Abstract
Drug delivery to the inner ear is an ideal method to treat a wide variety of otologic conditions. A broad range of potential applications is just beginning to be explored. New approaches combine principles of inner ear pharmacokinetics with emerging technologies of drug delivery including novel delivery systems, drug-device combinations, and new categories of drugs. Strategies include cell-specific targeting, manipulation of gene expression, local activation following systemic delivery, and use of stem cells, viral vectors, and gene editing systems. Translation of these therapies to the clinic remains challenging given the potential risks of intracochlear and intralabyrinthine trauma, our limited understanding of the etiologies of particular inner ear disorders, and paucity of accurate diagnostic tools at the cellular level. This review provides an overview of future methods, delivery systems, disease targets, and clinical considerations required for translation to clinical medicine.
1. Introduction
The delivery of reagents to accomplish inner ear therapy has been utilized for decades in the clinical environment, particularly with transtympanic injections delivered to the middle ear space to facilitate diffusion across the round window membrane into the cochlea. With the advent of the cochlear implant, intracochlear access has been frequently performed in humans via round window or basal turn cochleostomy. In animal models such as the guinea pig that provide easier access to various portions of the inner ear, additional routes of delivery have been explored, including a comparison of round window injections versus placement of drug-delivering materials on the surface of the round window membrane, intracochlear injections to the base, apex, or deep into the scala tympani, and intralabyrinthine injections to the semicircular canal or vestibule (Salt et al., 2009). In developing new inner ear therapies, the potential effect of the location of delivery on drug efficacy must be balanced against the risks of delivery to that location. In this review, additional potential methods of delivery will be discussed including scala media delivery, systemic delivery with local activation, and methods to mitigate risks of intracochlear and intralabyrinthine access by using automated systems such as robotic surgery and micro-infusion pumps.
Current reagents for inner ear therapy in the clinic primarily consist of pharmaceuticals such as aminoglycosides and steroids. However, in the laboratory where future therapies are being developed, many other reagents have been explored, including neurotrophins, small molecule drugs, small interfering RNA (siRNA), gene editing reagents, and viral vectors. The proposed treatments have been aimed at a wide variety of targets in the inner ear such as epithelial cells, neurons, glia, connective tissue, infectious pathogens, and immune-mediating cells. Many of these reagents await translation to the clinic, and are discussed in further detail below.
2. Future methods of delivery
2.1 Alternative routes of delivery
Much of the experimental work on inner ear drug delivery has been carried out in animal models. Each species has its own anatomical features, posing unique drug delivery possibilities and challenges. The inner ear of humans is larger than that of rodents commonly used in research, making some aspects of approach and injection easier. However, rodents such as guinea pigs and chinchillas have a much more accessible cochlea because the otic capsule protrudes into the middle ear space and is easily visualized when the middle ear is accessed.
Reagents placed into endolymph in the scala media are in direct contact with the luminal surfaces of the epithelial cells lining the cochlear duct including hair cells, supporting cells, and marginal cells of the stria vascularis. Several laboratories have obtained data in animal models demonstrating that adenovirus viral vectors injected into perilymph do not express reporter genes in the epithelium of the cochlear duct (Excoffon et al., 2006; Ishimoto et al., 2002; Venail et al., 2007). In contrast, when injected into the scala media, robust gene expression is seen in supporting cells of the mature ear (Atkinson et al., 2014; Ishimoto et al., 2002; Kawamoto et al., 2003a; Sheffield et al., 2011; Venail et al., 2007).
Supporting cells are endogenous cells that can transdifferentiate into new hair cells following depletion of the original hair cells, a process that occurs spontaneously in the hearing sensory epithelium of all vertebrates other than mammals (Brignull et al., 2009), and can be induced to transdifferentiate by manipulating gene expression in the living mammalian cochlea (Atkinson et al., 2014; Kawamoto et al., 2003a; Mizutari et al., 2013). This potential to manipulate gene expression in supporting cells is currently the most important rationale for injecting into the scala media, but access to the scala media in the human ear is difficult with current technology. Implementation and optimization of robotic surgery using personalized cochlear anatomic maps may make scala media injection feasible in the future.
2.2 Systemic delivery with local activation
For ease of delivery, it would be optimal if a therapeutic reagent could be given systemically, reach the ear at the same concentration as other organs, and yet act in the ear only. Dedicated research effort is needed to advance delivery technology in this direction, as currently this option has not yet been developed. For instance, ear-specific activation following systemic delivery could conceivably be achieved by harnessing features specific to the ear to activate or induce release of the active ingredients of therapeutic molecules. One specific ear feature is the unique chemical composition of endolymph characterized by high concentration of potassium and 80 mV positive direct current potential, which is not found elsewhere in the body. Should medicinal chemistry succeed in designing a compound that becomes active when immersed in such a fluid, it should be possible to accomplish ear-specific activation. Another feature of the ear is its ability to receive high intensity acoustic energy. With amplification provided by the ossicles in the middle ear, it is possible to vibrate the inner ear fluids at a specific frequency with high intensity acoustic signal, potentially leading to the activation of reagents designed for activation by such a physical phenomenon. Advances in the design of bioactive molecules may accomplish these tasks and provide ear-specific therapy following systemic administration. Such reagents are not currently available to our knowledge, but could be developed using advanced medicinal chemistry and related fields of research.
Specific delivery methods also will be needed to improve outcomes of cochlear implants. Cochlear implants are very successful and useful, but some of their features could still be improved. The two most important limitations are low speech intelligibility in noisy environments, and lack of music appreciation (Kohlberg et al., 2014; Zeitler et al., 2008). These limitations are partly due to the current spread of each electrode, resulting in relatively low signal quality (Caldwell et al., 2017). One way to address these limitations is to increase the number of stimulating channels via use of light instead of electric stimulation with optogenetics, whereby light-sensitive channel proteins are inserted into the auditory nerve and photons are converted to trigger a neural impulse. Unlike electrical fields that spread in all directions, light can be directed and even focused. Therefore, light stimulation can gain a significantly increased number of independent stimulating sites with high spatial resolution.
The field of optogenetics uses channelrhodopsins which are green algae photoconverting proteins, and other similar proteins (Cho et al., 2016). The channels would need to be expressed in spiral ganglion neurons (SGNs), which could be accomplished by insertion with a viral vector. Preliminary data show the feasibility of optical stimulation of the auditory nerve in the deafened rodent cochlea genetically modified to express a channelrhodopsin (Moser, 2015; Richter et al., 2013). Future enhancement in gene transfection technology and improvement in the design of more efficient light sources and sound-to-light algorithms will likely improve optogenetic technology, leading to enhanced outcomes of cochlear implantation. It appears that optical stimulation has the potential to target well-defined SGN populations, which would allow a much higher number of independent stimulation channels (Hernandez et al., 2014). While this optogenetic technology holds promise for creating an improved cochlear implant of the future, improved delivery methods for inserting the channelrhodopsins or other transgenes into SGNs and assuring long term gene expression will be necessary prior to transition into clinical settings.
3. Future drug delivery and targeting systems
3.1 Cell-specific targeting via gene expression
Manipulation of gene expression can be used to accomplish several potential goals through up- or down-regulation of expression. The goals of such manipulations include protection, repair, and regeneration of specific cell types and functions. In all cases it is necessary to verify that potential side effects, listed in a later section of this review, would not be greater than the potential benefit. Once the therapeutic reagent is in the inner ear, it is necessary to target its expression or activity to a specific type of cells. If the delivery shuttle is a viral vector, specificity can be accomplished by tropism of the virus to a specific cell type, and/or by using cell-specific promoters to drive expression. Small interfering RNA (siRNA) reagents are also useful for blocking expression of specific genes to accomplish therapeutic goals related to dominant negative mutations (Maeda et al., 2005), or to manipulate pathways for enhancing hair cell regeneration (Jung et al., 2013). siRNA reagents currently lack some of the specificity of viruses, but the ability to deliver these reagents using a virus may partly resolve this limitation.
The condition of cells in the inner ear also needs to be considered when designing therapies. Deaf ears or ears with partial pathology such as incomplete or localized hair cell loss may not necessarily respond to the presence of viruses in the same way as normal ears. Experimental animal models will need to be used to characterize and optimize delivery vehicles, cell specificity and side effects, along with efficacy and outcomes for addressing different stages of pathology.
Recent advances in understanding epigenetic changes in chromatin in general (Teschendorff et al., 2017) and in the inner ear (Doetzlhofer et al., 2017; Layman et al., 2014) increase and enhance the arsenal of possibilities for therapy, as seen by recent data on the protective effects of chromatin remodeling (Chen et al., 2009; Chen et al., 2016; He et al., 2015). Future ability to reverse epigenetic modifications could enhance the responsiveness of cells to exogenous reagents by revealing desirable binding sites on the chromatin. Such approaches could benefit attempts to induce regeneration or to correct mutations using phenotypic rescue approaches.
3.2 Increasing viral vector specificity
In many cases, viral vectors are the most efficient gene transfer shuttles, and their cell-specific uptake can be tailored to some extent (Heilbronn et al., 2010). A variety of viral vectors have been used in animal models for inner ear therapy. These include adeno-associated virus (AAV), bovine adeno-associated virus, lentivirus, adenovirus, and others, as recently reviewed (Lustig et al., 2012; Sacheli et al., 2013). Several aspects of viral gene transfer need further study to advance their translation to clinical applicability. One major task is to establish whether data obtained using rodent animal models translates to the human ear. Moving experimentation to primates will be an important step in this process. Even as studies in primates advance toward human applications, experiments in rodents and other animals will remain useful for first-line assessments of novel gene transfer methods.
The longevity and turnover of target cells also needs to be considered in further detail. For instance, a study evaluating the overexpression of neurotrophic factors for enhancing spiral ganglion survival and sprouting revealed that neurotrophin levels declined weeks after AAV injection (Budenz et al., 2015), possibly due to death of mesothelial cells infected by the virus. Targeting cell populations with low turnover may enhance long-term gene expression when it is needed, particularly for maintaining specific cell populations or for gene replacement therapy in cases of genetic deafness.
The immune response to the vector is another important aspect of viral-mediated gene delivery. Animals in the laboratory are usually specific pathogen free, namely, they have not been previously exposed to viruses, unlike most humans who are the targets for viral therapy. For safe therapy, most viruses used as gene delivery vectors in the laboratory must be engineered to reduce the severity of the immune response they elicit. AAV vectors may represent an exception due to their theoretically less severe immune response, as they appear to be well tolerated in the visual system (Simonelli et al., 2010) and are likely to also be successful in the human therapy in the ear and other organs (Kay, 2015).
AAV vectors injected into the perilymph of animal models lead to gene expression in epithelial cells of the cochlear duct, and transfected cells are usually hair cells (Konishi et al., 2008; Lalwani et al., 1996; Shibata et al., 2009; Stone et al., 2005). Therefore, hair cell-specific therapies are feasible, especially for curing genetic diseases involving hair cell mutations, as has been recently shown in several mouse models (Akil et al., 2012; Askew et al., 2015; Isgrig et al., 2017). One limitation of using AAV vectors for therapy is that their small size limits the genes that can be delivered, but the technology for enhancing the gene load in AAV vectors is rapidly developing, as discussed below. Another area requiring further development is the treatment of adult ears. In most of studies cited above, the virus was injected into immature ears; future experiments will need to extend the feasibility of these treatments to mature ears.
3.3 Sequential drug delivery
For some therapeutic goals, it may be necessary to sequentially deliver reagents at different time points. Attempts to induce hair cell regeneration or to implant stem cells and guide their differentiation may require more than one administration of therapeutics. Partial, inefficient regeneration of hair cells has been accomplished with the use of a single agent, as shown in experiments with DAPT, a gamma-secretase inhibitor leading to Notch pathway inhibition (Mizutari et al., 2013), and Math1 gene transfer via adenovirus, leading to Atoh1 overexpression (Kawamoto et al., 2003a). It is very likely that combination therapies utilizing more than one reagent with sequential administration will be required to enhance efficacy. To accomplish this, a cannula could be placed in the cochlea and connected to an external pump or injectable reservoir, which would facilitate reloading of reagents. Implanted mini-osmotic pumps may also serve an important purpose for future applications requiring continued or sequential administration of therapeutics.
Manipulation of membranous barriers may enhance penetration and access of therapeutics. Viral vectors placed on the round window membrane do not readily diffuse into perilymph, however partial digestion of the round window membrane facilitates AAV transfection of inner hair cells in an animal model (Wang et al., 2012). Once viral vectors are in perilymph, their access to different cell types, especially those lining the scala media, may still be restricted. Molecules that transiently enhance the permeability of internal cochlear membranes such as Reissner’s membrane may be useful for enhancing spread of vectors from the perilymph to target cells in other areas. This could be accomplished by use of sodium hyaluronate (Healon) (Shibata et al., 2012) or by reagents that transiently open adherens junctions (Park et al., 2014).
3.4 Drug-device combination therapy
Combining an implantable device with simultaneous single application drug delivery is a strategy that is already used clinically (Bento et al., 2016). Access to the cochlea is required for placement of cochlear implants, which concurrently provides an opportunity for additional drug delivery or other procedures directly to the cochlea. Healon and dexamethasone are examples of reagents placed along with the cochlear implant electrode. Implants eluting dexamethasone have been tested in animals and found to be effective in reducing fibrosis and impedance as compared to controls (Wilk et al., 2016). As such, these implants are likely to be transitioning to clinical trials in the near future for longer-term secretion into perilymph. Future treatments may include other reagents that can enhance the biological substrate of the ear that receives the implant. The main goals would be to enhance preservation of SGNs and to induce sprouting of neurons to an area closer to the electrodes. Experiments in animals show efficacy of treatment with neurotrophins, but variability in outcomes is a limitation (Pfingst et al., 2017; Pfingst et al., 2015; Ramekers et al., 2015; Wise et al., 2010; Wise et al., 2011). Use of viruses for overexpression may also have to await further improvement in vector technology. Further, a better understanding of the site of depolarization elicited by electrical stimulation will help to determine the importance of neurite sprouting in the direction of the cochlear implant electrode. Preliminary results on the improvement of several objective measurable parameters in guinea pigs provide rationale for proceeding with this approach (Pfingst et al., 2017).
Recent data on combining cochlear implantation with plasmid-based transgene expression of neurotrophins provides a potentially useful non-viral method for gene delivery (Pinyon et al., 2014). In these studies, the implant electrode was used for delivering electroporation current, facilitating uptake of naked DNA by cochlear cells and leading to neurotrophin overexpression.
In the more distant future, it is feasible that patients will be able to self-regulate therapy into the ear. When efficacious and safe treatments for tinnitus, Ménière’s disease, and other diseases are available through diffusible reagents, it is conceivable that delivery of therapeutic reagents via a patient-controlled, permanently implanted mini-osmotic pump or other controllable reservoir may become a reality. Therapeutics in such a device could be aimed at changing cochlear fluid pressure, the level of the endocochlear potential, the extent of outer hair cell motility, thresholds of neuronal firing, or the concentration of potassium in the scala media.
4. Future targets of inner ear therapy
With the advancement of technology to improve the process of drug delivery to the inner ear as well as the ability to target specific cell types, the prospect of treating the underlying causes of otologic and neurotologic diseases becomes more realistic. Several emerging techniques in molecular biology, particularly within the realm of personalized medicine for genetic disorders and neoplasms, can potentially be applied to the inner ear. Here, we review some of these techniques and discuss their application to otologic disease.
4.1 Genetic hearing loss
Approximately half of all cases of congenital hearing loss are genetic. Of these, approximately 37% are due to sporadic causes and 63% due to inherited causes (Marazita et al., 1993). The majority of non-syndromic hearing loss is autosomal recessive, approximately 75 to 80%; most of the remaining 20 to 25% are autosomal dominant, and 1 to 1.5% are X-linked or mitochondrial (Chang, 2015; Kenneson et al., 2002). Mutations in GJB2 and SLC26A4 are responsible for the majority of autosomal recessive cases, but no single gene has been associated with the majority of autosomal dominant cases.
Current strategies for curing genetic disease include use of embryonic or somatic stem cells, gene transfer, gene editing, and RNA modification (O’Connor et al., 2006). With increased efficiency of sequencing techniques, as well as the use of preimplantation genetic diagnosis, the correction of underlying genetic mutations to allow for normal protein production will become possible. Gene therapy can in principle involve insertion of a transgene or editing of the existing mutated gene. For the latter case, one emerging area of research involves using the CRISPR-Cas gene editing system to modify gene mutations (Gao et al., 2018; Liang et al., 2015; Ma et al., 2017); however, there are still challenges to overcome to ensure that the correct gene is reliably altered without producing off-target mutations or mosaic cell populations within the embryo (Ledford, 2017).
Fetal gene therapy is another area likely to see development in the near future. Fetal gene therapy will be extremely important for phenotypic rescue in cases where post-natal treatment is too late because the affected gene is required for development and/or because the affected cells die prenatally. Many of the transfer experiments conducted on mice carrying a mutant gene are performed on neonatal animals (Akil et al., 2012; Askew et al., 2015; Isgrig et al., 2017; Yu et al., 2014); the equivalent human developmental stage is earlier than mid-gestation. The feasibility of modulating gene transfer at even earlier time points during development has been demonstrated by injections into the mouse otocyst (Bedrosian et al., 2006; Brigande et al., 2009; Depreux et al., 2016; Gubbels et al., 2008). The ability of in utero gene therapy to prevent hearing loss in an affected individual (Miwa et al., 2013) provides a strong motivation for continued research in this area.
In addition to the need for further research and development regarding the above techniques, genetic therapies face regulatory, economic, societal, and political barriers given the ethical issues surrounding gene modification of embryos (O’Connor et al., 2006). These must be addressed prior to broader implementation.
4.2 Neoplasms of the temporal bone and lateral cranial base
Neoplasms of the external, middle, and inner ear, as well as the lateral cranial base, can alter or impair hearing and balance and pose other health risks. These tumors are typically treated with a combination of surgical resection, radiotherapy, stereotactic radiosurgery, and chemotherapy. Many benign and malignant neoplastic pathologies can present in this area, including cutaneous squamous cell carcinomas and basal cell carcinomas of the external ear, and squamous cell carcinomas, adenocarcinomas, and endolymphatic sac tumors in the middle and inner ear (Devaney et al., 2005). Vestibular schwannomas represent the majority of tumors arising within the cerebellopontine angle, and both sporadic and neurofibromatosis type 2 (NF2) cases have been linked to mutations in merlin (also known as schwannomin) (Sughrue et al., 2011). A better understanding of the molecular biology of vestibular schwannomas, both in the epidemiology of NF2 gene mutations and function of the merlin protein, will assist in the development of targeted therapies to help limit morbidity and address residual disease (Sughrue et al., 2011). Some of these treatments will necessitate specific delivery methods to facilitate targeted therapy.
In the field of cancer biology in general, significant advances have been made to individualize treatment based on the biology of a patient’s particular tumor, with the goal of targeting cancer cells while sparing the patient from side effects that affect normal cells. Immunotherapy and suicide gene therapy are two approaches that can be used to target cancer cells. In the field of immunotherapy, two main approaches have been studied: therapeutic monoclonal antibodies that prevent cancer cells from evading the immune response, and adoptive cell transfer-based immunotherapy to genetically engineer T-cells to attack specific tumor-associated antigens (Khalil et al., 2016; Restifo et al., 2012; Topalian et al., 2016). The Federal Drug Administration has recently approved monoclonal antibodies that target the immune checkpoints of cytotoxic T-lymphocyte associated antigen 4 and programmed cell death protein 1, with promising results in the treatment of advanced-stage melanoma and non-small-cell lung cancer (Khalil et al., 2016; Topalian et al., 2016). Suicide gene therapy is another approach that targets cancer cells. Vehicles such as viral vectors, liposomes, or nanoparticles can transport suicide-inducing genes to specific cell types via recognition of receptors expressed on cancer cells (Zarogoulidis et al., 2013). Once delivered to the cell, there are many potential mechanisms of action including silencing gene expression, expression of intracellular antibodies blocking vital cellular pathways, and expression of caspases and deoxyribonucleases. One advantage of these targeted therapies is that they inherently reduce the risk of side effects, allowing systemic administration of higher doses than might otherwise be used, but efficiency may further be improved by local delivery. These therapies have not yet been tested specifically for neoplasms of the inner ear or lateral skull base, but the general strategies appear to be applicable to a broad spectrum of tumors.
4.3 Diseases with no clear etiology
There are many otologic and neurotologic disorders with limited pharmaceutical treatment options. Some of these include Ménière’s disease, sudden sensorineural hearing loss, autoimmune inner ear disease, and acute-onset idiopathic facial nerve paralysis. A better understanding of the etiology and/or pathologic processes that drive these diseases is needed to develop targeted therapies. However, because these disorders are localized to the ear, it is feasible that therapy for these conditions will involve one or more of the local delivery methods described above, which will also reduce the risk of systemic side effects. Currently, high-dose oral corticosteroids are frequently prescribed to treat sudden sensorineural hearing loss, Ménière’s disease, autoimmune inner ear disease, and facial nerve paralysis, but these drugs can impose many unwanted systemic side effects including weight gain, hypertension, hyperglycemia, osteoporosis, immunosuppression, and adrenal suppression. Furthermore, systemic therapy is not consistently effective, as is the case with oral corticosteroid therapy. Transtympanic drug delivery has to some extent mitigated systemic toxicities, but its efficacy is also inconsistent. Once appropriate therapeutic targets can be determined, optimal delivery methods can be determined.
4.4 Diagnostic limitations and solutions
Diagnostic tools for assessing the condition of the cochlea in humans are limited at present, thereby preventing further development and optimization of therapies. Otoacoustic emissions can disclose if outer hair cell motility is driving the active cochlea normally or not, but in the case of negative results, it is not possible to distinguish whether outer hair cells are absent or dysfunctional. Increased auditory brainstem response thresholds cannot distinguish between absence or dysfunction of the auditory nerve. Tests to evaluate the condition of the stria vascularis have not yet been developed. Thus, without the ability to ascertain the presence and functionality of hair cells, neurons, or other cells within the cochlea, effective reparative or regenerative therapies will be difficult to develop.
Precision medicine, a constantly evolving and improving medical entity, will likely contribute to improved diagnosis and more personalized treatment that is based on the patient’s genetic and genomic profiles, in addition to their symptoms. Tools like the OtoSCOPE at the University of Iowa (Sloan-Heggen et al., 2016; Taylor et al., 2013) provide improved diagnosis for identification of mutations involved in hereditary hearing loss. OtoSCOPE now provides comprehensive genetic testing for all genes implicated in non-syndromic hearing loss, some syndromic hearing loss, and Usher mutations. To advance future therapies, additional information will be needed to diagnose the condition of ears with hearing loss due to aging, ototoxicity, acoustic trauma, infections, inflammation, and combinations of these causes. Improved diagnostics will provide much needed detail about the condition of the cochlear tissue as well as providing enhanced guidance for increasing accuracy of delivery and minimizing side effects. Without improved diagnostic tools, it will be difficult to select and implement future therapies for specific ears.
5. Translation of inner ear drug delivery to clinical application
5.1 Access to the inner ear in humans
We provide here a brief summary of current methods used for inner ear drug delivery, leading to a discussion of risks and potential side effects, and future technological advances that could reduce or eliminate these risks. As discussed previously, there are two principal approaches currently employed to access the inner ear for local drug delivery. The first is transtympanic delivery, which is typically performed in an in-office setting using topical anesthesia to the tympanic membrane (TM). Typically, 0.5–1 milliliter of drug solution is delivered through a small diameter needle through the posterior aspect of the TM to fill the middle ear space, and the solution is allowed to bathe the round window membrane for a period of time by optimizing patient positioning to avoid drug loss through the Eustachian tube. With this method, side effects of delivery are minimized and higher doses of anesthesia are avoided, allowing this procedure to be done on the same day as a clinic visit.
The second method is intracochlear delivery, which is a more involved approach most likely to be done in an operating room under a general or IV sedative anesthetic. After anesthesia has been established, the TM is elevated to access the middle ear space and lateral face of the otic capsule, similar to what is done in a transcanal stapedotomy procedure for otosclerosis. Canalplasty to facilitate additional bone removal from the canal may be required to provide visualization of the otic capsule. This approach would afford access for injecting the drug solution directly into the inner ear through the round window membrane, through the stapes footplate, or potentially through a basal turn cochleostomy. Much smaller volumes of drug solution would be needed for intracochlear delivery than with transtympanic injection. Injection of the drug solution could be performed directly into the cochlear fluids through one of the sites above, or by utilizing a round window membrane microperforator device to enhance drug entry to the cochlea, as effectively demonstrated in laboratory animals (Kelso et al., 2015). After returning the TM to a normal position, the patient would then be discharged home the same day or after a one-night hospital stay depending on the short-term side effects of the procedure.
Other approaches to the inner ear could be entertained such as a mastoidectomy approach through a postauricular incision. This type of approach would afford access to various intracochlear locations for injection such as the semicircular canals, vestibule, round window, and different compartments of the cochlea; however, given the additional effort, time, and risk involved, this type of approach seems less likely to be utilized for inner ear drug delivery if one of the above two methods can provide adequate access for delivery.
Both the transtympanic and intracochlear approaches for inner ear drug delivery have short- and long-term risks, as with any procedure. Many of these risks would be common to the two procedures; however, an intracochlear approach would be expected to have more potential side effects. Below we discuss these risks and the management of potential complications.
5.2 Potential risks of inner ear drug delivery
Short-term risks include bleeding, infection, TM perforation, facial nerve weakness or paralysis, dysgeusia (taste change), perilymph fistula, vestibular manifestations such as vertigo or dizziness, pain, and ear fullness. Inner ear inflammation, serous labyrinthitis, or reparative granuloma (exaggeration of the normal reparative process) may also occur. Potential long-term risks include hearing loss, vestibulopathy, or leakage of drug into the cerebrospinal and subarachnoid spaces. Such leakage can result in contralateral ear side effects as well as meningitis or encephalitis. Some of these risks could be mitigated by careful pre-procedural clinical examination with otomicroscopy and/or preoperative imaging to ensure normal anatomy and lack of existing TM perforation or middle ear inflammation. The administration of periprocedural steroids may help minimize intracochlear trauma and subsequent inflammation, as has been the experience with other inner ear procedural manipulations such as cochlear implantation and stapedotomy procedures (Eastwood et al., 2010; Honeder et al., 2015).
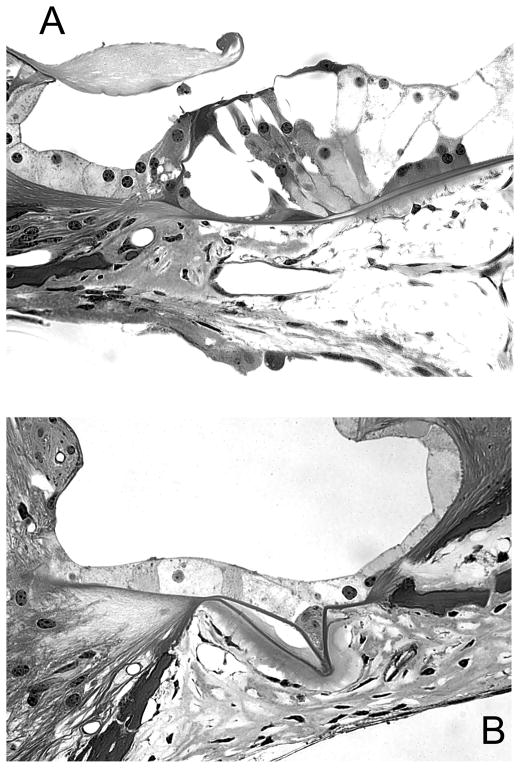
One common manifestation of adverse reaction of the cochlea to surgical manipulations is growth of connective tissue in the fluid spaces, also known as fibrosis (Fig. 1). Over many years of experimental procedures performed in our laboratory at Kresge Hearing Research Institute, we noted fibrosis in guinea pig ears following deafening, cochlear implant insertion, electrical stimulation, viral vector injection, and neurotrophin overexpression, alone, or in combination (Kang et al., 2010; Kawamoto et al., 2003b). The possibility that future methods performed on human ears may lead to similar fibrosis needs to be considered and addressed.
Figure 1.
Light micrographs of taken from the base of guinea pig cochleae that were implanted and stimulated without prior deafening (A) or following elimination of the hair cells (B). In both cases, fibrosis is seen in the scala tympani (with permission from JARO, Springer).
5.3 Future strategies to minimize risks
The short- and long-term risks associated with inner ear drug delivery may be further minimized by future technological or procedural developments. For example, optimization of drug delivery catheter design and size may help minimize intracochlear trauma and the size of the round window membrane/cochleostomy fenestration, which would reduce the risk of perilymph fistula. Similarly, the use of a minimally traumatic intracochlear catheter may allow for drug delivery to the more apical regions of the cochlear duct. In addition, a more potent biological effect could be obtained with the combination of a mini-osmotic pump for sustained delivery of the bioactive agent.
Another method to decrease the risk of intracochlear trauma and its downstream effects would be to curtail intracochlear pressure spikes upon infusion. Large intracochlear pressure spikes equivalent to a sound exposure of 115 dB have been demonstrated in studies of cochlear implant electrode insertion (Greene et al., 2016). Similar intracochlear pressure elevation could potentially be encountered with inner ear drug delivery. Robotic surgery is a promising method to minimize the risk of intracochlear trauma during cochleostomy, drug infusion, and device placement. Using preoperative high-resolution computed tomography for surgical planning and intraoperative navigation, the precise trajectory required for cochlear access can be planned by the surgeon, resulting in a minimally invasive surgical approach that minimizes trauma to the endosteal membrane and other cochlear tissues (Brett et al., 2007; Coulson et al., 2008; Majdani et al., 2009). In a comparison of manual versus robotic cochleostomy, there are significantly less intracochlear pressure disturbances using a robotic micro-drill (Assadi et al., 2013; Coulson et al., 2013; Dillon et al., 2016). A minimally traumatic cochleostomy can be achieved using an automatic or semi-automatic robotic drill, hand-guided robotic drill, or with laser (Brett et al., 2014; Hussong et al., 2008; Wimmer et al., 2014; Zhang et al., 2014). Robotic cochlear implant electrode insertion has also been validated in cadaveric models, demonstrating minimal insertion trauma and successful insertion in the majority of cases (McRackan et al., 2013; Schurzig et al., 2012; Venail et al., 2015). A robotic approach for cochlear implant insertion has already been tested in nine patients, with six radiographically confirmed insertions (Labadie et al., 2014). Unfortunately, one patient had permanent facial paralysis with this technique, which highlights the need for further refinement of targeting strategies prior to safe application of a robotic approach in cochlear implant insertions and other otological procedures. Further studies are required to refine the technique and test its broader applicability to a larger number of patients. Similarly, a robotic-assisted method for drug infusion and device placement could help mitigate the potentially damaging effects of intracochlear trauma.
With the refinement and increased availability of higher magnet strength MRI scanners, it may become possible to perform high resolution imaging of the cochlea after drug delivery. When paired with contrast administration, this could potentially identify evolving inner ear inflammation after drug delivery to enable prompt treatment with steroids and/or other anti-inflammatory medications, thereby reducing the risk of downstream adverse events.
In summary, there are numerous potential short- and long-term risks of inner ear drug delivery in clinical settings. Existing knowledge of how to minimize these risks, gained over decades of experience with stapedotomy and cochlear implantation, will be invaluable as novel inner ear biologics become a reality. In addition, novel technological developments in catheter design, micro-infusion pumps, robotic surgery, and high-resolution cochlear imaging may also provide alternative methods to ensure safety and optimize the efficacy of inner ear drug delivery in the future.
6. Conclusions
Inner ear delivery of a wider variety of reagents will provide a much broader range of treatment possibilities for otologic conditions.
There are several routes of delivery to the inner ear, most classically via a transtympanic or intracochlear approach, but many additional possibilities are being explored in animal models, including intralabyrinthine injections in various locations and direct injection into the scala media.
Many innovations in technology including optogenetics, cell-specific targeting, increased viral vector specificity, sequential drug-device combinations, gene editing, suicide gene therapy, and immunotherapy may be applied to the inner ear in the future.
Safer drug delivery may be accomplished with automated approaches such as robotic surgery and micro-infusion pumps, as well as with improved high-resolution imaging modalities for the inner ear.
In order to maximize potential therapies for the inner ear, future studies are needed to better understand the etiology of inner ear disorders such as Ménière’s disease and sudden sensorineural hearing loss, and to improve diagnostics of the condition of the cochlear cells, with details about their presence and functionality.
Supplementary Material
Inner ear delivery of a wider variety of reagents will provide a much broader range of treatment possibilities for otologic conditions.
Many innovations in may be applied to the inner ear in the future.
Safer drug delivery may benefit from automated approaches
In order to maximize potential therapies for the inner ear, future studies are needed to better understand the etiology of inner ear disorders such as Ménière’s disease and sudden sensorineural hearing loss, and to improve diagnostics of the condition of the cochlear cells, with details about their presence and functionality.
Acknowledgments
We thank Alex Arts, Noah Shohet and Don Swiderski for assistance with the manuscript. This work was supported by the R. Jamison and Betty Williams Professorship and NIH-NIDCD Grants T32 DC005356, R01-DC01634, R01-DC015809 R01-DC014832, R01-DC013912, R01-DC009410, R01-DC014456 and P30-DC05188.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Akil O, Seal RP, Burke K, Wang C, Alemi A, During M, Edwards RH, Lustig LR. Restoration of hearing in the VGLUT3 knockout mouse using virally mediated gene therapy. Neuron. 2012;75:283–93. doi: 10.1016/j.neuron.2012.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Askew C, Rochat C, Pan B, Asai Y, Ahmed H, Child E, Schneider BL, Aebischer P, Holt JR. Tmc gene therapy restores auditory function in deaf mice. Science translational medicine. 2015;7:295ra108. doi: 10.1126/scitranslmed.aab1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assadi MZ, Du X, Dalton J, Henshaw S, Coulson CJ, Reid AP, Proops DW, Brett PN. Comparison on intracochlear disturbances between drilling a manual and robotic cochleostomy. Proc Inst Mech Eng H. 2013;227:1002–8. doi: 10.1177/0954411913488507. [DOI] [PubMed] [Google Scholar]
- Atkinson PJ, Wise AK, Flynn BO, Nayagam BA, Richardson RT. Hair cell regeneration after ATOH1 gene therapy in the cochlea of profoundly deaf adult guinea pigs. PLoS One. 2014;9:e102077. doi: 10.1371/journal.pone.0102077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedrosian JC, Gratton MA, Brigande JV, Tang W, Landau J, Bennett J. In vivo delivery of recombinant viruses to the fetal murine cochlea: transduction characteristics and long-term effects on auditory function. Mol Ther. 2006;14:328–35. doi: 10.1016/j.ymthe.2006.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bento RF, Danieli F, Magalhaes AT, Gnansia D, Hoen M. Residual Hearing Preservation with the Evo(R) Cochlear Implant Electrode Array: Preliminary Results. Int Arch Otorhinolaryngol. 2016;20:353–358. doi: 10.1055/s-0036-1572530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brett P, Du X, Zoka-Assadi M, Coulson C, Reid A, Proops D. Feasibility study of a hand guided robotic drill for cochleostomy. BioMed research international. 2014;2014:656325. doi: 10.1155/2014/656325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brett PN, Taylor RP, Proops D, Coulson C, Reid A, Griffiths MV. A surgical robot for cochleostomy. Conf Proc IEEE Eng Med Biol Soc. 2007;2007:1229–32. doi: 10.1109/IEMBS.2007.4352519. [DOI] [PubMed] [Google Scholar]
- Brigande JV, Heller S. Quo vadis, hair cell regeneration? Nat Neurosci. 2009;12:679–685. doi: 10.1038/nn.2311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brignull HR, Raible DW, Stone JS. Feathers and fins: non-mammalian models for hair cell regeneration. Brain Res. 2009;1277:12–23. doi: 10.1016/j.brainres.2009.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budenz CL, Wong HT, Swiderski DL, Shibata SB, Pfingst BE, Raphael Y. Differential effects of AAV.BDNF and AAV.Ntf3 in the deafened adult guinea pig ear. Sci Rep. 2015;5:8619. doi: 10.1038/srep08619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldwell MT, Jiam NT, Limb CJ. Assessment and improvement of sound quality in cochlear implant users. Laryngoscope Investig Otolaryngol. 2017;2:119–124. doi: 10.1002/lio2.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang KW. Genetics of Hearing Loss--Nonsyndromic. Otolaryngol Clin North Am. 2015;48:1063–72. doi: 10.1016/j.otc.2015.06.005. [DOI] [PubMed] [Google Scholar]
- Chen FQ, Schacht J, Sha SH. Aminoglycoside-induced histone deacetylation and hair cell death in the mouse cochlea. J Neurochem. 2009;108:1226–36. doi: 10.1111/j.1471-4159.2009.05871.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Hill K, Sha SH. Inhibitors of Histone Deacetylases Attenuate Noise-Induced Hearing Loss. J Assoc Res Otolaryngol. 2016;17:289–302. doi: 10.1007/s10162-016-0567-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho YK, Li D. Optogenetics: Basic Concepts and Their Development. Methods Mol Biol. 2016;1408:1–17. doi: 10.1007/978-1-4939-3512-3_1. [DOI] [PubMed] [Google Scholar]
- Coulson CJ, Taylor RP, Reid AP, Griffiths MV, Proops DW, Brett PN. An autonomous surgical robot for drilling a cochleostomy: preliminary porcine trial. Clin Otolaryngol. 2008;33:343–7. doi: 10.1111/j.1749-4486.2008.01703.x. [DOI] [PubMed] [Google Scholar]
- Coulson CJ, Assadi MZ, Taylor RP, Du X, Brett PN, Reid AP, Proops DW. A smart micro-drill for cochleostomy formation: a comparison of cochlear disturbances with manual drilling and a human trial. Cochlear implants international. 2013;14:98–106. doi: 10.1179/1754762811Y.0000000018. [DOI] [PubMed] [Google Scholar]
- Depreux FF, Wang L, Jiang H, Jodelka FM, Rosencrans RF, Rigo F, Lentz JJ, Brigande JV, Hastings ML. Antisense oligonucleotides delivered to the amniotic cavity in utero modulate gene expression in the postnatal mouse. Nucleic Acids Res. 2016;44:9519–9529. doi: 10.1093/nar/gkw867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devaney KO, Boschman CR, Willard SC, Ferlito A, Rinaldo A. Tumours of the external ear and temporal bone. Lancet Oncol. 2005;6:411–20. doi: 10.1016/S1470-2045(05)70208-4. [DOI] [PubMed] [Google Scholar]
- Dillon NP, Fichera L, Wellborn PS, Labadie RF, Webster RJ., 3rd Making Robots Mill Bone More Like Human Surgeons: Using Bone Density and Anatomic Information to Mill Safely and Efficiently. Rep U S. 2016;2016:1837–1843. doi: 10.1109/IROS.2016.7759292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doetzlhofer A, Avraham KB. Insights into inner ear-specific gene regulation: Epigenetics and non-coding RNAs in inner ear development and regeneration. Semin Cell Dev Biol. 2017;65:69–79. doi: 10.1016/j.semcdb.2016.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eastwood H, Chang A, Kel G, Sly D, Richardson R, O’Leary SJ. Round window delivery of dexamethasone ameliorates local and remote hearing loss produced by cochlear implantation into the second turn of the guinea pig cochlea. Hear Res. 2010;265:25–9. doi: 10.1016/j.heares.2010.03.006. [DOI] [PubMed] [Google Scholar]
- Excoffon KJ, Avenarius MR, Hansen MR, Kimberling WJ, Najmabadi H, Smith RJ, Zabner J. The Coxsackievirus and Adenovirus Receptor: A new adhesion protein in cochlear development. Hear Res. 2006;215:1–9. doi: 10.1016/j.heares.2006.02.009. [DOI] [PubMed] [Google Scholar]
- Gao X, Tao Y, Lamas V, Huang M, Yeh WH, Pan B, Hu YJ, Hu JH, Thompson DB, Shu Y, Li Y, Wang H, Yang S, Xu Q, Polley DB, Liberman MC, Kong WJ, Holt JR, Chen ZY, Liu DR. Treatment of autosomal dominant hearing loss by in vivo delivery of genome editing agents. Nature. 2018;553:217–221. doi: 10.1038/nature25164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene NT, Mattingly JK, Banakis Hartl RM, Tollin DJ, Cass SP. Intracochlear Pressure Transients During Cochlear Implant Electrode Insertion. Otol Neurotol. 2016;37:1541–1548. doi: 10.1097/MAO.0000000000001232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gubbels SP, Woessner DW, Mitchell JC, Ricci AJ, Brigande JV. Functional auditory hair cells produced in the mammalian cochlea by in utero gene transfer. Nature. 2008;455:537–41. doi: 10.1038/nature07265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Y, Yu H, Cai C, Sun S, Chai R, Li H. Inhibition of H3K4me2 Demethylation Protects Auditory Hair Cells from Neomycin-Induced Apoptosis. Mol Neurobiol. 2015;52:196–205. doi: 10.1007/s12035-014-8841-3. [DOI] [PubMed] [Google Scholar]
- Heilbronn R, Weger S. Viral vectors for gene transfer: current status of gene therapeutics. Handbook of experimental pharmacology. 2010:143–70. doi: 10.1007/978-3-642-00477-3_5. [DOI] [PubMed] [Google Scholar]
- Hernandez VH, Gehrt A, Jing Z, Hoch G, Jeschke M, Strenzke N, Moser T. Optogenetic stimulation of the auditory nerve. Journal of visualized experiments: JoVE. 2014:e52069. doi: 10.3791/52069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honeder C, Landegger LD, Engleder E, Gabor F, Plasenzotti R, Plenk H, Kaider A, Hirtler L, Gstoettner W, Arnoldner C. Effects of intraoperatively applied glucocorticoid hydrogels on residual hearing and foreign body reaction in a guinea pig model of cochlear implantation. Acta Otolaryngol. 2015;135:313–9. doi: 10.3109/00016489.2014.986758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussong A, Rau T, Eilers H, Baron S, Heimann B, Leinung M, Lenarz T, Majdani O. Conception and design of an automated insertion tool for cochlear implants. Conf Proc IEEE Eng Med Biol Soc. 2008;2008:5593–6. doi: 10.1109/IEMBS.2008.4650482. [DOI] [PubMed] [Google Scholar]
- Isgrig K, Shteamer JW, Belyantseva IA, Drummond MC, Fitzgerald TS, Vijayakumar S, Jones SM, Griffith AJ, Friedman TB, Cunningham LL, Chien WW. Gene Therapy Restores Balance and Auditory Functions in a Mouse Model of Usher Syndrome. Mol Ther. 2017;25:780–791. doi: 10.1016/j.ymthe.2017.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishimoto S, Kawamoto K, Kanzaki S, Raphael Y. Gene transfer into supporting cells of the organ of Corti. Hear Res. 2002;173:187–97. doi: 10.1016/s0378-5955(02)00579-8. [DOI] [PubMed] [Google Scholar]
- Jung JY, Avenarius MR, Adamsky S, Alpert E, Feinstein E, Raphael Y. siRNA targeting Hes5 augments hair cell regeneration in aminoglycoside-damaged mouse utricle. Mol Ther. 2013;21:834–41. doi: 10.1038/mt.2013.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang SY, Colesa DJ, Swiderski DL, Su GL, Raphael Y, Pfingst BE. Effects of hearing preservation on psychophysical responses to cochlear implant stimulation. J Assoc Res Otolaryngol. 2010;11:245–65. doi: 10.1007/s10162-009-0194-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamoto K, Ishimoto S, Minoda R, Brough DE, Raphael Y. Math1 gene transfer generates new cochlear hair cells in mature guinea pigs in vivo. J Neurosci. 2003a;23:4395–400. doi: 10.1523/JNEUROSCI.23-11-04395.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamoto K, Yagi M, Stover T, Kanzaki S, Raphael Y. Hearing and hair cells are protected by adenoviral gene therapy with TGF-beta1 and GDNF. Mol Ther. 2003b;7:484–92. doi: 10.1016/s1525-0016(03)00058-3. [DOI] [PubMed] [Google Scholar]
- Kay MA. Selecting the Best AAV Capsid for Human Studies. Mol Ther. 2015;23:1800–1. doi: 10.1038/mt.2015.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelso CM, Watanabe H, Wazen JM, Bucher T, Qian ZJ, Olson ES, Kysar JW, Lalwani AK. Microperforations significantly enhance diffusion across round window membrane. Otol Neurotol. 2015;36:694–700. doi: 10.1097/MAO.0000000000000629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenneson A, Van Naarden Braun K, Boyle C. GJB2 (connexin 26) variants and nonsyndromic sensorineural hearing loss: a HuGE review. Genet Med. 2002;4:258–74. doi: 10.1097/00125817-200207000-00004. [DOI] [PubMed] [Google Scholar]
- Khalil DN, Smith EL, Brentjens RJ, Wolchok JD. The future of cancer treatment: immunomodulation, CARs and combination immunotherapy. Nat Rev Clin Oncol. 2016;13:394. doi: 10.1038/nrclinonc.2016.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohlberg G, Spitzer JB, Mancuso D, Lalwani AK. Does cochlear implantation restore music appreciation? Laryngoscope. 2014;124:587–8. doi: 10.1002/lary.24171. [DOI] [PubMed] [Google Scholar]
- Konishi M, Kawamoto K, Izumikawa M, Kuriyama H, Yamashita T. Gene transfer into guinea pig cochlea using adeno-associated virus vectors. The journal of gene medicine. 2008;10:610–8. doi: 10.1002/jgm.1189. [DOI] [PubMed] [Google Scholar]
- Labadie RF, Balachandran R, Noble JH, Blachon GS, Mitchell JE, Reda FA, Dawant BM, Fitzpatrick JM. Minimally invasive image-guided cochlear implantation surgery: first report of clinical implementation. Laryngoscope. 2014;124:1915–22. doi: 10.1002/lary.24520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lalwani AK, Walsh BJ, Reilly PG, Muzyczka N, Mhatre AN. Development of in vivo gene therapy for hearing disorders: introduction of adeno-associated virus into the cochlea of the guinea pig. Gene Ther. 1996;3:588–92. [PubMed] [Google Scholar]
- Layman WS, Zuo J. Epigenetic regulation in the inner ear and its potential roles in development, protection, and regeneration. Frontiers in cellular neuroscience. 2014;8:446. doi: 10.3389/fncel.2014.00446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledford H. CRISPR fixes disease gene in viable human embryos. Nature. 2017;548:13–14. doi: 10.1038/nature.2017.22382. [DOI] [PubMed] [Google Scholar]
- Liang P, Xu Y, Zhang X, Ding C, Huang R, Zhang Z, Lv J, Xie X, Chen Y, Li Y, Sun Y, Bai Y, Songyang Z, Ma W, Zhou C, Huang J. CRISPR/Cas9-mediated gene editing in human tripronuclear zygotes. Protein Cell. 2015;6:363–372. doi: 10.1007/s13238-015-0153-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lustig LR, Akil O. Cochlear gene therapy. Curr Opin Neurol. 2012;25:57–60. doi: 10.1097/WCO.0b013e32834f038c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma H, Marti-Gutierrez N, Park SW, Wu J, Lee Y, Suzuki K, Koski A, Ji D, Hayama T, Ahmed R, Darby H, Van Dyken C, Li Y, Kang E, Park AR, Kim D, Kim ST, Gong J, Gu Y, Xu X, Battaglia D, Krieg SA, Lee DM, Wu DH, Wolf DP, Heitner SB, Belmonte JCI, Amato P, Kim JS, Kaul S, Mitalipov S. Correction of a pathogenic gene mutation in human embryos. Nature. 2017;548:413–419. doi: 10.1038/nature23305. [DOI] [PubMed] [Google Scholar]
- Maeda Y, Fukushima K, Nishizaki K, Smith RJ. In vitro and in vivo suppression of GJB2 expression by RNA interference. Hum Mol Genet. 2005;14:1641–50. doi: 10.1093/hmg/ddi172. [DOI] [PubMed] [Google Scholar]
- Majdani O, Rau TS, Baron S, Eilers H, Baier C, Heimann B, Ortmaier T, Bartling S, Lenarz T, Leinung M. A robot-guided minimally invasive approach for cochlear implant surgery: preliminary results of a temporal bone study. Int J Comput Assist Radiol Surg. 2009;4:475–86. doi: 10.1007/s11548-009-0360-8. [DOI] [PubMed] [Google Scholar]
- Marazita ML, Ploughman LM, Rawlings B, Remington E, Arnos KS, Nance WE. Genetic epidemiological studies of early-onset deafness in the U.S. school-age population. Am J Med Genet. 1993;46:486–91. doi: 10.1002/ajmg.1320460504. [DOI] [PubMed] [Google Scholar]
- McRackan TR, Balachandran R, Blachon GS, Mitchell JE, Noble JH, Wright CG, Fitzpatrick JM, Dawant BM, Labadie RF. Validation of minimally invasive, image-guided cochlear implantation using Advanced Bionics, Cochlear, and Medel electrodes in a cadaver model. Int J Comput Assist Radiol Surg. 2013;8:989–95. doi: 10.1007/s11548-013-0842-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miwa T, Minoda R, Ise M, Yamada T, Yumoto E. Mouse otocyst transuterine gene transfer restores hearing in mice with connexin 30 deletion-associated hearing loss. Mol Ther. 2013;21:1142–50. doi: 10.1038/mt.2013.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizutari K, Fujioka M, Hosoya M, Bramhall N, Okano HJ, Okano H, Edge AS. Notch inhibition induces cochlear hair cell regeneration and recovery of hearing after acoustic trauma. Neuron. 2013;77:58–69. doi: 10.1016/j.neuron.2012.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moser T. Optogenetic stimulation of the auditory pathway for research and future prosthetics. Curr Opin Neurobiol. 2015;34:29–36. doi: 10.1016/j.conb.2015.01.004. [DOI] [PubMed] [Google Scholar]
- O’Connor TP, Crystal RG. Genetic medicines: treatment strategies for hereditary disorders. Nat Rev Genet. 2006;7:261–76. doi: 10.1038/nrg1829. [DOI] [PubMed] [Google Scholar]
- Park YH, Wilson KF, Ueda Y, Tung Wong H, Beyer LA, Swiderski DL, Dolan DF, Raphael Y. Conditioning the cochlea to facilitate survival and integration of exogenous cells into the auditory epithelium. Mol Ther. 2014;22:873–80. doi: 10.1038/mt.2013.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfingst BE, Colesa DJ, Swiderski DL, Hughes AP, Strahl SB, Sinan M, Raphael Y. Neurotrophin Gene Therapy in Deafened Ears with Cochlear Implants: Long-term Effects on Nerve Survival and Functional Measures. J Assoc Res Otolaryngol. 2017 doi: 10.1007/s10162-017-0633-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfingst BE, Zhou N, Colesa DJ, Watts MM, Strahl SB, Garadat SN, Schvartz-Leyzac KC, Budenz CL, Raphael Y, Zwolan TA. Importance of cochlear health for implant function. Hear Res. 2015;322:77–88. doi: 10.1016/j.heares.2014.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinyon JL, Tadros SF, Froud KE, ACYW, Tompson IT, Crawford EN, Ko M, Morris R, Klugmann M, Housley GD. Close-field electroporation gene delivery using the cochlear implant electrode array enhances the bionic ear. Science translational medicine. 2014;6:233ra54. doi: 10.1126/scitranslmed.3008177. [DOI] [PubMed] [Google Scholar]
- Ramekers D, Versnel H, Strahl SB, Klis SF, Grolman W. Temporary Neurotrophin Treatment Prevents Deafness-Induced Auditory Nerve Degeneration and Preserves Function. J Neurosci. 2015;35:12331–45. doi: 10.1523/JNEUROSCI.0096-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Restifo NP, Dudley ME, Rosenberg SA. Adoptive immunotherapy for cancer: harnessing the T cell response. Nat Rev Immunol. 2012;12:269–81. doi: 10.1038/nri3191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter CP, Rajguru S, Bendett M. Infrared neural stimulation in the cochlea. Proc SPIE Int Soc Opt Eng. 2013;8565:85651Y. doi: 10.1117/12.2010337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacheli R, Delacroix L, Vandenackerveken P, Nguyen L, Malgrange B. Gene transfer in inner ear cells: a challenging race. Gene Ther. 2013;20:237–47. doi: 10.1038/gt.2012.51. [DOI] [PubMed] [Google Scholar]
- Salt AN, Plontke SK. Principles of local drug delivery to the inner ear. Audiol Neurootol. 2009;14:350–60. doi: 10.1159/000241892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schurzig D, Labadie RF, Hussong A, Rau TS, Webster RJ., 3rd Design of a Tool Integrating Force Sensing With Automated Insertion in Cochlear Implantation. IEEE ASME Trans Mechatron. 2012;17:381–389. doi: 10.1109/TMECH.2011.2106795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheffield AM, Gubbels SP, Hildebrand MS, Newton SS, Chiorini JA, Di Pasquale G, Smith RJ. Viral vector tropism for supporting cells in the developing murine cochlea. Hear Res. 2011;277:28–36. doi: 10.1016/j.heares.2011.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibata SB, Di Pasquale G, Cortez SR, Chiorini JA, Raphael Y. Gene transfer using bovine adeno-associated virus in the guinea pig cochlea. Gene Ther. 2009;16:990–7. doi: 10.1038/gt.2009.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibata SB, Cortez SR, Wiler JA, Swiderski DL, Raphael Y. Hyaluronic acid enhances gene delivery into the cochlea. Hum Gene Ther. 2012;23:302–10. doi: 10.1089/hum.2011.086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonelli F, Maguire AM, Testa F, Pierce EA, Mingozzi F, Bennicelli JL, Rossi S, Marshall K, Banfi S, Surace EM, Sun J, Redmond TM, Zhu X, Shindler KS, Ying GS, Ziviello C, Acerra C, Wright JF, McDonnell JW, High KA, Bennett J, Auricchio A. Gene therapy for Leber’s congenital amaurosis is safe and effective through 1.5 years after vector administration. Mol Ther. 2010;18:643–50. doi: 10.1038/mt.2009.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sloan-Heggen CM, Smith RJ. Navigating genetic diagnostics in patients with hearing loss. Curr Opin Pediatr. 2016;28:705–712. doi: 10.1097/MOP.0000000000000410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone IM, Lurie DI, Kelley MW, Poulsen DJ. Adeno-associated virus-mediated gene transfer to hair cells and support cells of the murine cochlea. Mol Ther. 2005;11:843–8. doi: 10.1016/j.ymthe.2005.02.005. [DOI] [PubMed] [Google Scholar]
- Sughrue ME, Yeung AH, Rutkowski MJ, Cheung SW, Parsa AT. Molecular biology of familial and sporadic vestibular schwannomas: implications for novel therapeutics. J Neurosurg. 2011;114:359–66. doi: 10.3171/2009.10.JNS091135. [DOI] [PubMed] [Google Scholar]
- Taylor KR, Deluca AP, Shearer AE, Hildebrand MS, Black-Ziegelbein EA, Anand VN, Sloan CM, Eppsteiner RW, Scheetz TE, Huygen PL, Smith RJ, Braun TA, Casavant TL. AudioGene: predicting hearing loss genotypes from phenotypes to guide genetic screening. Hum Mutat. 2013;34:539–45. doi: 10.1002/humu.22268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teschendorff AE, Relton CL. Statistical and integrative system-level analysis of DNA methylation data. Nat Rev Genet. 2017 doi: 10.1038/nrg.2017.86. [DOI] [PubMed] [Google Scholar]
- Topalian SL, Taube JM, Anders RA, Pardoll DM. Mechanism-driven biomarkers to guide immune checkpoint blockade in cancer therapy. Nat Rev Cancer. 2016;16:275–87. doi: 10.1038/nrc.2016.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venail F, Wang J, Ruel J, Ballana E, Rebillard G, Eybalin M, Arbones M, Bosch A, Puel JL. Coxsackie adenovirus receptor and alpha nu beta3/alpha nu beta5 integrins in adenovirus gene transfer of rat cochlea. Gene Ther. 2007;14:30–7. doi: 10.1038/sj.gt.3302826. [DOI] [PubMed] [Google Scholar]
- Venail F, Bell B, Akkari M, Wimmer W, Williamson T, Gerber N, Gavaghan K, Canovas F, Weber S, Caversaccio M, Uziel A. Manual Electrode Array Insertion Through a Robot-Assisted Minimal Invasive Cochleostomy: Feasibility and Comparison of Two Different Electrode Array Subtypes. Otol Neurotol. 2015;36:1015–22. doi: 10.1097/MAO.0000000000000741. [DOI] [PubMed] [Google Scholar]
- Wang H, Murphy R, Taaffe D, Yin S, Xia L, Hauswirth WW, Bance M, Robertson GS, Wang J. Efficient cochlear gene transfection in guinea-pigs with adeno-associated viral vectors by partial digestion of round window membrane. Gene Ther. 2012;19:255–63. doi: 10.1038/gt.2011.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilk M, Hessler R, Mugridge K, Jolly C, Fehr M, Lenarz T, Scheper V. Impedance Changes and Fibrous Tissue Growth after Cochlear Implantation Are Correlated and Can Be Reduced Using a Dexamethasone Eluting Electrode. PLoS One. 2016;11:e0147552. doi: 10.1371/journal.pone.0147552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wimmer W, Venail F, Williamson T, Akkari M, Gerber N, Weber S, Caversaccio M, Uziel A, Bell B. Semiautomatic cochleostomy target and insertion trajectory planning for minimally invasive cochlear implantation. BioMed research international. 2014;2014:596498. doi: 10.1155/2014/596498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise AK, Hume CR, Flynn BO, Jeelall YS, Suhr CL, Sgro BE, O’Leary SJ, Shepherd RK, Richardson RT. Effects of localized neurotrophin gene expression on spiral ganglion neuron resprouting in the deafened cochlea. Mol Ther. 2010;18:1111–22. doi: 10.1038/mt.2010.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise AK, Tu T, Atkinson PJ, Flynn BO, Sgro BE, Hume C, O’Leary SJ, Shepherd RK, Richardson RT. The effect of deafness duration on neurotrophin gene therapy for spiral ganglion neuron protection. Hear Res. 2011;278:69–76. doi: 10.1016/j.heares.2011.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Q, Wang Y, Chang Q, Wang J, Gong S, Li H, Lin X. Virally expressed connexin26 restores gap junction function in the cochlea of conditional Gjb2 knockout mice. Gene Ther. 2014;21:71–80. doi: 10.1038/gt.2013.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarogoulidis P, Darwiche K, Sakkas A, Yarmus L, Huang H, Li Q, Freitag L, Zarogoulidis K, Malecki M. Suicide Gene Therapy for Cancer - Current Strategies. J Genet Syndr Gene Ther. 2013:4. doi: 10.4172/2157-7412.1000139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeitler DM, Kessler MA, Terushkin V, Roland TJ, Jr, Svirsky MA, Lalwani AK, Waltzman SB. Speech perception benefits of sequential bilateral cochlear implantation in children and adults: a retrospective analysis. Otol Neurotol. 2008;29:314–25. doi: 10.1097/mao.0b013e3181662cb5. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Pfeiffer T, Weller M, Wieser W, Huber R, Raczkowsky J, Schipper J, Worn H, Klenzner T. Optical coherence tomography guided laser cochleostomy: towards the accuracy on tens of micrometer scale. BioMed research international. 2014;2014:251814. doi: 10.1155/2014/251814. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.