Abstract
The Wnt signaling pathway is a highly conserved system that regulates complex biological processes across all metazoan species. At the cellular level, secreted Wnt proteins serve to break symmetry and provide cells with positional information that is critical to the patterning of the entire body plan. At the organismal level, Wnt signals are employed to orchestrate fundamental developmental processes, including the specification of the anterior–posterior body axis, induction of the primitive streak and ensuing gastrulation movements, and the generation of cell and tissue diversity. Wnt functions extend into adulthood where they regulate stem cell behavior, tissue homeostasis, and damage repair. Disruption of Wnt signaling activity during embryonic development or in adults results in a spectrum of abnormalities and diseases, including cancer. The molecular mechanisms that underlie the myriad of Wnt-regulated biological effects have been the subject of intense research for over three decades. This review is intended to summarize our current understanding of how Wnt signals are generated and interpreted.
This article is categorized under:
Biological Mechanisms > Cell Signaling
Developmental Biology > Stem Cell Biology and Regeneration
Keywords: beta-catenin, development, frizzled, signaling, stem cells, WNT
1 | INTRODUCTION
Since their discovery by Nusse and Varmus (1982), Wnt genes have captivated the attention of geneticists, protein biochemists, structural and cell biologists, embryologists, and stem and cancer cell scientists alike. The isolation and identification of the first Wnt gene was made possible by the insertion of the mouse mammary tumor virus, an oncogenic retrovirus, into a genomic locus then named integration site 1, or int1, an event that conferred a tumorigenic growth advantage on cells of the mammary gland. Low stringency hybridization of genomic DNA from multiple species with an int1 probe hinted at a high degree of conservation of these sequences from flies to humans (Nusse, van Ooyen, Cox, Fung, & Varmus, 1984). In an unrelated line of investigation, Drosophila geneticists studying mutants that produced so-called segment polarity phenotypes, identified a gene called wingless (wg; Nusslein-Volhard & Wieschaus, 1980; Sharma & Chopra, 1976), and molecular cloning of the wg and int1 genes revealed them to be homologs (Baker, 1987; Rijsewijk et al., 1987), an early example that underscored the close relationship between embryogenesis and tumorigenesis. With the realization that wg and int1 were members of a much larger gene family (Gavin, McMahon, & McMahon, 1990), the term Wnt was coined denoting “Wingless-related integration site” (Nusse et al., 1991).
Since these early days, research on Wnt genes has radiated into virtually every organism from hydra to humans and into every discipline of the life sciences from structural biology to medicine. With thousands of publications per year on Pubmed citing “Wnt” as a keyword, the field of Wnt biology is well established with regular international meetings dedicated to this broad topic. This review alone cannot encompass the entirety of this vast field, but rather will focus on our current understanding of how Wnt signals are produced and how they influence downstream molecular signaling events. For further information on Wnt and its biology, we encourage the reader to consult the primary literature, the many books and reviews focused on this topic as well as the Wnt homepage (http://web.stanford.edu/group/nusselab/cgi-bin/wnt/).
The biological effects of Wnt are vast and at first glance seemingly unrelated. The thread connecting the diversity of biological effects is Wnt’s property to break symmetry and establish body axes. This feature is apparent both at the cellular level, where Wnt signaling influences asymmetric cell divisions, and at the organismal level, where perturbations in Wnt signaling produce patterning defects. During adulthood, Wnt signaling plays critical roles in tissue homeostasis and wound healing, which involves the regulation and balance of tissue stem cells. Given the many essential roles of Wnt in the life of an organism, it is not surprising that alterations in Wnt signaling yield profound and often catastrophic effects, including developmental defects and cancer. This review aims to provide the reader with a foundation in the mechanisms by which Wnt signals are generated and interpreted at the cellular level.
2 | WNT: A UNIQUE CLASS OF SIGNALING MOLECULES
2.1 | Wnt proteins
Wnt genes encode secreted growth factors with short-range signaling activity. The mammalian genome contains at least 19 distinct Wnt genes, some of which express alternative transcripts that produce Wnts with variable activities (Bauer, Benard, Gaasterland, Willert, & Cappellen, 2013; Bunaciu, Tang, & Mao, 2008; Dichmann, Walentek, & Harland, 2015; Fear, Kelsell, Spurr, & Barnes, 2000; Katoh, Kirikoshi, Saitoh, Sagara, & Koike, 2000; Teh et al., 2007). Aside from the presence of a signal sequence, signifying entry into the secretory pathway, and a large number of invariantly spaced cysteine residues, the Wnt polypeptide sequence reveals little about its function and structure. Early biochemical experiments detected these proteins associated with the cell surface and extracellular matrix (Bradley & Brown, 1990; Brown, Papkoff, Fung, Shackleford, & Varmus, 1987; Burrus & McMahon, 1995; Papkoff, Brown, & Varmus, 1987) and, to a lesser extent, in the conditioned medium (Bradley & Brown, 1995; Shibamoto et al., 1998; van Leeuwen, Samos, & Nusse, 1994). Purification of biologically active Wnt proteins—first Wnt3a (Willert et al., 2003) and then Wnt5a (Mikels & Nusse, 2006)—revealed these proteins to be highly hydrophobic, a property afforded by a covalently attached lipid. This property still confounds isolation of other Wnt proteins, all of which exhibit slightly distinct biochemical properties. Furthermore, maintaining the solubility of purified Wnt proteins requires detergents, which are incompatible with in vivo systems, and dilution into nondetergent conditions often renders purified Wnts instantly insoluble and inactive. These obstacles have been partially overcome by preparing Wnt liposomes, which stabilize Wnt activity in the absence of detergents (Morrell et al., 2008; Tuysuz et al., 2017), though the feasibility of this process is not yet established for all Wnts.
X-ray crystallography revealed that Wnt proteins are composed of two domains that together form a structure resembling a hand (Janda, Waghray, Levin, Thomas, & Garcia, 2012). This hand-like molecule grasps the extracellular cysteine-rich domain (CRD) of Frizzled (Fzd), which is one of several receptors capable of interacting with Wnt proteins (see section “Wnt signal reception”). The amino (N)-terminal domain of Wnt, also referred to as D1, carries the lipid, which extends from a thumb-like extension, while the carboxy (C)-terminal domain, also referred to as D2, comprises an index finger-like portion (Bazan, Janda, & Garcia, 2012). Together the thumb and index finger engage the receptor with both lipid-and protein-mediated contacts. D1 resembles a saposin fold, which is a highly conserved four α-helix bundle that in many other proteins interacts with lipids. It has been proposed that over the course of evolution, the associated lipid came to be covalently attached to this fold. D2, on the other hand, resembles a cystine-knot cytokine, similar to those found in PDGF, IL17 and Noggin. The evolutionary origin of Wnt may have occurred from the fusion of these ancestral D1 and D2 precursors to create a signaling molecule unique to the metazoan lineage (Bazan et al., 2012).
2.2 | Wnt processing
As Wnts transit through the secretory pathway, they are modified in two important ways: acylation and glycosylation. Acylation, specifically the attachment of palmitoleic acid (PA), a monounsaturated 16-carbon lipid, to a conserved serine (Ser) residue (Takada et al., 2006), occurs early during Wnt processing, and is essential for activity as mutation of the Ser residue produces an inactive protein. Aside from being critical for activity, the covalently attached PA renders the Wnt protein highly hydrophobic and poorly soluble in an aqueous environment (Willert et al., 2003), thereby restricting Wnt’s signaling range to short distances. All Wnts tested to date (with the exception of the divergent Drosophila WntD) are lipidated at least on one site, a post-translational modification catalyzed by the endoplasmic reticulum (ER)-resident O-acyl transferase Porcupine (Porcn, Figure 1). Porcn’s primary role appears to be dedicated to the processing of Wnt proteins; however, the observation that a catalytically inactive Porcn promotes a rate-limiting signaling pathway in cancer cell growth hints at an alternate function for Porcn (Covey et al., 2012). Mutation of Porcn or treatment with small molecule inhibitors of Porcn (Chen et al., 2009; Dodge et al., 2012; Proffitt et al., 2013) creates a condition akin to an all-Wnt mutant, as no active Wnt proteins are secreted from the producing cell. This natural bottleneck in Wnt processing offers a unique opportunity to explore the requirement for Wnt signaling in diverse biological settings. For example, by using cells harboring mutations in the Porcn gene, we demonstrated that Wnt signaling is required for reprogramming of fibroblasts to an induced pluripotent stem cell state (Ross et al., 2014). Furthermore, blocking Wnt secretion may provide an opportunity to combat Wnt-driven tumors, and Porcn inhibitors are currently in clinical trial for the treatment of advanced solid tumors.
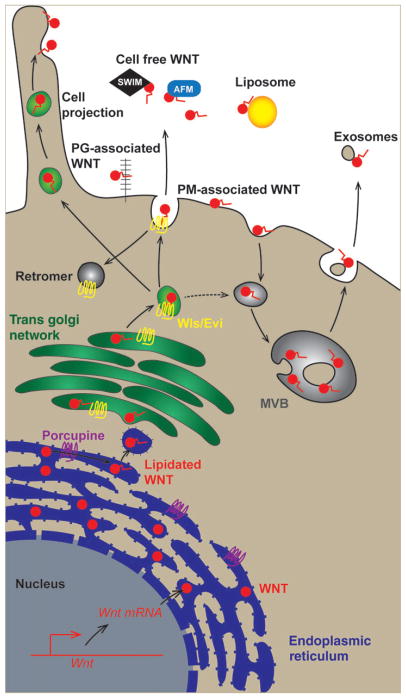
FIGURE 1.
Wnt secretion. Wnt proteins are translated into the endoplasmic reticulum where they are acylated by Porcupine (PORCN). Wntless (Wls/Evi) escorts acylated Wnt to the cell surface where it may associate with the plasma membrane (PM-associated Wnt) or with proteoglycans (PG-associated Wnt). Carrier proteins, such as SWIM and AFM, facilitate solubility of cell free Wnt proteins. Wnt proteins can signal at greater distances via cell projections (also known as filopodia, cytonemes, and nanotubes) or by associating with exosomes or liposomes
In contrast to this acylation, which occurs on at least one defined site, glycosylation of Wnt is more variable: for example, Wnt1 carries four and Wnt3a carries two N-linked glycosylations. Although mutation of individual glycosylation sites impairs Wnt’s secretion (Komekado, Yamamoto, Chiba, & Kikuchi, 2007; Kurayoshi, Yamamoto, Izumi, & Kikuchi, 2007; Mason, Kitajewski, & Varmus, 1992), it is not strictly required for activity; in fact in the case of Wingless (Wg, the Drosophila homolog of Wnt1), glycosylation is entirely dispensable for its activity (Tang et al., 2012).
In the next step of its maturation, Wnt associates with Wls (also known as Gpr177 or Evi, Figure 1), a trans-membrane protein required for movement of Wnt from the trans-Golgi network to the cell surface (Banziger et al., 2006; Bartscherer, Pelte, Ingelfinger, & Boutros, 2006; Goodman et al., 2006). This association of Wnt with Wls requires Porcn-mediated acylation (Herr & Basler, 2012; Tang et al., 2012). Furthermore, interfering with Wls function leads to retrograde Golgi-to-ER transport of Wnt and to ER stress (Zhang, Zhou, Pei, Lin, & Yuan, 2016). Upon release of Wnt at the cell surface, which requires vacuolar acidification (Coombs et al., 2010), Wls is recycled via the retromer complex to the Golgi, where it can facilitate secretion of newly synthesized and acylated Wnt proteins (Belenkaya et al., 2008; Coudreuse, Roel, Betist, Destree, & Korswagen, 2006; Harterink et al., 2011; Port et al., 2008; Prasad & Clark, 2006; Yang et al., 2008; Yu et al., 2014; Zhang et al., 2011). Taken together, Wls acts as a Wnt-specific chaperone essential for the proper transit of Wnt through the secretory pathway and eventual release from the cell. Mutations in Porcn and Wls produce similar gastrulation defects in the early mouse embryo (Biechele, Cox, & Rossant, 2011; Fu, Jiang, Mirando, Yu, & Hsu, 2009), resembling those observed in Wnt3 knockouts (Barrow et al., 2007; Liu et al., 1999), which highlights the essential roles these enzymes play in Wnt maturation.
2.3 | Wnt secretion
Release of Wnt from a cell has been the subject of extensive research, with multiple distinct mechanisms proposed for how Wnt may contact a neighboring cell (Figure 1). Although certain Wnt proteins have been isolated in a cell-free form (Mikels & Nusse, 2006; Shibamoto et al., 1998; van Leeuwen et al., 1994; Willert et al., 2003), Wnts remain active when immobilized (Habib et al., 2013), suggesting that Wnts may signal in a manner is more akin to Notch signaling than to conventional soluble growth factors. Most importantly, flies carrying a modified wg gene that produces a membrane-tethered protein develop normally with only a mild proliferative effect (Alexandre, Baena-Lopez, & Vincent, 2014), suggesting that all essential Wg activity is mediated by direct cell–cell contact.
Despite their close association with cells, Wnts have been observed at greater distances from their site of synthesis. The extreme hydrophobic nature of Wnts necessitates some type of association with other molecules or subcellular structures that shield the hydrophobic moiety in a largely aqueous environment. For example, chaperones that associate with Wnt and shield the lipid moiety have been identified, including secreted Wnt interacting molecule (SWIM; Mulligan et al., 2012) and Afamin (AFM; Mihara et al., 2016). Although these proteins clearly associate with Wnt and influence its secretion and solubility, their requirement in biological processes has not been rigorously established. Within the extracellular space, Wnts have been found to interact with various types of glycans, such as syndecans, glypicans, and biglycan (Alexander et al., 2000; Berendsen et al., 2011; Capurro, Martin, Shi, & Filmus, 2014; Lin & Perrimon, 1999), which regulate Wnt distribution and signaling range. Furthermore, several studies identified a series of genes encoding enzymes involved in heparan sulfate synthesis, including sugarless (homolog of human UGDH), sulfateless (NDST1), tout-velu (EXT1), and sister of tout-velu (EXT2), all of which regulate Wnt signaling (Binari et al., 1997; Bornemann, Duncan, Staatz, Selleck, & Warrior, 2004; Hacker, Lin, & Perrimon, 1997; Haerry, Heslip, Marsh, & O’Connor, 1997; Han et al., 2004; Khare & Baumgartner, 2000; Lin & Perrimon, 1999; Takei, Ozawa, Sato, Watanabe, & Tabata, 2004). The activity of these enzymes is not absolutely required for Wnt signaling, but rather serves to modulate Wnt distribution, receptor binding, and signaling.
Another mechanism by which Wnts may reach longer distances is through actin-based membrane protrusions, variably referred to as filopodia, cytonemes, or nanotubes (Hsiung, Ramirez-Weber, Iwaki, & Kornberg, 2005; Huang & Kornberg, 2015; Stanganello et al., 2015). In this mode of distribution, Wnt would remain associated with the plasma membrane of the Wnt expressing cell. The tips of these membrane protrusions may be reached either by lateral diffusion along the plasma membrane or by molecular motors that transport Wnt-containing vesicles along actin-based structures.
Additionally, several studies have identified Wnts on extracellular particles, including exosomes (Beckett et al., 2013; Chen, Takada, Noda, Kobayashi, & Takada, 2016; Gross, Chaudhary, Bartscherer, & Boutros, 2012; Harada et al., 2017; Korkut et al., 2009; Luga et al., 2012) and lipoprotein complexes (Neumann et al., 2009; Panakova, Sprong, Marois, Thiele, & Eaton, 2005), both of which would be capable of carrying Wnts long distances in bodily fluids, such as blood and cerebrospinal fluid. It also seems possible for Wnts to traverse through several cell boundaries in vesicles called argosomes (Greco, Hannus, & Argosomes, 2001). These various methods of Wnt protein distribution may provide a mechanism by which a membrane-tethered Wg retains biological activity (Alexandre et al., 2014). An unsolved mystery for all of these modes of Wnt transport is how Wnts are transferred from these carrier proteins and structures to their cognate receptor to initiate signaling in the target cell.
3 | WNT SIGNALING AT THE MEMBRANE
3.1 | Wnt signal reception
Several distinct Wnt receptors with signaling activities have been identified (Figure 2). The first Wnt receptor to be identified was Drosophila Frizzled 2 (Dfz2); overexpression of this cell surface protein conferred Wg binding and signaling, as monitored by stabilization of the Armadillo protein (Arm, the fly homolog of β-catenin; Bhanot et al., 1996). The role of Dfz2 in Wg signaling is functionally redundant with the activity of Fz, as only dfz2-fz double mutants produce the characteristic segment polarity phenotype of wg and porc (the fly homolog of Porcn) mutants (Bhanot et al., 1999; Bhat, 1998).
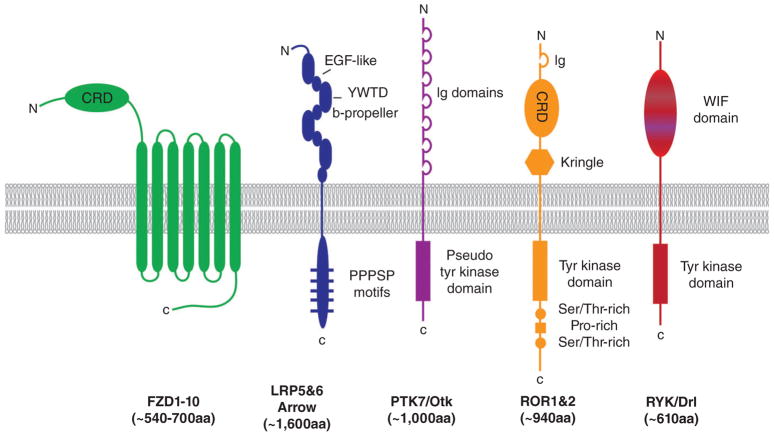
FIGURE 2.
Wnt receptors. Several cell surface proteins directly bind Wnt proteins, including Frizzled (FZD), LRP5 & 6 (fly homolog is Arrow), PTK7 (fly homolog is Otk), ROR1 & 2, and RYK (fly homolog is Drl). Abbreviations: aa = amino acids, CRD = cysteine-rich domain, C = carboxy terminus, Ig = immunoglobulin domain, N = amino terminus, WIF = Wnt inhibitory factor
The mammalian genome encodes 10 Frizzled (Fzd1–10) proteins. With their seven trans-membrane domain spanning topology, these Wnt receptors resemble G-protein coupled receptors (GPCRs, see section “Wnt and G-proteins”). A highly conserved extracellular CRD is required for Wnt binding. The structure of this CRD indicates that it is capable of forming a dimer (Dann et al., 2001), a property that may be promoted by Wnt, with its covalently attached unsaturated lipid moiety binding in a U-shaped hydrophobic groove formed by the two CRD molecules (Nile, Mukund, Stanger, Wang, & Hannoush, 2017). Additional data indicates that Fzd dimerization is mediated by interactions of trans-membrane α-helices 4 and 5 (Petersen et al., 2017). The precise mechanism by which Wnt binding to the CRD activates signaling inside the cell is not well understood and will require the complete structural analysis of a Fzd molecule.
The selectivity between Wnts and Fzds remains poorly understood. Biochemical studies with Wg revealed 10-fold higher affinity of Wg to Dfz2 than to Fz (Rulifson, Wu, & Nusse, 2000). Analysis of Wnt-Fzd interaction.s is confounded in large part by the scarcity of sufficiently purified and active Wnt proteins. Kinetic binding studies between a limited number of Wnts (Wnt3a, 4, 5a and 5b) and Fzd CRDs (Fzd1, 2, 4, 5, 7 and 8) revealed strong and weak associations, with dissociation constants (KD) ranging from less than 10 nM to greater than 100 nM (Dijksterhuis et al., 2015). Interestingly, Wnt3a binding to Fzd appeared to be highly promiscuous, a finding supported by Voloshanenko and colleagues who showed that Wnt3/3a coupled effectively to seven of nine Fzd receptors tested (Voloshanenko, Gmach, Winter, Kranz, & Boutros, 2017). Furthermore, Fzd5, Fzd8, and to a lesser extent Fzd4 were capable of transducing signals of multiple Wnts. Together, these studies provide somewhat limited insight into Wnt-Fzd specificities and may signify that Wnt-Fzd interactions are dictated by the tissue-specific expression patterns of these genes.
An important insight into Wnt signaling specificity came from the finding that the Drosophila gene arrow, which encodes a single-pass trans-membrane protein homologous to mammalian Lrp5 and Lrp6, was required for Wg signal reception (Wehrli et al., 2000). This finding contributed to the current model that Wnt promotes the heterodimerization of Fzd and Lrp5/6 and thereby activates downstream signaling (Pinson, Brennan, Monkley, Avery, & Skarnes, 2000; Tamai et al., 2000), which involves recruitment of intracellular signaling components, such as Axin, Disheveled (Dsh/Dvl) and glycogen synthase kinase 3 (GSK3), to the receptor complex to form the so-called signalosome (Bilic et al., 2007; Cliffe, Hamada, & Bienz, 2003; Davidson et al., 2005; Fiedler, Mendoza-Topaz, Rutherford, Mieszczanek, & Bienz, 2011; Zeng et al., 2005; Zeng et al., 2008). This mechanism of receptor heterodimerization and subsequent signal transduction was further confirmed by the use of bispecific binders, referred to as Wnt surrogates, that simultaneously bound Fzd and Lrp6 and activated signaling (Janda et al., 2017). The assignment of the terms receptor and coreceptor for Fzd and Lrp5/6, respectively, may be somewhat misleading as both components are capable of independently binding Wnt proteins (Bourhis et al., 2010). In this regard, it is noteworthy that overexpression of Fzd generally does not ectopically activate Wnt signaling whereas overexpression of Lrp5/6 does (Mao et al., 2001).
The extracellular domain of Lrp5/6 is comprised of four PE domains (YWTD β-propeller and EGF-like) that mediate interactions with several extracellular ligands, including Wnts (Bourhis et al., 2010; Mao et al., 2001; Pinson et al., 2000; Tamai et al., 2000) and the Wnt antagonists Dickkopf (Dkk; Bafico, Liu, Yaniv, Gazit, & Aaronson, 2001; Mao et al., 2001; Semenov et al., 2001) and Sclerostin (Sost; Li et al., 2005; Semenov, Tamai, & He, 2005). Analysis of the Lrp6 ectodomain using negative-stain electron microscopy revealed that each of these four PE domains forms a globular module with a flexible hinge connecting the second and third PE domain (Matoba et al., 2017), consistent with the structure solved by X-ray crystallography (Cheng et al., 2011). Binding of Dkk1 converts this flexible ectodomain into a compact conformation that likely precludes binding of Wnt, thus explaining the antagonistic activity of Dkk.
Although it is well established that a ternary complex of Wnt, Fzd and Lrp5/6 is essential to β-catenin signaling, mechanisms by which specificity is generated remain poorly understood. Recent studies on vascular development in the central nervous system shed new light on Wnt signaling specificity: the cell surface proteins Gpr124 (an orphan GPCR, gene name Adgra2) and Reck (Reversion-inducing-cysteine-rich protein with kazal motifs, a glycosylphosphatidylinositol [GPI]-anchored glycoprotein) act in a ternary complex with Fzd to enhance Wnt7/β-catenin signaling (Cho, Smallwood, & Nathans, 2017; Vanhollebeke et al., 2015). A distinct β-catenin signaling pathway in endothelial cells activated by Norrin (Ndp), a potent Fzd4-specific agonist, requires Tspan12 (Tetraspanin 12) for maximal signaling (Lai et al., 2017). Importantly, in this Ndp-Fzd4 context, Reck and Gpr124 have no augmenting activity. Similarly, our studies on hematopoietic stem cell development in zebrafish identified a highly specific requirement for Wnt9a: a Wnt9a morphant phenotype was only rescued by Wnt9a overexpression, but not by Wnt9b or the highly promiscuous Wnt3a (Grainger et al., 2016), suggesting the presence of additional components, such as coreceptors, that confer signaling specificity on individual Wnts. These observations hint at the potential complexity of Wnt signaling specificity: molecules distinct from Gpr124, Reck, and Tspan12 likely effect signaling specificity of other Wnt-regulated programs.
Aside from this central Fzd-Lrp5/6 complex, which stimulates Wnt/β-catenin signaling, several other Wnt receptors have been identified (Figure 2), including Ror1 and 2 (Receptor tyrosine kinase-like orphan receptors) and Ryk (Receptor-like tyrosine kinase), which promote alternate signaling cascades. The extracellular domains of Ror1/2 bear homology with the CRD of Fzd, indicating that Wnts engage these receptors in a similar manner to Fzd. Ror1 and 2 have been primarily characterized for their ability to bind Wnt5a (Fukuda et al., 2008; Liu, Rubin, Bodine, & Billiard, 2008; Mikels & Nusse, 2006; Oishi et al., 2003), however, interactions with other Wnt proteins have not been explored. Interestingly, Wnt5a is capable of mediating the heterodimerization of Ror1 and Ror2 (Yu et al., 2016), echoing Wnt’s ability to promote the dimerization of the Fzd CRDs via its lipid moiety (Nile et al., 2017).
The involvement of Ryk in Wnt signaling was first suggested by the homology of its extracellular domain to the Wnt-inhibitory-factor-1 (Wif-1; Patthy, 2000), a secreted protein that binds Wnts and inhibits their activity (Hsieh et al., 1999). Studies in Caenorhabditis elegans vulval development (Inoue et al., 2004) and in Drosophila axon guidance (Yoshikawa, McKinnon, Kokel, & Thomas, 2003) provided genetic evidence that Ryk (LIN-18 in worms and derailed/drl in flies) interacts with and transduces Wnt signals. Mammalian Ryk was shown to interact with Wnt1 and Wnt3a via its WIF domain and form a ternary complex with Fzd (Lu, Yamamoto, Ortega, & Baltimore, 2004).
A final cell surface protein deserving mention in the context of Wnt receptors is Ptk7 (Protein tyrosine kinase 7, also known as colon carcinoma kinase-4, CCK4), a catalytically inactive receptor tyrosine kinase that interacts with multiple Wnt components both in the extra- and intracellular space to regulate cell movements and participate in the establishment of planar cell polarity (PCP; Lu et al., 2004). Several Wnts, including mouse Wnt3a, 4, 5a, and 8, interact with the extracellular domain of Ptk7 (Martinez et al., 2015; Peradziryi et al., 2011), which is comprised of seven immunoglobulin domains. Interactions of Wnt with Ptk7 require the presence of other proteins: for example, Wnt3a-Ptk7 binding requires the CRD of Fzd7 (Berger et al., 2017). In flies, the Ptk7 homologs off track (Otk) and Otk2 form complexes with Fz, Dfz2, and Wnt2 to regulate development of the genital tract (Linnemannstons et al., 2014). Although Ptk7 has a clear role in regulating tissue architecture and cell movements (so-called noncanonical Wnt processes), its role in the well-established Wnt/β-catenin pathway remains controversial. For example, in formation of the Spemann organizer, which is critical to establishment of the vertebrate body axis, Ptk7-activated Wnt signaling (Puppo et al., 2011). In contrast, ptk7 mutant zebrafish showed an increase in expression of Wnt target genes, suggesting that Ptk7 acts to blunt Wnt signaling (Hayes, Naito, Daulat, Angers, & Ciruna, 2013). These controversial aspects of Wnt-Ptk7 aside, it is generally accepted that Ptk7 acts to fine-tune Wnt signaling, a process that is regulated by many other molecules as well.
3.2 | Tuning extracellular Wnt activity
The complexity of cell surface molecules that interact with Wnts and transduce their signals is matched by an equally diverse set of secreted and membrane bound proteins that modulate Wnt activity in the extracellular space. Wnt antagonists act at multiple levels to curtail Wnt’s signaling strength and range (Figure 3). Several secreted proteins, including secreted frizzled-related proteins (Sfrp; Finch et al., 1997; Leyns, Bouwmeester, Kim, Piccolo, & De Robertis, 1997; Rattner et al., 1997; Wang, Krinks, Lin, Luyten, & Moos Jr, 1997), Wnt-inhibitory factor 1 (Wif1; Hsieh et al., 1999) and Cerebus (Cer; Piccolo et al., 1999), act by directly binding to Wnt to block their ability to engage receptors. Sfrps carry a CRD homologous to the ligand-binding domain of Fzd, and the prevailing model has been that Sfrps antagonize Wnt signaling (Leyns et al., 1997; Wang et al., 1997). However, several studies have found that Sfrps do not always inhibit signaling (Galli et al., 2006), and at times even promote signaling (Xavier et al., 2014). Such biphasic effects may be explained by Sfrp dosage, Sfrp’s ability to homo- or heteromultimerize and cellular context.
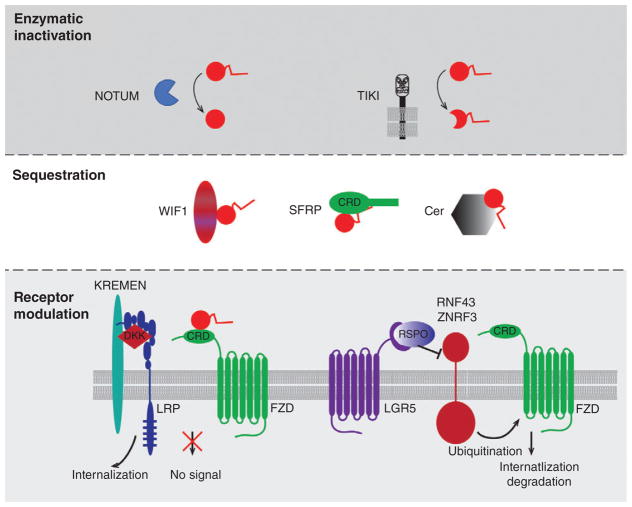
FIGURE 3.
Regulating extracellular Wnt activity. Wnt signaling activity is modulated by three main mechanisms: (a) enzymatic inactivation by NOTUM, which cleaves off the essential lipid moiety, or by TIKI, which removes a portion of the amino terminus, (b) sequestration of Wnt by proteins such as WIF, SFRP, and Cer, and (C) receptor modulation by DKK, which binds LRP and Kremen to prevent the formation of a Wnt-FZD-LRP5/6 receptor complex, and by the ubiquitin ligases ZNRF3 and RNF43, which act to down regulate cell surface expression of FZD. Binding of the secreted protein RSPO to LGR5 interferes with the ability of these ubiquitin ligases to target FZD protein for degradation, thereby increasing Wnt receptor availability on the cell surface
Wif1 is composed of a WIF domain (also found in Ryk/Drl) followed by five EGF-like domains. Binding studies revealed that Wif1 is capable of interacting with multiple Wnts (Hsieh et al., 1999; Surmann-Schmitt et al., 2009), and structural analysis indicates that both the WIF domain and EGF-like domains synergize to bind Wnt proteins (Malinauskas, Aricescu, Lu, Siebold, & Jones, 2011).
Cer, a secreted cystine-knot domain-containing protein, is highly expressed during gastrulation and is a potent inducer of the embryonic axis. It largely acts as an inhibitor of TGF-β signaling by binding to Nodal and BMP. Interestingly, in Xenopus Cer binds Wnt (Piccolo et al., 1999), an interaction that may not be conserved in other vertebrates, such as mice (Belo et al., 2000).
Aside from sequestering Wnt proteins through interactions with such molecules as Sfrp, Wif1, and Cer, Wnt proteins can also be inactivated enzymatically. Notum, which suppresses Wnt signaling in a wide variety of settings (Flowers, Topczewska, & Topczewski, 2012; Giraldez, Copley, & Cohen, 2002; Petersen & Reddien, 2011), encodes an extracellular deacylase that cleaves the essential PA moiety from Wnt, thereby rendering it inactive (Kakugawa et al., 2015; Zhang et al., 2015). In contrast, Tiki1 and Tiki2 (gene names Trabd2a and Trabd2b) encode trans-membrane Wnt-specific metalloproteases that cleave the N-termini of Wnts to inactivate them (Zhang et al., 2012; Zhang et al., 2016).
Wnt signaling activity is furthermore regulated by a host of cell surface molecules that regulate receptor availability. For example, the trans-membrane E3 ubiquitin ligases Znrf3 and Rnf43 ubiquitinate Fzd receptors and promote their internalization and subsequent degradation, thus desensitizing a cell to extracellular Wnt ligands (Hao et al., 2012; Koo et al., 2012). Importantly, binding of R-spondin (Rspo1–4), a secreted Wnt agonist, to the stem cell marker Lgr4/5/6, downregulates these ubiquitin ligases and thereby leads to an increase in Fzd proteins on the cell surface. Therefore, Rspo acts to augment or uncover Wnt signaling activity by increasing receptor availability, however, it should be noted that Rspo has no Wnt signaling activity in itself.
As mentioned above, other secreted proteins, including Dkk and Sost, directly bind to Lrp5/6 to preclude Wnt-mediated heterodimeriztion between Lrp5/6 and Fzd. In addition, Kremen1 and 2 interact with Dkk to form a ternary complex with Lrp6 and promote the rapid internalization of Lrp6 (Davidson, Mao, del Barco Barrantes, & Niehrs, 2002; Mao et al., 2002). Acting similarly, the extracellular portion of Apcdd1 directly interacts with Wnt3a and Lrp5 to preclude formation of a productive Wnt-Fzd-Lrp5/6 receptor complex (Shimomura et al., 2010).
A feature common to all signal transduction pathways is the activation of negative feedback loops, which serve to desensitize cells to the initial signaling input. Likewise, many of the above-mentioned Wnt antagonists are direct targets of Wnt signaling, including Dkk1 (Chamorro et al., 2005; Gonzalez-Sancho et al., 2005; Niida et al., 2004), Sfrp2 (Lescher, Haenig, & Kispert, 1998), Notum (Gerlitz & Basler, 2002; Torisu et al., 2008), Cer (Huggins et al., 2017), Apcdd1 (Takahashi et al., 2002), Znrf3, and Rnf43 (Hao et al., 2012; Van der Flier et al., 2007).
3.3 | Wnt and G-proteins
The seven-span trans-membrane topology of Fzd predicted that these Wnt receptors are GPCRs. Experimental evidence of G-protein involvement in Wnt signaling first surfaced in the study of noncanonical Wnt signaling where Wnt5a and Fzd synergized to increase the frequency of intracellular Ca2+ transients in zebrafish embryos (Slusarski, Corces, & Moon, 1997; Slusarski, Yang-Snyder, Busa, & Moon, 1997). Using a chimeric receptor that incorporated the ligand binding domains of the β2-adrenergic receptor into Fzd, Liu and colleagues showed that the β-adrenergic agonist isoproterenol was able to promote the stabilization of β-catenin, an effect that was blocked by addition of pertussis toxin and by depletion of Gαq and Gαo (Liu et al., 2001). Furthermore, Wnt5a binding to rat Fzd3 led to decreases in cyclic guanosine monophosphate (cGMP) levels and increases in inositol triphosphate (IP3) and diacylglycerol (DAG), both well-known downstream messengers of G-protein signaling (Ahumada et al., 2002). However, it should be noted that Wnt5a acts primarily through the Ror family of receptor tyrosine kinases as simultaneous disruption of Ror1 and Ror2 in mice cause embryonic defects that phenocopy loss of Wnt5a (Ho et al., 2012). A well-established in vitro assay for Wnt5a signaling is inhibition of Wnt/β-catenin signaling (Ishitani et al., 1999; Mikels & Nusse, 2006), and this pathway requires Ror expression, is pertussis toxin insensitive and has no effect on Ca2+ levels (Mikels & Nusse, 2006), observations inconsistent with the involvement of G-proteins downstream of Wnt5a-Ror signaling.
Nonetheless, several recent lines of research provide evidence for roles of G-proteins downstream of Wnt signaling. For example, genetic experiments in Drosophila showed that the Gαo subunit mediates signaling downstream of Fzd receptors (Katanaev, Ponzielli, Semeriva, & Tomlinson, 2005). Furthermore, in rat brain membranes and cultured cells, Wnt3a triggered Fzd-dependent guanine-nucleotide exchange in a Wnt antagonist and pertussis toxin sensitive manner (Koval & Katanaev, 2011). Nichols, Floyd, Bruinsma, Narzinski, and Baranski (2013) suggested that Fzd may act as nontraditional GPCRs by preferentially coupling to Gαs heterotrimeric G-proteins. Using fluorescence recovery after photobleaching (FRAP) and Förster resonance energy transfer (FRET) technology, Schulte and colleagues showed that Fzd complexes with Gαi and Gαq dissociated upon Wnt5a stimulation (Kilander et al., 2014; Kilander, Dahlstrom, & Schulte, 2014). Finally, a recent study demonstrated that Daple, a Dvl-binding protein, bridges Fzd-Gαi interactions to mediate G-protein activation by Wnt (Aznar et al., 2015). Taken together, mounting evidence points to a role of G-proteins downstream of Wnt in a manner somewhat distinct from traditional GPCRs.
4 | WNT SIGNALLING IN THE CYTOPLASM
Once a Wnt signal has been received and transduced across the membrane, a variety of signaling pathways may be activated. Most prominent among the various pathways is Wnt/β-catenin signaling, commonly referred to as “canonical” or “cell fate” Wnt signaling (Loh, van Amerongen, & Nusse, 2016). In addition to this pathway, which culminates in the nucleus to activate expression of target genes, several other cytoplasmic pathways and effectors, collectively termed “noncanonical,” “β-catenin independent” or “cell polarity” signals, have been described. Although these alternative signaling pathways likely comprise independent signaling events, they are not necessarily mutually exclusive from each other, a finding that is only beginning to be uncovered. For example, in the lung, the classic beta-catenin independent Wnt, Wnt5a acts upstream of Axin2, a beta-catenin target gene (Nabhan, Brownfield, Harbury, Krasnow, & Desai, 2018). In addition, particular Wnt-Fzd combinations may be able to activate multiple signaling cascades, though this is poorly understood. These noncanonical branches of Wnt signaling are beyond the scope of this review and will not be further discussed here, and have been reviewed elsewhere (van Amerongen, 2012).
4.1 | β-catenin: Adherens junction component versus transcriptional regulator
β-catenin acts as the primary mediator of Wnt signaling by either translocating to the nucleus to activate target gene expression, or being tagged and degraded constitutively. β-catenin’s critical role in Wnt signaling was first appreciated in Drosophila, where Armadillo (the fly homolog of β-catenin) acted in the same pathway as Wingless (a fly Wnt) to regulate segment polarity (Nusslein-Volhard & Wieschaus, 1980). Years later, a role for β-catenin in cell junctions was identified in Xenopus (McCrea, Turck, & Gumbiner, 1991). Therefore, there are two pools of β-catenin in the cell, one associated with adherens junctions, and another associated with the Wnt pathway, either in the cytosol, or the nucleus.
The factors affecting whether β-catenin acts as a transcriptional regulator, or in adherens junctions are poorly understood, but seem to involve a balance with other cadherins. It is possible that β-catenin exists in two pools, one involved in Wnt signaling, as a monomer, and one bound to α-catenin and existing as dimers in adherens junctions; the regulation of which form may be regulated by phosphorylation and conformational changes, although this is not entirely clear (Gottardi & Gumbiner, 2004). Overexpression of cadherins in Xenopus or Drosophila phenocopy loss of the Wnt/β-catenin signal, and can be rescued by β-catenin, but not by Xwnt-8, suggesting that cadherins negatively regulate the Wnt signal at the level of β-catenin (Heasman et al., 1994; Sanson, White, & Vincent, 1996). β-catenin’s interactions with the nuclear DNA-binding proteins encoded by the Tcf/Lef family seem to similarly compete with cadherins in the context of human cancer cell lines, as well as other tissue culture models (Gottardi, Wong, & Gumbiner, 2001; Kuphal & Behrens, 2006; Shtutman et al., 1999; Stockinger, Eger, Wolf, Beug, & Foisner, 2001). In addition, releasing β-catenin from adherens junctions with proteases, such as ADAM10, is sufficient to drive nuclear translocation of β-catenin and an increase in the Wnt signal (Maretzky et al., 2005; Reiss et al., 2005; Uemura et al., 2006), further supporting the notion that β-catenin exists in two pools, and shuttling between these regulates, at least in part, the Wnt signal. However, it is worthy of note that a loss of E-cadherin is not sufficient to affect the Wnt signal (Herzig, Savarese, Novatchkova, Semb, & Christofori, 2007); this may be due to compensatory loss of β-catenin, mediated for example, by the destruction complex.
4.2 | The β-catenin destruction complex
Evidence for how the Wnt signal is transduced from the membrane to the nucleus came from studies of Drosophila segmentation, and the Drosophila homolog wingless (wg) and its requirement for maintaining expression of the segment polarity gene engrailed (en; DiNardo, Sher, Heemskerk-Jongens, Kassis, & O’Farrell, 1988). The β-catenin homolog, armadillo (arm), was linked to wg since flies deficient for either wg or arm were phenotypically similar, and both transcripts are expressed in similar domains (Peifer, Rauskolb, Williams, Riggleman, & Wieschaus, 1991). In a similar study, it was observed that loss of zesty-white3 (zw3), the homolog to GSK3, phenocopies the loss of Wg, and leads to an activation engrailed, in a Wg-dependent manner (Siegfried, Chou, & Perrimon, 1992), providing the first mechanistic insight into β-catenin (arm) regulation. These studies built the foundation for our understanding of the most well-studied (canonical, or β-catenin-dependent) arm of the Wnt signaling cascade, where β-catenin operates as an inductive switch; its nuclear translocation results in the activation of target gene transcription (discussed below). To achieve this, the buildup of β-catenin in the cytoplasm, resulting in its nuclear translocation is tightly regulated by a group of proteins commonly called the “destruction complex.”
The destruction complex is comprised of several components, including Axin, adenomatous polyposis coli (APC), the serine/threonine kinases GSK3 and Casein kinase 1 (CK1), protein phosphatase 2A (PP2A), and beta-transducin repeat-containing E3 ubiquitin-protein ligase (βTrCP); together, these function to sequester and target β-catenin for degradation in the proteasome, downregulating the Wnt signal (Figure 4). Another component acting upstream of the destruction complex, called Disheveled (Dvl in mouse, Dsh in flies), was first identified in Drosophila as a segment polarity gene (Klingensmith, Nusse, & Perrimon, 1994), required for transmitting the Wg signal (Noordermeer, Klingensmith, Perrimon, & Nusse, 1994; Theisen et al., 1994). Dsh/Dvl is a phosphorylated protein present in the cytosol, which becomes hyperphosphorylated and associated with the membrane in response to Wnt signaling (Yanagawa, van Leeuwen, Wodarz, Klingensmith, & Nusse, 1995). In mice, this is more complicated, since there are three homologs to Dvl (Klingensmith et al., 1996; Sussman et al., 1994; Tsang et al., 1996; Willert, Brink, Wodarz, Varmus, & Nusse, 1997), which display some degree of functional overlap in some developmental contexts (Etheridge et al., 2008). Surprisingly, mice deficient for two of the three Dvl homologs have normal Wnt/β-catenin reporter activity, which is only abolished in triple mutants (Etheridge et al., 2008), confirming a requirement for Dvl in the Wnt signal in vertebrates, but suggesting considerable functional overlap as well as functional variation. Dvl interacts with Fzd at the C-terminal tail (Tauriello et al., 2012), and operates in both β-catenin dependent and independent pathways, and seems to play a role in determining whether or not the receptor complex is internalized (Jiang, Charlat, Zamponi, Yang, & Cong, 2015; Yu et al., 2007). The mechanism of regulation and differences in Dvl specificity are poorly understood, and may relate to different interacting partners, as reviewed in Mlodzik (2016). In the β-catenin signaling cascade, Dvl binds to Axin, Fzd and LRP5/6 in response to Wnt, to form signalosomes, which are necessary for Wnt signaling (Bilic et al., 2007; Zeng et al., 2008), although the mechanism of action beyond this is incompletely understood.
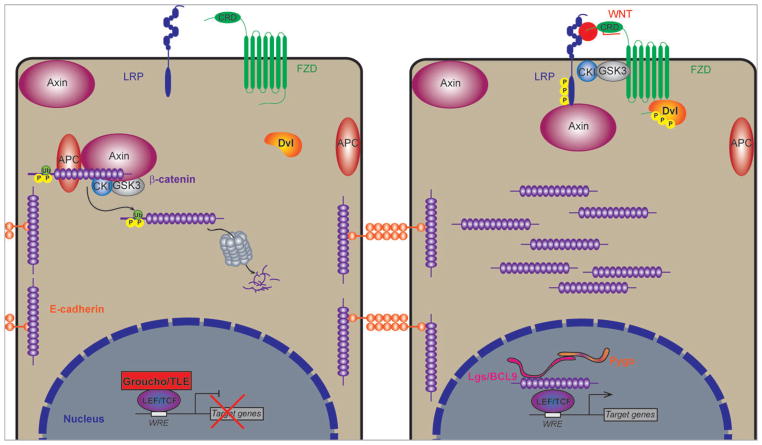
FIGURE 4.
β-catenin mediated Wnt signaling. In the absence of a Wnt ligand, β-catenin is sequestered by the multiprotein “destruction complex,” where it is phosphorylated and ubiquitinated, and targeted for proteasomal degradation. In the nucleus, LEF/TCF (lymphoid-enhancing factor/T-cell factor) transcription factors are resident on Wnt responsive elements (WREs), and recruit corepressors, such as Groucho/TLE. Upon Wnt-Fzd interaction, LRP oligomerizes with the receptor-ligand complex, and the destruction complex is dissociated. This leads to an accumulation of β-catenin in the cytosol, and eventual translocation to the nucleus, where it interacts directly with LEF/TCF transcription factors, and other transcriptional coactivators to initiate target gene expression
It is thought that one of the earliest steps in dissociation of the destruction complex is Axin association with Lrp5/6 (Mao et al., 2001; Tolwinski et al., 2003). Axin acts as a scaffolding anchor to the destruction complex; consistent with this, it is known to interact directly with APC, GSK3β, and β-catenin (Ikeda et al., 1998; Itoh, Krupnik, & Sokol, 1998; Kishida et al., 1998; Rubinfeld et al., 1996). Axin is thought to be the rate-limiting component (Lee, Salic, Kruger, Heinrich, & Kirschner, 2003), and its levels are tightly controlled by GSK-mediated phosphorylation (Yamamoto et al., 1999). Upon Wnt signal reception and formation of the signalosome, Axin is dephosphorylated by protein phosphatase 1 (PP1), reducing its association with β-catenin and consequently inhibiting β-catenin phosphorylation (Kim et al., 2013; Willert, Shibamoto, & Nusse, 1999), which leads to β-catenin stabilization and nuclear translocation. Axin levels are also regulated by SUMOylations at the C-terminus (Kim, Chia, & Costantini, 2008), which prevent its ubiquitin-mediated proteasomal degradation. On the other hand, this ubiquitination is balanced by the ubiquitin specific protease 34 (Lui et al., 2011). Additionally, Lrp5/6 regulates Axin levels: Lrp6 loss leads to an accumulation of Axin, suggesting that Lrp6 induces Axin degradation (Kofron et al., 2007), which is mediated, at least in part, by the E3 ubiquitin ligase Smurf (Smad ubiquitin regulatory factor; Fei et al., 2014; Kim & Jho, 2010). Finally, the poly-ADP ribosylating (PARsylating) enzyme Tankyrase promotes the degradation of Axin (Huang et al., 2009), through the E3 ubiquitin ligase RNF146 (Callow et al., 2011; Zhang et al., 2011). More recently, it has been demonstrated that there are likely two pools of Axin, with some residing near the membrane and being targeted for Tankyrase-dependent degradation, while another pool associates cytoplasmically with the β-catenin destruction complex; the localization seems to depend on the levels of APC, adding a further level of control to steady-state Axin levels (Wang, Tacchelly-Benites, Yang, & Ahmed, 2016). Altogether, these studies indicate that the tight control of Axin is a critical step in regulating the destruction complex and in controlling the Wnt signal output.
APC was first identified as the gene causative for familial adenomatous polyposis, a disease characterized by the formation of colorectal tumors (Kinzler et al., 1991; Nishisho et al., 1991). Screens for APC interactors identified β-catenin, which was thought at the time to be associated with a role for this pair in cell adhesion and migration (Rubinfeld et al., 1993; Su, Vogelstein, & Kinzler, 1993). It was not until later that mutational analysis of APC revealed that its loss leads to an accumulation of nuclear β-catenin, a driving mutation of colorectal tumorigenesis (Korinek et al., 1997; Morin et al., 1997; Rubinfeld, Albert, Porfiri, Munemitsu, & Polakis, 1997). APC is thought to operate as a scaffolding protein in the destruction complex and like Axin, APC is found in two distinct pools in the cell, either at the microtubules at the distal end of cell extensions (Nathke, Adams, Polakis, Sellin, & Nelson, 1996), or in distinct locations in the destruction complex (Penman, Leung, & Nathke, 2005). Axin recruits APC to the destruction complex in response to Wnt, and the Axin-binding domain is required for this redistribution of APC (Faux et al., 2008). Axin also plays a role in regulating APC levels in response to Wnt (Choi, Park, Costantini, Jho, & Joo, 2004). Although APC is traditionally thought to have a negative effect on the Wnt signal, it has also been shown to be a positive regulator in certain contexts (Brauburger et al., 2014; Takacs et al., 2008). Interestingly, some truncations in Axin and APC can be overcome, at least in vitro, and complemented by each other (Pronobis, Deuitch, Posham, Mimori-Kiyosue, & Peifer, 2017; Xu, Liu, Xu, Zhu, & Gao, 2017). Nevertheless, truncating mutations in APC that are common in cancer lead to an increase in nuclear β-catenin, leading to an increase in Wnt signal output, suggesting that these truncations are difficult to overcome in vivo.
β-catenin is targeted for degradation first by phosphorylation events, initially by the priming kinase CK1 at Serine 45, followed by GSK3 at Serines 33 and 37, and Threonine 41 (Amit et al., 2002; Peifer, Pai, & Casey, 1994; Yost et al., 1996); kinases are therefore important components of the destruction complex. There are at least two kinases, GSK3 and CKIα that are associated with the destruction complex. As discussed above, GSK3 (zw3) was first identified as a component of the wg signaling cascade in Drosophila (Siegfried et al., 1992). Further studies indicated that zw3 and wg have opposite effects on the distribution of the β-catenin homolog armadillo (Peifer, Sweeton, Casey, & Wieschaus, 1994), and that zw3 is required for armadillo phosphorylation (Peifer, Pai, & Casey, 1994), the first suggestions that destruction complex-mediated β-catenin phosphorylation leads to a decreased Wnt signal. There are two mammalian GSK3 homologs (α and β); these are functionally redundant, as shown by systematic allelic complementation in embryonic stem cells, where only double mutants affected kinase or Wnt activity (Doble, Patel, Wood, Kockeritz, & Woodgett, 2007). However, a recent study in mouse embryonic stem cells (mESCs) provided evidence for distinct roles for the two GSK3 homologs, with GSK3β maintaining mESC self-renewal and GSK3α promoting neural differentiation (Chen et al., 2017), indicating a greater complexity than previously appreciated.
APC, Axin, and LRP6 are also phosphorylated by GSK3, leading to a variety of cellular responses (Rubinfeld et al., 1996; Willert et al., 1999; Zeng et al., 2005). For example, GSK3-mediated phosphorylation of APC leads to enhanced binding to β-catenin (Rubinfeld et al., 1996), which is reversed by the phosphatase PP2A (Seeling et al., 1999). Axin phosphorylation by GSK3 increases its affinity for β-catenin (Willert et al., 1999). These phosphorylations can also be influenced by other members of the Wnt cascade; for example, LRP6 can directly inhibit GSK3 phosphorylation of β-catenin (Cselenyi et al., 2008). Inhibitors of GSK3 kinase activity, such as lithium, BIO (6-bromoindirubin-3′-oxime), CHIR98014, and CHIR99021, have proven to be potent activators of Wnt/β-catenin signaling. In fact, the mode of action of lithium in bipolar disorder may in part be mediated through its inhibition of GSK3 (Klein & Melton, 1996; Sato, Meijer, Skaltsounis, Greengard, & Brivanlou, 2004).
Another class of serine/threonine kinases is the casein kinase 1 (CK1) family, which encompasses several isoforms (alpha, beta, gamma 1–3 delta, epsilon), that play a myriad of roles in many cell types and have been reviewed elsewhere (Jiang, 2017). Of these, CK1 alpha, gamma, and delta have been implicated in Wnt signaling. Dvl, LRP5, TCF/LEF, Axin, β-catenin, and APC have all been shown to be phosphorylated by CK1, though the outcomes on the Wnt signal are varied (Cong, Schweizer, & Varmus, 2004; Gao, Seeling, Hill, Yochum, & Virshup, 2002; Kishida et al., 2001; Lee, Salic, & Kirschner, 2001; Liu et al., 2002; Peters, McKay, McKay, & Graff, 1999; Rubinfeld, Tice, & Polakis, 2001; Yanagawa et al., 1995; Zeng et al., 2005; Zhang et al., 2006). For example, overexpression of a dominant-negative CK1 results in a phenotype similar to loss of Wnt (Peters et al., 1999), indicating that CK1 is a positive regulator of the Wnt signal. CK1 associates with Dvl, and coexpression of these is sufficient to drive nuclear β-catenin accumulation (Kishida et al., 2001; Peters et al., 1999). CK1 expression leads to a decrease in β-catenin degradation, suggesting that it functions to destabilize the destruction complex, possibly through recruitment of PP2A (Gao et al., 2002). This is consistent with the finding that XWnt-8 or Wnt-3a induce CK1 activity (Swiatek et al., 2004). CK1 has been shown to be the “priming” kinase for GSK3 on β-catenin, leading to it being targeted for degradation (Liu et al., 2002). CK1 has also been implicated in negatively regulating Wnt signaling, by phosphorylating LEF-1, and inhibiting its interaction with β-catenin (Hammerlein, Weiske, & Huber, 2005). Altogether, these data indicate the importance of phosphorylation events in regulation of the destruction complex, and the Wnt signal.
In the absence of a Wnt ligand, β-catenin is targeted for degradation by the destruction complex (described below) in concert with a member of the Skp1-Cullin-F-box (SCF) E3 ubiquitin ligase complex, SCF-β-TRCP (Winston et al., 1999). This occurs in a series of events. First, β-catenin binds to Axin, which positions it for serial phosphorylations by CK1 and GSK3 (Amit et al., 2002; Liu et al., 2002). Following this, SCF-β-TRCP mediates the poly-ubiquitination of β-catenin, leading eventually to its degradation in the proteasome (Kitagawa et al., 1999; Lagna, Carnevali, Marchioni, & Hemmati-Brivanlou, 1999; Liu et al., 1999; Marikawa & Elinson, 1998). It is thought that these interactions between β-catenin and the destruction complex allow for carefully tuned control of Wnt-mediated transcription.
Since the destruction complex plays such an important role in regulating the Wnt signal, it is an attractive therapeutic target to treat diseases that have upregulated Wnt signaling levels, such as colorectal cancers. For instance, Tankyrase inhibitors such as IWR-1, JW74, and XAV939 stabilize the level of the rate-limiting member Axin, and reduce the Wnt signal (Chen et al., 2009; Huang et al., 2009; Stratford et al., 2014). CK1α has also been shown to be activated by pyrvinium, leading to an increase in β-catenin degradation, and a resultant loss of the Wnt signal (Thorne et al., 2010). A recent small molecule screen has identified a novel mechanism of Wnt regulation: Peptidylarginine deiminase 2 (PAD2) deaminates arginines to citrullines on β-catenin, which leads to its degradation. PAD2 activity can be increased by the small molecule nitazoxanide; Wnt signaling can therefore be blocked independent of APC (Qu et al., 2017). This line of targeting is downstream of the frequently mutated APC and β-catenin, and is therefore an attractive chemotherapeutic target.
5 | SIGNAL SIGNALING IN THE NUCLEUS
5.1 | β-catenin shuttling
In the presence of a Wnt ligand, the destruction complex is thought to be dissociated, allowing for the cytoplasmic buildup of β-catenin, followed by its import into the nucleus; how this import happens is poorly understood, since β-catenin does not have a nuclear import or export signal sequence. Assays conducted in vitro indicate that β-catenin docks directly to the nuclear pore machinery to facilitate its entry to the nucleus, independently of energy requirements (Fagotto, Gluck, & Gumbiner, 1998; Yokoya, Imamoto, Tachibana, & Yoneda, 1999). It is likely that β-catenin is sequestered in the cytoplasm, and allowed to move into the nucleus upon dissociation of the destruction complex, since loss of the structural components important for nuclear pore binding results in constitutive nuclear localization, and cadherins and Axin have both been shown to sequester β-catenin away from the nucleus (Fagotto, Funayama, Gluck, & Gumbiner, 1996; Gottardi et al., 2001; Heasman et al., 1994; Orsulic & Peifer, 1996; Sadot, Simcha, Shtutman, Ben-Ze’ev, & Geiger, 1998; Sanson et al., 1996; Shtutman et al., 1999; Tolwinski & Wieschaus, 2001). Upon entry into the nucleus, β-catenin can act as a transcriptional activator.
5.2 | LEF/TCF (lymphoid-enhancing factor/T-cell factor) transcription
The LEF/TCF family of transcription factors is encoded by four genes in mammals, LEF1, TCF7, TCF7L1, and TCF7L2 (formerly TCF1, TCF3, and TCF4, respectively; Korinek et al., 1997; Molenaar et al., 1996; Radler-Pohl, Pfeuffer, Karin, & Serfling, 1990; Travis, Amsterdam, Belanger, & Grosschedl, 1991). These bind to a plethora of degenerate Wnt responsive elements (WREs) on the DNA, through their HMG box DNA-binding domains (Badis et al., 2009). In the absence of a Wnt signal, LEF/TCFs are associated with transcriptional repressors, such as Groucho and transducing-like enhancer (Cavallo et al., 1998; Roose et al., 1998). In addition to these, LEF/TCFs are associated with chromatin modifiers that repress transcription, such as C-terminal-binding protein (CtBP1) and the Polycomb (PcG) complex (reviewed in (Chinnadurai, 2002). With the exception of TCF7L1, which seems to operate solely as a repressor (Dorsky, Itoh, Moon, & Chitnis, 2003; Kim et al., 2000; Liu, van den Broek, Destree, & Hoppler, 2005), upon Wnt activation, β-catenin interacts with these directly to transduce the Wnt signal through target gene activation (Behrens et al., 1996; Brunner, Peter, Schweizer, & Basler, 1997; Huber et al., 1996; Molenaar et al., 1996; van de Wetering et al., 1997). This interaction can be disrupted by the inhibitor of β-catenin and Tcf-4 (ICAT), a 9-kDa protein that sterically inhibits this interaction and antagonizes the Wnt signal (Tago et al., 2000). Target gene specificity among LEF/TCFs seems to be at least partially reliant on a domain adjacent to the HMG box, called the C-clamp, which has been reviewed elsewhere (Ramakrishnan & Cadigan, 2017).
Like other members of the Wnt cascade, LEF/TCFs are subject to post-translational modifications regulating their function. For example, the Nemo-like kinase (NLK) has been shown to phosphorylate TCF, leading to a decrease in the affinity of the TCF/β-catenin complex for DNA (Ishitani et al., 1999; Smit et al., 2004), a finding that has been also observed in multiple species, and with multiple kinases (Hammerlein et al., 2005; Hikasa et al., 2010; Hikasa & Sokol, 2011; Lee et al., 2001; Lo, Gay, Odom, Shi, & Lin, 2004). These observations highlight the complex nature of Wnt target gene regulation.
There are other nuclear factors required for the Wnt signal. For example, pygopus (pygo) and legless (lgs; BCL9 in vertebrates) were first identified in a Drosophila genetic screen for segment polarity genes (Kramps et al., 2002; Parker, Jemison, & Cadigan, 2002). These are required for transmission of the Wg signal throughout development; lgs recruits pygo to the TCF/β-catenin transcriptional complex, where it functions as a transcriptional coactivator (Kramps et al., 2002). In vertebrates, these are similarly required for β-catenin-mediated transcription (Belenkaya et al., 2002; Hoffmans & Basler, 2007; Thompson, Townsley, Rosin-Arbesfeld, Musisi, & Bienz, 2002), though some of these effects are context specific (Song et al., 2007; Sustmann, Flach, Ebert, Eastman, & Grosschedl, 2008). Lgs and Pygo are also implicated in the nuclear localization of β-catenin (Brembeck et al., 2004; Townsley, Cliffe, & Bienz, 2004). In addition to these, components of chromatin modifying complexes, such as polymerase-associated factor 1 (PAF1), are required for the Wnt transcriptional machinery (Mosimann, Hausmann, & Basler, 2006). TCF/β-catenin has also been shown to bind to the DNA helicase TIP49a/Pontin52, and members of the chromatin remodeling complex Brg-1, among others (Barker et al., 2001; Bauer et al., 2000). Further understanding of these interactions will allow us to target Wnt transcription. For example, Indocyanine Green (ICG)-001 is a small molecule inhibitor of the TCF/β-catenin complex, which is able to treat a variety of Wnt-mediated pathologies in vivo in mice, such as pulmonary fibrosis, sepsis following myocardial injury, and smooth muscle remodeling in asthma (Eguchi, Nguyen, Lee, & Kahn, 2005; Henderson Jr et al., 2010; Yousif, Hadi, & Hassan, 2017; Koopmans et al., 2016).
5.3 | Molecular targets of the Wnt signal
A variety Wnt target genes have been reported in a diverse set of biological processes, including stem cells self-renewal, maintenance, and proliferation. Many Wnt target genes are induced in a tissue and context-specific manner, although like other signaling cascades, Wnt signaling induces expression of negative regulators, such as SP5, Nkd1, and Axin2 (Huggins et al., 2017; Jho et al., 2002; Larraguibel et al., 2015; Yan et al., 2001). Wnt stimulation of cell lines, followed by mRNA analyses have uncovered several transcripts induced by Wnt (Gorrepati et al., 2015; Huggins et al., 2017; Jackson, Abete-Luzi, Krause, & Eisenmann, 2014; Maubant et al., 2015; Willert, Epping, Pollack, Brown, & Nusse, 2002). How these are regulated, however, remains elusive; a recent study found that only a subset of β-catenin-bound genomic loci are transcriptionally regulated by Wnt signaling, implying a further requirement for inputs to activate transcription (Nakamura, de Paiva Alves, Veenstra, & Hoppler, 2016).
Over the past decade, one of the best characterized context-specific target genes has been Leucine-rich containing G-protein coupled receptor 5 (Lgr5), originally identified in a screen for genes upregulated in the intestinal crypt in response to Wnt activation in APC-mutant mice (Barker et al., 2007). This GPCR has since been shown to be a marker of a variety of Wnt-responsive epithelial stem cell pools, including the small and large intestine, the stomach, the hair follicle and the mammary gland, among others (Barker et al., 2007, 2008, 2010; Barker & Clevers, 2010; de Visser et al., 2012; Jaks et al., 2008; Sato et al., 2009). In addition to the normal stem cell niche, Lgr5 is also a marker of cancer stem cells (Barker et al., 2009; Hirsch et al., 2014; Kemper et al., 2012; Merlos-Suarez et al., 2011; Schepers et al., 2012). These studies indicate the importance of the Wnt signal in stem cells and cancer stem cells, which has also been shown in other contexts. For example, Wnt signaling through the FZD7 receptor is required to maintain human embryonic stem cell pluripotency (Fernandez et al., 2014), and also for generating induced pluripotent stem cells (Ross et al., 2014). This is by no means an exhaustive list, as there are many examples of how Wnt regulates these process, reviewed elsewhere (Clevers & Nusse, 2012). A more comprehensive and continuously updated list of Wnt target genes can be found on the Wnt homepage (http://web.stanford.edu/group/nusselab/cgi-bin/wnt/). Improving our understanding of which targets Wnt regulates will improve our ability to target and treat Wnt-mediated diseases such as cancers.
6 | CONCLUSION
The intense research on the mechanisms of Wnt signal initiation and transduction, as summarized in this overview, has opened many new avenues of research and provided many opportunities for therapeutic intervention in currently incurable diseases, including neurodegeneration and cancer. Novel drugs that target specific points of Wnt signaling will likely be of significant clinical value: inhibition of Wnt signaling shows promise in the treatment of cancers, whereas activation of Wnt signaling will be useful in tissue engineering and regenerative medicine. However, as highlighted in this review, many key questions remain unanswered and await further probing. For example, with the large number of Wnt ligands, Fzd receptors and additional Wnt binding proteins, it remains unclear how signaling specificity is established. Recent studies hint at a much greater complexity in how specific Wnt signals are received and interpreted than previously appreciated. Critical to advancing, our understanding of this complex signaling module will be the development of new tools and assays, such as strategies to activate specific arms of the pathway and trigger desired biological outcomes.
Footnotes
CONFLICT OF INTEREST
The authors have declared no conflicts of interest for this article.
FURTHER READING
Loh, K. M., van Amerongen, R., & Nusse, R. (2016a). Generating cellular diversity and spatial form: Wnt signaling and the evolution of multicellular animals. Developmental Cell, 38(6), 643–655.
References
- Ahumada A, Slusarski DC, Liu X, Moon RT, Malbon CC, Wang HY. Signaling of rat Frizzled-2 through phosphodiesterase and cyclic GMP. Science. 2002;298:2006–2010. doi: 10.1126/science.1073776. [DOI] [PubMed] [Google Scholar]
- Alexander CM, Reichsman F, Hinkes MT, Lincecum J, Becker KA, Cumberledge S, Bernfield M. Syndecan-1 is required for Wnt-1-induced mammary tumorigenesis in mice. Nature Genetics. 2000;25:329–332. doi: 10.1038/77108. [DOI] [PubMed] [Google Scholar]
- Alexandre C, Baena-Lopez A, Vincent JP. Patterning and growth control by membrane-tethered wingless. Nature. 2014;505:180–185. doi: 10.1038/nature12879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amit S, Hatzubai A, Birman Y, Andersen JS, Ben-Shushan E, Mann M, … Alkalay I. Axin-mediated CKI phosphorylation of beta-catenin at Ser 45: A molecular switch for the Wnt pathway. Genes & Development. 2002;16:1066–1076. doi: 10.1101/gad.230302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aznar N, Midde KK, Dunkel Y, Lopez-Sanchez I, Pavlova Y, Marivin A, … Ghosh P. Daple is a novel non-receptor GEF required for trimeric G protein activation in Wnt signaling. eLife. 2015;4:e07091. doi: 10.7554/eLife.07091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badis G, Berger MF, Philippakis AA, Talukder S, Gehrke AR, Jaeger SA, … Bulyk ML. Diversity and complexity in DNA recognition by transcription factors. Science. 2009;324:1720–1723. doi: 10.1126/science.1162327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bafico A, Liu G, Yaniv A, Gazit A, Aaronson SA. Novel mechanism of Wnt signalling inhibition mediated by Dickkopf-1 interaction with LRP6/arrow. Nature Cell Biology. 2001;3:683–686. doi: 10.1038/35083081. [DOI] [PubMed] [Google Scholar]
- Baker NE. Molecular cloning of sequences from wingless, a segment polarity gene in Drosophila: The spatial distribution of a transcript in embryos. The EMBO Journal. 1987;6:1765–1773. doi: 10.1002/j.1460-2075.1987.tb02429.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banziger C, Soldini D, Schutt C, Zipperlen P, Hausmann G, Basler K. Wntless, a conserved membrane protein dedicated to the secretion of Wnt proteins from signaling cells. Cell. 2006;125:509–522. doi: 10.1016/j.cell.2006.02.049. [DOI] [PubMed] [Google Scholar]
- Barker N, Clevers H. Leucine-rich repeat-containing G-protein-coupled receptors as markers of adult stem cells. Gastroenterology. 2010;138:1681–1696. doi: 10.1053/j.gastro.2010.03.002. [DOI] [PubMed] [Google Scholar]
- Barker N, Huch M, Kujala P, van de Wetering M, Snippert HJ, van Es JH, … Clevers H. Lgr5(+ve) stem cells drive self-renewal in the stomach and build long-lived gastric units in vitro. Cell Stem Cell. 2010;6:25–36. doi: 10.1016/j.stem.2009.11.013. [DOI] [PubMed] [Google Scholar]
- Barker N, Hurlstone A, Musisi H, Miles A, Bienz M, Clevers H. The chromatin remodelling factor Brg-1 interacts with beta-catenin to promote target gene activation. The EMBO Journal. 2001;20:4935–4943. doi: 10.1093/emboj/20.17.4935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker N, Ridgway RA, van Es JH, van de Wetering M, Begthel H, van den Born M, … Clevers H. Crypt stem cells as the cells-of-origin of intestinal cancer. Nature. 2009;457:608–611. doi: 10.1038/nature07602. [DOI] [PubMed] [Google Scholar]
- Barker N, van Es JH, Jaks V, Kasper M, Snippert H, Toftgard R, Clevers H. Very long-term self-renewal of small intestine, colon, and hair follicles from cycling Lgr5+ve stem cells. Cold Spring Harbor Symposia on Quantitative Biology. 2008;73:351–356. doi: 10.1101/sqb.2008.72.003. [DOI] [PubMed] [Google Scholar]
- Barker N, van Es JH, Kuipers J, Kujala P, van den Born M, Cozijnsen M, … Clevers H. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature. 2007;449:1003–1007. doi: 10.1038/nature06196. [DOI] [PubMed] [Google Scholar]
- Barrow JR, Howell WD, Rule M, Hayashi S, Thomas KR, Capecchi MR, McMahon AP. Wnt3 signaling in the epiblast is required for proper orientation of the anteroposterior axis. Developmental Biology. 2007;312:312–320. doi: 10.1016/j.ydbio.2007.09.030. [DOI] [PubMed] [Google Scholar]
- Bartscherer K, Pelte N, Ingelfinger D, Boutros M. Secretion of Wnt ligands requires Evi, a conserved transmembrane protein. Cell. 2006;125:523–533. doi: 10.1016/j.cell.2006.04.009. [DOI] [PubMed] [Google Scholar]
- Bauer A, Chauvet S, Huber O, Usseglio F, Rothbacher U, Aragnol D, … Pradel J. Pontin52 and reptin52 function as antagonistic regulators of beta-catenin signalling activity. The EMBO Journal. 2000;19:6121–6130. doi: 10.1093/emboj/19.22.6121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer M, Benard J, Gaasterland T, Willert K, Cappellen D. WNT5A encodes two isoforms with distinct functions in cancers. PLoS One. 2013;8:e80526. doi: 10.1371/journal.pone.0080526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bazan JF, Janda CY, Garcia KC. Structural architecture and functional evolution of Wnts. Developmental Cell. 2012;23:227–232. doi: 10.1016/j.devcel.2012.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckett K, Monier S, Palmer L, Alexandre C, Green H, Bonneil E, … Vincent JP. Drosophila S2 cells secrete wingless on exosome-like vesicles but the wingless gradient forms independently of exosomes. Traffic. 2013;14:82–96. doi: 10.1111/tra.12016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behrens J, von Kries JP, Kuhl M, Bruhn L, Wedlich D, Grosschedl R, Birchmeier W. Functional interaction of beta-catenin with the transcription factor LEF-1. Nature. 1996;382:638–642. doi: 10.1038/382638a0. [DOI] [PubMed] [Google Scholar]
- Belenkaya TY, Han C, Standley HJ, Lin X, Houston DW, Heasman J. Pygopus encodes a nuclear protein essential for wingless/Wnt signaling. Development. 2002;129:4089–4101. doi: 10.1242/dev.129.17.4089. [DOI] [PubMed] [Google Scholar]
- Belenkaya TY, Wu Y, Tang X, Zhou B, Cheng L, Sharma YV, … Lin X. The retromer complex influences Wnt secretion by recycling wntless from endosomes to the trans-Golgi network. Developmental Cell. 2008;14:120–131. doi: 10.1016/j.devcel.2007.12.003. [DOI] [PubMed] [Google Scholar]
- Belo JA, Bachiller D, Agius E, Kemp C, Borges AC, Marques S, … De Robertis EM. Cerberus-like is a secreted BMP and nodal antagonist not essential for mouse development. Genesis. 2000;26:265–270. [PubMed] [Google Scholar]
- Berendsen AD, Fisher LW, Kilts TM, Owens RT, Robey PG, Gutkind JS, Young MF. Modulation of canonical Wnt signaling by the extracellular matrix component biglycan. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:17022–17027. doi: 10.1073/pnas.1110629108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger H, Breuer M, Peradziryi H, Podleschny M, Jacob R, Borchers A. PTK7 localization and protein stability is affected by canonical Wnt ligands. Journal of Cell Science. 2017;130:1890–1903. doi: 10.1242/jcs.198580. [DOI] [PubMed] [Google Scholar]
- Bhanot P, Brink M, Samos CH, Hsieh JC, Wang Y, Macke JP, … Nusse R. A new member of the frizzled family from Drosophila functions as a wingless receptor. Nature. 1996;382:225–230. doi: 10.1038/382225a0. [DOI] [PubMed] [Google Scholar]
- Bhanot P, Fish M, Jemison JA, Nusse R, Nathans J, Cadigan KM. Frizzled and Dfrizzled-2 function as redundant receptors for wingless during Drosophila embryonic development. Development. 1999;126:4175–4186. doi: 10.1242/dev.126.18.4175. [DOI] [PubMed] [Google Scholar]
- Bhat KM. Frizzled and frizzled 2 play a partially redundant role in wingless signaling and have similar requirements to wingless in neurogenesis. Cell. 1998;95:1027–1036. doi: 10.1016/s0092-8674(00)81726-2. [DOI] [PubMed] [Google Scholar]
- Biechele S, Cox BJ, Rossant J. Porcupine homolog is required for canonical Wnt signaling and gastrulation in mouse embryos. Developmental Biology. 2011;355:275–285. doi: 10.1016/j.ydbio.2011.04.029. [DOI] [PubMed] [Google Scholar]
- Bilic J, Huang YL, Davidson G, Zimmermann T, Cruciat CM, Bienz M, Niehrs C. Wnt induces LRP6 signalosomes and promotes dishevelled-dependent LRP6 phosphorylation. Science. 2007;316:1619–1622. doi: 10.1126/science.1137065. [DOI] [PubMed] [Google Scholar]
- Binari RC, Staveley BE, Johnson WA, Godavarti R, Sasisekharan R, Manoukian AS. Genetic evidence that heparin-like glycosaminoglycans are involved in wingless signaling. Development. 1997;124:2623–2632. doi: 10.1242/dev.124.13.2623. [DOI] [PubMed] [Google Scholar]
- Bornemann DJ, Duncan JE, Staatz W, Selleck S, Warrior R. Abrogation of heparan sulfate synthesis in Drosophila disrupts the wingless, hedgehog and decapentaplegic signaling pathways. Development. 2004;131:1927–1938. doi: 10.1242/dev.01061. [DOI] [PubMed] [Google Scholar]
- Bourhis E, Tam C, Franke Y, Bazan JF, Ernst J, Hwang J, … Hannoush RN. Reconstitution of a frizzled8.Wnt3a.LRP6 signaling complex reveals multiple Wnt and Dkk1 binding sites on LRP6. The Journal of Biological Chemistry. 2010;285:9172–9179. doi: 10.1074/jbc.M109.092130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley RS, Brown AM. The proto-oncogene int-1 encodes a secreted protein associated with the extracellular matrix. The EMBO Journal. 1990;9:1569–1575. doi: 10.1002/j.1460-2075.1990.tb08276.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley RS, Brown AM. A soluble form of Wnt-1 protein with mitogenic activity on mammary epithelial cells. Molecular and Cellular Biology. 1995;15:4616–4622. doi: 10.1128/mcb.15.8.4616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brauburger K, Akyildiz S, Ruppert JG, Graeb M, Bernkopf DB, Hadjihannas MV, Behrens J. Adenomatous polyposis coli (APC) membrane recruitment 3, a member of the APC membrane recruitment family of APC-binding proteins, is a positive regulator of Wnt-beta-catenin signalling. The FEBS Journal. 2014;281:787–801. doi: 10.1111/febs.12624. [DOI] [PubMed] [Google Scholar]
- Brembeck FH, Schwarz-Romond T, Bakkers J, Wilhelm S, Hammerschmidt M, Birchmeier W. Essential role of BCL9-2 in the switch between beta-catenin’s adhesive and transcriptional functions. Genes & Development. 2004;18:2225–2230. doi: 10.1101/gad.317604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown AM, Papkoff J, Fung YK, Shackleford GM, Varmus HE. Identification of protein products encoded by the proto-oncogene int-1. Molecular and Cellular Biology. 1987;7:3971–3977. doi: 10.1128/mcb.7.11.3971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunner E, Peter O, Schweizer L, Basler K. Pangolin encodes a Lef-1 homologue that acts downstream of armadillo to transduce the wingless signal in drosophila. Nature. 1997;385:829–833. doi: 10.1038/385829a0. [DOI] [PubMed] [Google Scholar]
- Bunaciu RP, Tang T, Mao CD. Differential expression of Wnt13 isoforms during leukemic cell differentiation. Oncology Reports. 2008;20:195–201. [PubMed] [Google Scholar]
- Burrus LW, McMahon AP. Biochemical analysis of murine Wnt proteins reveals both shared and distinct properties. Experimental Cell Research. 1995;220:363–373. doi: 10.1006/excr.1995.1327. [DOI] [PubMed] [Google Scholar]
- Callow MG, Tran H, Phu L, Lau T, Lee J, Sandoval WN, … Costa M. Ubiquitin ligase RNF146 regulates tankyrase and axin to promote Wnt signaling. PLoS One. 2011;6:e22595. doi: 10.1371/journal.pone.0022595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capurro M, Martin T, Shi W, Filmus J. Glypican-3 binds to frizzled and plays a direct role in the stimulation of canonical Wnt signaling. Journal of Cell Science. 2014;127:1565–1575. doi: 10.1242/jcs.140871. [DOI] [PubMed] [Google Scholar]
- Cavallo RA, Cox RT, Moline MM, Roose J, Polevoy GA, Clevers H, … Bejsovec A. Groucho interact to repress wingless signalling activity. Nature. 1998;395:604–608. doi: 10.1038/26982. [DOI] [PubMed] [Google Scholar]
- Chamorro MN, Schwartz DR, Vonica A, Brivanlou AH, Cho KR, Varmus HE. FGF-20 and DKK1 are transcriptional targets of beta-catenin and FGF-20 is implicated in cancer and development. The EMBO Journal. 2005;24:73–84. doi: 10.1038/sj.emboj.7600460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen B, Dodge ME, Tang W, Lu J, Ma Z, Fan CW, … Lum L. Small molecule-mediated disruption of Wnt-dependent signaling in tissue regeneration and cancer. Nature Chemical Biology. 2009;5:100–107. doi: 10.1038/nchembio.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Q, Takada R, Noda C, Kobayashi S, Takada S. Different populations of Wnt-containing vesicles are individually released from polarized epithelial cells. Scientific Reports. 2016;6:35562. doi: 10.1038/srep35562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Wang R, Liu X, Wu Y, Zhou T, Yang Y, … Ying QL. A chemical-genetic approach reveals the distinct roles of GSK3alpha and GSK3beta in regulating embryonic stem cell fate. Developmental Cell. 2017;43:563–576. e564. doi: 10.1016/j.devcel.2017.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Z, Biechele T, Wei Z, Morrone S, Moon RT, Wang L, Xu W. Crystal structures of the extracellular domain of LRP6 and its complex with DKK1. Nature Structural & Molecular Biology. 2011;18:1204–1210. doi: 10.1038/nsmb.2139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chinnadurai G. CtBP, an unconventional transcriptional corepressor in development and oncogenesis. Molecular Cell. 2002;9:213–224. doi: 10.1016/s1097-2765(02)00443-4. [DOI] [PubMed] [Google Scholar]
- Cho C, Smallwood PM, Nathans J. Reck and Gpr124 are essential receptor cofactors for Wnt7a/Wnt7b-specific signaling in mammalian CNS angiogen-esis and blood-brain barrier regulation. Neuron. 2017;95:1221–1225. doi: 10.1016/j.neuron.2017.08.032. [DOI] [PubMed] [Google Scholar]
- Choi J, Park SY, Costantini F, Jho EH, Joo CK. Adenomatous polyposis coli is down-regulated by the ubiquitin-proteasome pathway in a process facilitated by axin. The Journal of Biological Chemistry. 2004;279:49188–49198. doi: 10.1074/jbc.M404655200. [DOI] [PubMed] [Google Scholar]
- Clevers H, Nusse R. Wnt/beta-catenin signaling and disease. Cell. 2012;149:1192–1205. doi: 10.1016/j.cell.2012.05.012. [DOI] [PubMed] [Google Scholar]
- Cliffe A, Hamada F, Bienz M. A role of dishevelled in relocating axin to the plasma membrane during wingless signaling. Current Biology. 2003;13:960–966. doi: 10.1016/s0960-9822(03)00370-1. [DOI] [PubMed] [Google Scholar]
- Cong F, Schweizer L, Varmus H. Casein kinase Iepsilon modulates the signaling specificities of dishevelled. Molecular and Cellular Biology. 2004;24:2000–2011. doi: 10.1128/MCB.24.5.2000-2011.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coombs GS, Yu J, Canning CA, Veltri CA, Covey TM, Cheong JK, … Virshup DM. WLS-dependent secretion of WNT3A requires Ser209 acylation and vacuolar acidification. Journal of Cell Science. 2010;123:3357–3367. doi: 10.1242/jcs.072132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coudreuse DY, Roel G, Betist MC, Destree O, Korswagen HC. Wnt gradient formation requires retromer function in Wnt-producing cells. Science. 2006;312:921–924. doi: 10.1126/science.1124856. [DOI] [PubMed] [Google Scholar]
- Covey TM, Kaur S, Tan Ong T, Proffitt KD, Wu Y, Tan P, Virshup DM. PORCN moonlights in a Wnt-independent pathway that regulates cancer cell proliferation. PLoS One. 2012;7:e34532. doi: 10.1371/journal.pone.0034532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cselenyi CS, Jernigan KK, Tahinci E, Thorne CA, Lee LA, Lee E. LRP6 transduces a canonical Wnt signal independently of axin degradation by inhibiting GSK3’s phosphorylation of beta-catenin. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:8032–8037. doi: 10.1073/pnas.0803025105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dann CE, Hsieh JC, Rattner A, Sharma D, Nathans J, Leahy DJ. Insights into Wnt binding and signalling from the structures of two frizzled cysteine-rich domains. Nature. 2001;412:86–90. doi: 10.1038/35083601. [DOI] [PubMed] [Google Scholar]
- Davidson G, Mao B, del Barco Barrantes I, Niehrs C. Kremen proteins interact with Dickkopf1 to regulate anteroposterior CNS patterning. Development. 2002;129:5587–5596. doi: 10.1242/dev.00154. [DOI] [PubMed] [Google Scholar]
- Davidson G, Wu W, Shen J, Bilic J, Fenger U, Stannek P, … Niehrs C. Casein kinase 1 gamma couples Wnt receptor activation to cytoplasmic signal transduction. Nature. 2005;438:867–872. doi: 10.1038/nature04170. [DOI] [PubMed] [Google Scholar]
- de Visser KE, Ciampricotti M, Michalak EM, Tan DW, Speksnijder EN, Hau CS, … Jonkers J. Developmental stage-specific contribution of LGR5(+) cells to basal and luminal epithelial lineages in the postnatal mammary gland. The Journal of Pathology. 2012;228:300–309. doi: 10.1002/path.4096. [DOI] [PubMed] [Google Scholar]
- Dichmann DS, Walentek P, Harland RM. The alternative splicing regulator Tra2b is required for somitogenesis and regulates splicing of an inhibitory Wnt11b isoform. Cell Reports. 2015;10:527–536. doi: 10.1016/j.celrep.2014.12.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dijksterhuis JP, Baljinnyam B, Stanger K, Sercan HO, Ji Y, Andres O, … Schulte G. Systematic mapping of WNT-FZD protein interactions reveals functional selectivity by distinct WNT-FZD pairs. The Journal of Biological Chemistry. 2015;290:6789–6798. doi: 10.1074/jbc.M114.612648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiNardo S, Sher E, Heemskerk-Jongens J, Kassis JA, O’Farrell PH. Two-tiered regulation of spatially patterned engrailed gene expression during Drosophila embryogenesis. Nature. 1988;332:604–609. doi: 10.1038/332604a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doble BW, Patel S, Wood GA, Kockeritz LK, Woodgett JR. Functional redundancy of GSK-3alpha and GSK-3beta in Wnt/beta-catenin signaling shown by using an allelic series of embryonic stem cell lines. Developmental Cell. 2007;12:957–971. doi: 10.1016/j.devcel.2007.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodge ME, Moon J, Tuladhar R, Lu J, Jacob LS, Zhang LS, … Lum L. Diverse chemical scaffolds support direct inhibition of the membrane-bound O-acyltransferase porcupine. The Journal of Biological Chemistry. 2012;287:23246–23254. doi: 10.1074/jbc.M112.372029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorsky RI, Itoh M, Moon RT, Chitnis A. Two tcf3 genes cooperate to pattern the zebrafish brain. Development. 2003;130:1937–1947. doi: 10.1242/dev.00402. [DOI] [PubMed] [Google Scholar]
- Eguchi M, Nguyen C, Lee SC, Kahn M. ICG-001, a novel small molecule regulator of TCF/beta-catenin transcription. Medicinal Chemistry. 2005;1:467–472. doi: 10.2174/1573406054864098. [DOI] [PubMed] [Google Scholar]
- Etheridge SL, Ray S, Li S, Hamblet NS, Lijam N, Tsang M, … Wynshaw-Boris A. Murine dishevelled 3 functions in redundant pathways with dishevelled 1 and 2 in normal cardiac outflow tract, cochlea, and neural tube development. PLoS Genetics. 2008;4:e1000259. doi: 10.1371/journal.pgen.1000259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagotto F, Funayama N, Gluck U, Gumbiner BM. Binding to cadherins antagonizes the signaling activity of beta-catenin during axis formation in Xenopus. The Journal of Cell Biology. 1996;132:1105–1114. doi: 10.1083/jcb.132.6.1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagotto F, Gluck U, Gumbiner BM. Nuclear localization signal-independent and importin/karyopherin-independent nuclear import of beta-catenin. Current Biology. 1998;8:181–190. doi: 10.1016/s0960-9822(98)70082-x. [DOI] [PubMed] [Google Scholar]
- Faux MC, Coates JL, Catimel B, Cody S, Clayton AH, Layton MJ, Burgess AW. Recruitment of adenomatous polyposis coli and beta-catenin to axin-puncta. Oncogene. 2008;27:5808–5820. doi: 10.1038/onc.2008.205. [DOI] [PubMed] [Google Scholar]
- Fear MW, Kelsell DP, Spurr NK, Barnes MR. Wnt-16a, a novel Wnt-16 isoform, which shows differential expression in adult human tissues. Biochemical and Biophysical Research Communications. 2000;278:814–820. doi: 10.1006/bbrc.2000.3852. [DOI] [PubMed] [Google Scholar]
- Fei C, He X, Xie S, Miao H, Zhou Z, Li L. Smurf1-mediated axin ubiquitination requires Smurf1 C2 domain and is cell cycle-dependent. The Journal of Biological Chemistry. 2014;289:14170–14177. doi: 10.1074/jbc.M113.536714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez A, Huggins IJ, Perna L, Brafman D, Lu D, Yao S, … Willert K. The WNT receptor FZD7 is required for maintenance of the pluripotent state in human embryonic stem cells. Proceedings of the National Academy of Sciences. 2014;111:1409–1414. doi: 10.1073/pnas.1323697111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiedler M, Mendoza-Topaz C, Rutherford TJ, Mieszczanek J, Bienz M. Dishevelled interacts with the DIX domain polymerization interface of axin to interfere with its function in down-regulating beta-catenin. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:1937–1942. doi: 10.1073/pnas.1017063108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finch PW, He X, Kelley MJ, Uren A, Schaudies RP, Popescu NC, … Rubin JS. Purification and molecular cloning of a secreted, frizzled-related antagonist of Wnt action. Proceedings of the National Academy of Sciences of the United States of America. 1997;94:6770–6775. doi: 10.1073/pnas.94.13.6770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flowers GP, Topczewska JM, Topczewski J. A zebrafish notum homolog specifically blocks the Wnt/beta-catenin signaling pathway. Development. 2012;139:2416–2425. doi: 10.1242/dev.063206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu J, Jiang M, Mirando AJ, Yu HM, Hsu W. Reciprocal regulation of Wnt and Gpr177/mouse Wntless is required for embryonic axis formation. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:18598–18603. doi: 10.1073/pnas.0904894106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuda T, Chen L, Endo T, Tang L, Lu D, Castro JE, … Kipps TJ. Antisera induced by infusions of autologous Ad-CD154-leukemia B cells identify ROR1 as an oncofetal antigen and receptor for Wnt5a. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:3047–3052. doi: 10.1073/pnas.0712148105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galli LM, Barnes T, Cheng T, Acosta L, Anglade A, Willert K, … Burrus LW. Differential inhibition of Wnt-3a by Sfrp-1, Sfrp-2, and Sfrp-3. Developmental Dynamics. 2006;235:681–690. doi: 10.1002/dvdy.20681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao ZH, Seeling JM, Hill V, Yochum A, Virshup DM. Casein kinase I phosphorylates and destabilizes the beta-catenin degradation complex. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:1182–1187. doi: 10.1073/pnas.032468199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavin BJ, McMahon JA, McMahon AP. Expression of multiple novel Wnt-1/int-1-related genes during fetal and adult mouse development. Genes & Development. 1990;4:2319–2332. doi: 10.1101/gad.4.12b.2319. [DOI] [PubMed] [Google Scholar]
- Gerlitz O, Basler K. Wingful, an extracellular feedback inhibitor of wingless. Genes & Development. 2002;16:1055–1059. doi: 10.1101/gad.991802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giraldez AJ, Copley RR, Cohen SM. HSPG modification by the secreted enzyme notum shapes the wingless morphogen gradient. Developmental Cell. 2002;2:667–676. doi: 10.1016/s1534-5807(02)00180-6. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Sancho JM, Aguilera O, Garcia JM, Pendas-Franco N, Pena C, Cal S, … Munoz A. The Wnt antagonist DICKKOPF-1 gene is a downstream target of beta-catenin/TCF and is downregulated in human colon cancer. Oncogene. 2005;24:1098–1103. doi: 10.1038/sj.onc.1208303. [DOI] [PubMed] [Google Scholar]
- Goodman RM, Thombre S, Firtina Z, Gray D, Betts D, Roebuck J, … Selva EM. Sprinter: A novel transmembrane protein required for Wg secretion and signaling. Development. 2006;133:4901–4911. doi: 10.1242/dev.02674. [DOI] [PubMed] [Google Scholar]
- Gorrepati L, Krause MW, Chen W, Brodigan TM, Correa-Mendez M, Eisenmann DM. Identification of Wnt pathway target genes regulating the division and differentiation of larval seam cells and Vulval precursor cells in Caenorhabditis elegans. G3. 2015;5:1551–1566. doi: 10.1534/g3.115.017715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottardi CJ, Gumbiner BM. Distinct molecular forms of beta-catenin are targeted to adhesive or transcriptional complexes. The Journal of Cell Biology. 2004;167:339–349. doi: 10.1083/jcb.200402153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottardi CJ, Wong E, Gumbiner BM. E-cadherin suppresses cellular transformation by inhibiting beta-catenin signaling in an adhesion-independent manner. The Journal of Cell Biology. 2001;153:1049–1060. doi: 10.1083/jcb.153.5.1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grainger S, Richter J, Palazon RE, Pouget C, Lonquich B, Wirth S, … Willert K. Wnt9a is required for the aortic amplification of nascent hemato-poietic stem cells. Cell Reports. 2016;17:1595–1606. doi: 10.1016/j.celrep.2016.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greco V, Hannus M, Argosomes ES. A potential vehicle for the spread of morphogens through epithelia. Cell. 2001;106:633–645. doi: 10.1016/s0092-8674(01)00484-6. [DOI] [PubMed] [Google Scholar]
- Gross JC, Chaudhary V, Bartscherer K, Boutros M. Active Wnt proteins are secreted on exosomes. Nature Cell Biology. 2012;14:1036–1045. doi: 10.1038/ncb2574. [DOI] [PubMed] [Google Scholar]
- Habib SJ, Chen BC, Tsai FC, Anastassiadis K, Meyer T, Betzig E, Nusse R. A localized Wnt signal orients asymmetric stem cell division in vitro. Science. 2013;339:1445–1448. doi: 10.1126/science.1231077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hacker U, Lin X, Perrimon N. The Drosophila sugarless gene modulates wingless signaling and encodes an enzyme involved in polysaccharide biosynthesis. Development. 1997;124:3565–3573. doi: 10.1242/dev.124.18.3565. [DOI] [PubMed] [Google Scholar]
- Haerry TE, Heslip TR, Marsh JL, O’Connor MB. Defects in glucuronate biosynthesis disrupt wingless signaling in Drosophila. Development. 1997;124:3055–3064. doi: 10.1242/dev.124.16.3055. [DOI] [PubMed] [Google Scholar]
- Hammerlein A, Weiske J, Huber OA. Second protein kinase CK1-mediated step negatively regulates Wnt signalling by disrupting the lymphocyte enhancer factor-1/beta-catenin complex. Cellular and Molecular Life Sciences. 2005;62:606–618. doi: 10.1007/s00018-005-4507-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han C, Belenkaya TY, Khodoun M, Tauchi M, Lin X, Lin X. Distinct and collaborative roles of Drosophila EXT family proteins in morphogen signalling and gradient formation. Development. 2004;131:1563–1575. doi: 10.1242/dev.01051. [DOI] [PubMed] [Google Scholar]
- Hao HX, Xie Y, Zhang Y, Charlat O, Oster E, Avello M, … Cong F. ZNRF3 promotes Wnt receptor turnover in an R-spondin-sensitive manner. Nature. 2012;485:195–200. doi: 10.1038/nature11019. [DOI] [PubMed] [Google Scholar]
- Harada T, Yamamoto H, Kishida S, Kishida M, Awada C, Takao T, Kikuchi A. Wnt5b-associated exosomes promote cancer cell migration and proliferation. Cancer Science. 2017;108:42–52. doi: 10.1111/cas.13109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harterink M, Port F, Lorenowicz MJ, McGough IJ, Silhankova M, Betist MC, … Korswagen HC. A SNX3-dependent retromer pathway mediates retrograde transport of the Wnt sorting receptor Wntless and is required for Wnt secretion. Nature Cell Biology. 2011;13:914–923. doi: 10.1038/ncb2281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes M, Naito M, Daulat A, Angers S, Ciruna B. Ptk7 promotes non-canonical Wnt/PCP-mediated morphogenesis and inhibits Wnt/-beta-catenin-dependent cell fate decisions during vertebrate development. Development. 2013;140:1807–1818. doi: 10.1242/dev.090183. [DOI] [PubMed] [Google Scholar]
- Heasman J, Crawford A, Goldstone K, Garner-Hamrick P, Gumbiner B, McCrea P, … Wylie C. Overexpression of cadherins and underexpression of beta-catenin inhibit dorsal mesoderm induction in early Xenopus embryos. Cell. 1994;79:791–803. doi: 10.1016/0092-8674(94)90069-8. [DOI] [PubMed] [Google Scholar]
- Henderson WR, Jr, Chi EY, Ye X, Nguyen C, Tien YT, Zhou B, … Kahn M. Inhibition of Wnt/beta-catenin/CREB binding protein (CBP) signaling reverses pulmonary fibrosis. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:14309–14314. doi: 10.1073/pnas.1001520107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herr P, Basler K. Porcupine-mediated lipidation is required for Wnt recognition by Wls. Developmental Biology. 2012;361:392–402. doi: 10.1016/j.ydbio.2011.11.003. [DOI] [PubMed] [Google Scholar]
- Herzig M, Savarese F, Novatchkova M, Semb H, Christofori G. Tumor progression induced by the loss of E-cadherin independent of beta-catenin/Tcf-mediated Wnt signaling. Oncogene. 2007;26:2290–2298. doi: 10.1038/sj.onc.1210029. [DOI] [PubMed] [Google Scholar]
- Hikasa H, Ezan J, Itoh K, Li X, Klymkowsky MW, Sokol SY. Regulation of TCF3 by Wnt-dependent phosphorylation during vertebrate axis specification. Developmental Cell. 2010;19:521–532. doi: 10.1016/j.devcel.2010.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hikasa H, Sokol SY. Phosphorylation of TCF proteins by homeodomain-interacting protein kinase 2. The Journal of Biological Chemistry. 2011;286:12093–12100. doi: 10.1074/jbc.M110.185280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch D, Barker N, McNeil N, Hu Y, Camps J, McKinnon K, … Gaiser T. LGR5 positivity defines stem-like cells in colorectal cancer. Carcinogenesis. 2014;35:849–858. doi: 10.1093/carcin/bgt377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho HY, Susman MW, Bikoff JB, Ryu YK, Jonas AM, Hu L, … Greenberg ME. Wnt5a-Ror-dishevelled signaling constitutes a core developmental pathway that controls tissue morphogenesis. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:4044–4051. doi: 10.1073/pnas.1200421109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmans R, Basler K. BCL9-2 binds arm/beta-catenin in a Tyr142-independent manner and requires pygopus for its function in Wg/Wnt signaling. Mechanisms of Development. 2007;124:59–67. doi: 10.1016/j.mod.2006.09.006. [DOI] [PubMed] [Google Scholar]
- Hsieh JC, Kodjabachian L, Rebbert ML, Rattner A, Smallwood PM, Samos CH, … Nathans J. A new secreted protein that binds to Wnt proteins and inhibits their activities. Nature. 1999;398:431–436. doi: 10.1038/18899. [DOI] [PubMed] [Google Scholar]
- Hsiung F, Ramirez-Weber FA, Iwaki DD, Kornberg TB. Dependence of Drosophila wing imaginal disc cytonemes on decapentaplegic. Nature. 2005;437:560–563. doi: 10.1038/nature03951. [DOI] [PubMed] [Google Scholar]
- Huang H, Kornberg TB. Myoblast cytonemes mediate Wg signaling from the wing imaginal disc and Delta-Notch signaling to the air sac primordium. eLife. 2015;4:e06114. doi: 10.7554/eLife.06114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang SM, Mishina YM, Liu S, Cheung A, Stegmeier F, Michaud GA, … Cong F. Tankyrase inhibition stabilizes axin and antagonizes Wnt signalling. Nature. 2009;461:614–620. doi: 10.1038/nature08356. [DOI] [PubMed] [Google Scholar]
- Huber O, Korn R, McLaughlin J, Ohsugi M, Herrmann BG, Kemler R. Nuclear localization of beta-catenin by interaction with transcription factor LEF-1. Mechanisms of Development. 1996;59:3–10. doi: 10.1016/0925-4773(96)00597-7. [DOI] [PubMed] [Google Scholar]
- Huggins IJ, Bos T, Gaylord O, Jessen C, Lonquich B, Puranen A, … Willert K. The WNT target SP5 negatively regulates WNT transcriptional programs in human pluripotent stem cells. Nature Communications. 2017;8:1034. doi: 10.1038/s41467-017-01203-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda S, Kishida S, Yamamoto H, Murai H, Koyama S, Kikuchi A. Axin, a negative regulator of the Wnt signaling pathway, forms a complex with GSK-3beta and beta-catenin and promotes GSK-3beta-dependent phosphorylation of beta-catenin. The EMBO Journal. 1998;17:1371–1384. doi: 10.1093/emboj/17.5.1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue T, Oz HS, Wiland D, Gharib S, Deshpande R, Hill RJ, … Sternberg PW. C. elegans LIN-18 is a Ryk ortholog and functions in parallel to LIN-17/frizzled in Wnt signaling. Cell. 2004;118:795–806. doi: 10.1016/j.cell.2004.09.001. [DOI] [PubMed] [Google Scholar]
- Ishitani T, Ninomiya-Tsuji J, Nagai S, Nishita M, Meneghini M, Barker N, … Matsumoto K. The TAK1-NLK-MAPK-related pathway antagonizes signalling between beta-catenin and transcription factor TCF. Nature. 1999;399:798–802. doi: 10.1038/21674. [DOI] [PubMed] [Google Scholar]
- Itoh K, Krupnik VE, Sokol SY. Axis determination in Xenopus involves biochemical interactions of axin, glycogen synthase kinase 3 and beta-catenin. Current Biology. 1998;8:591–594. doi: 10.1016/s0960-9822(98)70229-5. [DOI] [PubMed] [Google Scholar]
- Jackson BM, Abete-Luzi P, Krause MW, Eisenmann DM. Use of an activated beta-catenin to identify Wnt pathway target genes in caenorhabditis elegans, including a subset of collagen genes expressed in late larval development. G3. 2014;4:733–747. doi: 10.1534/g3.113.009522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaks V, Barker N, Kasper M, van Es JH, Snippert HJ, Clevers H, Toftgard R. Lgr5 marks cycling, yet long-lived, hair follicle stem cells. Nature Genetics. 2008;40:1291–1299. doi: 10.1038/ng.239. [DOI] [PubMed] [Google Scholar]
- Janda CY, Dang LT, You C, Chang J, de Lau W, Zhong ZA, … Garcia KC. Surrogate Wnt agonists that phenocopy canonical Wnt and beta-catenin signalling. Nature. 2017;545:234–237. doi: 10.1038/nature22306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janda CY, Waghray D, Levin AM, Thomas C, Garcia KC. Structural basis of Wnt recognition by frizzled. Science. 2012;337:59–64. doi: 10.1126/science.1222879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jho EH, Zhang T, Domon C, Joo CK, Freund JN, Costantini F. Wnt/beta-catenin/Tcf signaling induces the transcription of Axin2, a negative regulator of the signaling pathway. Molecular and Cellular Biology. 2002;22:1172–1183. doi: 10.1128/MCB.22.4.1172-1183.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang J. CK1 in developmental signaling: Hedgehog and Wnt. Current Topics in Developmental Biology. 2017;123:303–329. doi: 10.1016/bs.ctdb.2016.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang X, Charlat O, Zamponi R, Yang Y, Cong F. Dishevelled promotes Wnt receptor degradation through recruitment of ZNRF3/RNF43 E3 ubiquitin ligases. Molecular Cell. 2015;58:522–533. doi: 10.1016/j.molcel.2015.03.015. [DOI] [PubMed] [Google Scholar]
- Kakugawa S, Langton PF, Zebisch M, Howell SA, Chang TH, Liu Y, … Vincent JP. Notum deacylates Wnt proteins to suppress signalling activity. Nature. 2015;519:187–192. doi: 10.1038/nature14259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katanaev VL, Ponzielli R, Semeriva M, Tomlinson A. Trimeric G protein-dependent frizzled signaling in Drosophila. Cell. 2005;120:111–122. doi: 10.1016/j.cell.2004.11.014. [DOI] [PubMed] [Google Scholar]
- Katoh M, Kirikoshi H, Saitoh T, Sagara N, Koike J. Alternative splicing of the WNT-2B/WNT-13 gene. Biochemical and Biophysical Research Communications. 2000;275:209–216. doi: 10.1006/bbrc.2000.3252. [DOI] [PubMed] [Google Scholar]
- Kemper K, Prasetyanti PR, De Lau W, Rodermond H, Clevers H, Medema JP. Monoclonal antibodies against Lgr5 identify human colorectal cancer stem cells. Stem Cells. 2012;30:2378–2386. doi: 10.1002/stem.1233. [DOI] [PubMed] [Google Scholar]
- Khare N, Baumgartner S. Dally-like protein, a new Drosophila glypican with expression overlapping with wingless. Mechanisms of Development. 2000;99:199–202. doi: 10.1016/s0925-4773(00)00502-5. [DOI] [PubMed] [Google Scholar]
- Kilander MB, Dahlstrom J, Schulte G. Assessment of frizzled 6 membrane mobility by FRAP supports G protein coupling and reveals WNT-frizzled selectivity. Cellular Signalling. 2014;26:1943–1949. doi: 10.1016/j.cellsig.2014.05.012. [DOI] [PubMed] [Google Scholar]
- Kilander MB, Petersen J, Andressen KW, Ganji RS, Levy FO, Schuster J, … Schulte G. Disheveled regulates precoupling of heterotrimeric G proteins to frizzled 6. The FASEB Journal. 2014;28:2293–2305. doi: 10.1096/fj.13-246363. [DOI] [PubMed] [Google Scholar]
- Kim CH, Oda T, Itoh M, Jiang D, Artinger KB, Chandrasekharappa SC, … Chitnis AB. Repressor activity of headless/Tcf3 is essential for vertebrate head formation. Nature. 2000;407:913–916. doi: 10.1038/35038097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim MJ, Chia IV, Costantini F. SUMOylation target sites at the C terminus protect axin from ubiquitination and confer protein stability. FASEB Journal: Official Publication of the Federation of American Societies for Experimental Biology. 2008;22:3785–3794. doi: 10.1096/fj.08-113910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S, Jho EH. The protein stability of axin, a negative regulator of Wnt signaling, is regulated by Smad ubiquitination regulatory factor 2 (Smurf2) The Journal of Biological Chemistry. 2010;285:36420–36426. doi: 10.1074/jbc.M110.137471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SE, Huang H, Zhao M, Zhang X, Zhang A, Semonov MV, … He X. Wnt stabilization of beta-catenin reveals principles for morphogen receptor-scaffold assemblies. Science. 2013;340:867–870. doi: 10.1126/science.1232389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinzler KW, Nilbert MC, Su LK, Vogelstein B, Bryan TM, Levy DB, … Nakamura Y. Identification of FAP locus genes from chromosome 5q21. Science. 1991;253:661–665. doi: 10.1126/science.1651562. [DOI] [PubMed] [Google Scholar]
- Kishida M, Hino S, Michiue T, Yamamoto H, Kishida S, Fukui A, … Kikuchi A. Synergistic activation of the Wnt signaling pathway by Dvl and casein kinase Iepsilon. The Journal of Biological Chemistry. 2001;276:33147–33155. doi: 10.1074/jbc.M103555200. [DOI] [PubMed] [Google Scholar]
- Kishida S, Yamamoto H, Ikeda S, Kishida M, Sakamoto I, Koyama S, Kikuchi A. Axin, a negative regulator of the Wnt signaling pathway, directly interacts with adenomatous polyposis coli and regulates the stabilization of β-catenin. Journal of Biological Chemistry. 1998;273:10823–10826. doi: 10.1074/jbc.273.18.10823. [DOI] [PubMed] [Google Scholar]
- Kitagawa M, Hatakeyama S, Shirane M, Matsumoto M, Ishida N, Hattori K, … Nakayama K. An F-box protein, FWD1, mediates ubiquitin-dependent proteolysis of beta-catenin. The EMBO Journal. 1999;18:2401–2410. doi: 10.1093/emboj/18.9.2401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein PS, Melton DA. A molecular mechanism for the effect of lithium on development. Proceedings of the National Academy of Sciences of the United States of America. 1996;93:8455–8459. doi: 10.1073/pnas.93.16.8455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klingensmith J, Nusse R, Perrimon N. The Drosophila segment polarity gene dishevelled encodes a novel protein required for response to the wingless signal. Genes & Development. 1994;8:118–130. doi: 10.1101/gad.8.1.118. [DOI] [PubMed] [Google Scholar]
- Klingensmith J, Yang Y, Axelrod JD, Beier DR, Perrimon N, Sussman DJ. Conservation of dishevelled structure and function between flies and mice: Isolation and characterization of Dvl2. Mechanisms of Development. 1996;58:15–26. doi: 10.1016/s0925-4773(96)00549-7. [DOI] [PubMed] [Google Scholar]
- Kofron M, Birsoy B, Houston D, Tao Q, Wylie C, Heasman J. Wnt11/beta-catenin signaling in both oocytes and early embryos acts through LRP6-mediated regulation of axin. Development. 2007;134:503–513. doi: 10.1242/dev.02739. [DOI] [PubMed] [Google Scholar]
- Komekado H, Yamamoto H, Chiba T, Kikuchi A. Glycosylation and palmitoylation of Wnt-3a are coupled to produce an active form of Wnt-3a. Genes to Cells. 2007;12:521–534. doi: 10.1111/j.1365-2443.2007.01068.x. [DOI] [PubMed] [Google Scholar]
- Koo BK, Spit M, Jordens I, Low TY, Stange DE, van de Wetering M, … Clevers H. Tumour suppressor RNF43 is a stem-cell E3 ligase that induces endocytosis of Wnt receptors. Nature. 2012;488:665–669. doi: 10.1038/nature11308. [DOI] [PubMed] [Google Scholar]
- Koopmans T, Crutzen S, Menzen MH, Halayko AJ, Hackett TL, Knight DA, Gosens R. Selective targeting of CREB-binding protein/beta-catenin inhibits growth of and extracellular matrix remodelling by airway smooth muscle. British Journal of Pharmacology. 2016;173:3327–3341. doi: 10.1111/bph.13620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korinek V, Barker N, Morin PJ, van Wichen D, de Weger R, Kinzler KW, … Clevers H. Constitutive transcriptional activation by a beta-catenin-Tcf complex in APC−/− colon carcinoma. Science. 1997;275:1784–1787. doi: 10.1126/science.275.5307.1784. [DOI] [PubMed] [Google Scholar]
- Korkut C, Ataman B, Ramachandran P, Ashley J, Barria R, Gherbesi N, Budnik V. Trans-synaptic transmission of vesicular Wnt signals through Evi/Wntless. Cell. 2009;139:393–404. doi: 10.1016/j.cell.2009.07.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koval A, Katanaev VL. Wnt3a stimulation elicits G-protein-coupled receptor properties of mammalian frizzled proteins. The Biochemical Journal. 2011;433:435–440. doi: 10.1042/BJ20101878. [DOI] [PubMed] [Google Scholar]
- Kramps T, Peter O, Brunner E, Nellen D, Froesch B, Chatterjee S, … Basler K. Wnt/wingless signaling requires BCL9/legless-mediated recruitment of pygopus to the nuclear beta-catenin-TCF complex. Cell. 2002;109:47–60. doi: 10.1016/s0092-8674(02)00679-7. [DOI] [PubMed] [Google Scholar]
- Kuphal F, Behrens J. E-cadherin modulates Wnt-dependent transcription in colorectal cancer cells but does not alter Wnt-independent gene expression in fibroblasts. Experimental Cell Research. 2006;312:457–467. doi: 10.1016/j.yexcr.2005.11.007. [DOI] [PubMed] [Google Scholar]
- Kurayoshi M, Yamamoto H, Izumi S, Kikuchi A. Post-translational palmitoylation and glycosylation of Wnt-5a are necessary for its signalling. The Biochemical Journal. 2007;402:515–523. doi: 10.1042/BJ20061476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagna G, Carnevali F, Marchioni M, Hemmati-Brivanlou A. Negative regulation of axis formation and Wnt signaling in Xenopus embryos by the F-box/WD40 protein beta TrCP. Mechanisms of Development. 1999;80:101–106. doi: 10.1016/s0925-4773(98)00208-1. [DOI] [PubMed] [Google Scholar]
- Lai MB, Zhang C, Shi J, Johnson V, Khandan L, McVey J, … Junge HJ. TSPAN12 is a Norrin co-receptor that amplifies frizzled 4 ligand selectivity and signaling. Cell Reports. 2017;19:2809–2822. doi: 10.1016/j.celrep.2017.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larraguibel J, Weiss AR, Pasula DJ, Dhaliwal RS, Kondra R, Van Raay TJ. Wnt ligand-dependent activation of the negative feedback regulator Nkd1. Molecular Biology of the Cell. 2015;26:2375–2384. doi: 10.1091/mbc.E14-12-1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee E, Salic A, Kirschner MW. Physiological regulation of [beta]-catenin stability by Tcf3 and CK1epsilon. The Journal of Cell Biology. 2001;154:983–993. doi: 10.1083/jcb.200102074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee E, Salic A, Kruger R, Heinrich R, Kirschner MW. The roles of APC and axin derived from experimental and theoretical analysis of the Wnt pathway. PLoS Biology. 2003;1:E10. doi: 10.1371/journal.pbio.0000010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lescher B, Haenig B, Kispert A. sFRP-2 is a target of the Wnt-4 signaling pathway in the developing metanephric kidney. Developmental Dynamics. 1998;213:440–451. doi: 10.1002/(SICI)1097-0177(199812)213:4<440::AID-AJA9>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Leyns L, Bouwmeester T, Kim SH, Piccolo S, De Robertis EM. Frzb-1 is a secreted antagonist of Wnt signaling expressed in the Spemann organizer. Cell. 1997;88:747–756. doi: 10.1016/s0092-8674(00)81921-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Zhang Y, Kang H, Liu W, Liu P, Zhang J, … Wu D. Sclerostin binds to LRP5/6 and antagonizes canonical Wnt signaling. The Journal of Biological Chemistry. 2005;280:19883–19887. doi: 10.1074/jbc.M413274200. [DOI] [PubMed] [Google Scholar]
- Lin X, Perrimon N. Dally cooperates with Drosophila frizzled 2 to transduce wingless signalling. Nature. 1999;400:281–284. doi: 10.1038/22343. [DOI] [PubMed] [Google Scholar]
- Linnemannstons K, Ripp C, Honemann-Capito M, Brechtel-Curth K, Hedderich M, Wodarz A. The PTK7-related transmembrane proteins off-track and off-track 2 are co-receptors for Drosophila Wnt2 required for male fertility. PLoS Genetics. 2014;10:e1004443. doi: 10.1371/journal.pgen.1004443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C, Kato Y, Zhang Z, Do VM, Yankner BA, He X. Beta-Trcp couples beta-catenin phosphorylation-degradation and regulates Xenopus axis formation. Proceedings of the National Academy of Sciences of the United States of America. 1999;96:6273–6278. doi: 10.1073/pnas.96.11.6273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C, Li Y, Semenov M, Han C, Baeg GH, Tan Y, … He X. Control of beta-catenin phosphorylation/degradation by a dual-kinase mechanism. Cell. 2002;108:837–847. doi: 10.1016/s0092-8674(02)00685-2. [DOI] [PubMed] [Google Scholar]
- Liu F, van den Broek O, Destree O, Hoppler S. Distinct roles for Xenopus Tcf/Lef genes in mediating specific responses to Wnt/beta-catenin signalling in mesoderm development. Development. 2005;132:5375–5385. doi: 10.1242/dev.02152. [DOI] [PubMed] [Google Scholar]
- Liu P, Wakamiya M, Shea MJ, Albrecht U, Behringer RR, Bradley A. Requirement for Wnt3 in vertebrate axis formation. Nature Genetics. 1999;22:361–365. doi: 10.1038/11932. [DOI] [PubMed] [Google Scholar]
- Liu T, DeCostanzo AJ, Liu X, Wang H, Hallagan S, Moon RT, Malbon CC. G protein signaling from activated rat frizzled-1 to the beta-catenin-Lef-Tcf pathway. Science. 2001;292:1718–1722. doi: 10.1126/science.1060100. [DOI] [PubMed] [Google Scholar]
- Liu Y, Rubin B, Bodine PV, Billiard J. Wnt5a induces homodimerization and activation of Ror2 receptor tyrosine kinase. Journal of Cellular Biochemistry. 2008;105:497–502. doi: 10.1002/jcb.21848. [DOI] [PubMed] [Google Scholar]
- Lo MC, Gay F, Odom R, Shi Y, Lin R. Phosphorylation by the beta-catenin/MAPK complex promotes 14-3-3-mediated nuclear export of TCF/-POP-1 in signal-responsive cells in C. elegans. Cell. 2004;117:95–106. doi: 10.1016/s0092-8674(04)00203-x. [DOI] [PubMed] [Google Scholar]
- Loh KM, van Amerongen R, Nusse R. Generating cellular diversity and spatial form: Wnt signaling and the evolution of multicellular animals. Developmental Cell. 2016;38:643–655. doi: 10.1016/j.devcel.2016.08.011. [DOI] [PubMed] [Google Scholar]
- Lu W, Yamamoto V, Ortega B, Baltimore D. Mammalian Ryk is a Wnt coreceptor required for stimulation of neurite outgrowth. Cell. 2004;119:97–108. doi: 10.1016/j.cell.2004.09.019. [DOI] [PubMed] [Google Scholar]
- Lu X, Borchers AG, Jolicoeur C, Rayburn H, Baker JC, Tessier-Lavigne M. PTK7/CCK-4 is a novel regulator of planar cell polarity in vertebrates. Nature. 2004;430:93–98. doi: 10.1038/nature02677. [DOI] [PubMed] [Google Scholar]
- Luga V, Zhang L, Viloria-Petit AM, Ogunjimi AA, Inanlou MR, Chiu E, … Wrana JL. Exosomes mediate stromal mobilization of autocrine Wnt-PCP signaling in breast cancer cell migration. Cell. 2012;151:1542–1556. doi: 10.1016/j.cell.2012.11.024. [DOI] [PubMed] [Google Scholar]
- Lui TT, Lacroix C, Ahmed SM, Goldenberg SJ, Leach CA, Daulat AM, Angers S. The ubiquitin-specific protease USP34 regulates axin stability and Wnt/beta-catenin signaling. Molecular and Cellular Biology. 2011;31:2053–2065. doi: 10.1128/MCB.01094-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malinauskas T, Aricescu AR, Lu W, Siebold C, Jones EY. Modular mechanism of Wnt signaling inhibition by Wnt inhibitory factor 1. Nature Structural & Molecular Biology. 2011;18:886–893. doi: 10.1038/nsmb.2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao B, Wu W, Davidson G, Marhold J, Li M, Mechler BM, … Niehrs C. Kremen proteins are Dickkopf receptors that regulate Wnt/beta-catenin signalling. Nature. 2002;417:664–667. doi: 10.1038/nature756. [DOI] [PubMed] [Google Scholar]
- Mao J, Wang J, Liu B, Pan W, Farr GH, 3rd, Flynn C, … Wu D. Low-density lipoprotein receptor-related protein-5 binds to axin and regulates the canonical Wnt signaling pathway. Molecular Cell. 2001;7:801–809. doi: 10.1016/s1097-2765(01)00224-6. [DOI] [PubMed] [Google Scholar]
- Maretzky T, Reiss K, Ludwig A, Buchholz J, Scholz F, Proksch E, … Saftig P. ADAM10 mediates E-cadherin shedding and regulates epithelial cell-cell adhesion, migration, and beta-catenin translocation. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:9182–9187. doi: 10.1073/pnas.0500918102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marikawa Y, Elinson RP. Beta-TrCP is a negative regulator of Wnt/beta-catenin signaling pathway and dorsal axis formation in Xenopus embryos. Mechanisms of Development. 1998;77:75–80. doi: 10.1016/s0925-4773(98)00134-8. [DOI] [PubMed] [Google Scholar]
- Martinez S, Scerbo P, Giordano M, Daulat AM, Lhoumeau AC, Thome V, … Borg JP. The PTK7 and ROR2 protein receptors interact in the vertebrate WNT/planar cell polarity (PCP) pathway. The Journal of Biological Chemistry. 2015;290:30562–30572. doi: 10.1074/jbc.M115.697615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason JO, Kitajewski J, Varmus HE. Mutational analysis of mouse Wnt-1 identifies two temperature-sensitive alleles and attributes of Wnt-1 protein essential for transformation of a mammary cell line. Molecular Biology of the Cell. 1992;3:521–533. doi: 10.1091/mbc.3.5.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matoba K, Mihara E, Tamura-Kawakami K, Miyazaki N, Maeda S, Hirai H, … Takagi J. Conformational freedom of the LRP6 ectodomain is regulated by N-glycosylation and the binding of the Wnt antagonist Dkk1. Cell Reports. 2017;18:32–40. doi: 10.1016/j.celrep.2016.12.017. [DOI] [PubMed] [Google Scholar]
- Maubant S, Tesson B, Maire V, Ye M, Rigaill G, Gentien D, … Dubois T. Transcriptome analysis of Wnt3a-treated triple-negative breast cancer cells. PLoS One. 2015;10:e0122333. doi: 10.1371/journal.pone.0122333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCrea PD, Turck CW, Gumbiner B. A homolog of the armadillo protein in Drosophila (plakoglobin) associated with E-cadherin. Science. 1991;254:1359–1361. doi: 10.1126/science.1962194. [DOI] [PubMed] [Google Scholar]
- Merlos-Suarez A, Barriga FM, Jung P, Iglesias M, Cespedes MV, Rossell D, et al. The intestinal stem cell signature identifies colorectal cancer stem cells and predicts disease relapse. Cell Stem Cell. 2011;8:511–524. doi: 10.1016/j.stem.2011.02.020. [DOI] [PubMed] [Google Scholar]
- Mihara E, Hirai H, Yamamoto H, Tamura-Kawakami K, Matano M, Kikuchi A, … Takagi J. Active and water-soluble form of lipidated Wnt protein is maintained by a serum glycoprotein afamin/alpha-albumin. eLife. 2016:5. doi: 10.7554/eLife.11621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikels AJ, Nusse R. Purified Wnt5a protein activates or inhibits beta-catenin-TCF signaling depending on receptor context. PLoS Biology. 2006;4:e115. doi: 10.1371/journal.pbio.0040115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mlodzik M. The dishevelled protein family: Still rather a mystery after over 20 years of molecular studies. Current Topics in Developmental Biology. 2016;117:75–91. doi: 10.1016/bs.ctdb.2015.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molenaar M, van de Wetering M, Oosterwegel M, Peterson-Maduro J, Godsave S, Korinek V, … Clevers H. XTcf-3 transcription factor mediates beta-catenin-induced axis formation in Xenopus embryos. Cell. 1996;86:391–399. doi: 10.1016/s0092-8674(00)80112-9. [DOI] [PubMed] [Google Scholar]
- Morin PJ, Sparks AB, Korinek V, Barker N, Clevers H, Vogelstein B, Kinzler KW. Activation of beta-catenin-Tcf signaling in colon cancer by mutations in beta-catenin or APC. Science. 1997;275:1787–1790. doi: 10.1126/science.275.5307.1787. [DOI] [PubMed] [Google Scholar]
- Morrell NT, Leucht P, Zhao L, Kim JB, ten Berge D, Ponnusamy K, … Nusse R. Liposomal packaging generates Wnt protein with in vivo biological activity. PLoS One. 2008;3:e2930. doi: 10.1371/journal.pone.0002930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosimann C, Hausmann G, Basler K. Parafibromin/hyrax activates Wnt/Wg target gene transcription by direct association with beta-catenin/armadillo. Cell. 2006;125:327–341. doi: 10.1016/j.cell.2006.01.053. [DOI] [PubMed] [Google Scholar]
- Mulligan KA, Fuerer C, Ching W, Fish M, Willert K, Nusse R. Secreted wingless-interacting molecule (Swim) promotes long-range signaling by maintaining wingless solubility. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:370–377. doi: 10.1073/pnas.1119197109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nabhan A, Brownfield DG, Harbury PB, Krasnow MA, Desai TJ. Single-cell Wnt signaling niches maintain stemness of alveolar type 2 cells. Science. 2018:eaam6603. doi: 10.1126/science.aam6603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura Y, de Paiva Alves E, Veenstra GJ, Hoppler S. Tissue- and stage-specific Wnt target gene expression is controlled subsequent to beta-catenin recruitment to cis-regulatory modules. Development. 2016;143:1914–1925. doi: 10.1242/dev.131664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathke IS, Adams CL, Polakis P, Sellin JH, Nelson WJ. The adenomatous polyposis coli tumor suppressor protein localizes to plasma membrane sites involved in active cell migration. The Journal of Cell Biology. 1996;134:165–179. doi: 10.1083/jcb.134.1.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann S, Coudreuse DY, van der Westhuyzen DR, Eckhardt ER, Korswagen HC, Schmitz G, Sprong H. Mammalian Wnt3a is released on lipoprotein particles. Traffic. 2009;10:334–343. doi: 10.1111/j.1600-0854.2008.00872.x. [DOI] [PubMed] [Google Scholar]
- Nichols AS, Floyd DH, Bruinsma SP, Narzinski K, Baranski TJ. Frizzled receptors signal through G proteins. Cellular Signalling. 2013;25:1468–1475. doi: 10.1016/j.cellsig.2013.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niida A, Hiroko T, Kasai M, Furukawa Y, Nakamura Y, Suzuki Y, … Akiyama T. DKK1, a negative regulator of Wnt signaling, is a target of the beta-catenin/TCF pathway. Oncogene. 2004;23:8520–8526. doi: 10.1038/sj.onc.1207892. [DOI] [PubMed] [Google Scholar]
- Nile AH, Mukund S, Stanger K, Wang W, Hannoush RN. Unsaturated fatty acyl recognition by frizzled receptors mediates dimerization upon Wnt ligand binding. Proceedings of the National Academy of Sciences of the United States of America. 2017;114:4147–4152. doi: 10.1073/pnas.1618293114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishisho I, Nakamura Y, Miyoshi Y, Miki Y, Ando H, Horii A, … Hedge P. Mutations of chromosome 5q21 genes in FAP and colorectal cancer patients. Science. 1991;253:665–669. doi: 10.1126/science.1651563. [DOI] [PubMed] [Google Scholar]
- Noordermeer J, Klingensmith J, Perrimon N, Nusse R. Dishevelled and armadillo act in the wingless signalling pathway in Drosophila. Nature. 1994;367:80–83. doi: 10.1038/367080a0. [DOI] [PubMed] [Google Scholar]
- Nusse R, Brown A, Papkoff J, Scambler P, Shackleford G, McMahon A, … Varmus H. A new nomenclature for int-1 and related genes: The Wnt gene family. Cell. 1991;64:231. doi: 10.1016/0092-8674(91)90633-a. [DOI] [PubMed] [Google Scholar]
- Nusse R, van Ooyen A, Cox D, Fung YK, Varmus H. Mode of proviral activation of a putative mammary oncogene (int-1) on mouse chromosome 15. Nature. 1984;307:131–136. doi: 10.1038/307131a0. [DOI] [PubMed] [Google Scholar]
- Nusse R, Varmus HE. Many tumors induced by the mouse mammary tumor virus contain a provirus integrated in the same region of the host genome. Cell. 1982;31:99–109. doi: 10.1016/0092-8674(82)90409-3. [DOI] [PubMed] [Google Scholar]
- Nusslein-Volhard C, Wieschaus E. Mutations affecting segment number and polarity in Drosophila. Nature. 1980;287:795–801. doi: 10.1038/287795a0. [DOI] [PubMed] [Google Scholar]
- Oishi I, Suzuki H, Onishi N, Takada R, Kani S, Ohkawara B, … Minami Y. The receptor tyrosine kinase Ror2 is involved in non-canonical Wnt5a/JNK signalling pathway. Genes to Cells. 2003;8:645–654. doi: 10.1046/j.1365-2443.2003.00662.x. [DOI] [PubMed] [Google Scholar]
- Orsulic S, Peifer M. An in vivo structure-function study of armadillo, the beta-catenin homologue, reveals both separate and overlapping regions of the protein required for cell adhesion and for wingless signaling. The Journal of Cell Biology. 1996;134:1283–1300. doi: 10.1083/jcb.134.5.1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panakova D, Sprong H, Marois E, Thiele C, Eaton S. Lipoprotein particles are required for hedgehog and wingless signalling. Nature. 2005;435:58–65. doi: 10.1038/nature03504. [DOI] [PubMed] [Google Scholar]
- Papkoff J, Brown AM, Varmus HE. The int-1 proto-oncogene products are glycoproteins that appear to enter the secretory pathway. Molecular and Cellular Biology. 1987;7:3978–3984. doi: 10.1128/mcb.7.11.3978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker DS, Jemison J, Cadigan KM. Pygopus, a nuclear PHD-finger protein required for wingless signaling in Drosophila. Development. 2002;129:2565–2576. doi: 10.1242/dev.129.11.2565. [DOI] [PubMed] [Google Scholar]
- Patthy L. The WIF module. Trends in Biochemical Sciences. 2000;25:12–13. doi: 10.1016/s0968-0004(99)01504-2. [DOI] [PubMed] [Google Scholar]
- Peifer M, Pai LM, Casey M. Phosphorylation of the Drosophila adherens junction protein armadillo: Roles for wingless signal and zeste-white 3 kinase. Developmental Biology. 1994;166:543–556. doi: 10.1006/dbio.1994.1336. [DOI] [PubMed] [Google Scholar]
- Peifer M, Rauskolb C, Williams M, Riggleman B, Wieschaus E. The segment polarity gene armadillo interacts with the wingless signaling pathway in both embryonic and adult pattern formation. Development. 1991;111:1029–1043. doi: 10.1242/dev.111.4.1029. [DOI] [PubMed] [Google Scholar]
- Peifer M, Sweeton D, Casey M, Wieschaus E. Wingless signal and Zeste-white 3 kinase trigger opposing changes in the intracellular distribution of armadillo. Development. 1994;120:369–380. doi: 10.1242/dev.120.2.369. [DOI] [PubMed] [Google Scholar]
- Penman GA, Leung L, Nathke IS. The adenomatous polyposis coli protein (APC) exists in two distinct soluble complexes with different functions. Journal of Cell Science. 2005;118:4741–4750. doi: 10.1242/jcs.02589. [DOI] [PubMed] [Google Scholar]
- Peradziryi H, Kaplan NA, Podleschny M, Liu X, Wehner P, Borchers A, Tolwinski NS. PTK7/Otk interacts with Wnts and inhibits canonical Wnt signalling. The EMBO Journal. 2011;30:3729–3740. doi: 10.1038/emboj.2011.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters JM, McKay RM, McKay JP, Graff JM. Casein kinase I transduces Wnt signals. Nature. 1999;401:345–350. doi: 10.1038/43830. [DOI] [PubMed] [Google Scholar]
- Petersen CP, Reddien PW. Polarized notum activation at wounds inhibits Wnt function to promote planarian head regeneration. Science. 2011;332:852–855. doi: 10.1126/science.1202143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen J, Wright SC, Rodriguez D, Matricon P, Lahav N, Vromen A, … Schulte G. Agonist-induced dimer dissociation as a macromolecular step in G protein-coupled receptor signaling. Nature Communications. 2017;8:226. doi: 10.1038/s41467-017-00253-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piccolo S, Agius E, Leyns L, Bhattacharyya S, Grunz H, Bouwmeester T, De Robertis EM. The head inducer Cerberus is a multifunctional antagonist of Nodal, BMP and Wnt signals. Nature. 1999;397:707–710. doi: 10.1038/17820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinson KI, Brennan J, Monkley S, Avery BJ, Skarnes WC. An LDL-receptor-related protein mediates Wnt signalling in mice. Nature. 2000;407:535–538. doi: 10.1038/35035124. [DOI] [PubMed] [Google Scholar]
- Port F, Kuster M, Herr P, Furger E, Banziger C, Hausmann G, Basler K. Wingless secretion promotes and requires retromer-dependent cycling of Wntless. Nature Cell Biology. 2008;10:178–185. doi: 10.1038/ncb1687. [DOI] [PubMed] [Google Scholar]
- Prasad BC, Clark SG. Wnt signaling establishes anteroposterior neuronal polarity and requires retromer in C. elegans. Development. 2006;133:1757–1766. doi: 10.1242/dev.02357. [DOI] [PubMed] [Google Scholar]
- Proffitt KD, Madan B, Ke Z, Pendharkar V, Ding L, Lee MA, … Virshup DM. Pharmacological inhibition of the Wnt acyltransferase PORCN prevents growth of WNT-driven mammary cancer. Cancer Research. 2013;73:502–507. doi: 10.1158/0008-5472.CAN-12-2258. [DOI] [PubMed] [Google Scholar]
- Pronobis MI, Deuitch N, Posham V, Mimori-Kiyosue Y, Peifer M. Reconstituting regulation of the canonical Wnt pathway by engineering a minimal beta-catenin destruction machine. Molecular Biology of the Cell. 2017;28:41–53. doi: 10.1091/mbc.E16-07-0557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puppo F, Thome V, Lhoumeau AC, Cibois M, Gangar A, Lembo F, et al. Protein tyrosine kinase 7 has a conserved role in Wnt/beta-catenin canonical signalling. EMBO Reports. 2011;12:43–49. doi: 10.1038/embor.2010.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu Y, Olsen JR, Yuan X, Cheng PF, Levesque MP, Brokstad KA, … Ke X. Small molecule promotes beta-catenin citrullination and inhibits Wnt signaling in cancer. Nature Chemical Biology. 2017;14:94–101. doi: 10.1038/nchembio.2510. [DOI] [PubMed] [Google Scholar]
- Radler-Pohl A, Pfeuffer I, Karin M, Serfling E. A novel T-cell trans-activator that recognizes a phorbol ester-inducible element of the interleukin-2 promoter. The New Biologist. 1990;2:566–573. [PubMed] [Google Scholar]
- Ramakrishnan AB, Cadigan KM. Wnt target genes and where to find them. F1000Research. 2017;6:746. doi: 10.12688/f1000research.11034.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rattner A, Hsieh JC, Smallwood PM, Gilbert DJ, Copeland NG, Jenkins NA, Nathans JA. Family of secreted proteins contains homology to the cysteine-rich ligand-binding domain of frizzled receptors. Proceedings of the National Academy of Sciences of the United States of America. 1997;94:2859–2863. doi: 10.1073/pnas.94.7.2859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiss K, Maretzky T, Ludwig A, Tousseyn T, de Strooper B, Hartmann D, Saftig P. ADAM10 cleavage of N-cadherin and regulation of cell-cell adhesion and beta-catenin nuclear signalling. The EMBO Journal. 2005;24:742–752. doi: 10.1038/sj.emboj.7600548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rijsewijk F, Schuermann M, Wagenaar E, Parren P, Weigel D, Nusse R. The Drosophila homolog of the mouse mammary oncogene int-1 is identical to the segment polarity gene wingless. Cell. 1987;50:649–657. doi: 10.1016/0092-8674(87)90038-9. [DOI] [PubMed] [Google Scholar]
- Roose J, Molenaar M, Peterson J, Hurenkamp J, Brantjes H, Moerer P, … Clevers H. The Xenopus Wnt effector XTcf-3 interacts with Groucho-related transcriptional repressors. Nature. 1998;395:608–612. doi: 10.1038/26989. [DOI] [PubMed] [Google Scholar]
- Ross J, Busch J, Mintz E, Ng D, Stanley A, Brafman D, … Willert K. A rare human syndrome provides genetic evidence that WNT signaling is required for reprogramming of fibroblasts to induced pluripotent stem cells. Cell Reports. 2014;9:1770–1780. doi: 10.1016/j.celrep.2014.10.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubinfeld B, Albert I, Porfiri E, Fiol C, Munemitsu S, Polakis P. Binding of GSK3beta to the APC-beta-catenin complex and regulation of complex assembly. Science. 1996;272:1023–1026. doi: 10.1126/science.272.5264.1023. [DOI] [PubMed] [Google Scholar]
- Rubinfeld B, Albert I, Porfiri E, Munemitsu S, Polakis P. Loss of beta-catenin regulation by the APC tumor suppressor protein correlates with loss of structure due to common somatic mutations of the gene. Cancer Research. 1997;57:4624–4630. [PubMed] [Google Scholar]
- Rubinfeld B, Souza B, Albert I, Muller O, Chamberlain SH, Masiarz FR, … Polakis P. Association of the APC gene product with beta-catenin. Science. 1993;262:1731–1734. doi: 10.1126/science.8259518. [DOI] [PubMed] [Google Scholar]
- Rubinfeld B, Tice DA, Polakis P. Axin-dependent phosphorylation of the adenomatous polyposis coli protein mediated by casein kinase 1epsilon. The Journal of Biological Chemistry. 2001;276:39037–39045. doi: 10.1074/jbc.M105148200. [DOI] [PubMed] [Google Scholar]
- Rulifson EJ, Wu CH, Nusse R. Pathway specificity by the bifunctional receptor frizzled is determined by affinity for wingless. Molecular Cell. 2000;6:117–126. [PubMed] [Google Scholar]
- Sadot E, Simcha I, Shtutman M, Ben-Ze’ev A, Geiger B. Inhibition of beta-catenin-mediated transactivation by cadherin derivatives. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:15339–15344. doi: 10.1073/pnas.95.26.15339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanson B, White P, Vincent JP. Uncoupling cadherin-based adhesion from wingless signalling in Drosophila. Nature. 1996;383:627–630. doi: 10.1038/383627a0. [DOI] [PubMed] [Google Scholar]
- Sato N, Meijer L, Skaltsounis L, Greengard P, Brivanlou AH. Maintenance of pluripotency in human and mouse embryonic stem cells through activation of Wnt signaling by a pharmacological GSK-3-specific inhibitor. Nature Medicine. 2004;10:55–63. doi: 10.1038/nm979. [DOI] [PubMed] [Google Scholar]
- Sato T, Vries RG, Snippert HJ, van de Wetering M, Barker N, Stange DE, … Clevers H. Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature. 2009;459:262–265. doi: 10.1038/nature07935. [DOI] [PubMed] [Google Scholar]
- Schepers AG, Snippert HJ, Stange DE, van den Born M, van Es JH, van de Wetering M, Clevers H. Lineage tracing reveals Lgr5+ stem cell activity in mouse intestinal adenomas. Science. 2012;337:730–735. doi: 10.1126/science.1224676. [DOI] [PubMed] [Google Scholar]
- Seeling JM, Miller JR, Gil R, Moon RT, White R, Virshup DM. Regulation of beta-catenin signaling by the B56 subunit of protein phosphatase 2A. Science. 1999;283:2089–2091. doi: 10.1126/science.283.5410.2089. [DOI] [PubMed] [Google Scholar]
- Semenov M, Tamai K, He X. SOST is a ligand for LRP5/LRP6 and a Wnt signaling inhibitor. The Journal of Biological Chemistry. 2005;280:26770–26775. doi: 10.1074/jbc.M504308200. [DOI] [PubMed] [Google Scholar]
- Semenov MV, Tamai K, Brott BK, Kuhl M, Sokol S, He X. Head inducer Dickkopf-1 is a ligand for Wnt coreceptor LRP6. Current Biology. 2001;11:951–961. doi: 10.1016/s0960-9822(01)00290-1. [DOI] [PubMed] [Google Scholar]
- Sharma RP, Chopra VL. Effect of the wingless (wg1) mutation on wing and haltere development in Drosophila melanogaster. Developmental Biology. 1976;48:461–465. doi: 10.1016/0012-1606(76)90108-1. [DOI] [PubMed] [Google Scholar]
- Shibamoto S, Higano K, Takada R, Ito F, Takeichi M, Takada S. Cytoskeletal reorganization by soluble Wnt-3a protein signalling. Genes to Cells. 1998;3:659–670. doi: 10.1046/j.1365-2443.1998.00221.x. [DOI] [PubMed] [Google Scholar]
- Shimomura Y, Agalliu D, Vonica A, Luria V, Wajid M, Baumer A, … Christiano AM. APCDD1 is a novel Wnt inhibitor mutated in hereditary hypotrichosis simplex. Nature. 2010;464:1043–1047. doi: 10.1038/nature08875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shtutman M, Zhurinsky J, Simcha I, Albanese C, D’Amico M, Pestell R, Ben-Ze’ev A. The cyclin D1 gene is a target of the beta-catenin/LEF-1 pathway. Proceedings of the National Academy of Sciences of the United States of America. 1999;96:5522–5527. doi: 10.1073/pnas.96.10.5522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegfried E, Chou TB, Perrimon N. Wingless signaling acts through zeste-white 3, the Drosophila homolog of glycogen synthase kinase-3, to regulate engrailed and establish cell fate. Cell. 1992;71:1167–1179. doi: 10.1016/s0092-8674(05)80065-0. [DOI] [PubMed] [Google Scholar]
- Slusarski DC, Corces VG, Moon RT. Interaction of Wnt and a frizzled homologue triggers G-protein-linked phosphatidylinositol signalling. Nature. 1997;390:410–413. doi: 10.1038/37138. [DOI] [PubMed] [Google Scholar]
- Slusarski DC, Yang-Snyder J, Busa WB, Moon RT. Modulation of embryonic intracellular Ca2+ signaling by Wnt-5A. Developmental Biology. 1997;182:114–120. doi: 10.1006/dbio.1996.8463. [DOI] [PubMed] [Google Scholar]
- Smit L, Baas A, Kuipers J, Korswagen H, van de Wetering M, Clevers H. Wnt activates the Tak1/Nemo-like kinase pathway. The Journal of Biological Chemistry. 2004;279:17232–17240. doi: 10.1074/jbc.M307801200. [DOI] [PubMed] [Google Scholar]
- Song N, Schwab KR, Patterson LT, Yamaguchi T, Lin X, Potter SS, Lang RA. Pygopus 2 has a crucial, Wnt pathway-independent function in lens induction. Development. 2007;134:1873–1885. doi: 10.1242/dev.001495. [DOI] [PubMed] [Google Scholar]
- Stanganello E, Hagemann AI, Mattes B, Sinner C, Meyen D, Weber S, … Scholpp S. Filopodia-based Wnt transport during vertebrate tissue patterning. Nature Communications. 2015;6:5846. doi: 10.1038/ncomms6846. [DOI] [PubMed] [Google Scholar]
- Stockinger A, Eger A, Wolf J, Beug H, Foisner R. E-cadherin regulates cell growth by modulating proliferation-dependent beta-catenin transcriptional activity. The Journal of Cell Biology. 2001;154:1185–1196. doi: 10.1083/jcb.200104036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stratford EW, Daffinrud J, Munthe E, Castro R, Waaler J, Krauss S, Myklebost O. The tankyrase-specific inhibitor JW74 affects cell cycle progression and induces apoptosis and differentiation in osteosarcoma cell lines. Cancer Medicine. 2014;3:36–46. doi: 10.1002/cam4.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su LK, Vogelstein B, Kinzler KW. Association of the APC tumor suppressor protein with catenins. Science. 1993;262:1734–1737. doi: 10.1126/science.8259519. [DOI] [PubMed] [Google Scholar]
- Surmann-Schmitt C, Widmann N, Dietz U, Saeger B, Eitzinger N, Nakamura Y, … Stock M. Wif-1 is expressed at cartilage-mesenchyme interfaces and impedes Wnt3a-mediated inhibition of chondrogenesis. Journal of Cell Science. 2009;122:3627–3637. doi: 10.1242/jcs.048926. [DOI] [PubMed] [Google Scholar]
- Sussman DJ, Klingensmith J, Salinas P, Adams PS, Nusse R, Perrimon N. Isolation and characterization of a mouse homolog of the Drosophila segment polarity gene dishevelled. Developmental Biology. 1994;166:73–86. doi: 10.1006/dbio.1994.1297. [DOI] [PubMed] [Google Scholar]
- Sustmann C, Flach H, Ebert H, Eastman Q, Grosschedl R. Cell-type-specific function of BCL9 involves a transcriptional activation domain that synergizes with beta-catenin. Molecular and Cellular Biology. 2008;28:3526–3537. doi: 10.1128/MCB.01986-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swiatek W, Tsai IC, Klimowski L, Pepler A, Barnette J, Yost HJ, Virshup DM. Regulation of casein kinase I epsilon activity by Wnt signaling. The Journal of Biological Chemistry. 2004;279:13011–13017. doi: 10.1074/jbc.M304682200. [DOI] [PubMed] [Google Scholar]
- Tago K, Nakamura T, Nishita M, Hyodo J, Nagai S, Murata Y, … Akiyama T. Inhibition of Wnt signaling by ICAT, a novel beta-catenin-interacting protein. Genes & Development. 2000;14:1741–1749. [PMC free article] [PubMed] [Google Scholar]
- Takacs CM, Baird JR, Hughes EG, Kent SS, Benchabane H, Paik R, Ahmed Y. Dual positive and negative regulation of wingless signaling by adenomatous polyposis coli. Science. 2008;319:333–336. doi: 10.1126/science.1151232. [DOI] [PubMed] [Google Scholar]
- Takada R, Satomi Y, Kurata T, Ueno N, Norioka S, Kondoh H, … Takada S. Monounsaturated fatty acid modification of Wnt protein: Its role in Wnt secretion. Developmental Cell. 2006;11:791–801. doi: 10.1016/j.devcel.2006.10.003. [DOI] [PubMed] [Google Scholar]
- Takahashi M, Fujita M, Furukawa Y, Hamamoto R, Shimokawa T, Miwa N, … Nakamura Y. Isolation of a novel human gene, APCDD1, as a direct target of the beta-catenin/T-cell factor 4 complex with probable involvement in colorectal carcinogenesis. Cancer Research. 2002;62:5651–5656. [PubMed] [Google Scholar]
- Takei Y, Ozawa Y, Sato M, Watanabe A, Tabata T. Three Drosophila EXT genes shape morphogen gradients through synthesis of heparan sulfate proteoglycans. Development. 2004;131:73–82. doi: 10.1242/dev.00913. [DOI] [PubMed] [Google Scholar]
- Tamai K, Semenov M, Kato Y, Spokony R, Liu C, Katsuyama Y, … He X. LDL-receptor-related proteins in Wnt signal transduction. Nature. 2000;407:530–535. doi: 10.1038/35035117. [DOI] [PubMed] [Google Scholar]
- Tang X, Wu Y, Belenkaya TY, Huang Q, Ray L, Qu J, Lin X. Roles of N-glycosylation and lipidation in Wg secretion and signaling. Developmental Biology. 2012;364:32–41. doi: 10.1016/j.ydbio.2012.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tauriello DV, Jordens I, Kirchner K, Slootstra JW, Kruitwagen T, Bouwman BA, … Maurice MM. Wnt/beta-catenin signaling requires interaction of the Dishevelled DEP domain and C terminus with a discontinuous motif in frizzled. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:E812–E820. doi: 10.1073/pnas.1114802109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teh MT, Blaydon D, Ghali LR, Briggs V, Edmunds S, Pantazi E, … Philpott MP. Role for WNT16B in human epidermal keratinocyte proliferation and differentiation. Journal of Cell Science. 2007;120:330–339. doi: 10.1242/jcs.03329. [DOI] [PubMed] [Google Scholar]
- Theisen H, Purcell J, Bennett M, Kansagara D, Syed A, Marsh JL. Dishevelled is required during wingless signaling to establish both cell polarity and cell identity. Development. 1994;120:347–360. doi: 10.1242/dev.120.2.347. [DOI] [PubMed] [Google Scholar]
- Thompson B, Townsley F, Rosin-Arbesfeld R, Musisi H, Bienz M. A new nuclear component of the Wnt signalling pathway. Nature Cell Biology. 2002;4:367–373. doi: 10.1038/ncb786. [DOI] [PubMed] [Google Scholar]
- Thorne CA, Hanson AJ, Schneider J, Tahinci E, Orton D, Cselenyi CS, … Lee E. Small-molecule inhibition of Wnt signaling through activation of casein kinase 1alpha. Nature Chemical Biology. 2010;6:829–836. doi: 10.1038/nchembio.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolwinski NS, Wehrli M, Rives A, Erdeniz N, DiNardo S, Wieschaus E. Wg/Wnt signal can be transmitted through arrow/LRP5,6 and axin independently of Zw3/Gsk3beta activity. Developmental Cell. 2003;4:407–418. doi: 10.1016/s1534-5807(03)00063-7. [DOI] [PubMed] [Google Scholar]
- Tolwinski NS, Wieschaus E. Armadillo nuclear import is regulated by cytoplasmic anchor axin and nuclear anchor dTCF/pan. Development. 2001;128:2107–2117. doi: 10.1242/dev.128.11.2107. [DOI] [PubMed] [Google Scholar]
- Torisu Y, Watanabe A, Nonaka A, Midorikawa Y, Makuuchi M, Shimamura T, … Aburatani H. Human homolog of NOTUM, overexpressed in hepa-tocellular carcinoma, is regulated transcriptionally by beta-catenin/TCF. Cancer Science. 2008;99:1139–1146. doi: 10.1111/j.1349-7006.2008.00814.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townsley FM, Cliffe A, Bienz M. Pygopus and legless target armadillo/beta-catenin to the nucleus to enable its transcriptional co-activator function. Nature Cell Biology. 2004;6:626–633. doi: 10.1038/ncb1141. [DOI] [PubMed] [Google Scholar]
- Travis A, Amsterdam A, Belanger C, Grosschedl R. LEF-1, a gene encoding a lymphoid-specific protein with an HMG domain, regulates T-cell receptor alpha enhancer function [corrected] Genes & Development. 1991;5:880–894. doi: 10.1101/gad.5.5.880. [DOI] [PubMed] [Google Scholar]
- Tsang M, Lijam N, Yang Y, Beier DR, Wynshaw-Boris A, Sussman DJ. Isolation and characterization of mouse dishevelled-3. Developmental Dynamics. 1996;207:253–262. doi: 10.1002/(SICI)1097-0177(199611)207:3<253::AID-AJA2>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Tuysuz N, van Bloois L, van den Brink S, Begthel H, Verstegen MM, Cruz LJ, … Ten Berge D. Lipid-mediated Wnt protein stabilization enables serum-free culture of human organ stem cells. Nature Communications. 2017;8:14578. doi: 10.1038/ncomms14578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uemura K, Kihara T, Kuzuya A, Okawa K, Nishimoto T, Bito H, … Shimohama S. Activity-dependent regulation of beta-catenin via epsilon-cleavage of N-cadherin. Biochemical and Biophysical Research Communications. 2006;345:951–958. doi: 10.1016/j.bbrc.2006.04.157. [DOI] [PubMed] [Google Scholar]
- van Amerongen R. Alternative Wnt pathways and receptors. Cold Spring Harbor Perspectives in Biology. 2012;4 doi: 10.1101/cshperspect.a007914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Wetering M, Cavallo R, Dooijes D, van Beest M, van Es J, Loureiro J, … Clevers H. Armadillo coactivates transcription driven by the product of the Drosophila segment polarity gene dTCF. Cell. 1997;88:789–799. doi: 10.1016/s0092-8674(00)81925-x. [DOI] [PubMed] [Google Scholar]
- Van der Flier LG, Sabates-Bellver J, Oving I, Haegebarth A, De Palo M, Anti M, … Clevers H. The intestinal Wnt/TCF signature. Gastroenterology. 2007;132:628–632. doi: 10.1053/j.gastro.2006.08.039. [DOI] [PubMed] [Google Scholar]
- van Leeuwen F, Samos CH, Nusse R. Biological activity of soluble wingless protein in cultured Drosophila imaginal disc cells. Nature. 1994;368:342–344. doi: 10.1038/368342a0. [DOI] [PubMed] [Google Scholar]
- Vanhollebeke B, Stone OA, Bostaille N, Cho C, Zhou Y, Maquet E, … Stainier DYR. Tip cell-specific requirement for an atypical Gpr124- and Reck-dependent Wnt/beta-catenin pathway during brain angiogenesis. eLife. 2015;4 doi: 10.7554/eLife.06489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voloshanenko O, Gmach P, Winter J, Kranz D, Boutros M. Mapping of Wnt-frizzled interactions by multiplex CRISPR targeting of receptor gene families. The FASEB Journal. 2017;31:4832–4844. doi: 10.1096/fj.201700144R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Krinks M, Lin K, Luyten FP, Moos M., Jr Frzb, a secreted protein expressed in the Spemann organizer, binds and inhibits Wnt-8. Cell. 1997;88:757–766. doi: 10.1016/s0092-8674(00)81922-4. [DOI] [PubMed] [Google Scholar]
- Wang Z, Tacchelly-Benites O, Yang E, Ahmed Y. Dual roles for membrane Association of Drosophila axin in Wnt signaling. PLoS Genetics. 2016;12:e1006494. doi: 10.1371/journal.pgen.1006494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wehrli M, Dougan ST, Caldwell K, O’Keefe L, Schwartz S, Vaizel-Ohayon D, … DiNardo S. Arrow encodes an LDL-receptor-related protein essential for wingless signalling. Nature. 2000;407:527–530. doi: 10.1038/35035110. [DOI] [PubMed] [Google Scholar]
- Willert J, Epping M, Pollack JR, Brown PO, Nusse R. A transcriptional response to Wnt protein in human embryonic carcinoma cells. BMC Developmental Biology. 2002;2:8. doi: 10.1186/1471-213x-2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willert K, Brink M, Wodarz A, Varmus H, Nusse R. Casein kinase 2 associates with and phosphorylates dishevelled. The EMBO Journal. 1997;16:3089–3096. doi: 10.1093/emboj/16.11.3089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willert K, Brown JD, Danenberg E, Duncan AW, Weissman IL, Reya T, … Nusse R. Wnt proteins are lipid-modified and can act as stem cell growth factors. Nature. 2003;423:448–452. doi: 10.1038/nature01611. [DOI] [PubMed] [Google Scholar]
- Willert K, Shibamoto S, Nusse R. Wnt-induced dephosphorylation of axin releases beta-catenin from the axin complex. Genes & Development. 1999;13:1768–1773. doi: 10.1101/gad.13.14.1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winston JT, Strack P, Beer-Romero P, Chu CY, Elledge SJ, Harper JW. The SCFbeta-TRCP-ubiquitin ligase complex associates specifically with phosphorylated destruction motifs in IkappaBalpha and beta-catenin and stimulates IkappaBalpha ubiquitination in vitro. Genes & Development. 1999;13:270–283. doi: 10.1101/gad.13.3.270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xavier CP, Melikova M, Chuman Y, Uren A, Baljinnyam B, Rubin JS. Secreted frizzled-related protein potentiation versus inhibition of Wnt3a/beta-catenin signaling. Cellular Signalling. 2014;26:94–101. doi: 10.1016/j.cellsig.2013.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu M, Liu X, Xu Y, Zhu S, Gao Y. Coexpression of axin and APC gene fragments inhibits colorectal cancer cell growth via regulation of the Wnt signaling pathway. Molecular Medicine Reports. 2017;16:3783–3790. doi: 10.3892/mmr.2017.7049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto H, Kishida S, Kishida M, Ikeda S, Takada S, Kikuchi A. Phosphorylation of axin, a Wnt signal negative regulator, by glycogen synthase kinase-3beta regulates its stability. The Journal of Biological Chemistry. 1999;274:10681–10684. doi: 10.1074/jbc.274.16.10681. [DOI] [PubMed] [Google Scholar]
- Yan D, Wallingford JB, Sun TQ, Nelson AM, Sakanaka C, Reinhard C, … Williams LT. Cell autonomous regulation of multiple Dishevelled-dependent pathways by mammalian Nkd. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:3802–3807. doi: 10.1073/pnas.071041898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanagawa S, van Leeuwen F, Wodarz A, Klingensmith J, Nusse R. The dishevelled protein is modified by wingless signaling in Drosophila. Genes & Development. 1995;9:1087–1097. doi: 10.1101/gad.9.9.1087. [DOI] [PubMed] [Google Scholar]
- Yang PT, Lorenowicz MJ, Silhankova M, Coudreuse DY, Betist MC, Korswagen HC. Wnt signaling requires retromer-dependent recycling of MIG-14/Wntless in Wnt-producing cells. Developmental Cell. 2008;14:140–147. doi: 10.1016/j.devcel.2007.12.004. [DOI] [PubMed] [Google Scholar]
- Yokoya F, Imamoto N, Tachibana T, Yoneda Y. Beta-catenin can be transported into the nucleus in a Ran-unassisted manner. Molecular Biology of the Cell. 1999;10:1119–1131. doi: 10.1091/mbc.10.4.1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshikawa S, McKinnon RD, Kokel M, Thomas JB. Wnt-mediated axon guidance via the Drosophila derailed receptor. Nature. 2003;422:583–588. doi: 10.1038/nature01522. [DOI] [PubMed] [Google Scholar]
- Yost C, Torres M, Miller JR, Huang E, Kimelman D, Moon RT. The axis-inducing activity, stability, and subcellular distribution of beta-catenin is regulated in Xenopus embryos by glycogen synthase kinase 3. Genes & Development. 1996;10:1443–1454. doi: 10.1101/gad.10.12.1443. [DOI] [PubMed] [Google Scholar]
- Yousif NG, Hadi NR, Hassan AM. Indocyanine Green-001 (ICG-001) attenuates Wnt/beta-catenin-induces myocardial injury following sepsis. Journal of Pharmacology and Pharmacotherapeutics. 2017;8:14–20. doi: 10.4103/jpp.JPP_153_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu A, Rual JF, Tamai K, Harada Y, Vidal M, He X, Kirchhausen T. Association of Dishevelled with the clathrin AP-2 adaptor is required for frizzled endocytosis and planar cell polarity signaling. Developmental Cell. 2007;12:129–141. doi: 10.1016/j.devcel.2006.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J, Chen L, Cui B, Widhopf GF, 2nd, Shen Z, Wu R, … Kipps TJ. Wnt5a induces ROR1/ROR2 heterooligomerization to enhance leukemia che-motaxis and proliferation. The Journal of Clinical Investigation. 2016;126:585–598. doi: 10.1172/JCI83535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J, Chia J, Canning CA, Jones CM, Bard FA, Virshup DM. WLS retrograde transport to the endoplasmic reticulum during Wnt secretion. Developmental Cell. 2014;29:277–291. doi: 10.1016/j.devcel.2014.03.016. [DOI] [PubMed] [Google Scholar]
- Zeng X, Huang H, Tamai K, Zhang X, Harada Y, Yokota C, … He X. Initiation of Wnt signaling: Control of Wnt coreceptor Lrp6 phosphorylatio-n/activation via frizzled, dishevelled and axin functions. Development. 2008;135:367–375. doi: 10.1242/dev.013540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng X, Tamai K, Doble B, Li S, Huang H, Habas R, … He X. A dual-kinase mechanism for Wnt co-receptor phosphorylation and activation. Nature. 2005;438:873–877. doi: 10.1038/nature04185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Jia J, Wang B, Amanai K, Wharton KA, Jr, Jiang J. Regulation of wingless signaling by the CKI family in Drosophila limb development. Developmental Biology. 2006;299:221–237. doi: 10.1016/j.ydbio.2006.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang P, Zhou L, Pei C, Lin X, Yuan Z. Dysfunction of Wntless triggers the retrograde Golgi-to-ER transport of wingless and induces ER stress. Scientific Reports. 2016;6:19418. doi: 10.1038/srep19418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Abreu JG, Yokota C, MacDonald BT, Singh S, Coburn KL, … He X. Tiki1 is required for head formation via Wnt cleavage-oxidation and inactivation. Cell. 2012;149:1565–1577. doi: 10.1016/j.cell.2012.04.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Cheong SM, Amado NG, Reis AH, MacDonald BT, Zebisch M, … He X. Notum is required for neural and head induction via Wnt deacylation, oxidation, and inactivation. Developmental Cell. 2015;32:719–730. doi: 10.1016/j.devcel.2015.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, MacDonald BT, Gao H, Shamashkin M, Coyle AJ, Martinez RV, He X. Characterization of Tiki, a new family of Wnt-specific metalloproteases. The Journal of Biological Chemistry. 2016;291:2435–2443. doi: 10.1074/jbc.M115.677807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Liu S, Mickanin C, Feng Y, Charlat O, Michaud GA, … Cong F. RNF146 is a poly(ADP-ribose)-directed E3 ligase that regulates axin degradation and Wnt signalling. Nature Cell Biology. 2011;13:623–629. doi: 10.1038/ncb2222. [DOI] [PubMed] [Google Scholar]