyespot of C. reinhardtii and are believed to be necessary for phototaxis. ChR2 includes seven transmembrane α-helices and the light-sensitive chromophore retinal (vitamin A aldehyde). Retinal is covalently attached to a lysine residue in the seventh helix via a Schiff base linkage. When ChR2 expressed on the cell membranes is illuminated with blue light, all-trans-retinal isomerizes to 13-cis-retinal; this leads to the conformational change of ChR2, allowing cations to pass through it. Without blue light, 13-cis-retinal quickly reverts back to all-trans-retinal, and the channel is closed.
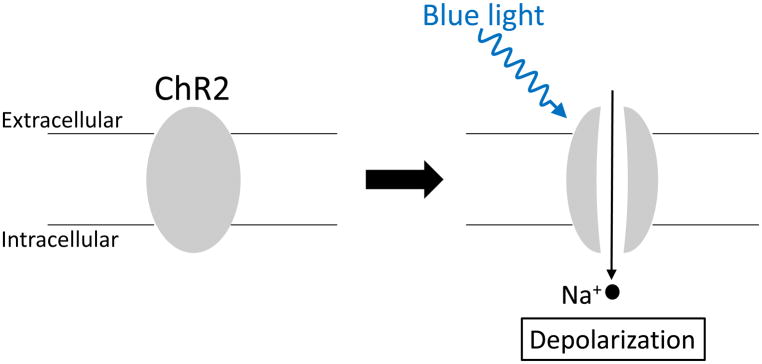
Karl Deisseroth applied these unique proteins in neuroscience for the first time [4]. He introduced ChR2 to mammalian hippocampal neurons in vitro using lentiviral ChR2 gene delivery and observed that action potentials were evoked just 1–2 ms after the illumination of these cells with blue light and that switching off the light immediately stopped firing. ChR2 expression itself does not affect the resting membrane potential of neurons because the gate is closed without the blue light. When the blue light is applied to ChR2-expressing neurons, the gate is opened, and Na+ enters the cells. If the amount of ChR2 and Na+ entry is large enough to reach the threshold, the voltage-gated Na+ channels open and evoke an action potential (Figure 1).
Figure 1.
Schematic of channelrhodopsin-2 (ChR2). ChR2 expression does not affect the resting membrane potential because the gate is closed without blue light. When ChR2 is illuminated with blue light, the gate is opened, and it functions as a nonselective cation channel, resulting in the depolarization of ChR2-expressing neurons mainly due to Na+ entry. If the Na+ entry is large enough to reach the threshold, voltage-gated Na+ channels open, evoking an action potential.
Since this first proof-of-concept experiment to manipulate neurons by light in 2005, optogenetics has been broadly utilized in vivo as an intervention tool. For in vivo experiments, viral vectors or transgenic mice are frequently used to introduce ChR2 to specific cell types. The specific expression of ChR2 is achieved by injecting viral vectors into the target region and/or by using the Cre-LoxP system. One example to demonstrate the usefulness of optogenetics is the role of orexin-producing neurons in the regulation of sleep–wakefulness cycle. Although the loss of orexin neurons in the hypothalamus had been linked to narcolepsy, it was unknown how these cells control sleep and wakefulness because electrical stimulation excites other neurons in the hypothalamus along with orexin neurons. By selectively introducing ChR2 to orexin neurons and directly delivering blue light to the hypothalamus of freely moving mice with an optical fiber, selective stimulation of orexin neurons clearly increased the probability of transition from sleep to wakefulness [9]. Conversely, selective acute inhibition of orexin neurons using halorhodopsin (NpHR, described below) induced sleep in mice [10]. Thus, optogenetics, which brought about a paradigm shift in neuroscience, was chosen as the Method of the Year in 2010.
The response characteristics of retinal to light are determined by the charge of amino acids surrounding retinal. Therefore, for the alteration of ChR2, point mutations targeting those amino acids have been used. Various mutations have been tried, and some were found to be useful in modulating the kinetic properties of ChR2. One of the most commonly used ChR2 variants is the H134R variant, in which the histidine residue at position 134 is mutated to an arginine. Compared with wild-type ChR2, ChR2 (H134R) causes larger stationary photocurrents [11]. To inhibit neuronal activity, NpHR, a light-sensitive inward chloride pump naturally expressed by the halobacterium Natronomonas pharaonis, can be used. When yellow light is applied to NpHR, Cl− enters the cells and the cells become hyperpolarized [12]. Another option is using archaerhodopsin, a light-sensitive outward proton pump [13]. The inhibition of neuronal firing using these pumps and their variants is another advantage of optogenetics because electrodes can excite neurons, but not inhibit them.
Neuromodulation: a promising therapeutic strategy for AKI
In mice, the electrical stimulation of cervical vagus afferent or efferent fibers before ischemia-reperfusion injury (IRI) significantly ameliorated kidney damage [14]. Additional experiments demonstrated that splenocytes expressing alpha7 nicotinic acetylcholine receptors are necessary for kidney protection, which is consistent with the activation of the cholinergic anti-inflammatory pathway (CAP) [15]. Vagus nerve stimulation (VNS) of brain-dead donor rats before kidney transplantation also resulted in better renal function in recipients [16]. Similar to VNS, pulsed ultrasound with a clinical ultrasound machine before IRI ameliorated mouse kidney injury, likely due to the activation of CAP [17]. Considering that VNS devices and ultrasound machines already have other clinical applications, they are promising candidates for an innovative therapeutic strategy for AKI, although precise mechanisms are yet to be determined.
Optogenetics may be a useful tool to investigate the mechanism by which VNS protects the kidney. Vagus afferent neurons were found to be divided into several subgroups based on their specific markers, and optogenetic stimulation of each subgroup affected the function of the gastrointestinal tract, lung, and heart in different ways [18,19]. These studies indicate that some subpopulation(s) of vagus afferent and probably efferent neurons may play important roles in the renoprotective effect of VNS. In addition, optogenetic stimulation of specific neurons in the brain ameliorated kidney IRI in mice [20]. C1 neurons, which are located in the medulla oblongata and innervate dorsal motor nucleus of the vagus nerve, sympathetic efferents, and paraventricular nucleus of the hypothalamus, mediate adaptive responses to various stressors such as hypotension and hypoxia. Optogenetic stimulation of C1 neurons protected the kidneys from IRI. Additional experiments suggested that this protective effect was through the activation of CAP, which occurred via the activation of a sympathetic route.
Future directions
In 2009, the optical control of intracellular signaling mediated by G-protein-coupled receptors (GPCRs) was reported [21]. OptoXRs are opsin/GPCR chimeras wherein the intracellular loops of rhodopsin are replaced with those from GPCRs. Green light illumination onto OptoXRs expressed in the cell membrane leads to the activation of G-protein-mediated signaling cascades, with no changes in the membrane potential. This technique opened a new avenue for the broader application of optogenetics to cells other than neurons.
Unfortunately, optogenetics cannot be a therapeutic tool in a clinical setting at this moment. For “optogenetic therapeutics,” opsins need to be delivered into the target tissues using a viral vector, which is not established in humans. Another problem is that delivered algal or bacterial opsins would trigger an immune response in human bodies. However, optogenetics is clearly a powerful tool to elucidate important roles of the nervous system in AKI pathophysiology.
Conclusion
The nervous system plays an important role in AKI pathophysiology; neuromodulation (e.g., VNS and pulsed ultrasound) is expected to be an innovative therapeutic strategy for AKI. Optogenetics helps us to stimulate or inhibit specific neurons using light. This technique enhances our understanding about the important roles of the nervous system in AKI pathophysiology, thus leading to a clinical application of neuromodulation to the treatment and prevention of AKI.
Key messages.
Optogenetics helps in stimulating or inhibiting specific neurons in vivo by light illumination with cellular and temporal precision.
Optogenetics is useful for studying the roles of the nervous system in AKI pathophysiology, thus leading to the possibility of the clinical application of neuromodulation to AKI treatment.
Acknowledgments
Research conducted for this publication was supported by the National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health (NIH) under award numbers R01DK085259, R01DK062324 and U18 EB021787.
Footnotes
Conflicts of Interest
Digestive and Kidney Diseases of the National Institutes of Health (NIH) under award numbers R01DK085259, R01DK062324 and U18 EB021787
References
- 1.Okusa MD, Rosner MH, Kellum JA, Ronco C Workgroup ADQIX. Therapeutic Targets of Human AKI: Harmonizing Human and Animal AKI. J Am Soc Nephrol. 2016;27:44–48. doi: 10.1681/ASN.2015030233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Okusa MD, Rosin DL, Tracey KJ. Targeting neural reflex circuits in immunity to treat kidney disease. Nat Rev Nephrol. 2017;13:669–680. doi: 10.1038/nrneph.2017.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Crick FH. Thinking about the brain. Sci Am. 1979;241:219–232. doi: 10.1038/scientificamerican0979-219. [DOI] [PubMed] [Google Scholar]
- 4.Boyden ES, Zhang F, Bamberg E, Nagel G, Deisseroth K. Millisecond-timescale, genetically targeted optical control of neural activity. Nat Neurosci. 2005;8:1263–1268. doi: 10.1038/nn1525. [DOI] [PubMed] [Google Scholar]
- 5.Nagel G, Ollig D, Fuhrmann M, Kateriya S, Musti AM, Bamberg E, Hegemann P. Channelrhodopsin-1: a light-gated proton channel in green algae. Science. 2002;296:2395–2398. doi: 10.1126/science.1072068. [DOI] [PubMed] [Google Scholar]
- 6.Sineshchekov OA, Jung KH, Spudich JL. Two rhodopsins mediate phototaxis to low- and high-intensity light in Chlamydomonas reinhardtii. Proc Natl Acad Sci U S A. 2002;99:8689–8694. doi: 10.1073/pnas.122243399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Suzuki T, Yamasaki K, Fujita S, Oda K, Iseki M, Yoshida K, Watanabe M, Daiyasu H, Toh H, Asamizu E, Tabata S, Miura K, Fukuzawa H, Nakamura S, Takahashi T. Archaeal-type rhodopsins in Chlamydomonas: model structure and intracellular localization. Biochem Biophys Res Commun. 2003;301:711–717. doi: 10.1016/s0006-291x(02)03079-6. [DOI] [PubMed] [Google Scholar]
- 8.Nagel G, Szellas T, Huhn W, Kateriya S, Adeishvili N, Berthold P, Ollig D, Hegemann P, Bamberg E. Channelrhodopsin-2, a directly light-gated cation-selective membrane channel. Proc Natl Acad Sci U S A. 2003;100:13940–13945. doi: 10.1073/pnas.1936192100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Adamantidis AR, Zhang F, Aravanis AM, Deisseroth K, de Lecea L. Neural substrates of awakening probed with optogenetic control of hypocretin neurons. Nature. 2007;450:420–424. doi: 10.1038/nature06310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tsunematsu T, Kilduff TS, Boyden ES, Takahashi S, Tominaga M, Yamanaka A. Acute optogenetic silencing of orexin/hypocretin neurons induces slow-wave sleep in mice. J Neurosci. 2011;31:10529–10539. doi: 10.1523/JNEUROSCI.0784-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nagel G, Brauner M, Liewald JF, Adeishvili N, Bamberg E, Gottschalk A. Light activation of channelrhodopsin-2 in excitable cells of Caenorhabditis elegans triggers rapid behavioral responses. Curr Biol. 2005;15:2279–2284. doi: 10.1016/j.cub.2005.11.032. [DOI] [PubMed] [Google Scholar]
- 12.Zhang F, Wang LP, Brauner M, Liewald JF, Kay K, Watzke N, Wood PG, Bamberg E, Nagel G, Gottschalk A, Deisseroth K. Multimodal fast optical interrogation of neural circuitry. Nature. 2007;446:633–639. doi: 10.1038/nature05744. [DOI] [PubMed] [Google Scholar]
- 13.Chow BY, Han X, Dobry AS, Qian X, Chuong AS, Li M, Henninger MA, Belfort GM, Lin Y, Monahan PE, Boyden ES. High-performance genetically targetable optical neural silencing by light-driven proton pumps. Nature. 2010;463:98–102. doi: 10.1038/nature08652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Inoue T, Abe C, Sung SS, Moscalu S, Jankowski J, Huang L, Ye H, Rosin DL, Guyenet PG, Okusa MD. Vagus nerve stimulation mediates protection from kidney ischemia-reperfusion injury through alpha7nAChR+ splenocytes. J Clin Invest. 2016;126:1939–1952. doi: 10.1172/JCI83658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tanaka S, Inoue T, Hossack JA, Okusa MD. Nonpharmacological, Biomechanical Approaches to Control Inflammation in Acute Kidney Injury. Nephron. 2017 doi: 10.1159/000477218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hoeger S, Fontana J, Jarczyk J, Selhorst J, Waldherr R, Kramer BK, Schnuelle P, Yard BA. Vagal stimulation in brain dead donor rats decreases chronic allograft nephropathy in recipients. Nephrol Dial Transplant. 2014;29:544–549. doi: 10.1093/ndt/gft451. [DOI] [PubMed] [Google Scholar]
- 17.Gigliotti JC, Huang L, Ye H, Bajwa A, Chattrabhuti K, Lee S, Klibanov AL, Kalantari K, Rosin DL, Okusa MD. Ultrasound prevents renal ischemia-reperfusion injury by stimulating the splenic cholinergic anti-inflammatory pathway. J Am Soc Nephrol. 2013;24:1451–1460. doi: 10.1681/ASN.2013010084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chang RB, Strochlic DE, Williams EK, Umans BD, Liberles SD. Vagal Sensory Neuron Subtypes that Differentially Control Breathing. Cell. 2015;161:622–633. doi: 10.1016/j.cell.2015.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Williams EK, Chang RB, Strochlic DE, Umans BD, Lowell BB, Liberles SD. Sensory Neurons that Detect Stretch and Nutrients in the Digestive System. Cell. 2016;166:209–221. doi: 10.1016/j.cell.2016.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Abe C, Inoue T, Inglis MA, Viar KE, Huang L, Ye H, Rosin DL, Stornetta RL, Okusa MD, Guyenet PG. C1 neurons mediate a stress-induced anti-inflammatory reflex in mice. Nat Neurosci. 2017;20:700–707. doi: 10.1038/nn.4526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Airan RD, Thompson KR, Fenno LE, Bernstein H, Deisseroth K. Temporally precise in vivo control of intracellular signalling. Nature. 2009;458:1025–1029. doi: 10.1038/nature07926. [DOI] [PubMed] [Google Scholar]