Abstract
Background:
Cardiovascular risk factors (CVRFs) and endothelial dysfunction have been associated independently with poorer neurocognition in middle-age adults, particularly on tests of frontal-lobe function. However, to our knowledge, no studies have examined markers of microvascular dysfunction and neurocognition or the potential interaction between macro and microvascular biomarkers on neurocognition. Therefore in a secondary analysis of a previously reported clinical trial, we examined this association in adults with major depressive disorder (MDD) in middle-aged and older adults.
Methods:
Participants included 202 adults with MDD and not receiving mental health treatment. Microvascular endothelial function was assessed using a non-invasive marker of forearm reactive hyperemia velocity while macrovascular endothelial function was assessed using flow mediated dilation (FMD) of the brachial artery. CVRFs were assessed using the Framingham Stroke Risk Profile and fasting lipid levels. A standardized neurocognitive assessment battery was used to assess three cognitive domains (Executive Function, Working Memory, and Verbal Memory).
Results:
Greater microvascular dysfunction was associated with poorer neurocognition across all three domains. In multiple regression analyses, microvascular function continued to predict Verbal Memory performance after accounting for background factors and CVRFs. Macro and microvascular function interacted to predict Working Memory performance (F = 4.511, 178, P = .035), with a similar non-significant association for Executive Function (F = 2.731, 178, P = .095), with moderate associations observed between microvascular function and neurocognition in the presence of preserved FMD (r61 = 0.40, P = .001), but not when FMD was impaired (r63 = −0.05, P = .675).
Conclusions:
Greater microvascular dysfunction is associated with poorer neurocognition among middle-aged and older adults. This association was strongest in participants with preserved macrovascular function, suggesting that it may provide a sensitive correlate of neurocognition that is superseded by systemic vascular dysfunction.
INTRODUCTION
Cardiovascular risk factors (CVRFs) and subclinical vascular disease are increasingly recognized as important contributors to cognitive decline and incident dementia.(1) CVRFs, particularly hypertension, are among the most modifiable risk factors for Alzheimer’s disease and vascular dementia.(2) In addition, CVRFs have been associated with subclinical cognitive decrements far preceding the development of clinically significant cognitive impairment. (3-7) Although the precise mechanisms underlying the association of CVRF and cognitive impairment have not been fully elucidated, microvascular disease has been suggested to play a central role in the pathogenesis of vascular cognitive impairment,(8) and recent studies have therefore begun to incorporate peripheral microvascular markers as predictors of neurocognitive performance.(9, 10)
Growing evidence suggests that peripheral markers of vascular dysfunction, including endothelial dysfunction, are associated with cognitive performance among middle-aged (11-13) and older adults (9, 14) with CVRFs. These associations may be particularly relevant for depressed adults, who exhibit a higher incidence of microvascular dysfunction (15, 16) and cognitive impairment (17). Peripheral markers of vascular dysfunction have been shown to be most closely associated with performance on cognitive tests of frontal-lobe functioning, including executive function and working memory.(11, 18, 19) Moreover, available evidence suggests that subtle impairments in endothelial dysfunction mediate the observed associations between CVRF and cognitive impairment among middle-aged adults.(11) In addition, preliminary evidence suggests that the influence of microvascular function on neurocognition may be moderated by systemic, macrovascular function, which may supersede the impact of microvascular dysfunction in some domains of cognitive function.(20) To our knowledge, no studies have examined the association between peripheral markers of microvascular dysfunction and cognitive performance. We previously reported that CVRFs and biomarkers of atherosclerosis were associated with impaired executive function and working memory (11, 12), as well as MRI markers of microvascular dysfunction (21), and that FMD mediated these associations. In the present study, we examined the association between microvascular dysfunction and neurocognition, as well as the potential influence of macrovascular function in a convenience sample of middle-aged and older adults with major depressive disorder and comorbid CVRFs. Specifically, we utilized data from the SMILE-II randomized trial: a 4-month randomized, parallel group, placebo-controlled trial of exercise and sertraline treatment for MDD.(22) Results revealed that aerobic exercise treatment was associated with comparable improvements in depression outcomes compared with Sertraline.(22) In addition to examining treatment changes in depression, measures of neurocognition were also obtained, although neurocognition did not improve with treatment.(23)
METHODS
The present study represents a secondary, post hoc analysis of novel microvascular endothelial markers and neurocognition from participants in the previously published SMILE-II randomized trial.(11, 21-24) We have previously examined markers of subclinical vascular disease and neurocognition (11, 21) in this cohort and the present study extends these findings by examining a novel, recently validated marker of microvascular functioning (25) that was unavailable at the time of our original analyses.(11) As described previously, (11, 22) participants were recruited between October 2000 and November 2005. In addition to the presence of MDD, eligibility criteria included age ≥40 years, not currently exercising, and no current psychiatric treatment. Exclusion criteria included the presence of another primary psychiatric diagnosis, such as a history of bipolar disorder or psychosis; and medical comorbidities that would preclude participation in the trial. All participants provided written informed consent and the protocol was approved by the Institutional Review Board at Duke University Medical Center. All data in the present analyses were taken from baseline assessments, collected 1-2 weeks prior to randomization.
Neurocognitive Performance
Details of our neurocognitive test battery have been reported in our prior publications utilizing neurocognitive outcomes from SMILE-II. (11, 23) Briefly, participants completed a neuropsychological test battery in order to assess performance in the domains of Executive Function and Psychomotor Speed. Neurocognitive tests were selected for the availability of multiple test versions, well-established psychometric properties, and accepted clinical utility.
Executive Function subtests included the Trail Making Test (B-A),(26) the Stroop Test (Word, Color, and Color-Word sections),(27) the Verbal Paired Associates test,(28) the Controlled Oral Word Association Test (COWAT),(29) the Digit Span test,(28) and the Verbal Fluency Test (Animal Naming).(29, 30) Psychomotor Speed subtests included the Ruff 2 & 7 Test,(31) and the Digit Symbol Substitution Test.(32) In addition, both the Stroop Test and the COWAT also loaded on our Psychomotor Speed variable. Details regarding the administration and scoring of these tests are provided elsewhere.(11, 23)
Cardiovascular Risk Factors
Details regarding the assessment of CVRFs have been previously reported.(11, 33) Briefly, our primary measure of CVD risk factors used the Framingham Stroke Risk Profile (FSRP), a risk assessment tool used to assess the 10-year incidence of stroke using systolic blood pressure, use of antihypertensive therapy, diabetes mellitus, cigarette smoking, cardiovascular disease, and atrial fibrillation.(34) (35) Because age served as a covariate in our final analyses, it was not included in calculating FSRP scores. We also included the more contemporary Atherosclerotic Disease Risk Score (ASCVD) in our cross-sectional analyses. (36).
Cardiovascular and Microvascular Biomarker Assessments
As previously reported, multiple measures of subclinical vascular disease were collected.(11, 33)
Flow mediated dilation (FMD) of the brachial artery was used to quantify both systemic, macrovascular dysfunction (FMD) and microvascular dysfunction (hyperemic velocity). FMD was assessed in the morning, after overnight fasting. Longitudinal B-mode ultrasound images of the brachial artery, 4 to 6 cm proximal to the antecubital crease, were obtained using an ultrasound platform (Aspen, Mountain View, CA) with a 10-MHz linear array transducer. Images were obtained after 10 minutes of supine relaxation and during reactive hyperemia, induced by 5 minutes of inflation of a pneumatic occlusion cuff placed around the forearm to a supra-systolic pressure (≥200 mm Hg). End-diastolic images were stored to a magnetic-optical disk and arterial diameters were measured as the distance between the proximal and distal arterial wall intima-media interfaces, using PC-based software (Brachial Analyzer Version 4.0, Medical Imaging Applications LLC, Iowa City, IA). Peak FMD response was assessed from 10 to 120 seconds post deflation of the cuff, with peak arterial diameter quantified using polynomial curve fitting. FMD, used as our measure of macrovascular function, was defined as the maximum percent change in arterial diameter relative to resting baseline.
Microvascular function was determined using hyperemic velocity change during FMD, which has recently been validated as a peripheral marker of systemic microvascular functioning.(25, 37) Pulsed Doppler flow signals in the brachial artery were recorded at baseline and for up to 15 seconds after cuff release. The velocity-time integral for baseline and reactive hyperemia was based upon the mean of triplicate pulsed-Doppler flow tracings recorded at each of these phases. Hyperemic velocity was derived by dividing the velocity-time integral by the inter-beat interval, and hyperemic flow was calculated from hyperemic velocity and brachial artery cross-sectional area.
Statistical Analyses
All analyses were performed in SAS 9.4 (Cary, NC). In order to minimize the number of statistical tests, we first combined subtests into domains to create a unit-weighted composite score, the details of which we have previously published.(11) In order to examine the independent association between CVRFs, microvascular function, and neurocognition, we first examined the unadjusted correlations between neurocognition and markers of microvascular function. We then conducted hierarchical regression analyses, in which 1) markers of microvascular function, 2) demographic characteristics, and 3) CVRFs (including HDL, LDL, BMI, and FSRP) were entered sequentially. We also examined the potential interaction between microvascular function (hyperemic velocity % change) and FMD on neurocognition in a secondary, exploratory step. Assumptions regarding linearity, additivity, and distribution of residuals were assessed and found to be adequate. All statistical tests were based on two-tailed probability values.
RESULTS
Participants
Four hundred fifty-seven participants were screened, of whom 202 met MDD inclusion criteria and were randomized. As shown in Table 1, participants were generally middle-aged and Caucasian, with relatively few CHD risk factors. Approximately one-third of participants were taking either antihypertensive or lipid lowering medications.
Table 1.
Background and demographic characteristics of the sample.
| Variable | Cohort (n = 202) |
|---|---|
| Age, years | 51.7 (7.6) |
| Female Gender, n (%) | 153 (76) |
| Race, n (%) Black Caucasian Other Ethnicity |
52 (26) 137 (68) 13 (6) |
| Hamilton Rating Scale for Depression | 16.8 (4.3) |
| Antihypertensive Medications, n (%) | 47 (32) |
| Diabetes, n (%) | 14 (7) |
| Current Tobacco Use, n (%) | 32 (16) |
| Systolic Blood Pressure, mm Hg | 124 (17) |
| Diastolic Blood Pressure, mm Hg | 79 (9) |
| Lipid-Lowering Medications, n (%) | 19 (9) |
| Total Cholesterol, ng/ml | 207 (40) |
| High Density Lipoprotein, ng/ml | 57 (16) |
| Low Density Lipoprotein, ng/ml | 122 (34) |
| Flow-Mediated Dilation, % | 5.9 (4.3) |
| Intima Medial Thickness, mm | 0.63 (0.13) |
| ASCVD Risk Score, 10-year % Risk | 4.7 (6.1) |
| Framingham Stroke Risk Profile | 3.9 (2.4) |
| Hyperemic Velocity Change, % | 728 (339) |
Correlates of Microvascular Function
As shown in Table 2, impaired microvascular function was associated with multiple demographic and CVD risk factors, including greater age, male gender, ASCVD and Framingham stroke risk scores, SBP, DBP, and lower HDL.
Table 2.
Associations between background characteristics microvascular function. Values represent unadjusted correlations between each variable and hyperemic velocity (degrees of freedom = 199).
| Variable | Hyperemic Velocity % |
|---|---|
| Age | −0.29** |
| Male Gender | −0.13† |
| Framingham Stroke Risk Score | −0.41** |
| ASCVD Risk Score | −0.43** |
| Systolic Blood Pressure | −0.40** |
| Diastolic Blood Pressure | −0.21** |
| High-Density Lipoprotein | 0.14* |
| Low-Density Lipoprotein | −0.07 |
| Intima Medial Thickness | −0.21** |
| Flow Mediated Dilation | 0.33** |
P<.10
P<.05
P<.01
Microvascular Function and Neurocognitive Performance
Examination of microvascular assessments and neurocognition demonstrated that poorer microvascular function was associated with poorer neurocognition across all domains in unadjusted analyses. Specifically, greater hyperemic velocity change was associated with better Verbal Memory (r199 = 0.18, P = .011), Executive Function (r199 = 0.17, P = .017), and Working Memory (r199 = 0.15, P = .031).
In order to examine the independent association between microvascular function after accounting for demographic and clinical characteristics, we conducted hierarchical regression models within each cognitive domain. As shown, hyperemic velocity was associated with better neurocognition across domains after controlling for baseline velocity. Accounting for demographic and CVD risk factors impacted the pattern of results differently depending on the domain. For Working Memory and Executive Function, the observed associations between hyperemic velocity and neurocognition were largely attenuated after accounting for demographic characteristics and completely attenuated after further controlling for CVD risk factors. In contrast, examination of Verbal Memory performance revealed that the association with hyperemic velocity was not only robust but tended to strengthen after controlling for demographic characteristics and CVD risk factors. Indeed, in contrast to Executive Function and Working Memory, Verbal Memory performance was more strongly associated with hyperemic velocity response than either age or CVD risk, both of which were strongly associated with performance in other cognitive domains (Table 3).
Table 3.
Hierarchical regression models demonstrating the associations between hyperemic velocity change and neurocognition. Values represent standardized regression coefficients. HRSD = Hamilton Rating Scale for
| Predictor | Model 1 | Model 2 | Model 3 |
|---|---|---|---|
| Working Memory | |||
| Age | ------ | −0.30** | −0.28** |
| Education | ------ | 0.41** | 0.33** |
| Gender | ------ | −0.01 | 0.01 |
| HRSD | ------ | 0.11† | 0.09 |
| HDL | ------ | ------ | 0.05 |
| LDL | ------ | ------ | 0.03 |
| Framingham Stroke Risk | ------ | ------ | −0.18* |
| Base Velocity | 0.19* | 0.17* | 0.14† |
| Hyperemic Velocity % | 0.25** | 0.15† | 0.06 |
| Executive Function | |||
| Age | ------ | −0.44** | −0.43** |
| Education | ------ | 0.30** | 0.23** |
| Gender | ------ | −0.09 | −0.05 |
| HRSD | ------ | 0.05 | 0.03 |
| HDL | ------ | ------ | 0.09 |
| LDL | ------ | ------ | 0.08 |
| Framingham Stroke Risk | ------ | ------ | −0.16* |
| Base Velocity | 0.22** | 0.17* | 0.14† |
| Hyperemic Velocity % | 0.28** | 0.11 | 0.03 |
| Verbal Memory | |||
| Age | ------ | 0.00 | 0.06 |
| Education | ------ | 0.23** | 0.20** |
| Gender | ------ | −0.03 | −0.04 |
| HRSD | ------ | −0.01 | −0.01 |
| HDL | ------ | ------ | −0.08 |
| LDL | ------ | ------ | −0.03 |
| Framingham Stroke Risk | ------ | ------ | 0.08 |
| Base Velocity | −0.05 | −0.06 | −0.04 |
| Hyperemic Velocity % | 0.15† | 0.15† | 0.21* |
Depression; HDL = high density lipoprotein; LDL = low density lipoprotein.
P<.10
P<.05
P<.01
Interaction of Macro and Microvascular Function on Neurocognition
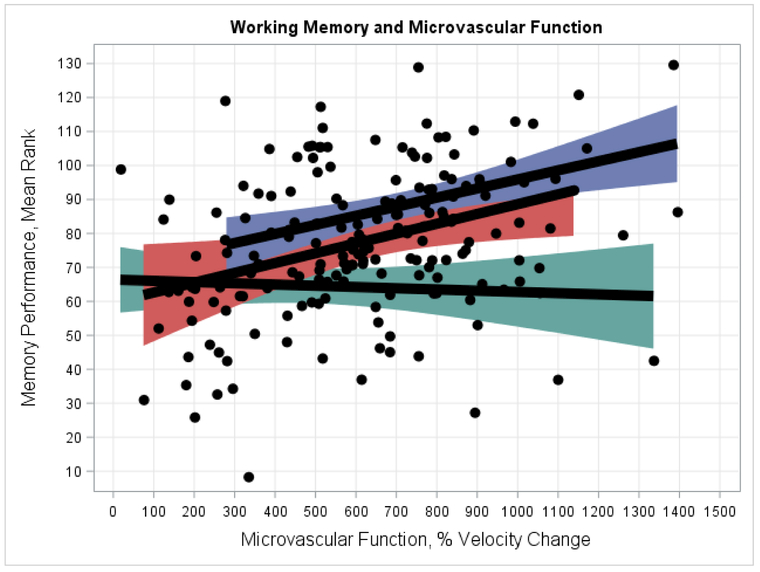
In exploratory analyses of potential interactions between hyperemic velocity change and FMD, we found that the association between microvascular function and Working Memory was moderated by FMD (F = 4.511, 178, P = .035), with a similar non-significant association for Executive Function (F = 2.731, 178, P = .095). Examination of this interaction revealed microvascular function was moderately associated with Working Memory (r61 = 0.40, P = .001 in highest FMD tertile) only among individuals with preserved FMD, whereas impaired FMD superseded any association between microvascular function and neurocognition (r63 = −0.05, P = .675 in lowest FMD tertile) (Figure 2). A similar association was observed for Executive Function, for which microvascular function was moderately associated (r61 = 0.34, P = .006) with neurocognition, but impaired FMD superseded this association (r63 = −0.04, P = .737 in lowest FMD tertile). In contrast, we found no evidence that the association between microvascular function and Verbal Memory was moderated by FMD ((F = 0.091, 178, P = .759)
Figure 2.
Interaction between FMD and microvascular function predicting Working Memory performance. As shown, the association between microvascular function and Working Memory varied by FMD level, with the strongest associations observed among individuals with preserved FMD. In contrast, individuals with impaired FMD showed no association with microvascular function: highest FMD tertile (blue: r = 0.40, P = .001), middle FMD tertile (red: r = 0.31, P = .022), and lowest FMD tertile (green: r = −0.05, P = .675).
DISCUSSION
Results from the present study suggest that a novel marker of peripheral microvascular function was associated with cognitive function, with the strongest associations observed with verbal memory. The associations between microvascular function and tests of frontal lobe function were largely mediated through greater age and CVRFs, whereas microvascular function was the factor most strongly associated with verbal memory performance. In addition, we found that macrovascular and microvascular function may interact to predict performance on frontal lobe tests, such that the association between microvascular function and neurocognition is eclipsed by systemic macrovascular dysfunction. However, among individuals with preserved macrovascular function, microvascular function was moderately associated with performance, suggesting it may provide important prognostic information in the earliest stages of CVD progression. These findings extend previous work demonstrating an association between peripheral markers of endothelial function and cognitive performance by demonstrating that markers of conduit artery function are more strongly associated with frontal lobe performance, whereas microvascular markers were more strongly associated with memory performance.
The present findings extend our prior work demonstrating that conduit artery assessments of endothelial function are associated with performance on cognitive tests assessing executive function and working memory, which we previously observed in patients with MDD (11) and hypertension.(12) These findings have subsequently been replicated, with systematic literature reviews demonstrating that flow-mediated dilation (FMD), a marker of endothelial function,(13, 38) demonstrates consistent inverse associations with frontal-lobe dependent test performance.(39) Consistent with the present findings, prior studies have reported that flow-mediated dilation is not related to memory performance,(39) despite numerous recent studies demonstrating that endothelial dysfunction is commonly observed among individuals with Alzheimer’s disease (8, 9, 40-42) and may play an important role in the pathogenesis of neurodegeneration. (8, 43, 44)
The finding that CVRFs and conduit artery endothelial function are more closely associated with frontal-lobe dependent tests may reflect the differing arterial distribution to neurocircuitry subserving these distinct neurocognitive functions. (45, 46) Executive function and working memory subtests are particularly reliant on subcortical-cortical functional connections, including projections between ‘watershed’ brain regions including the anterior cingulate,(47, 48) ventral striatum,(49) and dorsolateral prefrontal cortex.(50) Due to the relative lack of arterial redundancy in these regions, it is possible that they are more susceptible to perturbations in cerebral autoregulation or perfusion compared to mesial temporal lobe regions subserving memory functions, such as the hippocampal gyrus. Microvascular endothelial dysfunction has been shown to differentiate from macrovascular function to cause target organ damage in other disease processes.(37, 51) This is consistent with the finding that only memory performance was robustly associated with our most sensitive endothelial marker of microvascular function.
Limitations. The present study must be viewed with several limitations in mind. First, this was a cross-sectional analysis and future longitudinal studies are needed to replicate the observed pattern of findings. Second, we did not collect additional markers of microvascular disease, such as neuroimaging markers of white matter damage or ankle-brachial pressure indices. It should therefore be noted that, although our marker of microvascular endothelial function has been validated in other samples,{Dubin, 2016 #84114} future studies should attempt to further delineate peripheral markers of microvascular and macrovascular endothelial functioning. Finally, our study used a convenience sample of adults with MDD participating in an exercise and pharmacological intervention, such that individuals with ortopaedic comorbidities, taking psychotropic medications, engaging in exercise were excluded. It is therefore unclear to what extent the observed associations would be observed in a more representative sample of older adults with.
In conclusion, our findings extend prior work demonstrating that subtle perturbations in microvascular function may provide important information on risk of cognitive impairment. Future studies should attempt to replicate the present findings in larger, prospective studies utilizing sensitive neuroimaging markers. If replicated, peripheral markers of microvascular disease could represent a potential treatment target in efforts to reduce risk of vascular cognitive impairment.
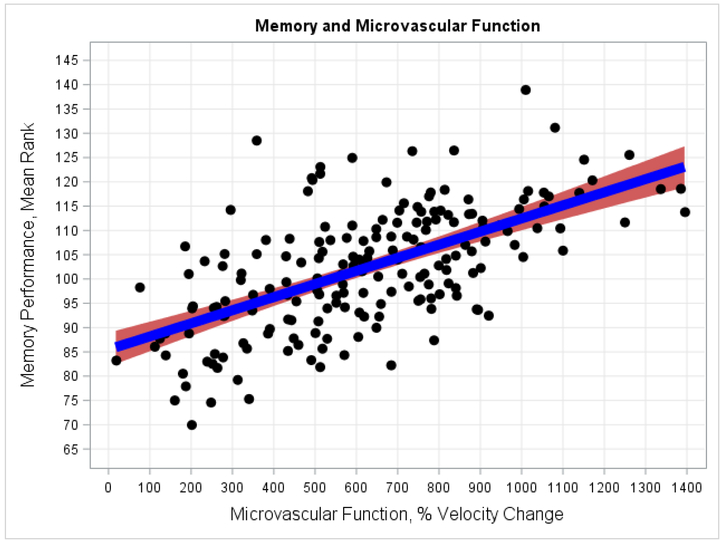
Figure 1.
Microvascular function and Verbal Memory performance. As shown, greater microvascular function was associated with better Verbal Memory performance after accounting for background characteristics and CVRFs.
Highlights:
The present findings are the first to demonstrate an association between peripheral microvascular endothelial function and neurocognition among adults with major depressive disorder
These findings extend previous findings by demonstrating an interaction between systemic vascular dysfunction and microvascular disease on neurocognition
Findings suggest that microvascular dysfunction is more closely associated with some aspects of neurocognition, including memory performance, than chronological age
Acknowledgments
Source of Funding: This study was supported by Grants MH49679, HL093374, HL101383 from the National Institutes of Health and National Institutes of Health Grant MO1-RR-30 from the National Center for Research Resources, Clinical Research Centers Program.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest: The authors declare there are no conflicts of interest
References
- 1.Gorelick PB, Scuteri A, Black SE, Decarli C, Greenberg SM, Iadecola C, Launer LJ, Laurent S, Lopez OL, Nyenhuis D, Petersen RC, Schneider JA, Tzourio C, Arnett DK, Bennett DA, Chui HC, Higashida RT, Lindquist R, Nilsson PM, Roman GC, Sellke FW, Seshadri S. Vascular contributions to cognitive impairment and dementia: a statement for healthcare professionals from the american heart association/american stroke association. Stroke. 2011;42:2672–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barnes DE, Yaffe K. The projected effect of risk factor reduction on Alzheimer’s disease prevalence. The Lancet Neurology. 2011;10:819–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stephan BCM, Harrison SL, Keage HAD, Babateen A, Robinson L, Siervo M. Cardiovascular Disease, the Nitric Oxide Pathway and Risk of Cognitive Impairment and Dementia. Current cardiology reports. 2017;19:87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Driscoll I, Gaussoin SA, Wassertheil-Smoller S, Limacher M, Casanova R, Yaffe K, Resnick SM, Espeland MA. Obesity and Structural Brain Integrity in Older Women: The Women’s Health Initiative Magnetic Resonance Imaging Study. J Gerontol A Biol Sci Med Sci. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chuang YF, An Y, Bilgel M, Wong DF, Troncoso JC, O’Brien RJ, Breitner JC, Ferruci L, Resnick SM, Thambisetty M. Midlife adiposity predicts earlier onset of Alzheimer’s dementia, neuropathology and presymptomatic cerebral amyloid accumulation. Mol Psychiatry. 2016;21:910–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Iadecola C, Yaffe K, Biller J, Bratzke LC, Faraci FM, Gorelick PB, Gulati M, Kamel H, Knopman DS, Launer LJ, Saczynski JS, Seshadri S, Zeki Al Hazzouri A, American Heart Association Council on H, Council on Clinical C, Council on Cardiovascular Disease in the Y, Council on C, Stroke N, Council on Quality of C, Outcomes R, Stroke C. Impact of Hypertension on Cognitive Function: A Scientific Statement From the American Heart Association. Hypertension (Dallas, Tex : 1979). 2016;68:e67–e94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lehert P, Villaseca P, Hogervorst E, Maki PM, Henderson VW. Individually modifiable risk factors to ameliorate cognitive aging: a systematic review and meta-analysis. Climacteric : the journal of the International Menopause Society. 2015;18:678–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Toth P, Tarantini S, Csiszar A, Ungvari Z. Functional vascular contributions to cognitive impairment and dementia: mechanisms and consequences of cerebral autoregulatory dysfunction, endothelial impairment, and neurovascular uncoupling in aging. American journal of physiology Heart and circulatory physiology. 2017;312:H1–H20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lim SL, Gao Q, Nyunt MSZ, Gong L, Lunaria JB, Lim ML, Ling A, Lam CS-P, Richards AM, Ling LH, Ng TP. Vascular Health Indices and Cognitive Domain Function: Singapore Longitudinal Ageing Studies. J Alzheimers Dis. 2016;50:27–40. [DOI] [PubMed] [Google Scholar]
- 10.Brant L, Bos D, Araujo LF, Ikram MA, Ribeiro AL, Barreto SM. Microvascular endothelial function and cognitive performance: The ELSA-Brasil cohort study. Vasc Med. 2018:1358863X18755004. [DOI] [PubMed] [Google Scholar]
- 11.Smith PJ, Blumenthal JA, Babyak MA, Hoffman BM, Doraiswamy PM, Waugh R, Hinderliter A, Sherwood A. Cerebrovascular risk factors, vascular disease, and neuropsychological outcomes in adults with major depression. Psychosom Med. 2007;69:578–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Smith PJ, Blumenthal JA, Babyak MA, Hinderliter A, Sherwood A. Association of vascular health and neurocognitive performance in overweight adults with high blood pressure. J Clin Exp Neuropsychol. 2011;33:559–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Haley AP, Tarumi T, Gonzales MM, Sugawara J, Tanaka H. Subclinical atherosclerosis is related to lower neuronal viability in middle-aged adults: a 1H MRS study. Brain Res. 2010;1344:54–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hoth KF, Tate DF, Poppas A, Forman DE, Gunstad J, Moser DJ, Paul RH, Jefferson AL, Haley AP, Cohen RA. Endothelial function and white matter hyperintensities in older adults with cardiovascular disease. Stroke. 2007;38:308–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.van Agtmaal MJM, Houben A, Pouwer F, Stehouwer CDA, Schram MT. Association of Microvascular Dysfunction With Late-Life Depression: A Systematic Review and Meta-analysis. JAMA Psychiatry. 2017;74:729–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aizenstein HJ, Baskys A, Boldrini M, Butters MA, Diniz BS, Jaiswal MK, Jellinger KA, Kruglov LS, Meshandin IA, Mijajlovic MD, Niklewski G, Pospos S, Raju K, Richter K, Steffens DC, Taylor WD, Tene O. Vascular depression consensus report - a critical update. BMC Med. 2016;14:161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bora E, Harrison BJ, Yucel M, Pantelis C. Cognitive impairment in euthymic major depressive disorder: a meta-analysis. Psychol Med. 2013;43:2017–26. [DOI] [PubMed] [Google Scholar]
- 18.Cohen RA, Poppas A, Forman DE, Hoth KF, Haley AP, Gunstad J, Jefferson AL, Tate DF, Paul RH, Sweet LH, Ono M, Jerskey BA, Gerhard-Herman M. Vascular and cognitive functions associated with cardiovascular disease in the elderly. JClinExpNeuropsychol. 2009;31:96–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Consoli D, Di CA, Inzitari D, De LD, Lamassa M, D’Avino M, Baldereschi M, Muto M, Mandarino A, Napolitano M, Romano MF, Caruso D. Subcortical ischaemic changes in young hypertensive patients: frequency, effect on cognitive performance and relationship with markers of endothelial and haemostatic activation. EurJNeurol. 2007;14:1222–9. [DOI] [PubMed] [Google Scholar]
- 20.Kim DH, Grodstein F, Newman AB, Chaves PHM, Odden MC, Klein R, Sarnak MJ, Lipsitz LA. Microvascular and Macrovascular Abnormalities and Cognitive and Physical Function in Older Adults: Cardiovascular Health Study. J Am Geriatr Soc. 2015;63:1886–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Smith PJ, Blumenthal JA, Babyak MA, Watkins LL, Hinderliter A, Hoffman BM, Steffens DC, Sherwood A, Doraiswamy PM. Cerebrovascular risk factors and cerebral hyperintensities among middle-aged and older adults with major depression. Am J Geriatr Psychiatry. 2010;18:848–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Blumenthal JA, Babyak MA, Doraiswamy PM, Watkins L, Hoffman BM, Barbour KA, Herman S, Craighead WE, Brosse AL, Waugh R, Hinderliter A, Sherwood A. Exercise and pharmacotherapy in the treatment of major depressive disorder. Psychosom Med. 2007;69:587–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hoffman BM, Blumenthal JA, Babyak MA, Smith PJ, Rogers SD, Doraiswamy PM, Sherwood A. Exercise fails to improve neurocognition in depressed middle-aged and older adults. Med Sci Sports Exerc. 2008;40:1344–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Smith PJ, Blumenthal JA, Babyak MA, Doraiswamy PM, Hinderliter A, Hoffman BM, Waugh R, Sherwood A. Intima-media thickness and age of first depressive episode. Biol Psychol. 2009;80:361–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Paine NJ, Hinderliter AL, Blumenthal JA, Adams KF Jr., Sueta CA, Chang PP, O’Connor CM, Sherwood A Reactive hyperemia is associated with adverse clinical outcomes in heart failure. Am Heart J. 2016;178:108–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reitan RM. Manual for administration of neuropsychological test batteries for adults and children: Tucson: Reitan Neuropsychological Laboratories, Inc.; 1979. [Google Scholar]
- 27.Stroop JR. Studies of interference in serial verbal reactions. J Exp Psychiat. 1935;18:643–62. [Google Scholar]
- 28.Wechsler D Wechsler Memory Scale-Revised. New York: Psychological Corp.; 1987. [Google Scholar]
- 29.Benton AL, Sivan A, de Hamsher KS. Multilingual Aphasia Examination. 1994. [Google Scholar]
- 30.Lezak MD, Howieson DB, Loring DW. Neuropsychological Assessment Batteries. Neuropsychological Assessment. Oxford, New York, Auckland, Bangkok, Buenos Aires, Cape Town, Chenna, Dar es Sallam, Delhi, Hong Kong, Istanbul, Karachi, Kolkata, Kuala Lumpur, Madrid, Melbourne, Mexico City, Mumbai, Nairobi, Sao Paulo, Shanghai, Taipei, Tokyo, Toronto: Oxford University Press; 2004. p. 647–95. [Google Scholar]
- 31.Ruff RM, Niemann H, Allen CC. The Ruff 2 and 7 Selective Attention Test: A neuropsychological application. Percept Mot Skills. 1992;75:1311–9. [DOI] [PubMed] [Google Scholar]
- 32.Wechsler D WMS-III Technical Manual. San Antonio, TX: Psychological Corporation; 1997. [Google Scholar]
- 33.Sherwood A, Blumenthal JA, Smith PJ, Watkins LL, Hoffman BM, Hinderliter AL. Effects of Exercise and Sertraline on Measures of Coronary Heart Disease Risk in Patients With Major Depression: Results From the SMILE-II Randomized Clinical Trial. Psychosom Med. 2016;78:602–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.D’Agostino RB, Wolf PA, Belanger AJ, Kannel WB. Stroke risk profile: adjustment for antihypertensive medication. The Framingham Study. Stroke. 1994;25:40–3. [DOI] [PubMed] [Google Scholar]
- 35.Wolf PA, D’Agostino RB, Belanger AJ, Kannel WB. Probability of stroke: a risk profile from the Framingham Study. Stroke. 1991;22:312–8. [DOI] [PubMed] [Google Scholar]
- 36.Goff DC Jr., Lloyd-Jones DM, Bennett G, Coady S, D’Agostino RB Sr., Gibbons R, Greenland P, Lackland DT, Levy D, O’Donnell CJ, Robinson JG, Schwartz JS, Shero ST, Smith SC Jr., Sorlie P, Stone NJ, Wilson PW, American College of Cardiology/American Heart Association Task Force on Practice G. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63:2935–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dubin RF, Guajardo I, Ayer A, Mills C, Donovan C, Beussink L, Scherzer R, Ganz P, Shah SJ. Associations of Macro- and Microvascular Endothelial Dysfunction With Subclinical Ventricular Dysfunction in End-Stage Renal Disease. Hypertension. 2016;68:913–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tarumi T, Gonzales MM, Fallow B, Nualnim N, Lee J, Pyron M, Tanaka H, Haley AP. Cerebral/Peripheral Vascular Reactivity and Neurocognition in Middle-Age Athletes. Medicine and science in sports and exercise. 2015;47:2595–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Naiberg MR, Newton DF, Goldstein BI. Flow-Mediated Dilation and Neurocognition: Systematic Review and Future Directions. Psychosom Med. 2016;78:192–207. [DOI] [PubMed] [Google Scholar]
- 40.Akinyemi RO, Mukaetova-Ladinska EB, Attems J, Ihara M, Kalaria RN. Vascular risk factors and neurodegeneration in ageing related dementias: Alzheimer’s disease and vascular dementia. Curr Alzheimer Res. 2013;10:642–53. [DOI] [PubMed] [Google Scholar]
- 41.Tarantini S, Tran CHT, Gordon GR, Ungvari Z, Csiszar A. Impaired neurovascular coupling in aging and Alzheimer’s disease: Contribution of astrocyte dysfunction and endothelial impairment to cognitive decline. Experimental gerontology. 2017;94:52–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tachibana H, Washida K, Kowa H, Kanda F, Toda T. Vascular Function in Alzheimer’s Disease and Vascular Dementia. American journal of Alzheimer’s disease and other dementias. 2016;31:437–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hohman TJ, Bell SP, Jefferson AL, Alzheimer’s Disease Neuroimaging I. The role of vascular endothelial growth factor in neurodegeneration and cognitive decline: exploring interactions with biomarkers of Alzheimer disease. JAMA Neurol. 2015;72:520–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Di Marco LY, Venneri A, Farkas E, Evans PC, Marzo A, Frangi AF. Vascular dysfunction in the pathogenesis of Alzheimer’s disease--A review of endothelium-mediated mechanisms and ensuing vicious circles. Neurobiology of disease. 2015;82:593–606. [DOI] [PubMed] [Google Scholar]
- 45.Jansen JFA, van Bussel FCG, van de Haar HJ, van Osch MJP, Hofman PAM, van Boxtel MPJ, van Oostenbrugge RJ, Schram MT, Stehouwer CDA, Wildberger JE, Backes WH. Cerebral blood flow, blood supply, and cognition in Type 2 Diabetes Mellitus. Sci Rep. 2016;6:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Alosco ML, Brickman AM, Spitznagel MB, Garcia SL, Narkhede A, Griffith EY, Raz N, Cohen R, Sweet LH, Colbert LH, Josephson R, Hughes J, Rosneck J, Gunstad J. Cerebral perfusion is associated with white matter hyperintensities in older adults with heart failure. Congestive heart failure (Greenwich, Conn). 2013;19:E29–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Anazodo UC, Shoemaker JK, Suskin N, Ssali T, Wang DJJ, St Lawrence KS. Impaired Cerebrovascular Function in Coronary Artery Disease Patients and Recovery Following Cardiac Rehabilitation. Frontiers in aging neuroscience. 2015;7:224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ding B, Ling H-w, Zhang Y, Huang J, Zhang H, Wang T, Yan FH. Pattern of cerebral hyperperfusion in Alzheimer’s disease and amnestic mild cognitive impairment using voxel-based analysis of 3D arterial spin-labeling imaging: initial experience. Clin Interv Aging. 2014;9:493–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Feekes JA, Cassell MD. The vascular supply of the functional compartments of the human striatum. Brain. 2006;129:2189–201. [DOI] [PubMed] [Google Scholar]
- 50.Thomas AJ, Perry R, Kalaria RN, Oakley A, McMeekin W, O’Brien JT. Neuropathological evidence for ischemia in the white matter of the dorsolateral prefrontal cortex in late-life depression. Int J Geriatr Psychiatry. 2003;18:7–13. [DOI] [PubMed] [Google Scholar]
- 51.Bordy R, Totoson P, Prati C, Marie C, Wendling D, Demougeot C. Microvascular endothelial dysfunction in rheumatoid arthritis. Nat Rev Rheumatol. 2018. [DOI] [PubMed] [Google Scholar]