Growing evidence suggests that many of the worldwide health concerns today can be impacted by omega-3 fatty acids. Indeed, dietary intake of omega-3 fatty acids, including eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA), is associated with protection against many significant pathologies, including ischemic and inflammatory diseases. EPA and DHA can be incorporated into cellular membranes to influence their biophysical properties, but can also be metabolized by enzymes such as cyclooxygenases (COXs), lipoxygenases (LOXs) and cytochromes P450 to a diverse collection of biologically active lipid mediators1. Discerning which of these lipid mediators are responsible for the beneficial effects of omega-3 fatty acid supplementation has been a daunting task.
DHA and EPA can be metabolized into subsets of molecules including resolvins, protectins, and maresins. Collectively termed specialized pro-resolving mediators (SPMs), these compounds promote resolution of inflammation in a variety of settings2,3. To date, more than 40 distinct SPMs have been identified. Minor differences in the regio- or stereochemical composition of the SPMs can significantly alter their potency and bioactivity, and influence the downstream signaling pathways activated by SPMs. SPMs are subsequently degraded to secondary metabolites which may or may not maintain pro-resolving properties4. Progress on the bioactions and functional relevance of individual SPMs has been hampered by the high cost related to the complex synthesis of these compounds, which are often not commercially available. As with all endogenous mediators, it is critical to validate the precise biochemical structures, quantify the endogenous levels, and elucidate the biological actions of these pro-resolving mediators. In the current issue of the Journal of Leukocyte Biology, Winkler et al. directly address these concerns and elucidate the endogenous production, metabolism and potent anti-inflammatory effects of Resolvin D4 (RvD4)5.
Like other D-series resolvins, RvD4 is formed by sequential enzymatic reactions in vivo; 15-Lipoxygenase metabolizes DHA to 17S-hydroxydocosahexaenoic acid (HDHA) and 5-lipoxygenase then generates 4S,5R,17S-trihydroxydocosahexaenoic acid (RvD4). Winkler et al. reported a method for commercial scale, total chemical synthesis of RvD4 and several of the RvD4 stereoisomers and endogenous metabolites. Using liquid chromatography, tandem mass spectrometry, they validated the synthetic RvD4 as identical to endogenous RvD4 produced in vivo in human bone marrow. Commercial scale production of RvD4 provided the capability to treat mice and cells with this compound to further elucidate its biological actions, downstream signaling pathways and utility as a possible therapeutic agent.
The authors found that RvD4 was abundantly produced in vivo. Mice treated with permanent ligation of the femoral artery exhibited pronounced production of RvD4 after 24 hours. Interestingly, post-ligation RvD4 levels were nearly 30-fold higher than those of its biosynthetic partner RvD3, which also has potent biological effects6. In a model of leukocyte-mediated secondary organ damage, pharmacological doses of RvD4 reduced markers of lung injury after temporary hind limb ischemia. RvD4 had similar potency to RvD3 in reducing neutrophil infiltration and levels of the proinflammatory eicosanoids LTB4, TXB2, PGE2 and PGF2α. While RvD4 prevented neutrophil infiltration in vivo, this appears to be an indirect effect, as it failed to regulate neutrophil chemotaxis in vitro. In contrast, RvD4 promoted bacterial phagocytosis by both macrophages and neutrophils in vitro. While the identity of the putative RvD4 receptor remains unknown, stimulation of macrophage phagocytosis by RvD4 was inhibited by cholera toxin, which suggests signaling through a Gs-coupled G-Protein-Coupled Receptor.
Winkler et al. also examined the metabolic inactivation of RvD4. Incubation of RvD4 with human bone marrow-derived leukocytes produced two major products: 17-oxo-RvD4 and 15,16-dihydro-RvD4. Eicosanoid oxidoreductase (EOR), also known as 15-hydroxyprostaglandin dehydrogenase (HPGD), was shown to metabolize RvD4 entirely to 17-oxo-RvD4. Importantly, conversion to 17-oxo-RvD4 abolished its ability to stimulate bacterial phagocytosis. Thus, confirmation of this inactivation pathway may enable development of potent SPM analogs that resist metabolic inactivation.
Commercial scale synthesis facilitated validation of RvD4 as a potent anti-inflammatory SPM. This critical first step opens the door to new avenues of investigation; however, many questions remain. To date, RvD4 has both distinct and overlapping features of other D-series resolvins. For example, like RvD4, RvD1 stimulates bacterial phagocytosis and suppresses inflammatory eicosanoid production7. Unlike RvD4, RvD1 directly regulates neutrophil migration. RvD1 and RvD2 have been widely studied; both reduce inflammation through suppression of NF-kB, histamine receptor or ERK signaling2. It remains to be determined whether RvD4 regulates any or all of these processes or signaling pathways in vivo.
The next critically important step will be to determine the cell surface receptors though which RvD4 signals. RvD1 binds to Lipoxin A4 receptors (ALX; also known as FPR2), which are found on neutrophils, eosinophils, monocytes, macrophages, T-cells, synovial fibroblasts, airway and intestinal epithelium, natural killer (NK) cells, and innate lymphoid cells. RvD1, RvD3 and RvD5 bind the D resolvin receptor 1 (DRV1; formerly GPR32), found on human neutrophils, lymphocytes, macrophages, monocytes and in vascular tissues. RvD2 binds to DRV2/GPR18 on neutrophils, monocytes, and macrophages6. Now that bioactive RvD4 can be readily produced, receptor binding assays can be performed and rigorous interrogation of the downstream signaling pathways which mediate its pro-resolving actions can begin.
This study focused on the metabolism and actions of 17S-RvD4, which is the product of 15-LOX and 5-LOX metabolism in vivo. A 17R-RvD4 stereoisomer (17-epi-RvD4) is also produced endogenously; CYP1 family enzymes produce 17R-hydroxydocosahexaenoic acid (HDHA), which is converted to 17R-RvD4 by 5-LOX8. Alternatively, aspirin acetylated COX‐2 and 5-LOX can produce large quantities of 17R-RvD4 in vivo1. While the 17R-RvD compounds often have similar efficacy as their 17S-RvD counterparts, they appear somewhat more resistant to degradation by EOR. Generation of 17R-RvD4 and comparison to 17S-RvD4 in various in vitro and in vivo models are warranted.
Together, the findings by Winkler et al. suggest that regulation of RvD4 levels could be beneficial for patients suffering from a variety of pathologies linked to excessive neutrophil-mediated tissue damage/organ injury or failure to resolve excessive inflammation. Inhibition of EOR would likely increase or prolong RvD4 bioactivity and signaling, but this might have undesirable consequences on the overall lipid profile. Since EOR is believed to be a tumor suppressor, long-term inhibition of EOR would also seem unwise9. Stable analogs which resist EOR degradation have been developed for other SPMs. For example, 18-p-fluorophenoxy-17-epi-RvD1 is stable and potently anti-inflammatory4. Development of similar RvD4 analogs, with a distinct profile of biological actions, might be a preferable approach for treatment of unresolved inflammation.
In summary, Winkler et al. demonstrate large scale, total chemical synthesis of RvD4 and its stable metabolites. Using the validated standard, they confirm endogenous production of RvD4 by bone marrow cells after ischemia which offers protection against secondary lung injury in mice. RvD4 actions are short-lived, likely due to rapid inactivation by EOR to 17-oxo-RvD4. These findings provide the structural basis to develop analogs that resist enzymatic degradation and offer a new approach for controlling pathologies linked to neutrophil-mediated tissue damage or excessive inflammation.
Figure 1. Transcellular metabolism and actions of RvD4.
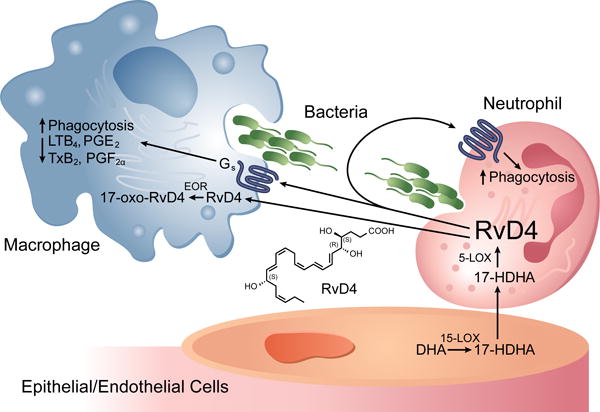
In endothelial cells, docosahexaenoic acid (DHA) is metabolized to 17(S)-hydroxydocosahexaenoic acid (HDHA) by 15-Lipoxygenase (15-LOX). 17(S)-HDHA undergoes transcellular trafficking to adjacent neutrophils where 5-lipoxygenase (5-LOX) can generate RvD4. RvD4 signals through an unknown, Gs-linked, G-Protein Coupled Receptor to promote bacterial phagocytosis by macrophages and neutrophils, and attenuate production of pro-inflammatory eicosanoids (LTB4, TXB2, PGE2 and PGF2α) by macrophages. RvD4 can be transported into cytosol of cells where it is metabolized by eicosanoid oxidoreductase (EOR) to less biologically active 17-oxo-RvD4.
Acknowledgments
This work was supported by the Intramural Research Program of the NIH, National Institute of Environmental Health Sciences (Z01 025034 to D.C.Z.).
Footnotes
AUTHORSHIP:
M.L.E. and D.C.Z wrote the paper.
DISCLOSURES:
The authors declare no conflict of interest.
References
- 1.Duvall MG, Levy BD. DHA- and EPA-derived resolvins, protectins, and maresins in airway inflammation. Eur J Pharmacol. 2016;785:144–55. doi: 10.1016/j.ejphar.2015.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Serhan CN. Pro-resolving lipid mediators are leads for resolution physiology. Nature. 2014;510:92–101. doi: 10.1038/nature13479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee CR, Zeldin DC. Resolvin Infectious Inflammation by Targeting the Host Response. N Engl J Med. 2015;373:2183–5. doi: 10.1056/NEJMcibr1511280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Serhan CN, Krishnamoorthy S, Recchiuti A, Chiang N. Novel anti-inflammatory–pro-resolving mediators and their receptors. Curr Top Med Chem. 2011;11:629–47. doi: 10.2174/1568026611109060629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Winkler JW, Libreros S, De La Rosa X, et al. Structural insights into Resolvin D4 actions and further metabolites via a new total organic synthesis and validation. Journal of Leukocyte Biology. 2018 doi: 10.1002/JLB.3MI0617-254R. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Basil MC, Levy BD. Specialized pro-resolving mediators: endogenous regulators of infection and inflammation. Nat Rev Immunol. 2016;16:51–67. doi: 10.1038/nri.2015.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Krishnamoorthy S, Recchiuti A, Chiang N, et al. Resolvin D1 binds human phagocytes with evidence for proresolving receptors. Proc Natl Acad Sci U S A. 2010;107:1660–5. doi: 10.1073/pnas.0907342107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Divanovic S, Dalli J, Jorge-Nebert LF, et al. Contributions of the three CYP1 monooxygenases to pro-inflammatory and inflammation-resolution lipid mediator pathways. J Immunol. 2013;191:3347–57. doi: 10.4049/jimmunol.1300699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wolf I, O’Kelly J, Rubinek T, et al. 15-hydroxyprostaglandin dehydrogenase is a tumor suppressor of human breast cancer. Cancer Res. 2006;66:7818–23. doi: 10.1158/0008-5472.CAN-05-4368. [DOI] [PubMed] [Google Scholar]


