Alcohol is the most ubiquitous recreational drug in the world, causing more than three million global deaths in 2012 (World Health Organization, 2014). Anxiety and stress disorders are highly comorbid with alcohol abuse (Lai et al, 2015), suggesting the possibility of common underlying mechanisms or related circuitry dysfunction. The close relationship between substance use, which acutely elicits feelings of euphoria, and the more unpleasant effects of stress, anxiety, or withdrawal from drug exposure reflects an important aspect of emotional information processing in the brain: the same circuitry can underlie both rewarding and aversive states. Consistent with this idea, the general experience of stress can also be either rewarding or aversive, depending on the type and duration of stress experienced and the state of the organism. The transition from alcohol use to alcohol abuse is characterized by a change in motivational state from drug reward-dependent intake early in alcohol use to intake motivated by withdrawal-induced aversion as the organism becomes increasingly dependent upon alcohol (Koob and Le Moal, 2001). Investigating the common cellular mechanisms of alcohol and stress in the brain allows for the dissection of specific circuits that underlie seemingly oppositional hedonic states and may provide insight into inherent vulnerability and/or new treatment directions for alcohol use and stress-related disorders as well as unique strategies for understanding their comorbidity.
The amygdala has emerged as a key brain region in the ontogeny of both alcoholism and stress/anxiety disorders. The amygdala is activated by alcohol-related cues in human participants (Dager et al, 2013) and by acute alcohol in rodent models (McBride, 2002), and has been shown to functionally regulate alcohol self-administration (Koob, 2003). Withdrawal from alcohol is associated with both changes in amygdala activity and anxiety-related behaviors (McCool, 2011; Overstreet et al, 2004). The relationship between fear/anxiety and the amygdala is robust. In both naïve rodents and healthy human participants, amygdala activity is associated with fear-paired cues during fear conditioning (Duvarci and Pare, 2014). In the context of anxiety disorders, amygdala activity both at rest and in response to anxiety-associated cues is enhanced in patients with post-traumatic stress disorder (PTSD), and similar amygdala responsivity has been observed in other anxiety disorders in humans (see VanElzakker et al., 2014 for review). The amygdala is therefore a region where alcohol and anxiety-associated circuitry converge and is closely associated with the behavioral manifestations of both disorders.
Elucidating the specific cellular mechanisms that underlie the role of the amygdala in alcoholism, anxiety and their comorbidity has been complicated by the heterogeneous nature of the amygdala. Comprising multiple subnuclei, each with a diversity of cell types and projections to and from other brain regions, the specific role of the amygdala in emotional or disease-related states depends upon the region and cell population. With the advent of transgenic mice, chemo- and optogenetics, and more precise tools for molecular mapping of individual neurons, the role of discrete neural circuits and cell populations within the amygdala is coming into focus. These techniques have indicated a critical role for cell type-specific neuroadaptations within the amygdala in the effects of both acute and chronic alcohol and anxiety-related behaviors. Precise local and projection-based control of specific neuronal populations is emerging as a key regulator of intra-amygdala activity, amygdala output, and behavior.
The Amygdala Complex
The amygdala complex is composed of distinct nuclei and subdivisions including the lateral (LA), basolateral (BL) and basomedial (BM) amygdala, the medial (Imp), lateral paracapsular (lpc) and main intercalated cell cluster (IN) and the lateral and medial central amygdala (CeA) (Pitkanen and Amaral, 1994; Sah et al, 2003). The main flow of information follows a lateral-to-medial path, with sensory information from the thalamus and cortex entering the amygdala by afferents synapsing in the LA, which sends glutamatergic projections throughout the amygdala including to the BM, lateral CeA, and to the Imp. The BM then sends glutamatergic projections to the medial CeA, while the Imp sends GABAergic projections to the lateral CeA, which forms an inhibitory microcircuit with the medial CeA. Both the lateral and medial CeA function as major output nuclei, sending projections to downstream regions regulating rewarding and/or aversive behaviors. The BLA is primarily composed of glutamatergic neurons that are under precisely regulated inhibitory control by lpc neurons and local interneurons (Marowsky et al, 2004; McDonald, 1982; McDonald and Betette, 2001; Muller et al, 2003). Although interneurons make up a small percentage of BLA neurons, the multiple levels of inhibitory control of pyramidal neurons demonstrate that interneurons have significant effects on output of the BLA. In contrast, the CeA is predominately GABAergic cells, (McDonald, 1985; McDonald and Augustine, 1993), which comprise the vast majority of both projection neurons and interneurons.
The circuitry of the amygdala has been implicated in a number of important brain functions, but is best known for its role in emotional processing, specifically in the interpretation of emotionally-relevant stimuli or the attachment of emotional relevance to otherwise neutral stimuli (Pitkanen et al, 1994). In particular, the amygdala nuclei mediate the negative association processing behind the acquisition and expression of conditioned fear (for review see Ehrlich 2009) and recent evidence suggests that amygdala microcircuits are also critical for the coding of both positive and negative-valence cues (Beyeler et al, 2018; Beyeler et al, 2016). The high degree of interconnectivity in the amygdala underlies the complex information processing that takes place as multi-modal input is received, integrated and processed to produce relevant behavioral output.
CRF and Amygdala Microcircuitry
Corticotropin releasing factor (CRF) is a neuropeptide that has a well-established role in the stress response of the mammalian central nervous system. CRF is one of the principal activators of the hypothalamic-pituitary-adrenal (HPA) axis, which is responsible for a host of physiological and behavioral responses to stress, including alterations in heart rate, blood flow, reactivity, locomotion and motivated behavior (Keller et al, 2006). CRF activation produces behaviors similar to those seen following acute and chronic stress. Transgenic mice with constitutive overproduction of CRF exhibit reduced locomotor activity in a novel environment and decreased time spent in the open arm of the elevated plus maze, consistent with an anxiogenic phenotype, an effect which was reversed with the intra-ventricular infusion of a CRF antagonist (Stenzel-Poore et al, 1994). Similarly, intraventricular infusion of CRF produces a variety of pro-anxiety behavioral responses in rodent models, including novelty reactivity (Britton et al, 1982), social conflict (Britton et al, 1985), acoustic startle (Swerdlow et al, 1986), and reduced time in the open arms on an elevated plus maze (Adamec et al, 1991). In human participants, CRF levels in the cerebrospinal fluid are elevated in patients with anxiety disorders relative to healthy controls (Baker et al, 1999; Bremner et al, 1997). Two G-protein coupled receptors for CRF have been identified in the mammalian brain, CRF-1 and CRF-2. CRF and the CRF receptors are expressed throughout the amygdala (Van Pett et al, 2000) and have been implicated in neuroplastic changes related to fear (Hubbard et al, 2007), anxiety (Overstreet et al, 2004; Rainnie et al, 2004) and alcohol exposure (Herman et al, 2013a; Lovinger and Roberto, 2013; Nie et al, 2004; Roberto et al, 2010).
Within the BLA, both CRF and the CRF1 receptor have been shown to functionally regulate synaptic activity. In vivo, CRF release into the BLA originates from projections from the CeA (Roozendaal et al, 2002). Central administration of both CRF and CRF1 agonists have been shown to activate the BLA and produce anxiogenic behavioral responses (Dube et al, 2000; Rainnie et al, 2004). Exogenous application of CRF peptide in BLA slices likewise increased the excitability of neurons (Ugolini et al, 2008), an effect which was diminished following chronic unpredictable stress and shown to be mediated via the CRF1 receptor (Sandi et al, 2008). Microinjection of CRF specifically within the BLA activated CaMKII-containing projection neurons but not GAD-containing interneurons (Rostkowski et al, 2013). Chronic administration of the CRF1 and CRF2 agonist urocortin produced long-lasting anxiety responses that required NMDA receptor and CaMKII activation and produced hyperexcitability of the BLA network due to reduced local inhibition (Rainnie et al, 2004; Sajdyk et al, 1999). Together, these findings indicate a general enhancement of excitatory synaptic transmission in the BLA following acute in vivo and ex vivo application of CRF that is blunted by chronic stress and chronic CRF activation.
The CRF system also plays an important role in the regulation of synaptic transmission in the central amygdala. Although the CeA receives input from CRF+ projections originating in the paraventricular hypothalamus (Hernandez et al, 2015) and the bed nucleus of the stria terminalis (Gungor et al, 2015), CRF function in the CeA is also driven by local microcircuitry (Sanford et al, 2017). CRF and GABA are colocalized within the CeA (Day et al, 1999), and CRF has been to shown to regulate GABAergic neurotransmission in this brain region. Exogenous application of CRF to CeA slices increases presynaptic GABA release in a CRF1, but not CRF2, -dependent manner (Kang-Park et al, 2015; Nie et al, 2004; Roberto et al, 2010). The effects of CRF on inhibitory neurotransmission in the CeA are regulated in part by protein kinase C-epsilon (PKCε); PKCε knockout mice exhibited increased baseline GABAergic tone in the CeA but were not responsive to the acute effects of exogenous CRF application, and PKCε inhibition occluded the effects of acute CRF in wild type mice (Bajo et al, 2008; Blasio et al, 2017). CRF has also been shown to alter excitatory transmission within the CeA. CRF application reduced evoked glutamate responses and enhanced glutamate release in the CeA of Sprague-Dawley rats (Liu et al, 2004; Varodayan et al, 2017) and C57BL/6 mice (Silberman et al, 2013a). In Wistar rats, CRF application had divergent effects on glutamatergic signaling, with a subset of CeA neurons exhibiting enhanced glutamate release following exogenous CRF application and a subset exhibiting reductions in glutamate release (Herman et al, 2016c).
Despite the expression of CRF and CRF1 in the amygdala and the relevance of the CRF system to behavioral conditions involving amygdala circuitry, how and where CRF1 fits into the known circuitry remains unclear. Previous technical limitations related to identifying CRF1 prevented the direct examination of CRF1-containing neurons in the amygdalar subnuclei and their place in the larger circuitry of the amygdala complex. This limitation had previously confounded the integration of cellular and behavioral data in determining the effects of CRF on specific circuitry within the amygdala and how alterations in the activity of that circuitry lead to adverse behavioral outcomes.
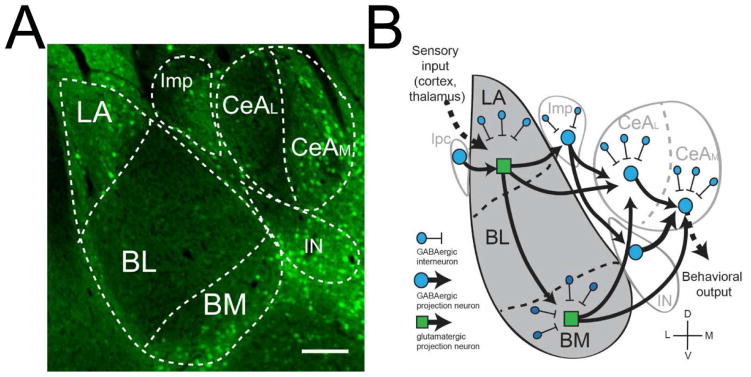
Previous limitations in identifying and targeting CRF1 neurons in the amygdala were overcome by the development of a BAC transgenic mouse line expressing green fluorescent protein (GFP) in CRF1-containing neurons (Justice et al, 2008). Immunohistochemical examination of GFP expression in the amygdala complex in this mouse indicates that CRF1 neurons makeup discrete cell populations with restrcied expression in discrete amygdala sub-nuclei (Figure 1A). Earlier work by our group utilized this transgenic mouse model approach to examine inhibitory transmission in CRF1-containing (CRF1+) and unlabeled (CRF1−) neurons in the CeA. We validated this model to establish that GFP expression was a reliable indicator of CRF1+ neurons in the amygdala and found that CRF1+ and CRF1− neurons exhibited distinct inhibitory characteristics. CRF1+ CeA neurons exhibited a significant ongoing tonic conductance mediated by GABAA receptors containing the α1 subunit. In contrast, unlabeled (CRF1−) CeA neurons possessed the potential for a tonic conductance mediated by δ subunit-containing GABAA receptors (Herman et al, 2013a). Paired recordings demonstrated that CRF1− neurons form local inhibitory synapses onto CRF1+ neurons, a portion of which project out of the CeA into the bed nucleus of the stria terminalis (Herman et al, 2016a). The specific role of CRF1+ and CRF1− cells in the CeA suggests that CRF1+ neurons may make up distinct neuronal populations in other amygdala nuclei, including the BLA, and that CRF1 neurons may display distinct patterns of inter- and intra-amygdalar connectivity (Figure 1B).
Figure 1.
CRF1 subpopulations in the amygdala complex. A. GFP expression in CRF1-containing neurons in the amygdala, including discrete populations in the LA. BM, Imp, IN, and CeAM. Scale bar = 400 μm. B. Schematic diagram of proposed CRF1 circuitry showing inter- and intranuclear connections. BL: basolateral; BM: basomedial; CeAL: lateral central amygdala; CeAM: medial central amygdala; Imp: medial paracapsular intercalated; IN main intercalated; LA lateral amygdala.
Alcohol and the Amygdala
Alcohol and GABAergic transmission
Although alcohol is known to engage many brain systems, GABAergic transmission is particularly sensitive to alcohol and is involved in the actions of acute alcohol as well as alcohol tolerance and dependence (Eckardt et al, 1998; Grobin et al, 1998). There are two main types of GABAA receptor transmission that function in a cell- and region-specific manner. Phasic signaling involves the generation of inhibitory postsynaptic currents (IPSCs) that are the result of ‘point to point’ transmission that occurs at synapses and results in 10 –100 ms of inhibition. Tonic signaling is characterized by the presence of persistent inhibitory currents that are the result of low levels of ambient GABA acting at highly sensitized extrasynaptic GABAA receptors (for review, see Brickley and Mody, 2012; Belelli et al. 2009;Glykys and Mody, 2007). The functional characteristics of GABAA receptors are determined by the subunit composition. Previous research suggests that extrasynaptic GABAA receptors containing the α4, α6, and/or δ subunit have an increased sensitivity to alcohol (Wallner et al, 2003; Wei et al, 2004), prompting some researchers to suggest that tonic receptors are the primary target for alcohol in the brain (Mody et al, 2007; Wallner et al, 2003), although the direct action of alcohol on extrasynaptic GABAA receptors remains controversial (Baur et al, 2009; Borghese and Harris, 2007; Botta et al, 2007).
Alcohol and the Basolateral Amygdala
Considering the known anxiolytic properties of acute alcohol (Koob and Britton, 1996; Kushner et al, 2000) and the increased anxiety phenotype associated with alcohol withdrawal (Menzaghi et al, 1994), it is not surprising that the BLA has been implicated in both the behavioral and cellular effects of acute and chronic alcohol. Previous studies have shown that glucose metabolism is increased in the BLA following alcohol self-administration in rats (Porrino et al, 1998), and more recently it has been shown that inactivation of the BLA disrupts the renewal of conditioned Pavlovian-alcohol seeking (Chaudhri et al, 2013) and inhibition of BLA pyramidal neurons by a serotonin type-2 receptor agonist suppressed alcohol seeking behavior (McCool et al, 2014). In addition, cellular studies have demonstrated that synaptic activity in the BLA is altered by both acute and chronic alcohol. Acute alcohol enhances both basal and evoked inhibitory currents in BLA pyramidal neurons (Samson et al, 2003; Zhu and Lovinger, 2006) from two distinct inhibitory pathways, the feed-forward inhibition provided by lpc neurons and the feedback inhibition provided by local interneurons (Silberman et al, 2008), although the effects of alcohol on local inhibitory transmission display an acute tolerance involving the GABAB receptor (Silberman et al, 2009; Silberman et al, 2008; Zhu et al, 2006). In contrast, acute alcohol has relatively minor effects on AMPA and NMDA-receptor mediated glutamatergic transmission, although it does decrease kainate receptor-mediated excitatory transmission onto BLA pyramidal neurons (Lack et al, 2009). Consistent with the effects on inhibitory and, to a lesser extent, the effects on excitatory transmission, acute alcohol has also been shown to decrease the firing of BLA pyramidal neurons projecting to the nucleus accumbens (Perra et al, 2008), possibly through increased inhibitory input and/or decreased excitatory input.
In contrast to the minimal effects of acute alcohol on glutamatergic transmission in BLA pyramidal neurons, chronic intermittent alcohol (CIE) and withdrawal (WD) produced anxiety behavior as well as significant increases in excitatory transmission mediated by both AMPA and NMDA receptors (Lack et al, 2007) and CIE, but not WD, increased kainate receptor-mediated glutamatergic transmission (Lack et al, 2009). Notably, CIE only produced increases in spontaneous action potential-dependent excitatory transmission, suggesting that CIE alters the activity of local intra-BLA glutamatergic neurons (Lack et al, 2008). The specific effects of CIE on BLA function may also vary based on synaptic connections; at BLA cells receiving input from the external capsule, CIA enhanced glutamatergic activity in a postsynaptic fashion, whereas cells connecting to stria terminalis afferents exhibited presynaptic enhancement of glutamate release (Morales et al, 2018). CIE and WD have also been shown to produce long-lasting structural and functional changes in inhibitory BLA circuitry. CIE increased the functional expression of GABAA receptor currents in BLA neurons and no tolerance to the acute effects of alcohol was observed (Diaz et al, 2011; McCool et al, 2003). CIE and WD also significantly dampened evoked feed-forward inhibitory input from distal lpc neurons, but did not alter action potential-independent presynaptic GABA release from local interneurons onto BLA pyramidal neurons (Diaz et al, 2011). CIE and WD also decreased total and surface levels of the α1 subunit and increased surface α4 subunit expression at lpc synapses, which was accompanied by decreased zolpidem sensitivity (Diaz et al, 2011).
Tonic inhibition has been described in principal cells of the BLA (McCool et al, 2003; Olmos-Serrano et al, 2010) and principal cells as well local interneurons in the LA (Marowsky et al, 2012). The tonic inhibition in the BLA is mediated by α3-subunit containing GABAA receptors but the receptor composition of neurons in the LA is unknown (Marowsky et al, 2012). The α1 GABAA receptor subunit displays selective expression in the LA (Marowsky et al, 2004; Wiltgen et al, 2009) and plays a critical role in LA plasticity and auditory fear learning (Wiltgen et al, 2009). The α4 and δ subunits are expressed in the LA and BLA, although expression is sparse (Pirker et al, 2000). Chronic alcohol has been shown to produce changes in α1 and α4 GABAA receptor subunit trafficking and expression in BLA neurons (Diaz et al, 2011) and chronic alcohol and withdrawal have been shown to produce changes in the surface expression of multiple GABAA receptor subunits as well as functional changes in the sensitivity to acute alcohol exposure in BLA neurons (Lindemeyer et al, 2014). However, alterations in tonic inhibitory conductance and subsequent changes in the excitability of distinct neuronal populations (i.e., CRF1-containing BLA neurons) following chronic alcohol exposure remains to be studied. As tonic signaling is a critical determinant of overall network activity and tone (Semyanov et al, 2004), differential tonic conductance in discrete populations of amygdala neurons would allow for multiple levels of network regulation and multiple sites for potential dysregulation by alcohol.
Much of the previous work on the effects of alcohol has focused on synaptic transmission in BLA pyramidal neurons, as the major source of output from the BLA. These studies provide compelling evidence of the dysregulating effects of alcohol on pyramidal BLA neurons, however the effects of acute and chronic alcohol on other BLA subpopulations including interneurons and local intra-BLA projection neurons that connect distinct BLA subnuclei (e.g. LA and BM) remains unclear. The BLA is known to have a heterogeneous population of interneurons that make synaptic connections with each other as well as purported pyramidal neurons (Muller et al, 2003) and provide important feed-forward and feedback inhibitory control of pyramidal neurons (Muller et al, 2003; Zhu et al, 2006). The contribution of alcohol-induced alterations in this inhibitory control on changes in pyramidal neuron activity in the BLA remains understudied. Recent work in the central amygdala highlights the importance of local inhibitory microcircuits in the processing of fear acquisition and expression (Ciocchi et al, 2010; Haubensak et al, 2010), but it is not clear if a similar system of local inhibitory microcircuitry is present in the BLA and if or how it contributes to the effects of acute and chronic alcohol.
Alcohol and the Central Amygdala
As the main output region of the amygdala, the CeA sends projections to brain regions known to mediate the rewarding/consummatory aspects of alcohol use (such as the nucleus accumbens) as well as structures that have been implicated in alcohol dependence and withdrawal (such as the bed nucleus of the stria terminalis). Connectivity with both of these critical limbic regions may underlie the role that the central amygdala plays in both hedonic and anhedonic aspects of alcohol abuse. In postmortem CeA tissue collected from human participants, AMPA, NMDA and GABAA receptor mRNA was altered in participants with a history of alcoholism (Jin et al, 2014). Similar alterations in AMPA (Cannady et al, 2017; Salling et al, 2016), NMDA (Roberto et al, 2006) and GABAA (Freeman et al, 2013) receptors have been observed in rodent models. Animal studies have also been used to establish a functional role for the CeA in alcohol self-administration behavior. Lesions to the CeA (Moller et al, 1997) and 5HT-3 antagonists microinjected into the CeA (Dyr and Kostowski, 1995) reduce voluntary alcohol consumption in non-dependent animals. Similarly, intra-CeA modulation of GABA signaling reduces operant alcohol self-administration in models of alcohol dependence (Hyytia and Koob, 1995; Roberts et al, 1996).
Like the BLA, the CeA has also been shown to respond to the presence of alcohol at the level of synaptic transmission. Acute alcohol enhances GABA signaling in CeA slices from alcohol-naïve rats via both presynaptic enhancement of GABA release and postsynaptic alterations in GABAA receptor function (Roberto et al, 2003). In terms of excitatory signaling, acute alcohol has been shown to increase spontaneous glutamate transmission in mice (Silberman et al, 2015; Silberman and Winder, 2013b), but suppress both action-potential dependent endogenous glutamatergic signaling and evoked glutamatergic transmission in rats (Roberto et al, 2004b; Zhu et al, 2007). These alterations in glutamatergic signaling are accompanied by mRNA and protein changes in subunits of the glutamatergic NMDA receptor (Roberto et al, 2006). However, alcohol-induced suppression of glutamatergic transmission is intact in the presence of NMDA receptor antagonists, indicating that non-NMDA glutamate receptors (presumably the AMPA receptor) also contribute to this effect (Zhu et al, 2007).
Models of chronic alcohol exposure have also identified significant adaptations in the CeA. Chronic alcohol exposure increases concentrations of glutamate in the CeA at baseline, and causes enhanced glutamate release into the CeA following acute alcohol infusion as compared with naïve rats (Roberto et al, 2004b), in contrast to the general suppression of glutamatergic signaling seen following acute alcohol exposure. These changes are accompanied by alterations in the NMDA receptor GluN2Bsubunit (Roberto et al, 2006). Interestingly, the effects of chronic alcohol exposure on inhibitory neurotransmission in the CeA appear to be similar to those of acute alcohol. Chronic alcohol consumption enhances presynaptic GABA release in the rat CeA and may also enhance GABAergic activity via postsynaptic mechanisms (Roberto et al, 2004a). The similarity of this effect following both acute and chronic alcohol exposure suggests a lack of tolerance to alcohol-induced enhancement of GABAergic activity (Roberto et al, 2012). These changes in GABAergic transmission are accompanied by alterations in GABAA receptor subunit expression in the amygdala as a whole (Papadeas et al, 2001), although cell type-specific alterations within the CeA remain to be explored. Alterations in GABAA mRNA have been reported in the CeA following withdrawal from chronic alcohol (Freeman et al, 2013). Tonic GABA conductance has also been reported in the CeA, with alcohol inducing a tonic current in CeA slices in a cell-type specific manner in both rats and mice (Herman et al, 2013a; Herman and Roberto, 2016b). Together, these findings suggest a general enhancement of GABAergic signaling in the CeA following both acute and chronic alcohol exposure.
Alcohol and the CRF1 System
Given the marked effects of alcohol on inhibitory neurotransmission in the amygdala and the regulation of amygdalar GABAergic activity by CRF and activity at the CRF1 receptor, the CRF system is a logical site for the actions of alcohol in this region. Human studies have indicated a potential link between crhr1, the human gene for the CRF1 receptor, polymorphisms and risk for developing AUD (Glaser et al, 2014; Ribbe et al, 2011; Treutlein et al, 2006). Indeed, withdrawal from alcohol is associated with both increases in anxiety-like behavior as well as increases in extracellular CRF concentration in the amygdala (Funk et al, 2006; Merlo Pich et al, 1995; Zorrilla et al, 2001). Chronic models of alcohol exposure have been shown to increase amygdalar CRF and CRF1 receptor expression (Roberto et al, 2010), and sub-chronic alcohol drinking has been shown to alter CRF immunoreactivity and mRNA, respectively, in the CeA of both adult (Lowery-Gionta et al, 2012; Zhou et al, 2013) and adolescent rats (Allen et al, 2011; Karanikas et al, 2013). Recently, the development of more precise pharmacological tools and transgenic mouse lines have facilitated the investigation of the specific role of the CRF1 receptor in the acute and chronic actions of alcohol in the amygdala.
Acute Alcohol and the CRF1 System
Consistent with the observed alterations in amygdalar CRF following acute alcohol exposure, CRF1 receptor signaling has been shown to mediate some of the effects of acute alcohol on amygdalar GABAergic activity. The alcohol-induced increase in GABA neurotransmission in the CeA is absent in CRF1 receptor knockout mice, and CRF1 receptor antagonists block this effect of alcohol in wild-type mice (Nie et al, 2004). These effects appear to be driven by presynaptic CRF1 receptors (Nie et al, 2009). Previous work by our group using CRF1-GFP transgenic reporter mice assessed the effects of acute alcohol on CRF1+ cells within the CeA (Herman et al, 2016a). Acute alcohol increased the firing discharge of CRF1+ neurons and decreased the firing discharge of CRF1− neurons. Further, CRF1+ cells exhibited basal tonic GABA conductance mediated by the α1 GABAA receptor subunit that was insensitive to acute alcohol application, whereas alcohol induced a δ subunit-dependent tonic conductance in CRF1− cells that lacked GABAA tone at baseline. As CRF1− cells were shown to form inhibitory synapses onto CRF1+ cells, a subset of which projected to the BNST, these findings revealed an inhibitory microcircuit within the CeA that is sensitive to the presence of alcohol. Increased inhibitory tone in CRF1− cells following alcohol exposure disinhibits CRF1+ cells, increasing their firing and enhancing CeA output into the BNST (Herman et al, 2016a). These findings add complexity to our understanding of the circuit-specific effects of ethanol and CRF in the CeA. Although our work thus far has investigated the effects of ethanol on CRF1-containing neurons, not on the direct actions of CRF on CRF1 receptors, it raises the possibility that CRF, like ethanol, has specific actions at distinct sites in CeA microcircuitry. The CRF1:GFP mice used in these studies only labels neurons containing CRF1, it does not offer information on the localization of these receptors. Additional studies investigating the functional effects of CRF and CRF1 activation in CeA microcircuitry are needed.
The majority of studies on the regulation of alcohol-induced alterations in GABAergic signaling by CRF1 have focused on the CeA. Like the CeA, the BLA does exhibit basal tonic inhibition and alcohol has been shown to potentiate phasic inhibition in the rat BLA (Silberman et al, 2008). The ability of CRF1 to modulate alcohol effects on tonic and phasic inhibition in the BLA has yet to be assessed.
The CRF1 receptor also plays a role in the effects of acute alcohol on excitatory synaptic transmission in the CeA. Several studies (Herman et al, 2016c; Silberman et al, 2015; Silberman et al, 2013b; Varodayan et al, 2017) have shown that acute alcohol can potentiate spontaneous glutamatergic EPSCs in the CeA. This potentiation was blocked by pretreatment with a CRF1 receptor antagonist, but persisted despite the ablation of CRF-containing neurons in the CeA and whole organism (Silberman et al, 2015). This finding suggests that the CRF1 receptor, but not CRF peptide, is required for the effects of alcohol on spontaneous excitatory transmission in the CeA. Other endogenous CRF1 agonists, such as the Urocortins, may be responsible for the role of CRF1 in these alcohol-induced effects. Prior work also indicates that acute alcohol decreases evoked EPSCs in the CeA (Herman et al, 2016c; Roberto et al, 2004b; Varodayan et al, 2017), which points to differences in spontaneous versus evoked glutamate activity in the context of alcohol. The ability of either CRF peptide or the CRF1 receptor to regulate the effects of alcohol on evoked EPSCs in CeA has not yet been established.
Evidence from behavioral models of acute alcohol drinking indicate a role for CRF1 within the extended amygdala and other limbic brain regions in non-dependent alcohol consumption. Microinjection of the CRF1 antagonist antalarmin into the CeA reduced binge alcohol consumption in adult rats with a history of early life stress (Gondre-Lewis et al, 2016) as well as C57BL/6J mice with no history of stress (Lowery-Gionta et al, 2012). Recently, CRF1 receptor inhibition specifically within the VTA reduced binge alcohol drinking, and chemogenetic inhibition of BNST CRF1+ neurons that project to VTA produced similar reductions in alcohol intake (Rinker et al, 2017).
Chronic Alcohol and the CRF1 System
Chronic alcohol exposure also impacts the CRF1 system within the CeA. A four-week history of voluntary alcohol drinking increased GABA release and blunted sensitivity to the CRF1 antagonist R121919 in Marchigian Sardinian (msP) rats (Herman et al, 2013b). These alcohol-preferring rats also exhibit elevated basal GABA release relative to outbred strains. In Sprague-Dawley rats, 2–4 weeks of chronic alcohol vapor enhanced sensitivity to the effects of CRF and CRF1 antagonists to alter CeA GABA release (Roberto et al, 2010). However, the degree to which chronic alcohol-induced increases in extracellular GABA depend upon CRF1 signaling mechanisms has yet to be conclusively established.
Behavioral pharmacology experiments have also indicated a role for the CRF1 receptor in discrete brain regions in chronic alcohol drinking. Chronic treatment with a CRF1 antagonist prevented the withdrawal-induced increase in alcohol drinking observed in dependent rats (Roberto et al, 2010). Similar reductions in alcohol drinking following CRF1 antagonism were reported during alcohol deprivation in mice with a history of heavy alcohol drinking, accompanied by alterations in CRF1 mRNA in the amygdala (Correia et al, 2015). Microinjection of the CRF1/2 antagonist D-Phe-CRF(12-41) into the CeA reduces alcohol intake in alcohol-dependent, but not non-dependent, rats (Funk et al, 2006). CRF1 activation has also been implicated in the anxiety behavior induced by alcohol withdrawal (Overstreet et al, 2004) but the mechanisms behind this effect are not known.
Due to the substantial preclinical literature suggesting a role for CRF1 signaling in alcoholism, CRF1 has become an attractive target for the treatment of AUD. However, two studies in human participants have failed to demonstrate an effect of centrally-active CRF1 antagonists on alcohol craving and stress-related behavior during abstinence (Kwako et al, 2015; Schwandt et al, 2016). The lack of translation of systemic CRF1 antagonism from rodent models to humans may speak to the greater complexity of these systems in the primate brain, the specific outcomes measured (craving, as opposed to relapse and alcohol intake), a relatively greater degree of individual differences in native CRF/CRF1 activity in human populations versus rodent studies (many of which use inbred strains with reduced intra-individual variation), or differences between the chronic stress models typically employed in preclinical studies versus the often dynamic role of stress in human conditions such as alcoholism (Koob and Zorrilla, 2012; Spierling and Zorrilla, 2017).
Together, a wealth of studies indicate that the amygdala plays a central role in the acute and chronic effects of alcohol and can functionally regulate alcohol drinking behavior in binge-like and dependent models of alcohol drinking. Further, CRF1 signaling in the CeA has emerged as an important mediator of the effects of acute and chronic alcohol in this brain region.
Fear and the Amygdala
The amygdala has been recognized as a major regulator of anxiety and fear-related behaviors for many years. Human participants with PTSD have been found to have reductions in amygdala volume compared with healthy controls (Starcevic et al, 2014), and PTSD has been shown to alter functional connectivity both within amygdalar subnuclei and between these regions and the cortex (Brown et al, 2014). Similar evidence for dysregulation of cortical connectivity with the amygdala has been demonstrated in generalized anxiety disorder patients (Etkin et al, 2009). Amygdala hyperexcitability is a particularly consistent feature of anxiety disorders in human functional neuroimaging studies (Shin and Liberzon, 2010). Increases in amygdala activity have also been observed after the presentation of stress or fear-related visual stimuli in social phobia (Stein et al, 2002), arachnophobia (Schienle et al, 2005) and PTSD (Liberzon and Sripada, 2008), although results from patients with generalized anxiety disorder have been mixed (Mochcovitch et al, 2014).
Animal models of anxiety-like behavior have revealed some of the underlying neurological mechanisms behind the alterations in amygdalar activity seen in human studies. Dysregulation of GABAergic inhibition of the BLA results in hyperexcitability (Muller et al, 2015; Truitt et al, 2009), which has been associated with anxiety in rodent models and humans (Nuss, 2015; Prager et al, 2016; Terburg et al, 2012). Lesions to the CeA prevent the expression of anxiety-related behavior in the elevated plus maze in response to acute stressors (Ventura-Silva et al, 2013). Optogenetic activation of cells projecting from BLA to CeA promotes anxiolysis and inhibition of these same cells produces anxiogenesis in the open-field and elevated plus maze tasks (Tye et al 2011). These studies suggest that changes in activity within the CeA contribute to the expression of anxiogenic behaviors.
The amygdala also plays a significant role in fear learning. Generally speaking, the LA has been recognized as a site for the acquisition of fear conditioning, whereas the CeA is involved in the expression of fear-related conditioned responses (Maren and Quirk, 2004). Inputs to the LA are thought to strengthen with repeated pairings of the unconditioned stimulus (US) with the conditioned stimulus (CS) and promote recruitment of downstream targets in the CeAM (Blair et al, 2001; Sigurdsson et al, 2007). CeAL neurons exhibit increased firing in response to fear conditioned stimuli, and activation of these cells triggers freezing behavior in mice (Ciocchi et al, 2010). Information relating to fear conditioning can relay from the LA to the CeAM via two pathways: an excitatory direct tract from the BLA, or an inhibitory indirect projection from the CeAL to the CeAM (Duvarci et al, 2014).
Amygdala Microcircuitry and Fear Learning
Recent work has begun to dissect functional amygdala circuits that are responsible for the acquisition and expression of fear learning. Ciocchi et al. (2010) identified separable populations of cells within the CeAL that have opposite responses to CS presentation, those that increase firing in response to the CS (CElon cells) and those that decrease firing in response to the CS (CEloff cells). In a related paper, Haubensak and colleagues (2010) demonstrated that the CEloff population are likely PKCδ-containing neurons that send a GABAergic inhibitory projection to the CeAM, regulating CeAM activity and thereby altering the expression of conditioned fear. Both studies also provided evidence of reciprocal inhibition of CElon and CEloff cells within the CeAL. Consistent with these findings, genetic silencing of PKCδ-containing cells increased freezing behavior in a subset of conditioned animals. Together, these results identified an inhibitory microcircuit within the CeAL that functionally regulates CeAM activity and may in turn alter the expression of conditioned fear.
Subsequently, Li et al. (2013) demonstrated plasticity of glutamatergic signaling onto the SOM+ GABAergic cells of the CeAL. This population is distinct from the PKCδ+ neurons characterized by Ciocchi and Haubensak and does not project to the CeAM; rather, these cells form local inhibitory microcircuits that gate activity of the CeAL. Stimulation of this SOM+ cell population triggered freezing behavior in naïve mice, and inhibition of this population reduced the expression of conditioned fear. Additional populations of SOM+ cells in the CeAL project to the periacqueductal gray and paraventricular nucleus of the hypothalamus, where they regulate freezing behavior (Penzo et al, 2014). More recent work from this group has demonstrated that SOM+ CeAL cells activate in real-time in response to external threats; activation of this population is associated with more passive defensive strategies such as freezing, whereas inhibition of this population promotes active avoidance behaviors (Yu et al, 2016). As a whole, these studies suggest that following fear conditioning, the LA can relay information to the CeAL, where activation of PKCδ+ cells that project to the CeAM triggers the expression of conditioned fear responses, and conversely activation of the local SOM+ CeAL cells inhibits PKCδ+ cells, reducing the expression of conditioned fear responses. However, the added complexity of local versus projection SOM+ neurons exerting opposite effects on freezing behavior, and recent reports of significant overlap at the level of mRNA between cell markers in the CeAL (McCullough et al, 2018), highlight the need for further characterization of specific cell populations recruited during fear-related behavioral tasks and the functional role of these circuits in the cellular and behavioral output of the amygdala as a whole.
CRF Involvement in Fear/Anxiety
Given the ability of CRF and CRF1+ cells to regulate BLA and CeA neuronal activity, it is unsurprising that the CRF system has also been shown to regulate fear and anxiety-like behaviors. Acute stress enhances CRF release in the amygdala (Koob and Heinrichs, 1999), and the systemic administration of CRF increases anxiety-like behavior in rodent models, including acoustic startle, elevated plus maze, forced swim test, and defensive withdrawal in an open field (see Dunn & Berridge, 1990 for review.) Restraint stress has also been shown to increase CRF1 receptor mRNA in the CeA and BLA of Wistar rats (Ciccocioppo et al, 2014). CRF1 agonists generally produce pro-stress behavioral effects, whereas CRF1 antagonists generally produce anxiolysis in behavioral tests of stress responsivity (for review, see Koob 2010). The Marchigian Sardinian P (msP) rat strain exhibits increased anxiety-like behaviors accompanied by constitutive increases in CRF1 expression within the CeA (Cippitelli et al, 2015; Natividad et al, 2017). CRF activity within the CeA has also been shown to functionally regulate anxiety-like behavior (Rassnick et al, 1993). In the laterocapsular division of the CeA, CRF peptide and the CRF1 receptor have also been shown to play a role in nonciception (Ji et al, 2013; Ji and Neugebauer, 2008), cellular adaptations to chronic pain (Fu and Neugebauer, 2008; Ji and Neugebauer, 2007b), and the expression of pain-related anxiety behaviors (Ji et al, 2007a).
To date, the role of CRF and CRF1+ neurons within the amygdala in the expression of anxiety-like behavior and conditioned fear, and where these cell populations fit into the fear-related microcircuitry already identified in the CeA, has not been fully characterized. Recent evidence supports a key role for the CRF1+ cell populations of the CeA in conditioned fear. One study has identified CRF1+ neurons in the rostral CeAL as a distinct population that does not overlap with PKCδ+ or SOM+ cells. These CRF1+ cells exhibit long-term potentiation following fear conditioning, and inactivation of these cells inhibits the acquisition, but not expression, of fear learning about weak threats (Sanford et al, 2017). Additional evidence suggests that the CRF1+ cells of the CeA regulate conditioned freezing behavior whereas the SOM+ cells of the CeA mediate conditioned escape behaviors, with reciprocal inhibitory connections between these populations gating the expression of passive or active defensive behavior (Fadok et al, 2017). However, the role of CRF1+ cells in the CeAM in the expression of fear conditioning to weak threats, and the alterations in CRF1+ cells in the CeAL following chronic intense fear conditioning, are unknown.
The CRF System, Fear and Alcohol in the Amygdala
Current studies in the BLA circuitry underlying specific fear behaviors provide a framework for an improved understanding of the neurobiological underpinnings of alcohol use disorders and dependence. Notably, work on the specific alterations in amygdala microcircuitry produced by fear conditioning can act as a model and as a point of comparison with the effects of alcohol on amygdala circuitry. As fear conditioning and alcohol dependence can both be conceptualized as examples of aversive conditioning and can be measured in terms of learned behavior, they may engage the circuitry in the same way and produce similar effects at the cellular and whole animal level. Conversely, if conditioned fear is viewed as a useful physiological function and alcohol dependence is viewed as a maladaptive pathological process, then while they may engage the same microcircuitry they will produce different (and possibly opposing) effects. This hypothesis is supported by studies demonstrating increased GABA release following acute alcohol (Zhu et al, 2006) and decreased GABA levels following fear conditioning (Stork et al, 2002). In addition, experimental evidence also indicates that binge-like alcohol exposure impairs fear conditioning in humans (Stephens et al, 2005) and that repeated alcohol withdrawal impairs the acquisition of fear conditioning in rats (Ripley et al, 2003). However, it has also been shown that the retrieval of fear memory increased alcohol consumption in alcohol-withdrawn rats (Bertotto et al, 2010), suggesting that there may be some mechanisms for behavioral overlap. One potential point of overlap is the CRF and CRF1 system, which has been implicated in both fear conditioning and alcohol dependence. Fear conditioned rats display increased CRF dialysate levels in the BLA that are positively correlated with the expression of fear behavior (Mountney et al, 2011), the CRF1 gene Crhr1 is upregulated in the BLA of dependent rats (Sommer et al, 2008) and selective deletion of the α1 GABAA receptor subunit in CRF+ neurons enhanced anxiety and impaired fear extinction (Gafford et al, 2012). Additionally, polymorphisms in the human crhr1 gene have been repeatedly associated with increased risk for alcoholism following stressful life events (Blomeyer et al, 2008; Ray et al, 2013; Schmid et al, 2010). The possible interference by alcohol on normal functioning of BLA microcircuitry adds a potential new layer to the existing knowledge on how alcohol alters normal brain function to contribute to the pathological phenotype of dependence. Impaired learning circuitry could explain why alcoholics continue to drink despite negative consequences, as well as why extinction therapy often fails in treating alcoholics. If chronic alcohol exposure alters amygdala circuitry that is critical for learned associations, then it could result in a compromised ability to process physiologically-relevant negative associations and/or generate appropriate behavioral responses to aversive conditions.
Although the findings detailed above provide ample evidence that the activity of the CRF1 receptor in the amygdala plays a role in the acute and chronic effects of alcohol exposure, several important areas of investigation remain understudied. The majority of the work on CRF1-alcohol interactions have been studied in the CeA, but the BLA clearly plays an important role in alcohol-related behaviors and undergoes significant molecular adaptations following alcohol exposure. Future work should examine the effects of alcohol on CRF1+ and CRF1− cells within the BLA to complement the existing work from the CeA. At present, work on the CRF system and alcohol in the amygdala has focused on three discrete facets of this system: CRF peptide, the CRF1/CRF2 receptors, and CRF1-containing cell populations. It will be critical for future work to establish clear roles for these various component of the CRF system in alcohol-related behaviors, and to understand how they interact with one another to produce alterations in amygdala activity and behavior. Additionally, a functional role for CRF1 activity on alcohol-induced alterations in CeA glutamatergic activity has not yet been established. Lastly, two studies (Herman et al, 2013a; Roberto et al, 2010) provide initial evidence of the impact of chronic alcohol exposure on the CRF system of the CeA, but specific circuit dissection and the identification of mechanisms behind this effect have not been performed.
Recently, PKCε has emerged as an important regulator of CeA GABAergic transmission, CRF1 activity, and alcohol effects in acute alcohol models. Studies utilizing systemic knockout and intra-amygdala knockdown of PKCε have resulted in reduced alcohol intake, reduced alcohol intoxication, and altered GABAergic transmission both at baseline and in response to alcohol (Choi et al, 2008; Lesscher et al, 2009). Binge-like alcohol drinking, in which non-dependent subjects achieve a high blood-alcohol concentration during a limited window of alcohol access, has been shown to increase expression of PKCε in the CeA, and inhibition of PKCε specifically within the CeA reduced alcohol consumption in the binge model (Cozzoli et al, 2016). Given that PKCε has been shown to mediate some of the effects of CRF and alcohol on CeA GABAergic activity (Bajo et al, 2008; Blasio et al, 2017), the relationship between PKCε, CRF1 signaling and alcohol consumption warrants further investigation. If indeed native PKCε serves to inhibit GABAergic activity in the CeA, the acute increase in PKCε expression following sub-chronic alcohol exposure may represent a compensatory mechanism to offset the acute enhancement of GABAergic signaling induced by alcohol. However, the effect of chronic alcohol on PKCε expression or activity, and the potential functions of PKCε in models of alcohol dependence, remain to be explored. PKC has also been implicated in the stress-inducing effects of intra-ventricular CRF infusion (Toth et al, 2013) and has a well-established role in learning and memory (Sun and Alkon, 2014). These features make PKCε a particularly attractive target for investigation of the overlap, or lack thereof, of amygdalar signaling mechanisms in anxiety and alcoholism. Inhibitors that are selective for PKCε may also represent a more fine-tuned approach to pharmacological intervention in AUD, given the failure of systemic CRF1 antagonists to reduce alcohol craving in human participants (Kwako et al, 2015; Schwandt et al, 2016).
Another important consideration in the ongoing quest to clarify the role of CRF1 signaling in alcoholism and anxiety is the role of developmental stage. Adolescence is a time of heightened emotionality and stress responsivity (Casey et al, 2010), accompanied by alterations in the HPA axis generally and the cortisol system specifically (Apter et al, 1979). Findings from studies utilizing models of adolescent social isolation stress have demonstrated alterations in alcohol intake, amygdala function and anxiety-like behaviors (Butler et al, 2016; Karkhanis et al, 2015; Rau et al, 2015; Skelly et al, 2015), suggesting the potential for overlapping circuitry for these behaviors during adolescent brain development. Exposure to alcohol (Grant and Dawson, 1997) and stress (Burke and Miczek, 2014) during adolescence has also been shown to substantially enhance the lifetime risk for alcoholism in human participants and increase adult alcohol consumption in rodent models. However, the specific molecular and cellular mechanisms responsible for this increased risk remain unclear. CRF represents a system where stress and alcoholism converge, and therefore may play a role in mediating the long-lasting effects of adolescent alcohol exposure. To date, this possibility has received relatively little investigation. Adolescent alcohol exposure has been shown to both increase (Karanikas et al, 2013) and decrease (Allen et al, 2011) CRF immunoreactivity in the CeA. Additionally, age and sex differences in the ontogeny of the CRF1 receptor have been catalogued in many limbic brain regions, including the hypothalamus, ventral tegmental area, nucleus accumbens and hippocampus (Lukkes et al, 2016; Weathington et al, 2014), but the development of this receptor in the amygdala has not been studied. Age differences in sensitivity to CRF1 antagonist effects on alcohol consumption and GABAergic neurotransmission in brain regions relevant to addiction and anxiety have also not been explored. Such studies would provide important insight into the progression of alcohol use disorders over the lifespan and may reveal potential molecular mechanisms underlying age differences in alcohol intake and lifetime risk of AUD.
Finally, the recent work to define the microcircuitry of the central amygdala and the role of specific cell populations in fear conditioning can stand as a useful model for advances in preclinical alcohol research. To date, the role of the amygdala in alcohol-related behaviors, and reward-related behaviors in general, has focused on projections between the amygdala and other limbic circuitry. For instance, several studies have identified a role for an excitatory projection from the BLA to the NAc in behavior related to both natural and drug reward. Stuber et al. (2011) characterized this cell population and demonstrated that activation of this projection was sufficient to maintain self-administration of optical stimulation. Further, inhibiting this pathway reduced cued sucrose intake. Two recent papers have established a role for this pathway in alcohol-related behaviors. Keistler et al. (2017) found that selective ablation of cells in the BLA to NAc projection reduces reinstatement of alcohol seeking in rats. Millan et al. (2017) demonstrated that activation of this pathway reduced alcohol drinking and cue-induced alcohol seeking. These findings provide compelling evidence of the central role the amygdala plays in the regulation of downstream brain regions and the expression of alcohol-related behaviors. However, the role of intra-amygdala microcircuits on the overall activity of amygdalar subnuclei and the ways in which this circuitry modulates alcohol seeking, consumption and withdrawal behaviors has not been assessed. The recent work to characterize local microcircuits within the CeA in the context of fear conditioning has identified several important cell populations, including the CRF1+, SOM+, and PKCδ+ cells of the CeAM and CeAL whose sensitivity to acute and chronic alcohol has received limited investigation. Differences in the functional role of these populations on alcohol-related behaviors remain to be studied. Engagement of this circuitry by alcohol may drive changes in amygdala activity associated with anxiety and stress, and conversely activation of some or all of these populations by stress may alter their sensitivity to the effects of alcohol. With the identification of these discrete cell populations within the amygdala, both in the context of fear conditioning and alcohol exposure, the stage is set for the thorough mechanistic evaluation of the functional role of the intra-amygdala architecture in the cellular, regional and behavioral effects of alcohol.
HIGHLIGHTS.
The amygdala is a brain region where alcohol and anxiety-associated circuitry converge and is closely associated with the behavioral manifestations of both disorders.
Precise local and projection-based control of specific neuronal populations is emerging as a key regulator of intra-amygdala activity, amygdala output, and behavior.
The corticotropin releasing factor (CRF) system is implicated in the effects of alcohol, stress, and anxiety in distinct amygdala nuclei, but the specific circuitry involved in these effects and the role of these circuits in stress and anxiety are only beginning to be understood.
Acknowledgments
Funding: This work was supported by the Bowles Center for Alcohol Studies and the National Institute of Health [grants # AA023002 and R13AA017581].
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adamec RE, Sayin U, Brown A. The effects of corticotrophin releasing factor (CRF) and handling stress on behavior in the elevated plus-maze test of anxiety. J Psychopharmacol. 1991;5(3):175–186. doi: 10.1177/026988119100500301. [DOI] [PubMed] [Google Scholar]
- Allen CD, Lee S, Koob GF, Rivier C. Immediate and prolonged effects of alcohol exposure on the activity of the hypothalamic-pituitary-adrenal axis in adult and adolescent rats. Brain Behav Immun. 2011;25(Suppl 1):S50–60. doi: 10.1016/j.bbi.2011.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apter D, Pakarinen A, Hammond GL, Vihko R. Adrenocortical function in puberty. serum ACTH, cortisol and dehydroepiandrosterone in girls and boys. Acta Paediatr Scand. 1979;68(4):599–604. doi: 10.1111/j.1651-2227.1979.tb05062.x. [DOI] [PubMed] [Google Scholar]
- Bajo M, Cruz MT, Siggins GR, Messing R, Roberto M. Protein kinase C epsilon mediation of CRF− and ethanol-induced GABA release in central amygdala. Proc Natl Acad Sci U S A. 2008;105(24):8410–8415. doi: 10.1073/pnas.0802302105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker DG, West SA, Nicholson WE, Ekhator NN, Kasckow JW, Hill KK, et al. Serial CSF corticotropin-releasing hormone levels and adrenocortical activity in combat veterans with posttraumatic stress disorder. Am J Psychiatry. 1999;156(4):585–588. doi: 10.1176/ajp.156.4.585. [DOI] [PubMed] [Google Scholar]
- Baur R, Kaur KH, Sigel E. Structure of alpha6 beta3 delta GABA(A) receptors and their lack of ethanol sensitivity. J Neurochem. 2009;111(5):1172–1181. doi: 10.1111/j.1471-4159.2009.06387.x. [DOI] [PubMed] [Google Scholar]
- Belelli D, Harrison NL, Maguire J, Macdonald RL, Walker MC, Cope DW. Extrasynaptic GABAA receptors: form, pharmacology, and function. J Neurosci. 2009;29(41):12757–12763. doi: 10.1523/JNEUROSCI.3340-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertotto ME, Bussolino DF, Molina VA, Martijena ID. Increased voluntary ethanol consumption and c-Fos expression in selected brain areas induced by fear memory retrieval in ethanol withdrawn rats. Eur Neuropsychopharmacol. 2010;20(8):568–581. doi: 10.1016/j.euroneuro.2010.02.014. [DOI] [PubMed] [Google Scholar]
- Beyeler A, Chang CJ, Silvestre M, Leveque C, Namburi P, Wildes CP, et al. Organization of Valence-Encoding and Projection-Defined Neurons in the Basolateral Amygdala. Cell Rep. 2018;22(4):905–918. doi: 10.1016/j.celrep.2017.12.097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beyeler A, Namburi P, Glober GF, Simonnet C, Calhoon GG, Conyers GF, et al. Divergent Routing of Positive and Negative Information from the Amygdala during Memory Retrieval. Neuron. 2016;90(2):348–361. doi: 10.1016/j.neuron.2016.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair HT, Schafe GE, Bauer EP, Rodrigues SM, LeDoux JE. Synaptic plasticity in the lateral amygdala: a cellular hypothesis of fear conditioning. Learn Mem. 2001;8(5):229–242. doi: 10.1101/lm.30901. [DOI] [PubMed] [Google Scholar]
- Blasio A, Wang J, Wang D, Varodayan FP, Pomrenze MB, Miller J, et al. Novel Small-Molecule Inhibitors of Protein Kinase C Epsilon Reduce Ethanol Consumption in Mice. Biol Psychiatry. 2017 doi: 10.1016/j.biopsych.2017.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blomeyer D, Treutlein J, Esser G, Schmidt MH, Schumann G, Laucht M. Interaction between CRHR1 gene and stressful life events predicts adolescent heavy alcohol use. Biol Psychiatry. 2008;63(2):146–151. doi: 10.1016/j.biopsych.2007.04.026. [DOI] [PubMed] [Google Scholar]
- Borghese CM, Harris RA. Studies of ethanol actions on recombinant delta-containing gamma-aminobutyric acid type A receptors yield contradictory results. Alcohol. 2007;41(3):155–162. doi: 10.1016/j.alcohol.2007.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botta P, Mameli M, Floyd KL, Radcliffe RA, Valenzuela CF. Ethanol sensitivity of GABAergic currents in cerebellar granule neurons is not increased by a single amino acid change (R100Q) in the alpha6 GABAA receptor subunit. J Pharmacol Exp Ther. 2007;323(2):684–691. doi: 10.1124/jpet.107.127894. [DOI] [PubMed] [Google Scholar]
- Bremner JD, Licinio J, Darnell A, Krystal JH, Owens MJ, Southwick SM, et al. Elevated CSF corticotropin-releasing factor concentrations in posttraumatic stress disorder. Am J Psychiatry. 1997;154(5):624–629. doi: 10.1176/ajp.154.5.624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brickley SG, Mody I. Extrasynaptic GABA(A) receptors: their function in the CNS and implications for disease. Neuron. 2012;73(1):23–34. doi: 10.1016/j.neuron.2011.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britton DR, Koob GF, Rivier J, Vale W. Intraventricular corticotropin-releasing factor enhances behavioral effects of novelty. Life Sci. 1982;31(4):363–367. doi: 10.1016/0024-3205(82)90416-7. [DOI] [PubMed] [Google Scholar]
- Britton KT, Morgan J, Rivier J, Vale W, Koob GF. Chlordiazepoxide attenuates response suppression induced by corticotropin-releasing factor in the conflict test. Psychopharmacology (Berl) 1985;86(1–2):170–174. doi: 10.1007/BF00431704. [DOI] [PubMed] [Google Scholar]
- Brown VM, LaBar KS, Haswell CC, Gold AL, Mid-Atlantic MW, McCarthy G, et al. Altered resting-state functional connectivity of basolateral and centromedial amygdala complexes in posttraumatic stress disorder. Neuropsychopharmacology. 2014;39(2):351–359. doi: 10.1038/npp.2013.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke AR, Miczek KA. Stress in adolescence and drugs of abuse in rodent models: role of dopamine, CRF, and HPA axis. Psychopharmacology (Berl) 2014;231(8):1557–1580. doi: 10.1007/s00213-013-3369-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler TR, Karkhanis AN, Jones SR, Weiner JL. Adolescent Social Isolation as a Model of Heightened Vulnerability to Comorbid Alcoholism and Anxiety Disorders. Alcohol Clin Exp Res. 2016;40(6):1202–1214. doi: 10.1111/acer.13075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannady R, Fisher KR, Graham C, Crayle J, Besheer J, Hodge CW. Potentiation of amygdala AMPA receptor activity selectively promotes escalated alcohol self-administration in a CaMKII-dependent manner. Addict Biol. 2017;22(3):652–664. doi: 10.1111/adb.12357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey BJ, Jones RM, Levita L, Libby V, Pattwell SS, Ruberry EJ, et al. The storm and stress of adolescence: insights from human imaging and mouse genetics. Dev Psychobiol. 2010;52(3):225–235. doi: 10.1002/dev.20447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhri N, Woods CA, Sahuque LL, Gill TM, Janak PH. Unilateral inactivation of the basolateral amygdala attenuates context-induced renewal of Pavlovian-conditioned alcohol-seeking. Eur J Neurosci. 2013;38(5):2751–2761. doi: 10.1111/ejn.12278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi DS, Wei W, Deitchman JK, Kharazia VN, Lesscher HM, McMahon T, et al. Protein kinase Cdelta regulates ethanol intoxication and enhancement of GABA-stimulated tonic current. J Neurosci. 2008;28(46):11890–11899. doi: 10.1523/JNEUROSCI.3156-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciccocioppo R, de Guglielmo G, Hansson AC, Ubaldi M, Kallupi M, Cruz MT, et al. Restraint stress alters nociceptin/orphanin FQ and CRF systems in the rat central amygdala: significance for anxiety-like behaviors. J Neurosci. 2014;34(2):363–372. doi: 10.1523/JNEUROSCI.2400-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciocchi S, Herry C, Grenier F, Wolff SB, Letzkus JJ, Vlachos I, et al. Encoding of conditioned fear in central amygdala inhibitory circuits. Nature. 2010;468(7321):277–282. doi: 10.1038/nature09559. [DOI] [PubMed] [Google Scholar]
- Cippitelli A, Ayanwuyi LO, Barbier E, Domi E, Lerma-Cabrera JM, Carvajal F, et al. Polymorphism in the corticotropin-releasing factor receptor 1 (CRF1-R) gene plays a role in shaping the high anxious phenotype of Marchigian Sardinian alcohol-preferring (msP) rats. Psychopharmacology (Berl) 2015;232(6):1083–1093. doi: 10.1007/s00213-014-3743-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Correia D, Martynhak BJ, Pereira M, Siba IP, Ribeiro AF, Camarini R, et al. Reduction of ethanol intake by corticotropin-releasing factor receptor-1 antagonist in “heavy-drinking” mice in a free-choice paradigm. Psychopharmacology (Berl) 2015;232(15):2731–2739. doi: 10.1007/s00213-015-3909-y. [DOI] [PubMed] [Google Scholar]
- Cozzoli DK, Courson J, Rostock C, Campbell RR, Wroten MG, McGregor H, et al. Protein Kinase C Epsilon Activity in the Nucleus Accumbens and Central Nucleus of the Amygdala Mediates Binge Alcohol Consumption. Biol Psychiatry. 2016;79(6):443–451. doi: 10.1016/j.biopsych.2015.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dager AD, Anderson BM, Stevens MC, Pulido C, Rosen R, Jiantonio-Kelly RE, et al. Influence of alcohol use and family history of alcoholism on neural response to alcohol cues in college drinkers. Alcohol Clin Exp Res. 2013;37(Suppl 1):E161–171. doi: 10.1111/j.1530-0277.2012.01879.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day HE, Curran EJ, Watson SJ, Jr, Akil H. Distinct neurochemical populations in the rat central nucleus of the amygdala and bed nucleus of the stria terminalis: evidence for their selective activation by interleukin-1beta. J Comp Neurol. 1999;413(1):113–128. [PubMed] [Google Scholar]
- Diaz MR, Christian DT, Anderson NJ, McCool BA. Chronic ethanol and withdrawal differentially modulate lateral/basolateral amygdala paracapsular and local GABAergic synapses. J Pharmacol Exp Ther. 2011;337(1):162–170. doi: 10.1124/jpet.110.177121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dube T, Brunson T, Nehlig A, Baram TZ. Activation of specific neuronal circuits by corticotropin releasing hormone as indicated by c-fos expression and glucose metabolism. J Cereb Blood Flow Metab. 2000;20(10):1414–1424. doi: 10.1097/00004647-200010000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn AJ, Berridge CW. Physiological and behavioral responses to corticotropin-releasing factor administration: is CRF a mediator of anxiety or stress responses? Brain Res Brain Res Rev. 1990;15(2):71–100. doi: 10.1016/0165-0173(90)90012-d. [DOI] [PubMed] [Google Scholar]
- Duvarci S, Pare D. Amygdala microcircuits controlling learned fear. Neuron. 2014;82(5):966–980. doi: 10.1016/j.neuron.2014.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyr W, Kostowski W. Evidence that the amygdala is involved in the inhibitory effects of 5-HT3 receptor antagonists on alcohol drinking in rats. Alcohol. 1995;12(4):387–391. doi: 10.1016/0741-8329(95)00023-k. [DOI] [PubMed] [Google Scholar]
- Eckardt MJ, File SE, Gessa GL, Grant KA, Guerri C, Hoffman PL, et al. Effects of moderate alcohol consumption on the central nervous system. Alcohol Clin Exp Res. 1998;22(5):998–1040. doi: 10.1111/j.1530-0277.1998.tb03695.x. [DOI] [PubMed] [Google Scholar]
- Ehrlich I, Humeau Y, Grenier F, Ciocchi S, Herry C, Luthi A. Amygdala inhibitory circuits and the control of fear memory. Neuron. 2009;62(6):757–771. doi: 10.1016/j.neuron.2009.05.026. [DOI] [PubMed] [Google Scholar]
- Etkin A, Prater KE, Schatzberg AF, Menon V, Greicius MD. Disrupted amygdalar subregion functional connectivity and evidence of a compensatory network in generalized anxiety disorder. Arch Gen Psychiatry. 2009;66(12):1361–1372. doi: 10.1001/archgenpsychiatry.2009.104. [DOI] [PubMed] [Google Scholar]
- Fadok JP, Krabbe S, Markovic M, Courtin J, Xu C, Massi L, et al. A competitive inhibitory circuit for selection of active and passive fear responses. Nature. 2017;542(7639):96–100. doi: 10.1038/nature21047. [DOI] [PubMed] [Google Scholar]
- Freeman K, Staehle MM, Vadigepalli R, Gonye GE, Ogunnaike BA, Hoek JB, et al. Coordinated dynamic gene expression changes in the central nucleus of the amygdala during alcohol withdrawal. Alcohol Clin Exp Res. 2013;37(Suppl 1):E88–100. doi: 10.1111/j.1530-0277.2012.01910.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu Y, Neugebauer V. Differential mechanisms of CRF1 and CRF2 receptor functions in the amygdala in pain-related synaptic facilitation and behavior. J Neurosci. 2008;28(15):3861–3876. doi: 10.1523/JNEUROSCI.0227-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funk CK, O’Dell LE, Crawford EF, Koob GF. Corticotropin-releasing factor within the central nucleus of the amygdala mediates enhanced ethanol self-administration in withdrawn, ethanol-dependent rats. J Neurosci. 2006;26(44):11324–11332. doi: 10.1523/JNEUROSCI.3096-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gafford GM, Guo JD, Flandreau EI, Hazra R, Rainnie DG, Ressler KJ. Cell-type specific deletion of GABA(A)alpha1 in corticotropin-releasing factor-containing neurons enhances anxiety and disrupts fear extinction. Proc Natl Acad Sci U S A. 2012;109(40):16330–16335. doi: 10.1073/pnas.1119261109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glaser YG, Zubieta JK, Hsu DT, Villafuerte S, Mickey BJ, Trucco EM, et al. Indirect effect of corticotropin-releasing hormone receptor 1 gene variation on negative emotionality and alcohol use via right ventrolateral prefrontal cortex. J Neurosci. 2014;34(11):4099–4107. doi: 10.1523/JNEUROSCI.3672-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glykys J, Mody I. Activation of GABAA receptors: views from outside the synaptic cleft. Neuron. 2007;56(5):763–770. doi: 10.1016/j.neuron.2007.11.002. [DOI] [PubMed] [Google Scholar]
- Gondre-Lewis MC, Warnock KT, Wang H, June HL, Jr, Bell KA, Rabe H, et al. Early life stress is a risk factor for excessive alcohol drinking and impulsivity in adults and is mediated via a CRF/GABA(A) mechanism. Stress. 2016;19(2):235–247. doi: 10.3109/10253890.2016.1160280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant BF, Dawson DA. Age at onset of alcohol use and its association with DSM-IV alcohol abuse and dependence: results from the National Longitudinal Alcohol Epidemiologic Survey. J Subst Abuse. 1997;9:103–110. doi: 10.1016/s0899-3289(97)90009-2. [DOI] [PubMed] [Google Scholar]
- Grobin AC, Matthews DB, Devaud LL, Morrow AL. The role of GABA(A) receptors in the acute and chronic effects of ethanol. Psychopharmacology (Berl) 1998;139(1–2):2–19. doi: 10.1007/s002130050685. [DOI] [PubMed] [Google Scholar]
- Gungor NZ, Yamamoto R, Pare D. Optogenetic study of the projections from the bed nucleus of the stria terminalis to the central amygdala. J Neurophysiol. 2015;114(5):2903–2911. doi: 10.1152/jn.00677.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haubensak W, Kunwar PS, Cai H, Ciocchi S, Wall NR, Ponnusamy R, et al. Genetic dissection of an amygdala microcircuit that gates conditioned fear. Nature. 2010;468(7321):270–276. doi: 10.1038/nature09553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman MA, Contet C, Justice NJ, Vale W, Roberto M. Novel subunit-specific tonic GABA currents and differential effects of ethanol in the central amygdala of CRF receptor-1 reporter mice. J Neurosci. 2013a;33(8):3284–3298. doi: 10.1523/JNEUROSCI.2490-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman MA, Contet C, Roberto M. A Functional Switch in Tonic GABA Currents Alters the Output of Central Amygdala Corticotropin Releasing Factor Receptor-1 Neurons Following Chronic Ethanol Exposure. J Neurosci. 2016a;36(42):10729–10741. doi: 10.1523/JNEUROSCI.1267-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman MA, Kallupi M, Luu G, Oleata CS, Heilig M, Koob GF, et al. Enhanced GABAergic transmission in the central nucleus of the amygdala of genetically selected Marchigian Sardinian rats: alcohol and CRF effects. Neuropharmacology. 2013b;67:337–348. doi: 10.1016/j.neuropharm.2012.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman MA, Roberto M. Cell-type-specific tonic GABA signaling in the rat central amygdala is selectively altered by acute and chronic ethanol. Addict Biol. 2016b;21(1):72–86. doi: 10.1111/adb.12181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman MA, Varodayan FP, Oleata CS, Luu G, Kirson D, Heilig M, et al. Glutamatergic transmission in the central nucleus of the amygdala is selectively altered in Marchigian Sardinian alcohol-preferring rats: Alcohol and CRF effects. Neuropharmacology. 2016c;102:21–31. doi: 10.1016/j.neuropharm.2015.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez VS, Vazquez-Juarez E, Marquez MM, Jauregui-Huerta F, Barrio RA, Zhang L. Extra-neurohypophyseal axonal projections from individual vasopressin-containing magnocellular neurons in rat hypothalamus. Front Neuroanat. 2015;9:130. doi: 10.3389/fnana.2015.00130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbard DT, Nakashima BR, Lee I, Takahashi LK. Activation of basolateral amygdala corticotropin-releasing factor 1 receptors modulates the consolidation of contextual fear. Neuroscience. 2007;150(4):818–828. doi: 10.1016/j.neuroscience.2007.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyytia P, Koob GF. GABAA receptor antagonism in the extended amygdala decreases ethanol self-administration in rats. Eur J Pharmacol. 1995;283(1–3):151–159. doi: 10.1016/0014-2999(95)00314-b. [DOI] [PubMed] [Google Scholar]
- Ji G, Fu Y, Adwanikar H, Neugebauer V. Non-pain-related CRF1 activation in the amygdala facilitates synaptic transmission and pain responses. Mol Pain. 2013;9:2. doi: 10.1186/1744-8069-9-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji G, Fu Y, Ruppert KA, Neugebauer V. Pain-related anxiety-like behavior requires CRF1 receptors in the amygdala. Mol Pain. 2007a;3:13. doi: 10.1186/1744-8069-3-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji G, Neugebauer V. Differential effects of CRF1 and CRF2 receptor antagonists on pain-related sensitization of neurons in the central nucleus of the amygdala. J Neurophysiol. 2007b;97(6):3893–3904. doi: 10.1152/jn.00135.2007. [DOI] [PubMed] [Google Scholar]
- Ji G, Neugebauer V. Pro- and anti-nociceptive effects of corticotropin-releasing factor (CRF) in central amygdala neurons are mediated through different receptors. J Neurophysiol. 2008;99(3):1201–1212. doi: 10.1152/jn.01148.2007. [DOI] [PubMed] [Google Scholar]
- Jin Z, Bhandage AK, Bazov I, Kononenko O, Bakalkin G, Korpi ER, et al. Expression of specific ionotropic glutamate and GABA-A receptor subunits is decreased in central amygdala of alcoholics. Front Cell Neurosci. 2014;8:288. doi: 10.3389/fncel.2014.00288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Justice NJ, Yuan ZF, Sawchenko PE, Vale W. Type 1 corticotropin-releasing factor receptor expression reported in BAC transgenic mice: implications for reconciling ligand-receptor mismatch in the central corticotropin-releasing factor system. J Comp Neurol. 2008;511(4):479–496. doi: 10.1002/cne.21848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang-Park M, Kieffer BL, Roberts AJ, Siggins GR, Moore SD. Interaction of CRF and kappa opioid systems on GABAergic neurotransmission in the mouse central amygdala. J Pharmacol Exp Ther. 2015;355(2):206–211. doi: 10.1124/jpet.115.225870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karanikas CA, Lu YL, Richardson HN. Adolescent drinking targets corticotropin-releasing factor peptide-labeled cells in the central amygdala of male and female rats. Neuroscience. 2013;249:98–105. doi: 10.1016/j.neuroscience.2013.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karkhanis AN, Alexander NJ, McCool BA, Weiner JL, Jones SR. Chronic social isolation during adolescence augments catecholamine response to acute ethanol in the basolateral amygdala. Synapse. 2015;69(8):385–395. doi: 10.1002/syn.21826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keistler CR, Hammarlund E, Barker JM, Bond CW, DiLeone RJ, Pittenger C, et al. Regulation of Alcohol Extinction and Cue-Induced Reinstatement by Specific Projections among Medial Prefrontal Cortex, Nucleus Accumbens, and Basolateral Amygdala. J Neurosci. 2017;37(17):4462–4471. doi: 10.1523/JNEUROSCI.3383-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller PA, McCluskey A, Morgan J, O’Connor SM. The role of the HPA axis in psychiatric disorders and CRF antagonists as potential treatments. Arch Pharm (Weinheim) 2006;339(7):346–355. doi: 10.1002/ardp.200600021. [DOI] [PubMed] [Google Scholar]
- Koob G, Britton K. Neurobiological substrates for the anti-anxiety effects of ethanol. The pharmacology of alcohol and alcohol dependence. 1996:477–506. [Google Scholar]
- Koob GF. Neuroadaptive mechanisms of addiction: studies on the extended amygdala. Eur Neuropsychopharmacol. 2003;13(6):442–452. doi: 10.1016/j.euroneuro.2003.08.005. [DOI] [PubMed] [Google Scholar]
- Koob GF. The role of CRF and CRF-related peptides in the dark side of addiction. Brain Res. 2010;1314:3–14. doi: 10.1016/j.brainres.2009.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Heinrichs SC. A role for corticotropin releasing factor and urocortin in behavioral responses to stressors. Brain Res. 1999;848(1–2):141–152. doi: 10.1016/s0006-8993(99)01991-5. [DOI] [PubMed] [Google Scholar]
- Koob GF, Le Moal M. Drug addiction, dysregulation of reward, and allostasis. Neuropsychopharmacology. 2001;24(2):97–129. doi: 10.1016/S0893-133X(00)00195-0. [DOI] [PubMed] [Google Scholar]
- Koob GF, Zorrilla EP. Update on corticotropin-releasing factor pharmacotherapy for psychiatric disorders: a revisionist view. Neuropsychopharmacology. 2012;37(1):308–309. doi: 10.1038/npp.2011.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kushner MG, Abrams K, Borchardt C. The relationship between anxiety disorders and alcohol use disorders: a review of major perspectives and findings. Clin Psychol Rev. 2000;20(2):149–171. doi: 10.1016/s0272-7358(99)00027-6. [DOI] [PubMed] [Google Scholar]
- Kwako LE, Spagnolo PA, Schwandt ML, Thorsell A, George DT, Momenan R, et al. The corticotropin releasing hormone-1 (CRH1) receptor antagonist pexacerfont in alcohol dependence: a randomized controlled experimental medicine study. Neuropsychopharmacology. 2015;40(5):1053–1063. doi: 10.1038/npp.2014.306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lack AK, Ariwodola OJ, Chappell AM, Weiner JL, McCool BA. Ethanol inhibition of kainate receptor-mediated excitatory neurotransmission in the rat basolateral nucleus of the amygdala. Neuropharmacology. 2008;55(5):661–668. doi: 10.1016/j.neuropharm.2008.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lack AK, Christian DT, Diaz MR, McCool BA. Chronic ethanol and withdrawal effects on kainate receptor-mediated excitatory neurotransmission in the rat basolateral amygdala. Alcohol. 2009;43(1):25–33. doi: 10.1016/j.alcohol.2008.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lack AK, Diaz MR, Chappell A, DuBois DW, McCool BA. Chronic ethanol and withdrawal differentially modulate pre- and postsynaptic function at glutamatergic synapses in rat basolateral amygdala. J Neurophysiol. 2007;98(6):3185–3196. doi: 10.1152/jn.00189.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai HM, Cleary M, Sitharthan T, Hunt GE. Prevalence of comorbid substance use, anxiety and mood disorders in epidemiological surveys, 1990–2014: A systematic review and meta-analysis. Drug Alcohol Depend. 2015;154:1–13. doi: 10.1016/j.drugalcdep.2015.05.031. [DOI] [PubMed] [Google Scholar]
- Lesscher HM, Wallace MJ, Zeng L, Wang V, Deitchman JK, McMahon T, et al. Amygdala protein kinase C epsilon controls alcohol consumption. Genes Brain Behav. 2009;8(5):493–499. doi: 10.1111/j.1601-183X.2009.00485.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Penzo MA, Taniguchi H, Kopec CD, Huang ZJ, Li B. Experience-dependent modification of a central amygdala fear circuit. Nat Neurosci. 2013;16(3):332–339. doi: 10.1038/nn.3322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liberzon I, Sripada CS. The functional neuroanatomy of PTSD: a critical review. Prog Brain Res. 2008;167:151–169. doi: 10.1016/S0079-6123(07)67011-3. [DOI] [PubMed] [Google Scholar]
- Lindemeyer AK, Liang J, Marty VN, Meyer EM, Suryanarayanan A, Olsen RW, et al. Ethanol-induced plasticity of GABAA receptors in the basolateral amygdala. Neurochem Res. 2014;39(6):1162–1170. doi: 10.1007/s11064-014-1297-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Yu B, Neugebauer V, Grigoriadis DE, Rivier J, Vale WW, et al. Corticotropin-releasing factor and Urocortin I modulate excitatory glutamatergic synaptic transmission. J Neurosci. 2004;24(16):4020–4029. doi: 10.1523/JNEUROSCI.5531-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovinger DM, Roberto M. Synaptic effects induced by alcohol. Curr Top Behav Neurosci. 2013;13:31–86. doi: 10.1007/7854_2011_143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowery-Gionta EG, Navarro M, Li C, Pleil KE, Rinker JA, Cox BR, et al. Corticotropin releasing factor signaling in the central amygdala is recruited during binge-like ethanol consumption in C57BL/6J mice. J Neurosci. 2012;32(10):3405–3413. doi: 10.1523/JNEUROSCI.6256-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukkes JL, Norman KJ, Meda S, Andersen SL. Sex differences in the ontogeny of CRF receptors during adolescent development in the dorsal raphe nucleus and ventral tegmental area. Synapse. 2016;70(3):125–132. doi: 10.1002/syn.21882. [DOI] [PubMed] [Google Scholar]
- Maren S, Quirk GJ. Neuronal signalling of fear memory. Nat Rev Neurosci. 2004;5(11):844–852. doi: 10.1038/nrn1535. [DOI] [PubMed] [Google Scholar]
- Marowsky A, Fritschy JM, Vogt KE. Functional mapping of GABA A receptor subtypes in the amygdala. Eur J Neurosci. 2004;20(5):1281–1289. doi: 10.1111/j.1460-9568.2004.03574.x. [DOI] [PubMed] [Google Scholar]
- Marowsky A, Rudolph U, Fritschy JM, Arand M. Tonic inhibition in principal cells of the amygdala: a central role for alpha3 subunit-containing GABAA receptors. J Neurosci. 2012;32(25):8611–8619. doi: 10.1523/JNEUROSCI.4404-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBride WJ. Central nucleus of the amygdala and the effects of alcohol and alcohol-drinking behavior in rodents. Pharmacol Biochem Behav. 2002;71(3):509–515. doi: 10.1016/s0091-3057(01)00680-3. [DOI] [PubMed] [Google Scholar]
- McCool BA. Ethanol modulation of synaptic plasticity. Neuropharmacology. 2011;61(7):1097–1108. doi: 10.1016/j.neuropharm.2010.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCool BA, Christian DT, Fetzer JA, Chappell AM. Lateral/basolateral amygdala serotonin type-2 receptors modulate operant self-administration of a sweetened ethanol solution via inhibition of principal neuron activity. Front Integr Neurosci. 2014;8:5. doi: 10.3389/fnint.2014.00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCool BA, Frye GD, Pulido MD, Botting SK. Effects of chronic ethanol consumption on rat GABA(A) and strychnine-sensitive glycine receptors expressed by lateral/basolateral amygdala neurons. Brain Res. 2003;963(1–2):165–177. doi: 10.1016/s0006-8993(02)03966-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCullough KM, Morrison FG, Hartmann J, Carlezon WA, Jr, Ressler KJ. Quantified Coexpression Analysis of Central Amygdala Subpopulations. eNeuro. 2018;5(1) doi: 10.1523/ENEURO.0010-18.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald AJ. Neurons of the lateral and basolateral amygdaloid nuclei: a Golgi study in the rat. J Comp Neurol. 1982;212(3):293–312. doi: 10.1002/cne.902120307. [DOI] [PubMed] [Google Scholar]
- McDonald AJ. Immunohistochemical identification of gamma-aminobutyric acid-containing neurons in the rat basolateral amygdala. Neurosci Lett. 1985;53(2):203–207. doi: 10.1016/0304-3940(85)90186-7. [DOI] [PubMed] [Google Scholar]
- McDonald AJ, Augustine JR. Localization of GABA-like immunoreactivity in the monkey amygdala. Neuroscience. 1993;52(2):281–294. doi: 10.1016/0306-4522(93)90156-a. [DOI] [PubMed] [Google Scholar]
- McDonald AJ, Betette RL. Parvalbumin-containing neurons in the rat basolateral amygdala: morphology and co-localization of Calbindin-D(28k) Neuroscience. 2001;102(2):413–425. doi: 10.1016/s0306-4522(00)00481-4. [DOI] [PubMed] [Google Scholar]
- Menzaghi F, Rassnick S, Heinrichs S, Baldwin H, Pich EM, Weiss F, et al. The role of corticotropin-releasing factor in the anxiogenic effects of ethanol withdrawal. Ann N Y Acad Sci. 1994;739:176–184. doi: 10.1111/j.1749-6632.1994.tb19819.x. [DOI] [PubMed] [Google Scholar]
- Merlo Pich E, Lorang M, Yeganeh M, Rodriguez de Fonseca F, Raber J, Koob GF, et al. Increase of extracellular corticotropin-releasing factor-like immunoreactivity levels in the amygdala of awake rats during restraint stress and ethanol withdrawal as measured by microdialysis. J Neurosci. 1995;15(8):5439–5447. doi: 10.1523/JNEUROSCI.15-08-05439.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millan EZ, Kim HA, Janak PH. Optogenetic activation of amygdala projections to nucleus accumbens can arrest conditioned and unconditioned alcohol consummatory behavior. Neuroscience. 2017;360:106–117. doi: 10.1016/j.neuroscience.2017.07.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mochcovitch MD, da Rocha Freire RC, Garcia RF, Nardi AE. A systematic review of fMRI studies in generalized anxiety disorder: evaluating its neural and cognitive basis. J Affect Disord. 2014;167:336–342. doi: 10.1016/j.jad.2014.06.041. [DOI] [PubMed] [Google Scholar]
- Mody I, Glykys J, Wei W. A new meaning for “Gin & Tonic”: tonic inhibition as the target for ethanol action in the brain. Alcohol. 2007;41(3):145–153. doi: 10.1016/j.alcohol.2007.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moller C, Wiklund L, Sommer W, Thorsell A, Heilig M. Decreased experimental anxiety and voluntary ethanol consumption in rats following central but not basolateral amygdala lesions. Brain Res. 1997;760(1–2):94–101. doi: 10.1016/s0006-8993(97)00308-9. [DOI] [PubMed] [Google Scholar]
- Morales M, McGinnis MM, Robinson SL, Chappell AM, McCool BA. Chronic Intermittent Ethanol Exposure Modulation of Glutamatergic Neurotransmission in Rat Lateral/Basolateral Amygdala is Duration-, Input-, and Sex-Dependent. Neuroscience. 2018;371:277–287. doi: 10.1016/j.neuroscience.2017.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mountney C, Anisman H, Merali Z. In vivo levels of corticotropin-releasing hormone and gastrin-releasing peptide at the basolateral amygdala and medial prefrontal cortex in response to conditioned fear in the rat. Neuropharmacology. 2011;60(2–3):410–417. doi: 10.1016/j.neuropharm.2010.10.013. [DOI] [PubMed] [Google Scholar]
- Muller I, Caliskan G, Stork O. The GAD65 knock out mouse - a model for GABAergic processes in fear- and stress-induced psychopathology. Genes Brain Behav. 2015;14(1):37–45. doi: 10.1111/gbb.12188. [DOI] [PubMed] [Google Scholar]
- Muller JF, Mascagni F, McDonald AJ. Synaptic connections of distinct interneuronal subpopulations in the rat basolateral amygdalar nucleus. J Comp Neurol. 2003;456(3):217–236. doi: 10.1002/cne.10435. [DOI] [PubMed] [Google Scholar]
- Natividad LA, Buczynski MW, Herman MA, Kirson D, Oleata CS, Irimia C, et al. Constitutive Increases in Amygdalar Corticotropin-Releasing Factor and Fatty Acid Amide Hydrolase Drive an Anxious Phenotype. Biol Psychiatry. 2017;82(7):500–510. doi: 10.1016/j.biopsych.2017.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nie Z, Schweitzer P, Roberts AJ, Madamba SG, Moore SD, Siggins GR. Ethanol augments GABAergic transmission in the central amygdala via CRF1 receptors. Science. 2004;303(5663):1512–1514. doi: 10.1126/science.1092550. [DOI] [PubMed] [Google Scholar]
- Nie Z, Zorrilla EP, Madamba SG, Rice KC, Roberto M, Siggins GR. Presynaptic CRF1 receptors mediate the ethanol enhancement of GABAergic transmission in the mouse central amygdala. Scientific World Journal. 2009;9:68–85. doi: 10.1100/tsw.2009.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuss P. Anxiety disorders and GABA neurotransmission: a disturbance of modulation. Neuropsychiatr Dis Treat. 2015;11:165–175. doi: 10.2147/NDT.S58841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olmos-Serrano JL, Paluszkiewicz SM, Martin BS, Kaufmann WE, Corbin JG, Huntsman MM. Defective GABAergic neurotransmission and pharmacological rescue of neuronal hyperexcitability in the amygdala in a mouse model of fragile X syndrome. J Neurosci. 2010;30(29):9929–9938. doi: 10.1523/JNEUROSCI.1714-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overstreet DH, Knapp DJ, Breese GR. Modulation of multiple ethanol withdrawal-induced anxiety-like behavior by CRF and CRF1 receptors. Pharmacol Biochem Behav. 2004;77(2):405–413. doi: 10.1016/j.pbb.2003.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papadeas S, Grobin AC, Morrow AL. Chronic ethanol consumption differentially alters GABA(A) receptor alpha1 and alpha4 subunit peptide expression and GABA(A) receptor-mediated 36 Cl(−) uptake in mesocorticolimbic regions of rat brain. Alcohol Clin Exp Res. 2001;25(9):1270–1275. [PubMed] [Google Scholar]
- Penzo MA, Robert V, Li B. Fear conditioning potentiates synaptic transmission onto long-range projection neurons in the lateral subdivision of central amygdala. J Neurosci. 2014;34(7):2432–2437. doi: 10.1523/JNEUROSCI.4166-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perra S, Pillolla G, Luchicchi A, Pistis M. Alcohol inhibits spontaneous activity of basolateral amygdala projection neurons in the rat: involvement of the endocannabinoid system. Alcohol Clin Exp Res. 2008;32(3):443–449. doi: 10.1111/j.1530-0277.2007.00588.x. [DOI] [PubMed] [Google Scholar]
- Pirker S, Schwarzer C, Wieselthaler A, Sieghart W, Sperk G. GABA(A) receptors: immunocytochemical distribution of 13 subunits in the adult rat brain. Neuroscience. 2000;101(4):815–850. doi: 10.1016/s0306-4522(00)00442-5. [DOI] [PubMed] [Google Scholar]
- Pitkanen A, Amaral DG. The distribution of GABAergic cells, fibers, and terminals in the monkey amygdaloid complex: an immunohistochemical and in situ hybridization study. J Neurosci. 1994;14(4):2200–2224. doi: 10.1523/JNEUROSCI.14-04-02200.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porrino LJ, Williams-Hemby L, Whitlow C, Bowen C, Samson HH. Metabolic mapping of the effects of oral alcohol self-administration in rats. Alcohol Clin Exp Res. 1998;22(1):176–182. [PubMed] [Google Scholar]
- Prager EM, Bergstrom HC, Wynn GH, Braga MF. The basolateral amygdala gamma-aminobutyric acidergic system in health and disease. J Neurosci Res. 2016;94(6):548–567. doi: 10.1002/jnr.23690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rainnie DG, Bergeron R, Sajdyk TJ, Patil M, Gehlert DR, Shekhar A. Corticotrophin releasing factor-induced synaptic plasticity in the amygdala translates stress into emotional disorders. J Neurosci. 2004;24(14):3471–3479. doi: 10.1523/JNEUROSCI.5740-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rassnick S, Heinrichs SC, Britton KT, Koob GF. Microinjection of a corticotropin-releasing factor antagonist into the central nucleus of the amygdala reverses anxiogenic-like effects of ethanol withdrawal. Brain Res. 1993;605(1):25–32. doi: 10.1016/0006-8993(93)91352-s. [DOI] [PubMed] [Google Scholar]
- Rau AR, Chappell AM, Butler TR, Ariwodola OJ, Weiner JL. Increased Basolateral Amygdala Pyramidal Cell Excitability May Contribute to the Anxiogenic Phenotype Induced by Chronic Early-Life Stress. J Neurosci. 2015;35(26):9730–9740. doi: 10.1523/JNEUROSCI.0384-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray LA, Sehl M, Bujarski S, Hutchison K, Blaine S, Enoch MA. The CRHR1 gene, trauma exposure, and alcoholism risk: a test of G x E effects. Genes Brain Behav. 2013;12(4):361–369. doi: 10.1111/gbb.12032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribbe K, Ackermann V, Schwitulla J, Begemann M, Papiol S, Grube S, et al. Prediction of the risk of comorbid alcoholism in schizophrenia by interaction of common genetic variants in the corticotropin-releasing factor system. Arch Gen Psychiatry. 2011;68(12):1247–1256. doi: 10.1001/archgenpsychiatry.2011.100. [DOI] [PubMed] [Google Scholar]
- Rinker JA, Marshall SA, Mazzone CM, Lowery-Gionta EG, Gulati V, Pleil KE, et al. Extended Amygdala to Ventral Tegmental Area Corticotropin-Releasing Factor Circuit Controls Binge Ethanol Intake. Biol Psychiatry. 2017;81(11):930–940. doi: 10.1016/j.biopsych.2016.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ripley TL, O’Shea M, Stephens DN. Repeated withdrawal from ethanol impairs acquisition but not expression of conditioned fear. Eur J Neurosci. 2003;18(2):441–448. doi: 10.1046/j.1460-9568.2003.02759.x. [DOI] [PubMed] [Google Scholar]
- Roberto M, Bajo M, Crawford E, Madamba SG, Siggins GR. Chronic ethanol exposure and protracted abstinence alter NMDA receptors in central amygdala. Neuropsychopharmacology. 2006;31(5):988–996. doi: 10.1038/sj.npp.1300840. [DOI] [PubMed] [Google Scholar]
- Roberto M, Cruz MT, Gilpin NW, Sabino V, Schweitzer P, Bajo M, et al. Corticotropin releasing factor-induced amygdala gamma-aminobutyric Acid release plays a key role in alcohol dependence. Biol Psychiatry. 2010;67(9):831–839. doi: 10.1016/j.biopsych.2009.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberto M, Gilpin NW, Siggins GR. The central amygdala and alcohol: role of gamma-aminobutyric acid, glutamate, and neuropeptides. Cold Spring Harb Perspect Med. 2012;2(12):a012195. doi: 10.1101/cshperspect.a012195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberto M, Madamba SG, Moore SD, Tallent MK, Siggins GR. Ethanol increases GABAergic transmission at both pre- and postsynaptic sites in rat central amygdala neurons. Proc Natl Acad Sci U S A. 2003;100(4):2053–2058. doi: 10.1073/pnas.0437926100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberto M, Madamba SG, Stouffer DG, Parsons LH, Siggins GR. Increased GABA release in the central amygdala of ethanol-dependent rats. J Neurosci. 2004a;24(45):10159–10166. doi: 10.1523/JNEUROSCI.3004-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberto M, Schweitzer P, Madamba SG, Stouffer DG, Parsons LH, Siggins GR. Acute and chronic ethanol alter glutamatergic transmission in rat central amygdala: an in vitro and in vivo analysis. J Neurosci. 2004b;24(7):1594–1603. doi: 10.1523/JNEUROSCI.5077-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts AJ, Cole M, Koob GF. Intra-amygdala muscimol decreases operant ethanol self-administration in dependent rats. Alcohol Clin Exp Res. 1996;20(7):1289–1298. doi: 10.1111/j.1530-0277.1996.tb01125.x. [DOI] [PubMed] [Google Scholar]
- Roozendaal B, Brunson KL, Holloway BL, McGaugh JL, Baram TZ. Involvement of stress-released corticotropin-releasing hormone in the basolateral amygdala in regulating memory consolidation. Proc Natl Acad Sci U S A. 2002;99(21):13908–13913. doi: 10.1073/pnas.212504599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rostkowski AB, Leitermann RJ, Urban JH. Differential activation of neuronal cell types in the basolateral amygdala by corticotropin releasing factor. Neuropeptides. 2013;47(4):273–280. doi: 10.1016/j.npep.2012.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sah P, Faber ES, Lopez De Armentia M, Power J. The amygdaloid complex: anatomy and physiology. Physiol Rev. 2003;83(3):803–834. doi: 10.1152/physrev.00002.2003. [DOI] [PubMed] [Google Scholar]
- Sajdyk TJ, Schober DA, Gehlert DR, Shekhar A. Role of corticotropin-releasing factor and urocortin within the basolateral amygdala of rats in anxiety and panic responses. Behav Brain Res. 1999;100(1–2):207–215. doi: 10.1016/s0166-4328(98)00132-6. [DOI] [PubMed] [Google Scholar]
- Salling MC, Faccidomo SP, Li C, Psilos K, Galunas C, Spanos M, et al. Moderate Alcohol Drinking and the Amygdala Proteome: Identification and Validation of Calcium/Calmodulin Dependent Kinase II and AMPA Receptor Activity as Novel Molecular Mechanisms of the Positive Reinforcing Effects of Alcohol. Biol Psychiatry. 2016;79(6):430–442. doi: 10.1016/j.biopsych.2014.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samson RD, Dumont EC, Pare D. Feedback inhibition defines transverse processing modules in the lateral amygdala. J Neurosci. 2003;23(5):1966–1973. doi: 10.1523/JNEUROSCI.23-05-01966.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandi C, Cordero MI, Ugolini A, Varea E, Caberlotto L, Large CH. Chronic stress-induced alterations in amygdala responsiveness and behavior--modulation by trait anxiety and corticotropin-releasing factor systems. Eur J Neurosci. 2008;28(9):1836–1848. doi: 10.1111/j.1460-9568.2008.06451.x. [DOI] [PubMed] [Google Scholar]
- Sanford CA, Soden ME, Baird MA, Miller SM, Schulkin J, Palmiter RD, et al. A Central Amygdala CRF Circuit Facilitates Learning about Weak Threats. Neuron. 2017;93(1):164–178. doi: 10.1016/j.neuron.2016.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schienle A, Schafer A, Walter B, Stark R, Vaitl D. Brain activation of spider phobics towards disorder-relevant, generally disgust- and fear-inducing pictures. Neurosci Lett. 2005;388(1):1–6. doi: 10.1016/j.neulet.2005.06.025. [DOI] [PubMed] [Google Scholar]
- Schmid B, Blomeyer D, Treutlein J, Zimmermann US, Buchmann AF, Schmidt MH, et al. Interacting effects of CRHR1 gene and stressful life events on drinking initiation and progression among 19-year-olds. Int J Neuropsychopharmacol. 2010;13(6):703–714. doi: 10.1017/S1461145709990290. [DOI] [PubMed] [Google Scholar]
- Schwandt ML, Cortes CR, Kwako LE, George DT, Momenan R, Sinha R, et al. The CRF1 Antagonist Verucerfont in Anxious Alcohol-Dependent Women: Translation of Neuroendocrine, But not of Anti-Craving Effects. Neuropsychopharmacology. 2016;41(12):2818–2829. doi: 10.1038/npp.2016.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semyanov A, Walker MC, Kullmann DM, Silver RA. Tonically active GABA A receptors: modulating gain and maintaining the tone. Trends Neurosci. 2004;27(5):262–269. doi: 10.1016/j.tins.2004.03.005. [DOI] [PubMed] [Google Scholar]
- Shin LM, Liberzon I. The neurocircuitry of fear, stress, and anxiety disorders. Neuropsychopharmacology. 2010;35(1):169–191. doi: 10.1038/npp.2009.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigurdsson T, Doyere V, Cain CK, LeDoux JE. Long-term potentiation in the amygdala: a cellular mechanism of fear learning and memory. Neuropharmacology. 2007;52(1):215–227. doi: 10.1016/j.neuropharm.2006.06.022. [DOI] [PubMed] [Google Scholar]
- Silberman Y, Ariwodola OJ, Weiner JL. Differential effects of GABAB autoreceptor activation on ethanol potentiation of local and lateral paracapsular GABAergic synapses in the rat basolateral amygdala. Neuropharmacology. 2009;56(5):886–895. doi: 10.1016/j.neuropharm.2009.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silberman Y, Fetterly TL, Awad EK, Milano EJ, Usdin TB, Winder DG. Ethanol produces corticotropin-releasing factor receptor-dependent enhancement of spontaneous glutamatergic transmission in the mouse central amygdala. Alcohol Clin Exp Res. 2015;39(11):2154–2162. doi: 10.1111/acer.12881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silberman Y, Matthews RT, Winder DG. A corticotropin releasing factor pathway for ethanol regulation of the ventral tegmental area in the bed nucleus of the stria terminalis. J Neurosci. 2013a;33(3):950–960. doi: 10.1523/JNEUROSCI.2949-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silberman Y, Shi L, Brunso-Bechtold JK, Weiner JL. Distinct mechanisms of ethanol potentiation of local and paracapsular GABAergic synapses in the rat basolateral amygdala. J Pharmacol Exp Ther. 2008;324(1):251–260. doi: 10.1124/jpet.107.128728. [DOI] [PubMed] [Google Scholar]
- Silberman Y, Winder DG. Corticotropin releasing factor and catecholamines enhance glutamatergic neurotransmission in the lateral subdivision of the central amygdala. Neuropharmacology. 2013b;70:316–323. doi: 10.1016/j.neuropharm.2013.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skelly MJ, Chappell AE, Carter E, Weiner JL. Adolescent social isolation increases anxiety-like behavior and ethanol intake and impairs fear extinction in adulthood: Possible role of disrupted noradrenergic signaling. Neuropharmacology. 2015;97:149–159. doi: 10.1016/j.neuropharm.2015.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommer WH, Rimondini R, Hansson AC, Hipskind PA, Gehlert DR, Barr CS, et al. Upregulation of voluntary alcohol intake, behavioral sensitivity to stress, and amygdala crhr1 expression following a history of dependence. Biol Psychiatry. 2008;63(2):139–145. doi: 10.1016/j.biopsych.2007.01.010. [DOI] [PubMed] [Google Scholar]
- Spierling SR, Zorrilla EP. Don’t stress about CRF: assessing the translational failures of CRF1antagonists. Psychopharmacology (Berl) 2017;234(9–10):1467–1481. doi: 10.1007/s00213-017-4556-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starcevic A, Postic S, Radojicic Z, Starcevic B, Milovanovic S, Ilankovic A, et al. Volumetric analysis of amygdala, hippocampus, and prefrontal cortex in therapy-naive PTSD participants. Biomed Res Int. 2014;2014:968495. doi: 10.1155/2014/968495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein MB, Goldin PR, Sareen J, Zorrilla LT, Brown GG. Increased amygdala activation to angry and contemptuous faces in generalized social phobia. Arch Gen Psychiatry. 2002;59(11):1027–1034. doi: 10.1001/archpsyc.59.11.1027. [DOI] [PubMed] [Google Scholar]
- Stenzel-Poore MP, Heinrichs SC, Rivest S, Koob GF, Vale WW. Overproduction of corticotropin-releasing factor in transgenic mice: a genetic model of anxiogenic behavior. J Neurosci. 1994;14(5 Pt 1):2579–2584. doi: 10.1523/JNEUROSCI.14-05-02579.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens DN, Ripley TL, Borlikova G, Schubert M, Albrecht D, Hogarth L, et al. Repeated ethanol exposure and withdrawal impairs human fear conditioning and depresses long-term potentiation in rat amygdala and hippocampus. Biol Psychiatry. 2005;58(5):392–400. doi: 10.1016/j.biopsych.2005.04.025. [DOI] [PubMed] [Google Scholar]
- Stork O, Ji FY, Obata K. Reduction of extracellular GABA in the mouse amygdala during and following confrontation with a conditioned fear stimulus. Neurosci Lett. 2002;327(2):138–142. doi: 10.1016/s0304-3940(02)00387-7. [DOI] [PubMed] [Google Scholar]
- Stuber GD, Sparta DR, Stamatakis AM, van Leeuwen WA, Hardjoprajitno JE, Cho S, et al. Excitatory transmission from the amygdala to nucleus accumbens facilitates reward seeking. Nature. 2011;475(7356):377–380. doi: 10.1038/nature10194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun MK, Alkon DL. The “memory kinases”: roles of PKC isoforms in signal processing and memory formation. Prog Mol Biol Transl Sci. 2014;122:31–59. doi: 10.1016/B978-0-12-420170-5.00002-7. [DOI] [PubMed] [Google Scholar]
- Swerdlow NR, Geyer MA, Vale WW, Koob GF. Corticotropin-releasing factor potentiates acoustic startle in rats: blockade by chlordiazepoxide. Psychopharmacology (Berl) 1986;88(2):147–152. doi: 10.1007/BF00652231. [DOI] [PubMed] [Google Scholar]
- Terburg D, Morgan BE, Montoya ER, Hooge IT, Thornton HB, Hariri AR, et al. Hypervigilance for fear after basolateral amygdala damage in humans. Transl Psychiatry. 2012;2:e115. doi: 10.1038/tp.2012.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toth M, Gresack JE, Hauger RL, Halberstadt AL, Risbrough VB. The role of PKC signaling in CRF-induced modulation of startle. Psychopharmacology (Berl) 2013;229(4):579–589. doi: 10.1007/s00213-013-3114-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treutlein J, Kissling C, Frank J, Wiemann S, Dong L, Depner M, et al. Genetic association of the human corticotropin releasing hormone receptor 1 (CRHR1) with binge drinking and alcohol intake patterns in two independent samples. Mol Psychiatry. 2006;11(6):594–602. doi: 10.1038/sj.mp.4001813. [DOI] [PubMed] [Google Scholar]
- Truitt WA, Johnson PL, Dietrich AD, Fitz SD, Shekhar A. Anxiety-like behavior is modulated by a discrete subpopulation of interneurons in the basolateral amygdala. Neuroscience. 2009;160(2):284–294. doi: 10.1016/j.neuroscience.2009.01.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ugolini A, Sokal DM, Arban R, Large CH. CRF1 receptor activation increases the response of neurons in the basolateral nucleus of the amygdala to afferent stimulation. Front Behav Neurosci. 2008;2:2. doi: 10.3389/neuro.08.002.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Pett K, Viau V, Bittencourt JC, Chan RK, Li HY, Arias C, et al. Distribution of mRNAs encoding CRF receptors in brain and pituitary of rat and mouse. J Comp Neurol. 2000;428(2):191–212. doi: 10.1002/1096-9861(20001211)428:2<191::aid-cne1>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- VanElzakker MB, Dahlgren MK, Davis FC, Dubois S, Shin LM. From Pavlov to PTSD: the extinction of conditioned fear in rodents, humans, and anxiety disorders. Neurobiol Learn Mem. 2014;113:3–18. doi: 10.1016/j.nlm.2013.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varodayan FP, Correia D, Kirson D, Khom S, Oleata CS, Luu G, et al. CRF modulates glutamate transmission in the central amygdala of naive and ethanol-dependent rats. Neuropharmacology. 2017;125:418–428. doi: 10.1016/j.neuropharm.2017.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ventura-Silva AP, Melo A, Ferreira AC, Carvalho MM, Campos FL, Sousa N, et al. Excitotoxic lesions in the central nucleus of the amygdala attenuate stress-induced anxiety behavior. Front Behav Neurosci. 2013;7:32. doi: 10.3389/fnbeh.2013.00032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallner M, Hanchar HJ, Olsen RW. Ethanol enhances alpha 4 beta 3 delta and alpha 6 beta 3 delta gamma-aminobutyric acid type A receptors at low concentrations known to affect humans. Proc Natl Acad Sci U S A. 2003;100(25):15218–15223. doi: 10.1073/pnas.2435171100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weathington JM, Hamki A, Cooke BM. Sex- and region-specific pubertal maturation of the corticotropin-releasing factor receptor system in the rat. J Comp Neurol. 2014;522(6):1284–1298. doi: 10.1002/cne.23475. [DOI] [PubMed] [Google Scholar]
- Wei W, Faria LC, Mody I. Low ethanol concentrations selectively augment the tonic inhibition mediated by delta subunit-containing GABAA receptors in hippocampal neurons. J Neurosci. 2004;24(38):8379–8382. doi: 10.1523/JNEUROSCI.2040-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiltgen BJ, Godsil BP, Peng Z, Saab F, June HL, Linn ML, et al. The alpha1 subunit of the GABA(A) receptor modulates fear learning and plasticity in the lateral amygdala. Front Behav Neurosci. 2009;3:37. doi: 10.3389/neuro.08.037.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization. Global Status Report on Alcohol Use and Health. 2014. [Google Scholar]
- Yu K, Garcia da Silva P, Albeanu DF, Li B. Central Amygdala Somatostatin Neurons Gate Passive and Active Defensive Behaviors. J Neurosci. 2016;36(24):6488–6496. doi: 10.1523/JNEUROSCI.4419-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Colombo G, Gessa GL, Kreek MJ. Effects of voluntary alcohol drinking on corticotropin-releasing factor and preprodynorphin mRNA levels in the central amygdala of Sardinian alcohol-preferring rats. Neurosci Lett. 2013;554:110–114. doi: 10.1016/j.neulet.2013.08.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu PJ, Lovinger DM. Ethanol potentiates GABAergic synaptic transmission in a postsynaptic neuron/synaptic bouton preparation from basolateral amygdala. J Neurophysiol. 2006;96(1):433–441. doi: 10.1152/jn.01380.2005. [DOI] [PubMed] [Google Scholar]
- Zhu W, Bie B, Pan ZZ. Involvement of non-NMDA glutamate receptors in central amygdala in synaptic actions of ethanol and ethanol-induced reward behavior. J Neurosci. 2007;27(2):289–298. doi: 10.1523/JNEUROSCI.3912-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zorrilla EP, Valdez GR, Weiss F. Changes in levels of regional CRF-like-immunoreactivity and plasma corticosterone during protracted drug withdrawal in dependent rats. Psychopharmacology (Berl) 2001;158(4):374–381. doi: 10.1007/s002130100773. [DOI] [PubMed] [Google Scholar]