Abstract
Objective
To examine the relation between use of antibiotics in a cohort of preschool children and nasal carriage of resistant strains of pneumococcus.
Design and participants
Prospective cohort study over two years of 461 children aged under 4 years living in Canberra, Australia.
Main outcome measures
Use of drugs, respiratory symptoms, and visits to doctors were documented in a daily diary by parents of the children during 25 months of observation. Isolates of pneumococci, which were cultured from nasal swabs collected approximately six monthly, were tested for antibiotic resistance.
Results
From the four swab collections 631 positive pneumococcal isolates from 461 children were found, of which 13.6% were resistant to penicillin. Presence of penicillin resistant pneumococci was significantly associated with children's use of a β lactam antibiotic in the two months before each swab collection (odds ratio 2.03 (95% confidence interval 1.15 to 3.56, P=0.01)). The odds ratio of the association remained >1 (though did not reach significance at the 0.05 level) for use in the six months before swab collection. The association was seen in children who received only penicillin or only cephalosporin antibiotics in that period. The odds ratio was 4.67 (1.29 to 17.09, P=0.02) in children who had received both types of β lactam in the two months before their nasal swab. The modelled odds of carrying penicillin resistant pneumococcus was 4% higher for each additional day of use of β lactam antibiotics in the six months before swab collection.
Conclusions
Reduction in β lactam use could quickly reduce the carriage rates of penicillin resistant pneumococci in early childhood. In view of the propensity of these organisms to be spread among children in the community, the prevalence of penicillin resistant organisms may fall as a consequence.
What is already known on this topic
Resistance to pneumococcal antibiotics is increasing worldwide
One possible cause of resistance is the excessive use of antibiotics in children with respiratory symptoms
Few cross sectional studies have looked at the association between antibiotic use and subsequent carriage of organisms resistant to penicillin
What this study adds
Carriage of pneumococcus is high in preschool Australian children throughout the year and highest in winter
The likelihood of carrying penicillin resistant pneumococcus is doubled in children who have used any β lactam antibiotic in the two months before testing
The likelihood of a child carrying a penicillin resistant pneumococcus is increased by 4% for each additional day of β lactam use in the six months before testing
Introduction
The worldwide increase in pneumococcal resistance to antibiotics is worrying.1,2 An important contributor to antibiotic resistance is the excessive use of antibiotics in young children with respiratory symptoms.3 It is widely accepted that antibiotic resistance is related to both the extent and amount of antibiotics used. However, there are few prospective data to support these presumptions. A recent study showed that the carriage of resistant pneumococci in children was related to the duration of antibiotic use, for periods of >7 days, in the preceding month.4 Carriage of resistant pneumococci in childhood has been associated with younger age, attendance at daycare centres, and previous use of antibiotics.5 A cross sectional study showed that the carriage of resistant organisms correlated with previous exposure and total consumption of antibiotics.6 In our two year prospective study in a community setting we studied the nasal carriage of pneumococci, their sensitivity profile, and the intake of antibiotics by preschool children in Canberra, Australia.
Methods
A cohort of 484 children from Canberra participated in the study from September 1997 to September 1999. General practitioners were first recruited into the study, then these doctors were asked to recruit the first 15 children who visited their practice for any reason. The parents of all participants kept a daily diary of the children's respiratory symptoms, visits to the doctor, and use of drugs, with detailed information on antibiotic use, including the name of the drug, duration of use, and the reason for taking it.
At the children's entry into the study their parents completed a questionnaire on demographic, environmental, and health related information on the children. Informed consent was obtained from the parents during recruitment.
Nasal swabs were collected from the children up to four times over the 25 months of the study. Pneumococci were identified by colony characteristics on blood agar plates, Gram staining, bile solubility, and optochin disc susceptibility. The predominant type of colony in each isolate was tested for susceptibility to penicillin by using Etest strips (AB Biodisk, Solna, Sweden) on Mueller-Hinton agar with 5% blood. We interpreted susceptibility according to the breakpoints for minimal inhibitory concentration published by the National Committee for Clinical Laboratory Standards.7 The isolates were defined as sensitive to penicillin when the minimal inhibitory concentration was ⩽0.064 mg/l, of intermediate resistance when it was >0.064 mg/l but ⩽1 mg/l, and highly resistant when it was >1 mg/l. We reported both intermediate and highly resistant strains as penicillin resistant.
The study period was divided into 12 periods of 60 days each. To limit misclassification of antibiotic use we considered only those children whose diary observations were complete for at least 75% of observation days. We also excluded from the main analysis swabs that were collected from children who were receiving continuous antibiotic therapy.
We defined penicillin as including penicillin V, amoxicillin, and amoxicillin clavulate. Cephalosporin refers also to cefalexin and ceclor. The term β lactam refers to either penicillin or cephalosporin.
We used two sided Fisher's exact tests for our univariate analyses and multiple logistic regression models to model the odds of carriage of a resistant organism. We accounted for correlation between repeated observations on the same child by using the Huber-White robust variance estimator,8,9 which is available in STATA (version 6; Statacorp, College Station, TX) using the cluster option of the logistic regression command. We assessed a range of covariates as possible confounders of the association between antibiotic use and carriage of a resistant organism. A potential confounder was entered into the logistic model if adjustment for the variable resulted in a distortion of the odds ratio of ⩾10%.10
Results
From the four nasal swab collections 631 pneumococcal isolates from 461 children were found—37%, 46%, 39%, and 46% of the swabs collected on each occasion (the higher percentages were from the swabs collected in winter). Of the 631 pneumococcal isolates, 86 (13.6%) were resistant to penicillin, and six isolates were highly resistant.
Elsewhere we have described in detail the pattern of antibiotic resistance in the isolates collected from these children.11 Over half (334 (52.9%)) the isolates were resistant to at least one of the six antibiotics for which sensitivity was tested (penicillin, erythromycin, co-trimoxazole, tetracycline, chloramphenicol, and cefotaxime); 119 (18.9%) were resistant to two or more antibiotics; and 5 (0.8%) were resistant to all six. Across the two years the rate of penicillin resistance remained constant, but the rate of resistance to co-trimoxazole rose from 45% to 55%, although use of co-trimoxazole by the children was relatively low in this period.
Of the 631 pneumococcal isolates, 456 were collected from the children whose diary records of drug treatment and medical visits were at least 75% complete in the six months before collection of the swab. Of these isolates 68 (14.9%) were resistant to penicillin. These children took β lactam antibiotics for 0 to 85 days. Of the 456 isolates, 199 (43.6%) were from children with at least one day of β lactam use in the previous six months.
Over three quarters of the children (355 (76.9%)) received an antibiotic at some point in the two year study period, and the overall mean period of antibiotic use per child per year was 17.6 days: penicillin derivatives were taken on 6.5 days and cephalosporins on 6.1 days, with other antibiotics, including macrolides, co-trimoxazole, and chloramphenicol eye or ear drops, accounting for the other 5.0 days per child per year.11
To consider the relation between antibiotic use and resistance, we used a logistic model to assess the odds of carrying a penicillin resistant pneumococcal isolate against β lactam use in each two month period before swab collection over the 24 months of the study. Age, sex, number of siblings, type of day care, duration of day care, and any hospital admission were assessed for their possible confounding effects. The use of β lactam antibiotics in the two months before swab collection was significantly associated with isolation of a penicillin resistant pneumococcus, and the relation was not confounded by the variables tested (adjusted odds ratio 2.03 (95% confidence interval 1.15 to 3.56; P=0.01)). The odds ratio adjusted for the clustering effect of multiple swabs in any one child was slightly >1 for β lactam prescriptions in the penultimate and last two month periods before swabbing (odds ratios of 1.27 and 1.25, respectively), but the 95% confidence intervals of these estimates included 1. The odds ratio for use of antibiotics in each of the six separate two month periods from 18 months to seven months before swabbing was less than 1, but the 95% confidence intervals all included 1 (these odds ratios were 0.87, 0.66, 0.75, 0.71, 0.76, and 0.36.) If children had received both a penicillin and a cephalosporin preparation in the two month period before swabbing, the odds ratio of carrying a resistant organism was 4.67 (1.27 to 17.09; P=0.02).
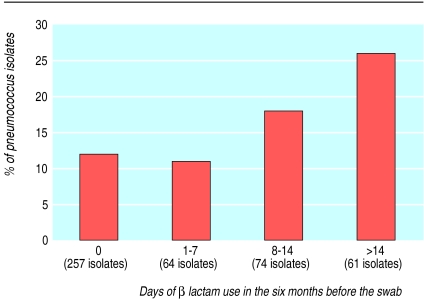
To determine whether greater antibiotic use would increase the likelihood of pneumococcal resistance to antibiotics, we stratified children by duration of β lactam use in the six months before swab collection (figure). The percentages of penicillin resistant isolates were similar in the group that had no β lactam use (12% (32/257)) and the group that had only a relatively short course of β lactam (11% (7/64)), but this percentage increased as the number of days of use increased beyond seven days, to 26% (16/61) in children who had >14 days' use.
Although the children who received β lactam antibiotics for >7 days in the six months before swab collection were more likely to carry a penicillin resistant pneumococcus than the children who had not received β lactam preparations in that period, this association was significant only in children who had received >14 days of β lactam (table).
We further explored the association between duration of β lactam use and penicillin resistance by using duration of use as a continuous rather than a categorical variable. Using a logistic regression model adjusted for the effect of clustering within children, we found that for each additional day of use in the six months before the swab collection, the odds of a child carrying a penicillin resistant pneumococcus increased by 4% (adjusted odds ratio 1.04 (1.01 to 1.06; P=0.001)).
Discussion
Several cross sectional and retrospective studies have shown an association between use of antibiotics and the carriage of penicillin resistant organisms. The opportunity to study this issue prospectively, and in a community setting, has provided some new insights.
Carriage of pneumococcus in these preschool Australian children was high, with the highest rate (46% of swabs) seen in winter. Because of the extensive use of β lactam antibiotics in this population and the similarity of the mechanism of penicillin resistance in cephalosporins and the penicillin group of drugs,12 we paid particular attention to penicillin resistance and previous use of β lactam antibiotics.
In these children, the likelihood that the carried pneumococcus would be partially or highly resistant to penicillin was increased if the child had taken any β lactam antibiotic in the two months before swabbing. This odds ratio was increased by 4% for each additional day of β lactam antibiotic use in the six months before swab collection. The odds ratio nearly quintupled if the children had taken both penicillin and cephalosporin in that period, though this observation was based on small numbers.
The odds ratio that a penicillin resistant pneumococcus would be carried remained >1 for six months after β lactam use; however, it fell to <1 for the second and third periods of six months after β lactam use. This may reflect the fact that spontaneous termination of carriage of resistant strains occurs after some weeks, when the child seems to develop immunity to the serotype of pneumococcus that has been carried.13 Such immunity would make a child less likely than non-immune children to be colonised by that particular strain in later months if it was encountered again. As we did not serotype these pneumococcal strains or measure antibodies to them, we cannot do more than speculate on the mechanism for this observation.
Resistant strains were often found in children who had not taken any antibiotics in the six months before swab collection. It thus seems likely that many of these children acquired their resistant strains through transmission from other children in the community, such as in daycare centres, rather than as a result of earlier use of β lactams.
Conclusions
Our results show that the likelihood of children carrying a resistant organism is in the short term related to the amount of β lactam recently taken. Therefore if the amount of β lactam prescribed could be reduced, it follows that selection and transmission of resistant strains would occur less often.
Antibiotics are being overused in children of this age group in Australia. We found that 47% of all episodes of respiratory symptoms resulted in a visit to the general practitioner and that up to 48% of children who visited their general practitioner received an antibiotic on their first visit. Elsewhere we have examined the severity of symptoms in children who received antibiotics and those who did not receive them, and we did not find any difference between the two groups.11 This accords with the growing evidence from randomised controlled trials that the benefits of antibiotics in most early childhood respiratory illnesses are trivial or non-existent.14,15 The likelihood that antibiotic use will, in the short term, result in carriage of a resistant organism needs to be built into clinical decision making.
A substantial reduction of β lactam use in preschool children could quickly result in reduced carriage of penicillin resistant pneumococci. A recent randomised trial compared dosages of 90 mg/day of amoxicillin for five days and 40 mg/day for 10 days in children and found that the higher dose of shorter duration was associated with lesser subsequent carriage of resistant pneumococci.16This supports our findings on the duration of β lactam use. If these drugs are to retain their clinical usefulness, new prescribing policies are needed in community practice.
Figure.
Penicillin resistance in pneumococcus isolates taken from children, and β lactam use in the six months before swab collection
Table.
Effect on penicillin resistance in pneumococcus isolates (n=456) of duration of β lactam use in the six months before swab collection. No β lactam use is the reference group
| No of days of β lactam use | Odds ratio | 95% CI | P value |
|---|---|---|---|
| 0 | (reference group) | ||
| 1-7 | 0.86 | 0.37 to 2.02 | 0.73 |
| 8-14 | 1.50 | 0.73 to 3.06 | 0.27 |
| >14 | 2.50 | 1.30 to 4.82 | 0.006 |
Acknowledgments
We acknowledge the enthusiastic support of 50 general practitioners in Canberra and the parents of the children who participated in the study, and we thank Dr Rennie D'Souza for comments on an early manuscript and Anna Wilkinson for contacting parents.
Footnotes
Funding: The study was funded by research grants to RMD from the general practice evaluation programme of the Australian Department of Health and Aged Care and from SmithKline Beecham Pharmaceuticals to RMD.
Competing interests: None declared.
References
- 1.Linares J, Pallares R, Alonso T, Perez JL, Ayats J, Gudiol F, et al. Trends in antimicrobial resistance of clinical isolates of Streptococcus pneumoniae in Bellvitge Hospital, Barcelona, Spain (1979-1990) Clin Infect Dis. 1992;15:99–105. doi: 10.1093/clinids/15.1.99. [DOI] [PubMed] [Google Scholar]
- 2.Turnidge JD, Bell JM, Collignon PJ. Rapidly emerging antimicrobial resistance in Streptococcus pneumoniae in Australia. Med J Aust. 1999;170:152–155. doi: 10.5694/j.1326-5377.1999.tb127710.x. [DOI] [PubMed] [Google Scholar]
- 3.Low DE, Scheld WM. Strategies for stemming the tide of antimicrobial resistance. JAMA. 1998;279:394–395. doi: 10.1001/jama.279.5.394. [DOI] [PubMed] [Google Scholar]
- 4.Guillemot D, Carbon C, Balkau B, Geslin P, Lecoeur H, Vauzelle-Kervroedan F, et al. Low dosage and long treatment duration of beta-lactam: risk factors for carriage of penicillin-resistant Streptococcus pneumoniae. JAMA. 1998;279:365–370. doi: 10.1001/jama.279.5.365. [DOI] [PubMed] [Google Scholar]
- 5.Reichler MR, Allphin AA, Breiman RF, Schreiber JR, Ernold JE, McDougal LK, et al. The spread of multiply resistant Streptococcus pneumoniae at a day care center in Ohio. J Infect Dis. 1992;166:1346–1353. doi: 10.1093/infdis/166.6.1346. [DOI] [PubMed] [Google Scholar]
- 6.Arason VA, Kristinsson KG, Sigurdsson JA, Stefansdottir G, Molstad S, Gudmundsson S. Do antimicrobials increase the carriage rate of penicillin resistant pneumococci in children? Cross sectional prevalence study. BMJ. 1996;313:387–391. doi: 10.1136/bmj.313.7054.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.National Committee for Clinical Laboratory Standards. Performance standards for antimicrobial susceptibility testing. 5th informational supplement. Villanova, PN: NCCLS; 1994. . (Document M100-S5.) [Google Scholar]
- 8.Huber PJ. The behavior of maximum likelihood estimates under non-standard conditions. Proceedings of the 5th Berkeley symposium on mathematical statistics and probability. Berkeley, CA: University of California Press; 1967. p. 1. :221-33. [Google Scholar]
- 9.White H. Maximum likelihood estimates of misspecified models. Econometrics. 1982;50:1–25. [Google Scholar]
- 10.Hennekens CH, Buring JE. Epidemiology in medicine. 1st ed. Boston, MA and Toronto: Little, Brown; 1987. [Google Scholar]
- 11.Nasrin D. Effects of antibiotic use on respiratory illness and on antibiotic resistance in children [doctoral dissertation]. Canberra, Australia: Australian National University; 2000. [Google Scholar]
- 12.Klugman KP. Pneumococcal resistance to antibiotics. Clin Microb Rev. 1990;3:171–196. doi: 10.1128/cmr.3.2.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Douglas RM, Hansman D, Miles H, Paton JC. Pneumococcal carriage and type specific antibody: failure of a 14-valent vaccine to reduce carriage in healthy children. Am J Dis Child. 1986;140:1183–1185. doi: 10.1001/archpedi.1986.02140250109044. [DOI] [PubMed] [Google Scholar]
- 14.Del Mar CB, Glasziou PP, Hayem M. Are antibiotics indicated as initial treatment for children with acute otitis media? A meta-analysis. BMJ. 1997;314:1526–1529. doi: 10.1136/bmj.314.7093.1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Del Mar C, Glasziou PP, Spinks P. Antibiotics for sore throat. Cochrane Database Syst Rev 2000;(2):CD000023. [DOI] [PubMed]
- 16.Schrag SJ, Pena C, Fernandez J, Sanchez J, Gomez V, Perez E, Feris JM, Besser RE. Effect of short-course, high-dose amoxicillin therapy on resistant pneumococcal carriage: a randomized trial. JAMA. 2001;286:49–56. doi: 10.1001/jama.286.1.49. [DOI] [PubMed] [Google Scholar]