Abstract
Innate immune cells are an essential part of the host defense response, promoting inflammation through release of pro-inflammatory cytokines or formation of neutrophil extracellular traps. While these processes are important for defense against infectious agents or injury, aberrant activation potentiates pathologic inflammatory disease. Thus, understanding regulatory mechanisms that limit neutrophil extracellular traps formation and cytokine release is of therapeutic interest for targeting pathologic diseases. Activated protein C is an endogenous serine protease with anticoagulant activity as well as anti-inflammatory and cytoprotective functions, the latter of which are mediated through binding cell surface receptors and inducing intracellular signaling. In this review, we discuss certain leukocyte functions, namely neutrophil extracellular traps formation and cytokine release, and the inhibition of these processes by activated protein C.
Keywords: Neutrophils, activated protein C, APC, sepsis, Mac-1, EPCR, PAR1, PAR3
Introduction
Neutrophils, a type of innate immune cell, are among the first responders to sites of infection and injury, and they are indispensable for the initiation, amplification and resolution of inflammation [1–4]. Inflammation, for better or worse, is promoted by release of pro-inflammatory cytokines [5] and formation of DNA-rich neutrophil extracellular traps (NETs) [6,7]. For the better, these processes are invaluable for successful host defense. For the worse, cytokine-driven inflammation and NETs have each been implicated in a multitude of acute and chronic inflammatory diseases [7–14]. Thus, new understandings of the regulatory mechanisms in NETs formation and cytokine release can provide insights into host defense and identify potential therapeutic targets when host defense mechanisms become pathologic.
The crosstalk between leukocytes, especially neutrophils, and coagulation serine proteases is a broad area that has been extensively reviewed [15–17]. Here, we focus on the crosstalk between leukocytes and the serine protease activated protein C (APC). Endogenous regulation of the coagulation cascade occurs, in part, through the protein C system in which the trypsin-like serine protease APC catalyzes inactivation of the activated coagulation factors Va and VIIIa to reduce thrombin generation [18,19]. APC is also well recognized to have cell signaling actions whereby it can exert a spectrum of cytoprotective effects [20–22]. The goal of this review is to present a summary of certain leukocyte functions, namely NETs formation and inflammation, and to discuss the regulatory role of APC and the protein C pathway on these leukocytic processes.
Leukocyte Cell Functions
Leukocytes are essential for successful host defense, but excessive activity and resultant inflammation contribute to serious infectious or autoinflammatory diseases [3,11,23–25]. Pathologic leukocyte activation also contributes to increased thrombotic risk [15,17,26]; however, the complex topic of immunothrombosis will not be addressed in this review. As part of the normal host defense response to pathogen-associated or damage-associated molecular patterns (PAMPs and DAMPs, respectively), pattern recognition receptors (PRRs) are activated to elicit rapid responses either against foreign pathogens or to endogenous host-derived signals [3,23,27,28]. The ability of leukocytes to sense and respond to their microenvironment is tightly regulated by a series of receptor-mediated oxidative and nonoxidative signaling pathways that control the assembly of intracellular proinflammatory complexes, degranulation, chemotaxis, cell death, and resolution [4,23,24,29–31].
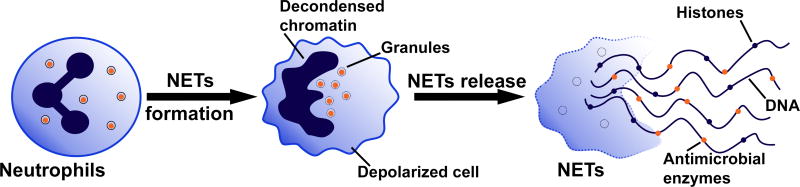
In 2004, it was first described that neutrophils form NETs [6], and this process can cause suicidal NETosis, an alternative form of cell death. Suicidal NETosis involves a general pro-inflammatory mechanism whereby in response to various stimuli, intracellular signaling results in decondensation and extrusion of chromatin decorated with antimicrobial enzymes, including myeloperoxidase (MPO) and neutrophil elastase (NE) (Figure 1) [7,32–34]. Vital NETosis has also been reported whereby nuclear or mitochondrial DNA is extruded without cell membrane lysis, such that the resulting anucleate cytoplasm retains cellular functions [35–37]. Clearly, continued characterization of the stimuli and mechanisms driving vital and suicidal NETosis is required to resolve their respective roles in the host response. Limited, early formation of NETs is considered beneficial in select disease states like sepsis to promote pathogen degradation and clearance [35,38]. On the pathologic side, aberrant extracellular trap formation is associated with a multitude of chronic and acute diseases [39–43].
Figure 1. Neutrophil Extracellular Trap formation.
In response to inflammatory stimuli, neutrophils are activated and can undergo neutrophil extracellular trap (NETs) formation whereby the cell depolarizes and chromatin comprised of histones and DNA is ejected from the cell. NETs are decorated with antimicrobial enzymes, including myeloperoxidase and neutrophil elastase. Figure adapted from [7,26].
As one of their pro-inflammatory responses, leukocytes release the pro-inflammatory cytokines interleukin (IL)-1β and IL-18 [44]. These cytokines are generated from their pro-IL precursors by caspase-1, which itself is generated by activation of pro-caspase-1 by inflammasomes. Inflammasomes are intracellular aggregates of multiple proteins, most commonly driven by one protein, nucleotide-binding domain and leucine-rich repeat-containing protein 3 (NLRP3, also known as cyropyrin). Inflammasome signaling has been reviewed in detail elsewhere [14,45,46]. In general, canonical inflammasome assembly, activation and signaling require multiple signals and steps, including initiation of signaling by cell surface receptors, upregulation of transcription of select inflammasome components, multi-protein inflammasome assembly, and, finally, inflammasome activation resulting in activated caspase-1. The identification of activating missense mutations in cryopyrin results in a constitutively active protein and causes chronic autoinflammation in humans [47]. This discovery highlighted the importance of tight regulation of inflammasomes for healthy host defense. Aberrant activation of the inflammasome may be associated with, inter alia, multiple sclerosis, type 2 diabetes, and atherosclerosis (see Inflammation subsection below) [13,14,44].
The Protein C System
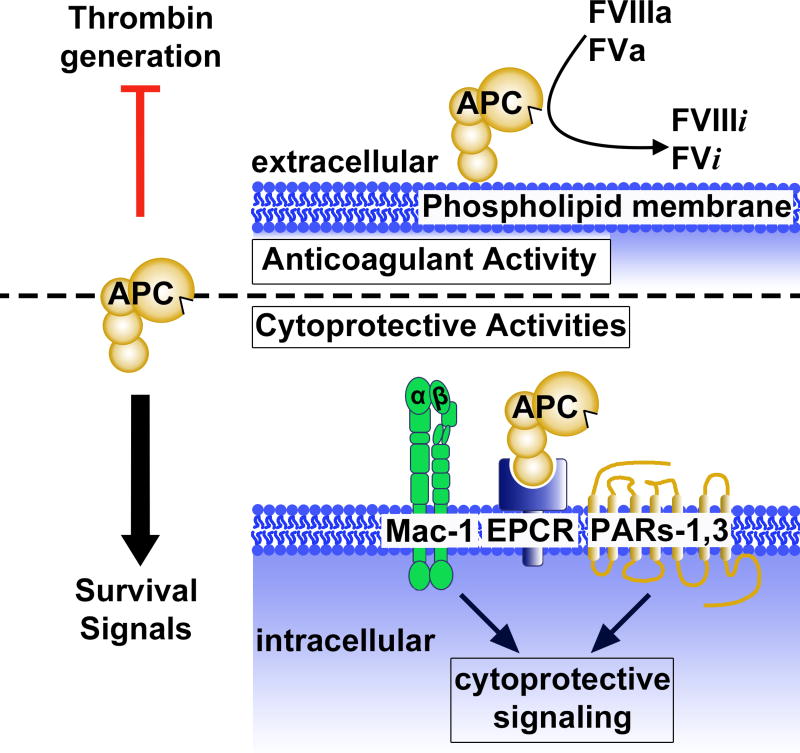
APC is a multi-functional plasma serine protease with potent anticoagulant activity as well as multiple cell signaling activities that are protective against diverse injuries [20–22] (Figure 2). For activation of the inactive zymogen protein C (PC), it binds to endothelial protein C receptor (EPCR) to form a complex, whereby it is cleaved by thrombomodulin-bound thrombin to generate the active protease, APC [18,48,49]. In its capacity as an anticoagulant, APC dampens thrombin generation through irreversible proteolytic inactivation of coagulation factors Va and VIIIa (Figure 2) [18,50,51]. To initiate intracellular signaling that can limit activation of inflammatory pathways and enable cell survival in the face of pro-apoptotic challenges, APC binds several receptors and can then activate the G-protein coupled receptors (GPCRs) protease activated receptor (PAR)1 and/or PAR3 (Figure 2). Intracellular mediators of APC-induced signaling include β-arrestin-2, PI(3)K/Akt and Rac1 [52–56]. Other key receptors that can bind APC and that may mediate APC’s signaling include β1-3 integrins, especially Mac-1 [57–60], apolipoprotein E receptor 2 (ApoER2) [61,62], and Tie2 [63].
Figure 2. Anticoagulant and cytoprotective functions of activated protein C (APC).
APC has potent anticoagulant and cell survival effects. Thrombin generation is inhibited by APC on phospholipid membranes via proteolytic irreversible conversion of activated coagulation factors FVIIIa and FVa into their inactive forms, FVIIIi and FVi, respectively. APC also exerts diverse cytoprotective activities, including promotion of cell survival, by binding to select cell surface receptors, including Mac-1 (CD11b/CD18) and endothelial protein C receptor (EPCR), and by subsequent activation of protease activated receptors (PAR)1 and PAR3 to promote intracellular cytoprotective signaling. Figure adapted from [20].
APC’s multiple cytoprotective activities [20–22] have been demonstrated on a variety of cell types including but not limited to endothelial cells [55,64], monocytes [62,65–67], macrophages [65,68] and neutrophils [58,60,69–72]. The beneficial effects of APC on immune system cells are summarized in Table 1 [20,73,74]. Highly relevant for immune system cells is the recent discovery that APC inhibits development of the activated inflammasome [68] which is discussed in a separate inflammation subsection below.
TABLE 1.
Innate immune cells regulated by APC.
| Cell type | Cell line | Receptors | Inhibitory effects | References |
|---|---|---|---|---|
| Monocytes | Primary [Human] (blood) | Unknown | Reduction in MIP-1-α release in septic patients and healthy volunteers | [65,66] |
| Reduction in expression of CD14, CD11b and CD18 induced by INF-γ or PMA | ||||
|
|
||||
| THP-1 U937 | EPCR, PAR1, ApoER2 | Reduction in MIP-1-α, MCP-1, TNF-α release ± LPS | [62,65,67] | |
| CAM-induced apoptosis | ||||
| Dab1-dependent signaling | ||||
|
| ||||
| Macrophages | Primary (alveolar) | Unknown | Reduction in TNF-α release in response to LPS | [66,68] |
| [Mouse] (BMDM) | PAR 1 | Suppression of NLRP3 inflammasome activation | ||
|
| ||||
| Neutrophils | Primary (blood) | EPCR, integrins, PAR3 | Reduction in chemotaxis | [58,60,69–72] |
| No effect on spontaneous apoptosis | ||||
| APC cleavage of extracellular histones (in vitro and in vivo) | ||||
| Inhibition of NET s formation | ||||
Footnote. Table Abbreviations. APC: Activated protein C; BMDM: bone marrow-derived macrophages; THP-1: monocytic cell line; U937: monocytic cell line; EPCR: endothelial protein C receptor; PARs-1,3: protease activated receptor-1/3; NLRP3: NOD-like receptor 3; ApoER2: Apolipoprotein E receptor 2; MIP-1-α: macrophage inhibitory protein 1-α; INF-γ: Interferon-γ; PMA: phorbol 12-myristate 13-acetate; MCP-1: monocyte chemoattractant protein-1; TNF-α: tumor necrosis factor-α; LPS: lipopolysaccharide; CAM: camptothecin.
APC and sepsis
Following the success of the Recombinant Human Protein C Worldwide Evaluation in Severe Sepsis (PROWESS) trial in 2001 for recombinant wt-APC for adult severe sepsis, wt-APC was approved for this indication [75]. The subsequent PROWESS-SHOCK trial was completed in 2011 and failed to show benefit for wt-APC for severe adult sepsis [76], resulting in withdrawal of the drug from the market. Despite this, controversy remains, as meta-analysis and meta-regression of the effectiveness and safety of wt-APC for sepsis covering >40,000 patients concluded that real-life use of wt-APC was more in line with the PROWESS trial rather than the PROWESS-SHOCK trial [77,78]. Unfortunately, the rationale and design for the PROWESS trial and subsequent trials of APC for sepsis lacked a true understanding of APC’s mechanisms of action in preclinical sepsis models and thus of its potential mechanisms of action in humans. Indeed, as of now, abundant evidence from preclinical models emphasizes the major role for APC’s cell-signaling actions rather than its anticoagulant actions for reducing death in sepsis [73,79–84]. Cytoprotective-selective APC mutants reduce death in a murine pneumonia sepsis model due to APC’s biased signaling via cleavage of PAR1 at Arg46 [85]. One such APC mutant, 3K3A-APC, is safe in humans when given as a high-dose bolus [86], and a recently completed clinical trial for treatment of ischemic stroke (RHAPSODY trial) showed that this APC mutant is safe in stroke patients (P. Lyden, unpublished data). Based on these observations and on a wealth of preclinical data, we believe that there is now a compelling rationale for development of cytoprotective-selective APC mutants such as 3K3A-APC for novel sepsis therapies that employ bolus dosing.
NETs
Sepsis
Severe sepsis is defined as a systemic inflammatory host response presenting with infection and acute organ dysfunction [16,27,87,88]. In the development of sepsis, inflammation is initiated in response to infection, activating the immune response and the blood coagulation cascade, the latter of which can exacerbate inflammation. Crosstalk between the immune response and the coagulation cascade results in the consumption of immune cells and coagulation factors, exhausting the immune and coagulation responses and increasing the risk for adverse bleeding events or thrombosis as well as death.
The formation of NETs in the pulmonary airways and liver microvasculature was initially observed using in vivo mouse models of sepsis, and NETs formation was determined to be partly beneficial for survival by capturing pathogens and promoting their clearance [35,38]. However, the observation that degradation of NETs by DNases reduced intravascular coagulation and reduced organ damage associated with sepsis and septicemia highlighted the delicate balance of the immune response between successful normal host defense and harmful pathological responses [38,89].
NETs and Activated Protein C (APC)
Genetic overexpression of APC in mice decreases neutrophil influx into lung tissue and bronchial lavage fluid during pneumococcal pneumonia [90]. The additional findings that APC cleaved extracellular histones in baboon [72] and mouse [70] models of sepsis raised the hypothesis that one or more of APC’s cytoprotective or anti-inflammatory actions might include regulation of neutrophil function and NETs formation. As low dose infusions of recombinant wt-APC initially succeeded but later failed to provide an overall benefit in adult severe sepsis trials (see above), studies of APC’s cell signaling effects on neutrophils acquired greater relevance.
Receptors on the neutrophil surface that can bind APC and influence neutrophil migration include the canonical APC receptor EPCR [71] as well as the neutrophil β1-3 integrins [57,58] and VLA-3 (α3β1; CD49c/CD29) [60]. Notably, APC inhibits induction of NETs by phorbol 12-myristate 13-acetate (PMA) or by autologous platelet secretome, and this effect of APC requires the neutrophil receptors EPCR, PAR3, and Mac-1 (αMβ2; CD11b/CD18) [69]. The signaling-selective APC mutant 3K3A-APC demonstrated a similar degree of inhibition of NETs generation as did wt-APC, further supporting the immuno-regulatory potential of cytoprotective-selective APC mutants. Evidence for APC’s in vivo beneficial effects on neutrophils in sepsis came from a baboon model of E. coli-induced sepsis in which infusion of APC reduced levels of myeloperoxidase, a marker for neutrophil activation linked to NETs formation [69]. Currently, much remains to be clarified regarding receptor interactions and intracellular signaling pathways that mediate APC’s anti-inflammatory activities involving neutrophils.
Inflammation and Inflammasomes
Inflammation is mediated by the release of cytokines and chemokines that help to drive innate immune cell responses and mobilization of the adaptive immune system. Many view IL-1β as the quintessential proinflammatory mediator in acute and chronic inflammation and one of the most powerful inducers of the innate immune response [5,91,92]. The inflammasome, a source for IL-1β, is a primary driver of inflammation and is best characterized in macrophages [9,25,45,46,93], but is present in other cell types, including epithelial cells [94] and neutrophils [95,96]. Neutralization of IL-1β by a monoclonal antibody in the Canakinumab for Atherosclerotic Disease (CANTOS) trial recently demonstrated that reduction of inflammation without concomitant change in lipid levels reduces the risk of atherothrombosis [13], and is strong evidence pointing to the broad reach of inflammation, especially a pathologic role for IL-1β in cardiovascular disease. Additional in vivo evidence shows that myocardial ischemia reperfusion injury involves the NLRP3 inflammasome. Recent studies showed that development of the inflammasome and injury was suppressed by APC treatment [68], raising the broad question of whether APC’s inhibition of inflammasome development in many cell types partially explains APC’s multiple effects in a large number of preclinical injury models [20]. Future studies of the regulation of inflammasomes in neutrophils and many other cell types will help broaden our understanding of inflammation in its multiple manifestations.
Concluding Remarks
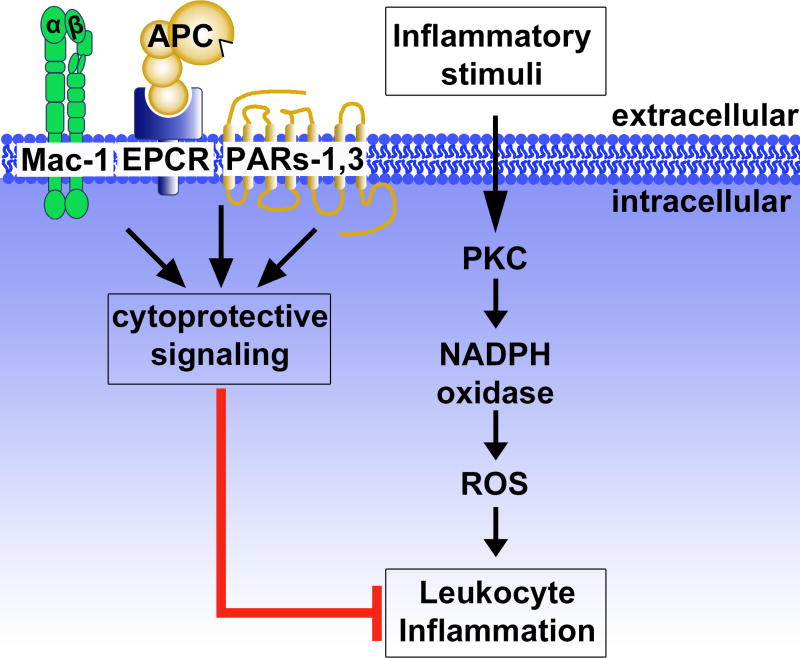
The literature reviewed above highlights the crosstalk between leukocyte-driven inflammation and APC, which has notable therapeutic potential for its anti-inflammatory functions. Specific to neutrophils, APC initiates cytoprotective signaling through select cell surface receptors, including EPCR, Mac-1 and PAR3, which results in inhibition of NETs formation (Figure 3). Nonetheless, much remains to be learned concerning the mechanisms that regulate inflammation and NETs formation, knowledge that will hopefully lead to methods limiting the progression of inflammatory pathologies.
Figure 3. Activated protein C (APC) and leukocyte inflammation.
Inflammatory signaling by leukocytes is driven in part via protein kinase C (PKC) signaling to generate reactive oxygen species (ROS) through NADPH oxidase, resulting in development of an activated inflammasome and/or formation of NETs. APC inhibits leukocyte inflammatory processes in a receptor-dependent manner, requiring Mac-1 (CD11b/CD18), endothelial protein C receptor (EPCR), PAR1 and PAR3, inducing intracellular cytoprotective signaling. Figure adapted from [69].
Summary sentence.
This review focuses on select leukocytic functions and how they are regulated by the anti-inflammatory protease, activated protein C.
Acknowledgments
This work was supported by grants from the National Institutes of Health (R01HL101972 and R01GM116184 to O.J.T.M.; T32AI007472 to L.D.H; HL052246 and HL133728 to J.H.G) and the American Heart Association (13EIA12630000 to O.J.T.M.). R.A.R. is a Whitaker International Fellow.
Abbreviations page
- APC
Activated protein C
- ApoER2
Apolipoprotein E receptor 2
- BMDM
Bone marrow-derived macrophages
- CAM
Camptothecin
- CANTOS
Canakinumab for atherosclerotic disease
- DAMPs
Damage-associated molecular patterns
- EPCR
Endothelial protein C receptor
- GPCR
G protein-coupled receptors
- IL-1β & IL-18
Interleukin-1β & -18
- INF-γ
Interferon-γ
- LPS
Lipopolysaccharide
- Mac-1
Macrophage Antigen-1; αMβ2; CD11b/CD18
- MCP-1
Monocyte chemoattractant protein-1
- MIP-1-α
Macrophage inhibitory protein 1-α
- MPO
Myeloperoxidase
- NE
Neutrophil elastase
- NETs
Neutrophil extracellular traps
- NLRP
Nucleotide-binding domain and leucine-rich repeat-containing proteins; NOD-like receptor protein
- PAMPs
Pathogen-associated molecular patterns
- PAR1,3
Protease activated receptors-1,3
- PC
Protein C
- PKC
Protein kinase C
- PMA
Phorbol 12-myristate 13-acetate
- PROWESS
Recombinant Human Protein C Worldwide Evaluation in Severe Sepsis
- PRRs
Pattern recognition receptors
- ROS
Reactive oxygen species
- THP-1
Monocytic cell line
- TNF-α
Tumor necrosis factor-α
- U937
Monocytic cell line
- VLA-3
Very Late Antigen-3; α3β1; CD49c/CD29
Footnotes
Authorship
L.D.H., J.H.G. and O.J.T.M. planned, researched and wrote the review. R.A.R. participated in writing and critical editing of the manuscript.
Disclosures
The authors declare no conflicts of interest.
References
- 1.Borregaard N. Neutrophils, from Marrow to Microbes. Immunity. 2010;33:657–70. doi: 10.1016/j.immuni.2010.11.011. [DOI] [PubMed] [Google Scholar]
- 2.Ley K, Laudanna C, Cybulsky MI, Nourshargh S. Getting to the site of inflammation: the leukocyte adhesion cascade updated. Nat Rev Immunol. 2007;7:678. doi: 10.1038/nri2156. [DOI] [PubMed] [Google Scholar]
- 3.Mócsai A. Diverse novel functions of neutrophils in immunity, inflammation, and beyond. J Exp Med. 2013;210:1283–99. doi: 10.1084/jem.20122220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nauseef WM, Borregaard N. Neutrophils at work. Nat Immunol. 2014;15:602–11. doi: 10.1038/ni.2921. [DOI] [PubMed] [Google Scholar]
- 5.Dinarello CA, Simon A, van der Meer JWM. Treating inflammation by blocking interleukin-1 in a broad spectrum of diseases. Nat Rev Drug Discov. 2012;11:633–52. doi: 10.1038/nrd3800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brinkmann V, Reichard U, Goosmann C, Fauler B, Uhlemann Y, Weiss DS, Weinrauch Y, Zychlinsky A. Neutrophil extracellular traps kill bacteria. Science. 2004;303:1532–5. doi: 10.1126/science.1092385. [DOI] [PubMed] [Google Scholar]
- 7.Papayannopoulos V. Neutrophil extracellular traps in immunity and disease. Nat Rev Immunol. 2017 doi: 10.1038/nri.2017.105. Advance Online Publication. [DOI] [PubMed] [Google Scholar]
- 8.Brinkmann V, Zychlinsky A. Beneficial suicide: why neutrophils die to make NETs. Nat Rev Microbiol. 2007;5:577–82. doi: 10.1038/nrmicro1710. [DOI] [PubMed] [Google Scholar]
- 9.Broderick L, De Nardo D, Franklin BS, Hoffman HM, Latz E. The inflammasomes and autoinflammatory syndromes. Annu Rev Pathol. 2015;10:395–424. doi: 10.1146/annurev-pathol-012414-040431. [DOI] [PubMed] [Google Scholar]
- 10.Jorch SK, Kubes P. An emerging role for neutrophil extracellular traps in noninfectious disease. Nat Med. 2017;23:279–87. doi: 10.1038/nm.4294. [DOI] [PubMed] [Google Scholar]
- 11.Nauseef WM, Kubes P. Pondering neutrophil extracellular traps with healthy skepticism. Cell Microbiol. 2016;18:1349–57. doi: 10.1111/cmi.12652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Papayannopoulos V, Zychlinsky A. NETs: a new strategy for using old weapons. Trends Immunol. 2009;30:513–21. doi: 10.1016/j.it.2009.07.011. [DOI] [PubMed] [Google Scholar]
- 13.Ridker PM, Everett BM, Thuren T, MacFadyen JG, Chang WH, Ballantyne C, Fonseca F, Nicolau J, Koenig W, Anker SD, Kastelein JJP, Cornel JH, Pais P, Pella D, Genest J, Cifkova R, Lorenzatti A, Forster T, Kobalava Z, Vida-Simiti L, et al. Antiinflammatory Therapy with Canakinumab for Atherosclerotic Disease. N Engl J Med. 2017;377:1119–31. doi: 10.1056/NEJMoa1707914. [DOI] [PubMed] [Google Scholar]
- 14.Schroder K, Tschopp J. The inflammasomes. Cell. 2010;140:821–32. doi: 10.1016/j.cell.2010.01.040. [DOI] [PubMed] [Google Scholar]
- 15.Engelmann B, Massberg S. Thrombosis as an intravascular effector of innate immunity. Nat Rev Immunol. 2013;13:34–45. doi: 10.1038/nri3345. [DOI] [PubMed] [Google Scholar]
- 16.Esmon CT. Inflammation and the Activated Protein C Anticoagulant Pathway. Semin Thromb Hemost. 2006;32:049–60. doi: 10.1055/s-2006-939554. [DOI] [PubMed] [Google Scholar]
- 17.Swystun LL, Liaw PC. The role of leukocytes in thrombosis. Blood. 2016;128:753–62. doi: 10.1182/blood-2016-05-718114. [DOI] [PubMed] [Google Scholar]
- 18.Esmon CT, Stenflo J, Suttie JW. A new vitamin K-dependent protein. A phospholipid-binding zymogen of a serine esterase. J Biol Chem. 1976;251:3052–6. [PubMed] [Google Scholar]
- 19.Walker FJ, Sexton PW, Esmon CT. The inhibition of blood coagulation by activated Protein C through the selective inactivation of activated Factor V. Biochim Biophys Acta. 1979;571:333–42. doi: 10.1016/0005-2744(79)90103-7. [DOI] [PubMed] [Google Scholar]
- 20.Griffin JH, Zlokovic BV, Mosnier LO. Activated protein C: biased for translation. Blood. 2015;125:2898–907. doi: 10.1182/blood-2015-02-355974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mosnier LO, Zlokovic BV, Griffin JH. The cytoprotective protein C pathway. Blood. 2007;109:3161–72. doi: 10.1182/blood-2006-09-003004. [DOI] [PubMed] [Google Scholar]
- 22.Rezaie AR. Regulation of the Protein C Anticoagulant and Antiinflammatory Pathways. Curr Med Chem. 2010;17:2059–69. doi: 10.2174/092986710791233706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hyun Y-M, Hong C-W. Deep insight into neutrophil trafficking in various organs. J Leukoc Biol. 2017;102:617–29. doi: 10.1189/jlb.1RU1216-521R. [DOI] [PubMed] [Google Scholar]
- 24.Kourtzelis I, Mitroulis I, von Renesse J, Hajishengallis G, Chavakis T. From leukocyte recruitment to resolution of inflammation: the cardinal role of integrins. J Leukoc Biol. 2017;102:677–83. doi: 10.1189/jlb.3MR0117-024R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee C, Geng S, Zhang Y, Rahtes A, Li L. Programming and memory dynamics of innate leukocytes during tissue homeostasis and inflammation. J Leukoc Biol. 2017;102:719–26. doi: 10.1189/jlb.6MR0117-027RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Martinod K, Wagner DD. Thrombosis: tangled up in NETs. Blood. 2014;123:2768–76. doi: 10.1182/blood-2013-10-463646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ito T. PAMPs and DAMPs as triggers for DIC. J Intensive Care. 2014;2:67. doi: 10.1186/s40560-014-0065-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kolaczkowska E, Kubes P. Neutrophil recruitment and function in health and inflammation. Nat Rev Immunol. 2013;13:159–75. doi: 10.1038/nri3399. [DOI] [PubMed] [Google Scholar]
- 29.Gordon S. Phagocytosis: An Immunobiologic Process. Immunity. 2016;44:463–75. doi: 10.1016/j.immuni.2016.02.026. [DOI] [PubMed] [Google Scholar]
- 30.Lee W-Y, Kubes P. Leukocyte adhesion in the liver: distinct adhesion paradigm from other organs. J Hepatol. 2008;48:504–12. doi: 10.1016/j.jhep.2007.12.005. [DOI] [PubMed] [Google Scholar]
- 31.Serhan CN, Brain SD, Buckley CD, Gilroy DW, Haslett C, O’Neill LAJ, Perretti M, Rossi AG, Wallace JL. Resolution of inflammation: state of the art, definitions and terms. FASEB J Off Publ Fed Am Soc Exp Biol. 2007;21:325–32. doi: 10.1096/fj.06-7227rev. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kolaczkowska E, Jenne CN, Surewaard BGJ, Thanabalasuriar A, Lee W-Y, Sanz M-J, Mowen K, Opdenakker G, Kubes P. Molecular mechanisms of NET formation and degradation revealed by intravital imaging in the liver vasculature. Nat Commun. 2015;6:6673. doi: 10.1038/ncomms7673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Papayannopoulos V, Metzler KD, Hakkim A, Zychlinsky A. Neutrophil elastase and myeloperoxidase regulate the formation of neutrophil extracellular traps. J Cell Biol. 2010;191:677–91. doi: 10.1083/jcb.201006052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Noubouossie DF, Whelihan MF, Yu Y-B, Sparkenbaugh E, Pawlinski R, Monroe DM, Key NS. In vitro activation of coagulation by human neutrophil DNA and histone proteins but not neutrophil extracellular traps. Blood. 2017;129:1021–59. doi: 10.1182/blood-2016-06-722298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Clark SR, Ma AC, Tavener SA, McDonald B, Goodarzi Z, Kelly MM, Patel KD, Chakrabarti S, McAvoy E, Sinclair GD, Keys EM, Allen-Vercoe E, Devinney R, Doig CJ, Green FHY, Kubes P. Platelet TLR4 activates neutrophil extracellular traps to ensnare bacteria in septic blood. Nat Med. 2007;13:463–9. doi: 10.1038/nm1565. [DOI] [PubMed] [Google Scholar]
- 36.Ingelsson B, Söderberg D, Strid T, Söderberg A, Bergh A-C, Loitto V, Lotfi K, Segelmark M, Spyrou G, Rosén A. Lymphocytes eject interferogenic mitochondrial DNA webs in response to CpG and non-CpG oligodeoxynucleotides of class C. Proc Natl Acad Sci. 2018;115:E478–487. doi: 10.1073/pnas.1711950115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yipp BG, Petri B, Salina D, Jenne CN, Scott BNV, Zbytnuik LD, Pittman K, Asaduzzaman M, Wu K, Meijndert HC, Malawista SE, de Boisfleury Chevance A, Zhang K, Conly J, Kubes P. Infection-induced NETosis is a dynamic process involving neutrophil multitasking in vivo. Nat Med. 2012;18:1386–93. doi: 10.1038/nm.2847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McDonald B, Urrutia R, Yipp BG, Jenne CN, Kubes P. Intravascular Neutrophil Extracellular Traps Capture Bacteria from the Bloodstream during Sepsis. Cell Host Microbe. 2012;12:324–33. doi: 10.1016/j.chom.2012.06.011. [DOI] [PubMed] [Google Scholar]
- 39.Brill A, Fuchs TA, Savchenko AS, Thomas GM, Martinod K, De Meyer SF, Bhandari AA, Wagner DD. Neutrophil extracellular traps promote deep vein thrombosis in mice. J Thromb Haemost JTH. 2012;10:136–44. doi: 10.1111/j.1538-7836.2011.04544.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Demers M, Wagner DD. NETosis: a new factor in tumor progression and cancer-associated thrombosis. Semin Thromb Hemost. 2014;40:277–83. doi: 10.1055/s-0034-1370765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Khandpur R, Carmona-Rivera C, Vivekanandan-Giri A, Gizinski A, Yalavarthi S, Knight JS, Friday S, Li S, Patel RM, Subramanian V, Thompson P, Chen P, Fox DA, Pennathur S, Kaplan MJ. NETs are a source of citrullinated autoantigens and stimulate inflammatory responses in rheumatoid arthritis. Sci Transl Med. 2013;5:178ra40. doi: 10.1126/scitranslmed.3005580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Martinod K, Witsch T, Erpenbeck L, Savchenko A, Hayashi H, Cherpokova D, Gallant M, Mauler M, Cifuni SM, Wagner DD. Peptidylarginine deiminase 4 promotes age-related organ fibrosis. J Exp Med. 2017;214:439–58. doi: 10.1084/jem.20160530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Narasaraju T, Yang E, Samy RP, Ng HH, Poh WP, Liew A-A, Phoon MC, van Rooijen N, Chow VT. Excessive neutrophils and neutrophil extracellular traps contribute to acute lung injury of influenza pneumonitis. Am J Pathol. 2011;179:199–210. doi: 10.1016/j.ajpath.2011.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Guo H, Callaway JB, Ting JP-Y. Inflammasomes: mechanism of action, role in disease, and therapeutics. Nat Med. 2015;21:677–87. doi: 10.1038/nm.3893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lamkanfi M, Dixit VM. Mechanisms and functions of inflammasomes. Cell. 2014;157:1013–22. doi: 10.1016/j.cell.2014.04.007. [DOI] [PubMed] [Google Scholar]
- 46.Ong JDH, Mansell A, Tate MD. Hero turned villain: NLRP3 inflammasome-induced inflammation during influenza A virus infection. J Leukoc Biol. 2017;101:863–74. doi: 10.1189/jlb.4MR0616-288R. [DOI] [PubMed] [Google Scholar]
- 47.Hoffman HM, Mueller JL, Broide DH, Wanderer AA, Kolodner RD. Mutation of a new gene encoding a putative pyrin-like protein causes familial cold autoinflammatory syndrome and Muckle-Wells syndrome. Nat Genet. 2001;29:301–5. doi: 10.1038/ng756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Esmon CT. The protein c pathway*. Chest. 2003;124:26S–32S. doi: 10.1378/chest.124.3_suppl.26s. [DOI] [PubMed] [Google Scholar]
- 49.Esmon CT, Owen WG. Identification of an endothelial cell cofactor for thrombin-catalyzed activation of protein C. Proc Natl Acad Sci U S A. 1981;78:2249–52. doi: 10.1073/pnas.78.4.2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gale AJ, Tsavaler A, Griffin JH. Molecular characterization of an extended binding site for coagulation factor Va in the positive exosite of activated protein C. J Biol Chem. 2002;277:28836–40. doi: 10.1074/jbc.M204363200. [DOI] [PubMed] [Google Scholar]
- 51.Gale AJ, Cramer TJ, Rozenshteyn D, Cruz JR. Detailed Mechanisms of the Inactivation of Factor VIIIa by Activated Protein C in the Presence of Its Cofactors, Protein S and Factor V. J Biol Chem. 2008;283:16355–62. doi: 10.1074/jbc.M708985200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Burnier L, Mosnier LO. Novel mechanisms for activated protein C cytoprotective activities involving noncanonical activation of protease-activated receptor 3. Blood. 2013;122:807–16. doi: 10.1182/blood-2013-03-488957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Coughlin SR. Thrombin signalling and protease-activated receptors. Nature. 2000;407:258–64. doi: 10.1038/35025229. [DOI] [PubMed] [Google Scholar]
- 54.Mosnier LO, Sinha RK, Burnier L, Bouwens EA, Griffin JH. Biased agonism of protease-activated receptor 1 by activated protein C caused by noncanonical cleavage at Arg46. Blood. 2012;120:5237–46. doi: 10.1182/blood-2012-08-452169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Riewald M, Petrovan RJ, Donner A, Mueller BM, Ruf W. Activation of endothelial cell protease activated receptor 1 by the protein C pathway. Science. 2002;296:1880–2. doi: 10.1126/science.1071699. [DOI] [PubMed] [Google Scholar]
- 56.Soh UJK, Trejo J. Activated protein C promotes protease-activated receptor-1 cytoprotective signaling through β-arrestin and dishevelled-2 scaffolds. Proc Natl Acad Sci. 2011;108:E1372–80. doi: 10.1073/pnas.1112482108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cao C, Gao Y, Li Y, Antalis TM, Castellino FJ, Zhang L. The efficacy of activated protein C in murine endotoxemia is dependent on integrin CD11b. J Clin Invest. 2010;120:1971–80. doi: 10.1172/JCI40380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Elphick GF, Sarangi PP, Hyun Y-M, Hollenbaugh JA, Ayala A, Biffl WL, Chung H-L, Rezaie AR, McGrath JL, Topham DJ, Reichner JS, Kim M. Recombinant human activated protein C inhibits integrin-mediated neutrophil migration. Blood. 2009;113:4078–85. doi: 10.1182/blood-2008-09-180968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kurosawa S, Esmon CT, Stearns-Kurosawa DJ. The Soluble Endothelial Protein C Receptor Binds to Activated Neutrophils: Involvement of Proteinase-3 and CD11b/CD18. J Immunol. 2000;165:4697–703. doi: 10.4049/jimmunol.165.8.4697. [DOI] [PubMed] [Google Scholar]
- 60.Sarangi PP, Lee H-W, Lerman YV, Trzeciak A, Harrower EJ, Rezaie AR, Kim M. Activated Protein C Attenuates Severe Inflammation by Targeting VLA-3(high) Neutrophil Subpopulation in Mice. J Immunol. 2017;199:2930–6. doi: 10.4049/jimmunol.1700541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.White-Adams TC, Berny MA, Tucker EI, Gertz JM, Gailani D, Urbanus RT, de Groot PG, Gruber A, McCarty OJT. Identification of coagulation factor XI as a ligand for platelet apolipoprotein E receptor 2 (ApoER2) Arterioscler Thromb Vasc Biol. 2009;29:1602–7. doi: 10.1161/ATVBAHA.109.187393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yang XV, Banerjee Y, Fernández JA, Deguchi H, Xu X, Mosnier LO, Urbanus RT, Groot PGde, White-Adams TC, McCarty OJT, Griffin JH. Activated protein C ligation of ApoER2 (LRP8) causes Dab1-dependent signaling in U937 cells. Proc Natl Acad Sci. 2009;106:274–9. doi: 10.1073/pnas.0807594106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Xue M, Chow S-O, Dervish S, Chan Y-KA, Julovi SM, Jackson CJ. Activated protein C enhances human keratinocyte barrier integrity via sequential activation of epidermal growth factor receptor and Tie2. J Biol Chem. 2011;286:6742–50. doi: 10.1074/jbc.M110.181388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Joyce DE, Gelbert L, Ciaccia A, DeHoff B, Grinnell BW. Gene Expression Profile of Antithrombotic Protein C Defines New Mechanisms Modulating Inflammation and Apoptosis. J Biol Chem. 2001;276:11199–203. doi: 10.1074/jbc.C100017200. [DOI] [PubMed] [Google Scholar]
- 65.Brueckmann M, Hoffmann U, De Rossi L, Weiler HM, Liebe V, Lang S, Kaden JJ, Borggrefe M, Haase KK, Huhle G. Activated protein C inhibits the release of macrophage inflammatory protein-1-alpha from THP-1 cells and from human monocytes. Cytokine. 2004;26:106–13. doi: 10.1016/j.cyto.2004.01.004. [DOI] [PubMed] [Google Scholar]
- 66.Grey ST, Tsuchida A, Hau H, Orthner CL, Salem HH, Hancock WW. Selective inhibitory effects of the anticoagulant activated protein C on the responses of human mononuclear phagocytes to LPS, IFN-gamma, or phorbol ester. J Immunol. 1994;153:3664–72. [PubMed] [Google Scholar]
- 67.Stephenson DA, Toltl LJ, Beaudin S, Liaw PC. Modulation of monocyte function by activated protein C, a natural anticoagulant. J Immunol Baltim Md 1950. 2006;177:2115–22. doi: 10.4049/jimmunol.177.4.2115. [DOI] [PubMed] [Google Scholar]
- 68.Nazir S, Gadi I, Al-Dabet MM, Elwakiel A, Kohli S, Ghosh S, Manoharan J, Ranjan S, Bock F, Braun-Dullaeus RC, Esmon CT, Huber TB, Camerer E, Dockendorff C, Griffin JH, Isermann B, Shahzad K. Cytoprotective activated protein C averts Nlrp3 inflammasome induced ischemia reperfusion injury via mTORC1 inhibition. Blood. 2017;130:2664–77. doi: 10.1182/blood-2017-05-782102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Healy LD, Puy C, Fernández JA, Mitrugno A, Keshari RS, Taku NA, Chu TT, Xu X, Gruber A, Lupu F, Griffin JH, McCarty OJT. Activated protein C inhibits neutrophil extracellular trap formation in vitro and activation in vivo. J Biol Chem. 2017;292:8616–29. doi: 10.1074/jbc.M116.768309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Iba T, Nagakari K. The effect of plasma-derived activated protein C on leukocyte cell-death and vascular endothelial damage. Thromb Res. 2015;135:963–9. doi: 10.1016/j.thromres.2015.03.012. [DOI] [PubMed] [Google Scholar]
- 71.Sturn DH, Kaneider NC, Feistritzer C, Djanani A, Fukudome K, Wiedermann CJ. Expression and function of the endothelial protein C receptor in human neutrophils. Blood. 2003;102:1499–505. doi: 10.1182/blood-2002-12-3880. [DOI] [PubMed] [Google Scholar]
- 72.Xu J, Zhang X, Pelayo R, Monestier M, Ammollo CT, Semeraro F, Taylor FB, Esmon NL, Lupu F, Esmon CT. Extracellular histones are major mediators of death in sepsis. Nat Med. 2009;15:1318–21. doi: 10.1038/nm.2053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.McDonnell CJ, Soule EE, Walsh PT, O’Donnell JS, Preston RJS. The Immunoregulatory Activities of Activated Protein C in Inflammatory Disease. Semin Thromb Hemost. 2017 doi: 10.1055/s-0037-1608910. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 74.Rezaie AR. Protease-activated receptor signalling by coagulation proteases in endothelial cells. Thromb Haemost. 2014;112:876–82. doi: 10.1160/TH14-02-0167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bernard GR, Vincent J-L, Laterre P-F, LaRosa SP, Dhainaut J-F, Lopez-Rodriguez A, Steingrub JS, Garber GE, Helterbrand JD, Ely EW, Fisher CJJ. Efficacy and Safety of Recombinant Human Activated Protein C for Severe Sepsis. N Engl J Med. 2001;344:699–709. doi: 10.1056/NEJM200103083441001. [DOI] [PubMed] [Google Scholar]
- 76.Ranieri VM, Thompson BT, Barie PS, Dhainaut J-F, Douglas IS, Finfer S, Gårdlund B, Marshall JC, Rhodes A, Artigas A, Payen D, Tenhunen J, Al-Khalidi HR, Thompson V, Janes J, Macias WL, Vangerow B, Williams MD. PROWESS-SHOCK Study Group. Drotrecogin alfa (activated) in adults with septic shock. N Engl J Med. 2012;366:2055–64. doi: 10.1056/NEJMoa1202290. [DOI] [PubMed] [Google Scholar]
- 77.Christiaans SC, Wagener BM, Esmon CT, Pittet JF. Protein C and acute inflammation: a clinical and biological perspective. Am J Physiol Lung Cell Mol Physiol. 2013;305:L455–466. doi: 10.1152/ajplung.00093.2013. [DOI] [PubMed] [Google Scholar]
- 78.Kalil AC, LaRosa SP. Effectiveness and safety of drotrecogin alfa (activated) for severe sepsis: a meta-analysis and metaregression. Lancet Infect Dis. 2012;12:678–86. doi: 10.1016/S1473-3099(12)70157-3. [DOI] [PubMed] [Google Scholar]
- 79.Guo H, Liu D, Gelbard H, Cheng T, Insalaco R, Fernández JA, Griffin JH, Zlokovic BV. Activated Protein C Prevents Neuronal Apoptosis via Protease Activated Receptors 1 and 3. Neuron. 2004;41:563–72. doi: 10.1016/s0896-6273(04)00019-4. [DOI] [PubMed] [Google Scholar]
- 80.Kerschen E, Hernandez I, Zogg M, Jia S, Hessner MJ, Fernandez JA, Griffin JH, Huettner CS, Castellino FJ, Weiler H. Activated protein C targets CD8+ dendritic cells to reduce the mortality of endotoxemia in mice. J Clin Invest. 2010;120:3167–78. doi: 10.1172/JCI42629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kerschen EJ, Fernandez JA, Cooley BC, Yang XV, Sood R, Mosnier LO, Castellino FJ, Mackman N, Griffin JH, Weiler H. Endotoxemia and sepsis mortality reduction by non-anticoagulant–activated protein C. J Exp Med. 2007;204:2439–48. doi: 10.1084/jem.20070404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Mosnier LO, Gale AJ, Yegneswaran S, Griffin JH. Activated protein C variants with normal cytoprotective but reduced anticoagulant activity. Blood. 2004;104:1740–4. doi: 10.1182/blood-2004-01-0110. [DOI] [PubMed] [Google Scholar]
- 83.Mosnier LO, Zampolli A, Kerschen EJ, Schuepbach RA, Banerjee Y, Fernández JA, Yang XV, Riewald M, Weiler H, Ruggeri ZM, Griffin JH. Hyperantithrombotic, noncytoprotective Glu149Ala-activated protein C mutant. Blood. 2009;113:5970–8. doi: 10.1182/blood-2008-10-183327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Preston RJS, Ajzner E, Razzari C, Karageorgi S, Dua S, Dahlbäck B, Lane DA. Multifunctional specificity of the protein C/activated protein C Gla domain. J Biol Chem. 2006;281:28850–7. doi: 10.1074/jbc.M604966200. [DOI] [PubMed] [Google Scholar]
- 85.Sinha RK, Wang Y, Zhao Z, Xu X, Burnier L, Gupta N, Fernandez JA, Martin G, Kupriyanov S, Mosnier LO, Zlokovic BV, Griffin JH. PAR1 Biased Signaling is Required for Activated Protein C In Vivo Benefits in Sepsis and Stroke. Blood. 2018 doi: 10.1182/blood-2017-10-810895. Prepublished online. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lyden P, Levy H, Weymer S, Pryor K, Kramer W, Griffin JH, Davis TP, Zlokovic B. Phase 1 Safety, Tolerability and Pharmacokinetics of 3K3A-APC in Healthy Adult Volunteers. Curr Pharm Des. 2013;19:7479–85. doi: 10.2174/1381612819666131230131454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Esmon CT. The normal role of Activated Protein C in maintaining homeostasis and its relevance to critical illness. Crit Care. 2001;5:S7. doi: 10.1186/cc1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Esmon CT, Taylor FB, Snow TR. Inflammation and coagulation: linked processes potentially regulated through a common pathway mediated by protein C. Thromb Haemost. 1991;66:160–5. [PubMed] [Google Scholar]
- 89.Jiménez-Alcázar M, Rangaswamy C, Panda R, Bitterling J, Simsek YJ, Long AT, Bilyy R, Krenn V, Renné C, Renné T, Kluge S, Panzer U, Mizuta R, Mannherz HG, Kitamura D, Herrmann M, Napirei M, Fuchs TA. Host DNases prevent vascular occlusion by neutrophil extracellular traps. Science. 2017;358:1202–6. doi: 10.1126/science.aam8897. [DOI] [PubMed] [Google Scholar]
- 90.de Boer JD, Kager LM, Roelofs JJTH, Meijers JCM, de Boer OJ, Weiler H, Isermann B, van ’t Veer C, van der Poll T. Overexpression of activated protein C hampers bacterial dissemination during pneumococcal pneumonia. BMC Infect Dis. 2014;14:559. doi: 10.1186/s12879-014-0559-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Dinarello CA. Interleukin-1 in the pathogenesis and treatment of inflammatory diseases. Blood. 2011;117:3720–32. doi: 10.1182/blood-2010-07-273417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ridker PM. From C-Reactive Protein to Interleukin-6 to Interleukin-1: Moving Upstream To Identify Novel Targets for Atheroprotection. Circ Res. 2016;118:145–56. doi: 10.1161/CIRCRESAHA.115.306656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Mathur A, Hayward JA, Man SM. Molecular mechanisms of inflammasome signaling. J Leukoc Biol. 2017 doi: 10.1189/jlb.3MR0617-250R. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 94.Chung H, Vilaysane A, Lau A, Stahl M, Morampudi V, Bondzi-Simpson A, Platnich JM, Bracey NA, French M-C, Beck PL, Chun J, Vallance BA, Muruve DA. NLRP3 regulates a non-canonical platform for caspase-8 activation during epithelial cell apoptosis. Cell Death Differ. 2016;23:1331–46. doi: 10.1038/cdd.2016.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Bakele M, Joos M, Burdi S, Allgaier N, Pöschel S, Fehrenbacher B, Schaller M, Marcos V, Kümmerle-Deschner J, Rieber N, Borregaard N, Yazdi A, Hector A, Hartl D. Localization and functionality of the inflammasome in neutrophils. J Biol Chem. 2014;289:5320–9. doi: 10.1074/jbc.M113.505636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Karmakar M, Katsnelson MA, Dubyak GR, Pearlman E. Neutrophil P2X7 receptors mediate NLRP3 inflammasome-dependent IL-1β secretion in response to ATP. Nat Commun. 2016;7:10555. doi: 10.1038/ncomms10555. [DOI] [PMC free article] [PubMed] [Google Scholar]