Abstract
With the growing wait list of individuals waiting for kidney transplantation, there has been renewed interest in organ donor quality and function. In particular, concern about the development of delayed allograft function (DGF) after transplantation continues to lead to avoidance of donor organs offered to a transplant center. DGF is associated with worse short and long-term outcomes and associated with higher rejection rates. There are no FDA-approved therapies to mitigate the ischemic injury that occurs. Risk factors include both donor and recipient characteristics, although their prediction is not precise. With new understanding about mechanisms of injury and new focus on the function of the deceased donor, there is opportunity to identify not only novel therapies to improve allograft function but to identify potential biomarkers of DGF. Delayed graft function remains a significant factoring impacting kidney transplant outcome and finding biomarkers will assist in the development and approval of novel agents to ameliorate early and later injury. This mini-review highlights our presentation at the 23rd International Conference on Advances in Critical Care Nephrology and UAB/UCSD O’Brien Center Acute Kidney Injury Pre-Meeting.
Keywords: transplant, AKI, outcomes, allograft, chemokine
Background
While short-term kidney transplant outcomes are outstanding, long-term allograft survival continues to be a challenge. A significant proportion of kidney transplants rely on brain dead donors, although they have inferior outcomes compared to kidneys from living donors. In part, these outcomes relate to donor quality, as living donors are intensively screened for medical excellence and a lack of medical co-morbidites, as well as ample renal function. Moreover, the nephrectomy is a carefully timed procedure that limits both warm and cold ischemia. In contrast, deceased donors, while medically screened, are limited to known medical history, and the events leading to brain death may lead to functional impairment. Also, underpinning deceased donor selection is the intense scarcity of organs available for transplant procedures and a waiting list that has been growing on a geometric basis over the last decade leading to less than optimal selection. Finally, brain death in and of itself, induces an intense pro-inflammatory state, which may impact recipient immunity and graft function after kidney transplantation (1).
Delayed graft function (DGF) refers to the acute kidney injury that occurs in the first week of kidney transplantation that necessitates dialysis intervention. DGF is associated with higher rates of acute cellular rejection and shorter graft survivals (reviewed in (2). It often results in changes in maintenance immunosuppression therapy, specifically calcineurin inhibitor use. The incidence of DGF has varied, and on average is 31% in US transplant centers. While prolonged cold ischemic time (CIT) is associated with DGF, risk factors are primarily clinically based. We will review the key features and risks for DGF, mechanistic insights, therapies, and possible biomarkers. We propose that the current clinical features are not specific enough to predict DGF post-transplantation, and that better identification of the mechanisms of DGF are needed to guide therapeutic trials and identify biomarkers in this field.
Clinical Implications of DGF
The kidney donor profile index (KDPI)is a clinical measure of donor quality utilized in the kidney allocation scheme in US transplant centers and is based on age, height, weight, ethnicity, history of hypertension or diabetes, cause of death, serum creatinine, hepatitis C virus (HCV), and donation after cardiac death (DCD). Of deceased donors, the vast majority are brain dead while a smaller proportion are from DCD donors. DGF rate is about 30.8% in US deceased donors (3) which is significantly higher in DCDs (45-55.1%) (4). The incidence of DGF is dependent not only on length of cold ischemia to the organ, but the extent of warm ischemic injury, which tends to be lengthier in DCD by the nature of the induction of cardiac death, as well as other factors such as the KDPI. Alternatively, traumatic brain death may be accompanied by a syndrome of thrombotic microangiopathy that can be additive to kidney injury and may be accelerated or supported by the use of calcineurin inhibitors (CNI) and/or mTOR inhibitors used for maintenance therapy (reviewed in (2).
The development of DGF is associated with worse baseline kidney transplant function and shorter graft survivals, anywhere from 3-5 year shorter graft half-life as demonstrated in a recent single center analysis (4). Poor function in the immediate post-operative period necessitates the use of dialysis from anywhere from days to months which adds a significant cost impact to patient management (5), as well as complicating post-transplant management as an outpatient. Immunosuppressive therapy, particularly the maintenance use of calcineurin inhibitors, which in and of themselves associated with acute nephrotoxicity, are frequently minimized or not utilized, suggesting one mechanism tying DGF to early rejection, that is, under-immunosuppression. There has not been clear-cut evidence to the impact of induction antibody therapy; although North American transplant centers typically use rabbit anti-thymocyte globulin to minimize CNI dosing early on, while across the globe, anti-CD25 monoclonal therapy is the primary induction agent.
Mechanisms of AKI /DGF
A thorough and in depth discussion can be found in (2). Much like AKI seen in native kidneys, DGF is associated with innate immune activation, with complement activation and released damage associated molecular patterns (DAMPs) such as hypomethylated DNA, hyaluronic acid, HMGB1, heparin sulfate, fibrinogen and heat shock proteins. These molecules transmit their signals through toll-like receptors particularly in the proximal tubular epithelium (6). Engagement of TLR activates the NFkB pathway through the adapter protein MyD88, and this leads to the induction of inflammatory cytokines such as IL-1, IL-6, TNFα and IFNβ. Furthermore, TLR activation directly stimulates macrophage iNOS and as well as activation of the NADPH oxidase system. Finally these interactions can lead to activation of interstitial dendritic cells with upregulation of CCR7 promoting cell migration to secondary lymph nodes, upregulation of expression of costimulatory molecules (CD80/B7-1, CD86/B7-2), and production of IL-12, the latter leading to Th1 cell differentiation. This latter event in particular is essential in the priming of antigen-reactive T cell responses including responses to allografts and leads to crosstalk with the adaptive immune response. Thus, there is a potential for recipient immune activation and acute rejection of the graft.
A number of groups have examined the role of innate immune molecules in preclinical in αvivo models of AKI. Such work has identified complement activation as a key feature of ischemic kidney injury (2). Such studies have found upregulation of interleukin-6 levels and hepatocyte growth factor, and disruption of such pathways has been associated with mitigation of injury histologically and functionally. However, pre-clinical studies have not been entirely accurate in predicting responses in human treatment trials; for example, infusion of superoxide dismutase, a free radical scavenger, blockade of ICAM-1, or treatment with pentoxyfyline to inhibit TNFα have not been consistent in terms of mitigating DGF as they have been in rodents. Such studies highlight the differences in rodent and human inflammatory responses and the genetic diversity inherent in man that influence ischemic innate injury and alloimmune responses.
Risk Factors for DGF
There are multiple studies identifying risk factors for DGF. These are summarized in Table 1. With the complexity and interplay of both donor and recipient factors, a number of groups, a number of groups have created risk factor prediction models using large data registries that are publically available. The most widely used, with about 70% accuracy, is the Irish risk calculator This calculator is based on 20 independent recipient- and donor-related risk factors and identified the most significant factors associated as cold ischemic time, donor terminal creatinine, donor body mass index, donation after cardiac death, and donor age (7). However, these is still disagreement about its ability to predict clinical events, in part, due to the limitations of granularity in registry data.
Table I.
Risk factors for the development of delayed graft function, with bold-faced characteristics identified in multiple studies (adapted from (18).
| Donor-related | Recipient-related |
|---|---|
|
| |
| Female gender | Male gender |
| Increasing age | Black race |
| Body mass index | Body mass index |
| Deceased vs living donation | Previous transplant |
| Donation after cardiac death | Diabetes |
| Increasing donor serum creatinine | Pre-transplant dialysis versus none |
| Cause of death (anoxia vs cerebrovascular) | Duration and type of dialysis |
| Duration of intensive care stay | Residual diuresis |
| Duration of brain death | Pre-transplant transfusion |
| Diabetes | Pre-transplant blood pressure |
| History of hypertension | Pre-transplant lipid profile |
| Graft atherosclerosis | Cardiac function |
|
| |
| Preservation | Transplant-related |
|
| |
| Cold ischemic time (CIT) | Sensitization |
| Warm ischemic time | HLA mismatches |
| ABO incompatibility | |
Are there relevant biomarkers?
The study of such mechanisms plays potential not only for therapeutic innovation and allows for identifying biomarkers of irreparable injury. Moreover, the imprecise nature of both donor and recipient clinical factors to predict DGF development indicates an unmet need of biomarkers of injury. To this end, higher urinary NGAL and IL-18 levels in recipients on the first post-transplant day were associated with higher DGF rates and poor allograft function at 12 months post transplantation, in a prospective study involving a number of US transplant centers (8). Similarly, higher DBD urinary NGAL concentrations was associated with DGF with the highest versus lowest NGAL tertile relative risk of 1.21 (95% confidence interval 1.02 to 1.43) (9). Recent studies by Pianta et al. have identified urinary expression of clusterin, IL-18, and KIM-1 as well as urinary tissue inhibitor of metalloproteinases-2 (TIMP-2) and insulin-like growth factor-binding protein 7 (IGFBP7) (10, 11), as additive to clinical features in predicting early DGF. These studies focus on recipient expression, and not that of the deceased donor.
We have recently initiated a prospective study of BDD at our donor center with the goal of correlating donor management and clinical features, with possible biomarkers of DGF in recipients. As elevated levels of recipient urinary monocyte chemoattractant protein-1 (MCP-1), a powerful chemoattractant for monocytes and macrophages, have been associated with interstitial macrophage infiltration, interstitial fibrosis and inflammation, and DGF (12), we first assessed levels of MCP-1 in the donor. While we found no differences in systemic levels of MCP-1, MCP-1 was elevated in both the renal parenchyma and urine, and was associated with delayed graft function (Figure 1). Along these lines, Mansour et al have shown that DBD urine MCP-1 associated with AKI, which occurred in only 9% of recipients studied, although sample collection was inconsistent across donor hospitals and facilities (13). These studies highlight the potential of BDD biosamples as adjunct to more precisely predict those at risk for DGF.
Figure 1.
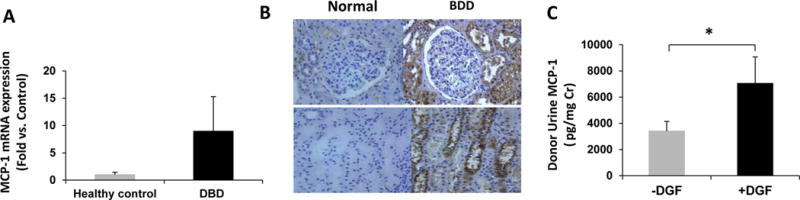
MCP-1 expression in BD donor serum, urine and kidney biopsies. A. Gene expression of MCP-1 in BDD kidney biopsies (n=29) was upregulated compared to normal, healthy controls (n=4), although this was not statistically significant (p=0.649). Values are fold induction compared to healthy controls, mean ± SEM. B. Representative immunohistochemical staining for MCP-1 in kidney biopsies from BDD and healthy controls (upper panels 100×, lower panels 200×). Expression was localized to tubular epithelium. C. Urine MCP-1 levels stratified by recipients with DGF (+DGF) and those without (−DGF). DGF was associated with significantly higher urine levels in BDD (*p=0.0498).
Treatment
There have been investigations in both deceased donors and recipients to mitigate the ischemic injury but to date, there is no FDA-approved therapy. Based on work by Moers et al, hypothermic pump perfusion is utilized by organ procurement centers to minimize DGF (14). Interventions in recipients have not been entirely successful in mitigation DGF such as dopamine and superoxide dismutase infusions, fenoldapam, and anti-ICAM1 treatment (reviewed in (2). As yet unreported phase III studies that include the use of anti-C5 antibody and silencing RNA to p53 to limit cellular apoptosis. There are single center phase I studies that are encouraging in terms of limiting ischemic renal injury through the inhibition of IL-6 and HGF. More definitive clinical studies in large volume transplant centers are currently underway.
Donor management investigation has been less well studied but may be a critical opportunity to improve organ quality. In this landmark study in donor management, Niemann demonstrated that modest donor hypothermia significantly reduced DGF rates from 39% to 28% (15). While these differences are modest, they do demonstrate improvement in transplanted organs and could translate into more usable organs and organs that have less damage to begin with. Critically in this work is the complex process of experimental management whose risks and benefits may extend beyond the sole purpose of better kidney function, and further complicate the process of organ allocation, and in particular utilization, particularly when recipient informed consent is required. Such complexities, which are a major hurdle to donor management therapies was the focus of the 2017 Institute of Medicine report (16).
The Future
It is important to reiterate that clinical features of the brain dead donor and renal function are not precise predictors of the development of DGF. Husain and colleagues explored the clinical characteristics of unilateral kidney transplant recipients stratified by the reason the partner kidney was discarded. They demonstrated that the rate of DGF was 40% when there was extended ischemia, 38% when donor function was felt to be insufficient, and 37% when a kidney was discarded due to concerns of donor biopsy pathology (17). This indicates the imprecise nature of the assessment of clinical outcome following kidney implantation and calls for the need for new tools to predict later function and transplant excellence.
References
- 1.Watts RP, Thom O, Fraser JF. Inflammatory signalling associated with brain dead organ donation: from brain injury to brain stem death and posttransplant ischaemia reperfusion injury. J Transplant. 2013;2013:521369. doi: 10.1155/2013/521369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Siedlecki A, Irish W, Brennan DC. Delayed Graft Function in the Kidney Transplant. Am J Transplant. 2011;11(11):2279–96. doi: 10.1111/j.1600-6143.2011.03754.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang CJ, Wetmore JB, Israni AK. Old versus new: Progress in reaching the goals of the new kidney allocation system. Human Immunol. 2017;78(1):9–15. doi: 10.1016/j.humimm.2016.08.007. [DOI] [PubMed] [Google Scholar]
- 4.Zens TJ, Danobeitia JS, Leverson G, Chlebeck PJ, Zitur LJ, Redfield RR, et al. The impact of kidney donor profile index on delayed graft function and transplant outcomes: A single-center analysis. Clin Transplant. 2018;32(3):e13190. doi: 10.1111/ctr.13190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Buchanan P, Schnitzler M, Axelrod D, Salvaggio P. The Clinical and Financial Burden of Early Dialysis After Deceased Donor Kidney Transplantation. J Nephrol Therapeutic. 2011;S4:001. doi: 10.4172/2161-0959.s4-001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rosin DL, Okusa MD. Dangers within: DAMP responses to damage and cell death in kidney disease. J Am Soc Nephrol. 2011;22(3):416–25. doi: 10.1681/ASN.2010040430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Irish WD, Ilsley JN, Schnitzler MA, Feng S, Brennan DC. A risk prediction model for delayed graft function in the current era of deceased donor renal transplantation. Am J Transplant. 2010;10(10):2279–86. doi: 10.1111/j.1600-6143.2010.03179.x. [DOI] [PubMed] [Google Scholar]
- 8.Hall IE, Yarlagadda SG, Coca SG, Wang Z, Doshi M, Devarajan P, et al. IL-18 and urinary NGAL predict dialysis and graft recovery after kidney transplantation. J Am Soc Nephrol. 2010;21(1):189–97. doi: 10.1681/ASN.2009030264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Reese PP, Hall IE, Weng FL, Schroppel B, Doshi MD, Hasz RD, et al. Associations between Deceased-Donor Urine Injury Biomarkers and Kidney Transplant Outcomes. J Am Soc Nephrol. 2016;27(5):1534–43. doi: 10.1681/ASN.2015040345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pianta TJ, Peake PW, Pickering JW, Kelleher M, Buckley NA, Endre ZH. Evaluation of biomarkers of cell cycle arrest and inflammation in prediction of dialysis or recovery after kidney transplantation. Transpl Int. 2015;28(12):1392–404. doi: 10.1111/tri.12636. [DOI] [PubMed] [Google Scholar]
- 11.Pianta TJ, Peake PW, Pickering JW, Kelleher M, Buckley NA, Endre ZH. Clusterin in kidney transplantation: novel biomarkers versus serum creatinine for early prediction of delayed graft function. Transplantation. 2015;99(1):171–9. doi: 10.1097/TP.0000000000000256. [DOI] [PubMed] [Google Scholar]
- 12.Ho J, Wiebe C, Gibson IW, Hombach-Klonisch S, Gao A, Rigatto C, et al. Elevated urinary CCL2: Cr at 6 months is associated with renal allograft interstitial fibrosis and inflammation at 24 months. Transplantation. 2014;98(1):39–46. doi: 10.1097/01.TP.0000442776.40295.73. [DOI] [PubMed] [Google Scholar]
- 13.Mansour SG, Puthumana J, Reese PP, Hall IE, Doshi MD, Weng FL, et al. Associations between Deceased-Donor Urine MCP-1 and Kidney Transplant Outcomes. Kidney international reports. 2017;2(4):749–58. doi: 10.1016/j.ekir.2017.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moers C, Smits JM, Maathuis MH, Treckmann J, van Gelder F, Napieralski BP, et al. Machine perfusion or cold storage in deceased-donor kidney transplantation. N Engl J Med. 2009;360(1):7–19. doi: 10.1056/NEJMoa0802289. [DOI] [PubMed] [Google Scholar]
- 15.Niemann CU, Feiner J, Swain S, Bunting S, Friedman M, Crutchfield M, et al. Therapeutic Hypothermia in Deceased Organ Donors and Kidney-Graft Function. N Engl J Med. 2015;373(5):405–14. doi: 10.1056/NEJMoa1501969. [DOI] [PubMed] [Google Scholar]
- 16.James FC, Sarah D, Catharyn TL, editors. National Academies of Sciences E, Medicine Opportunities for Organ Donor Intervention Research: Saving Lives by Improving the Quality and Quantity of Organs for Transplantation. Washington, DC: The National Academies Press; 2017. [PubMed] [Google Scholar]
- 17.Husain SA, Chiles MC, Lee S, Pastan SO, Patzer RE, Tanriover B, et al. Characteristics and Performance of Unilateral Kidney Transplants from Deceased Donors. Clin J Am Soc Nephrol. 2018;13(1):118–27. doi: 10.2215/CJN.06550617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nashan B, Abbud-Filho M, Citterio F. Prediction, prevention, and management of delayed graft function: where are we now? Clinical Transplant. 2016;30(10):1198–208. doi: 10.1111/ctr.12832. [DOI] [PubMed] [Google Scholar]


