Abstract
Objective
Fetal intelligent navigation echocardiography (FINE) is a novel method that automatically generates and displays 9 standard fetal echocardiographic views in normal hearts by applying intelligent navigation technology to spatiotemporal image correlation (STIC) volume data sets. The main objective was to determine the sensitivity and specificity of FINE in the prenatal detection of congenital heart disease (CHD).
Methods
A case‐control study was conducted in 50 fetuses with a broad spectrum of CHD (cases) and 100 fetuses with normal hearts (controls) in the second and third trimesters. Using 4‐dimensional ultrasound with STIC technology, volume data sets were acquired. After all identifying information was removed, the data sets were randomly distributed to a different investigator for analysis using FINE. The sensitivity and specificity for the prenatal detection of CHD, as well as positive and negative likelihood ratios were determined.
Results
The diagnostic performance of FINE for the prenatal detection of CHD was: sensitivity of 98% (49 of 50), specificity of 93% (93 of 100), positive likelihood ratio of 14, and negative likelihood ratio of 0.02. Among cases with confirmed CHD, the diagnosis with use of FINE completely matched the final diagnosis in 74% (37 of 50); minor discrepancies were seen in 12% (6 of 50), and major discrepancies were seen in 14% (7 of 50).
Conclusions
This is the first time the sensitivity and specificity of the FINE method in fetuses with normal hearts and CHD in the second and third trimesters has been reported. Because FINE identifies a broad spectrum of CHD with 98% sensitivity, this method could be used prenatally to screen for and diagnose CHD.
Keywords: cardiac, fetal heart, 4‐dimensional, prenatal diagnosis, spatiotemporal image correlation, ultrasound
Abbreviations
- CHD
congenital heart disease
- FINE
fetal intelligent navigation echocardiography
- 4D
4‐dimensional
- STIC
spatiotemporal image correlation
- VIS‐Assistance
Virtual Intelligent Sonographer Assistance
Congenital heart disease (CHD) is the most common birth defect,1 as well as the leading cause of infant morbidity and mortality from congenital malformations.2 Screening of all pregnancies is required to detect CHD prenatally, since up to 90% of cases occur in pregnancies without high‐risk features.3 Yet the prenatal detection of CHD has remained challenging, with the sensitivity of sonography ranging from 15% to 39%.4, 5, 6, 7, 8, 9, 10, 11, 12 This has been attributed mainly to operator skill and expertise13, 14, 15, 16, 17, 18; however, other factors include the complex anatomy, motion, and small size of the fetal heart. The lack of prenatal identification of CHD is associated with adverse consequences for the neonate, including abnormal neurologic development and even death.19, 20, 21 In contrast, the prenatal diagnosis of specific cardiac anomalies improves the preoperative condition,22, 23, 24, 25, 26 presurgical mortality rate,27, 28, 29 survival after surgery,19, 22, 30, 31 and long‐term neurocognitive function and outcome.32, 33
Four‐dimensional (4D) ultrasound with spatiotemporal image correlation (STIC) facilitates fetal cardiac examination34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55, 56, 57, 58, 59, 60, 61, 62, 63, 64, 65 and has the potential to increase the detection rate of CHD,34, 66, 67 leading some to propose its clinical application in both prenatal cardiac screening and diagnosis of CHD.68, 69, 70, 71, 72, 73, 74 STIC technology allows the acquisition of a volume data set of the fetal heart, in which cardiac planes may be extracted and displayed in any orientation.42, 54 However, the use of software to perform manual navigation76 through such volume data sets is time‐consuming, highly operator dependent, and requires a thorough knowledge of cardiac anatomy.76, 77, 78 As a result, this process is difficult and impractical, especially when the fetal heart is abnormal.79
Fetal intelligent navigation echocardiography (FINE) is a novel method developed recently that automatically generates and displays 9 standard fetal echocardiographic views in normal hearts by applying “intelligent navigation” technology to STIC volume data sets.65, 75, 76, 77, 78, 79 Therefore, this method can simplify examination of the fetal heart and reduce operator dependency. Indeed, studies have reported that FINE applied to STIC volume data sets can successfully generate 9 fetal echocardiographic views in 96% to 100%78 and 98% to 100%76, 77 of normal hearts in the second and third trimesters. As a result, FINE has been suggested as a method to screen for CHD.77, 78 However, to be clinically useful, a screening test should demonstrate both good specificity and sensitivity.80 Therefore, we conducted this study to determine the sensitivity and specificity of FINE in the prenatal detection of CHD.
Materials and Methods
Study Participants
A case‐control study of 150 pregnant women having a singleton fetus with a normal heart (100 controls) or confirmed CHD (50 cases) in the second or third trimester was conducted. Patients were examined at the Detroit Medical Center/Wayne State University and the Perinatology Research Branch of the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD), National Institutes of Health (NIH), Department of Health and Human Services. All women had been enrolled in research protocols approved by the Institutional Review Board of NICHD, NIH, and by the Human Investigation Committee of Wayne State University. All participants provided written informed consent for the use of sonographic images for research purposes. The final diagnosis of CHD was confirmed by neonatal echocardiography and/or other ancillary diagnostic procedures (eg, surgery, cardiac catheterization, and autopsy). In cases when such information was not available, the final diagnosis of CHD was made on the basis of the results of fetal echocardiography performed by an expert.
Spatiotemporal Image Correlation Volume Data Sets
Using STIC technology, sonographic volume data sets of the fetal heart containing grayscale information were acquired from a 4‐chamber view by transverse sweeps through the fetal chest in patients examined at our unit. The acquisition time ranged from 10 to 12.5 seconds, depending on fetal motion, and the acquisition angle ranged from 20 ° to 40 °, depending on gestational age.
Volume data sets were selected for analysis using FINE if they met the following criteria: (1) fetal spine located between the 4‐ and 8‐o'clock positions (reducing the possibility of shadowing from the ribs or spine); (2) upper fetal mediastinum and stomach included and visible in the STIC volume; (3) adequate image quality such that fetal anatomy could be visualized; and (4) absence of excessive motion artifacts that could distort anatomic structures. Only a single STIC volume per patient was analyzed.
The following 7 major types of CHD were the focus of this study: (1) complete transposition of the great vessels; (2) tetralogy of Fallot; (3) coarctation of the aorta; (4) pulmonary atresia with intact ventricular septum; (5) double‐outlet right ventricle; (6) hypoplastic left heart; and (7) atrioventricular canal defect. This is justified because such CHDs are difficult to diagnose; prenatal diagnosis improves the outcome; and some represent a neonatal emergency. However, a broad spectrum of CHD types overall was included to evaluate the diagnostic performance of FINE.
Training on the FINE Method
One of the investigators (S.L.) who had no prior familiarity with the FINE method underwent formal training by the same investigators who invented FINE. Such training occurred in a 2‐day period and included attending didactic lectures on FINE and review of 16 previously acquired STIC volume data sets of normal fetal hearts using the FINE method. Specifically, the following features of FINE were reviewed during the training period (Table 1) 54, 75, 76, 78. (1) intelligent navigation technology; (2) STICLoopand STICLoop criteria; (3) Anatomic Box (learning how to identify and mark anatomic structures of the fetal heart); (4) diagnostic planes; (5) automatic labeling and its features; (6) Virtual Intelligent Sonographer Assistance (VIS‐Assistance) and its advantages in providing additional information, as well as how to perform double and triple VIS‐Assistance79; and (7) intelligent and marking alerts. Moreover, the same investigator was provided a written manual on FINE and underwent a practical session on how to technically use the system (eg, review of buttons and screens). It is noteworthy that during the training period, no STIC volumes of CHD cases were reviewed using the FINE method.
Table 1.
Features of the FINE Method
| Feature | Definition |
|---|---|
| Intelligent navigation technology | New method of interrogation of a volume data set whereby identification and selection of key anatomic landmarks allows the system to (1) generate a geometrical reconstruction of the organ of interest (eg, fetal heart) and (2) automatically navigate, find, extract, and display specific diagnostic planes |
| STICLoop |
2‐dimensional cine loop tool developed to aid the user in determining the appropriateness of STIC volume data sets before applying the FINE method to such volumes STICLoop criteria: 1. Fetal spine located between the 5‐ and 7‐o'clock positions (reducing the possibility of shadowing from the ribs or spine) 2. Minimal or absent shadowing (including the 3 vessels and trachea view), which could obscure visualization of cardiac anatomy 3. Adequate image quality 4. Upper mediastinum and stomach included within the volume and clearly visible 5. Minimal or no motion artifacts observed in the STICLoop (ie, smooth sweep without evidence of abrupt jumps or discontinuous movements) 6. Chest circumference contained within the region of interest 7. Sequential axial planes parallel to each other, similar to a sliced loaf of bread (ie, no “drifting spine” from the 4‐chamber view down to the stomach) 8. No azimuthal issues observed (ie, atria/ventricles do not appear foreshortened in the 4‐chamber view) 9. Minimal or no motion artifacts observed in the sagittal plane |
| Anatomic Box |
Tool used to mark 7 anatomic structures of the fetal heart within the STIC volume to generate a geometrical model of the fetal heart: 1. Cross‐section of the aorta at the level of the stomach 2. Cross‐section of the aorta at the level of the 4‐chamber view 3. Crux 4. Right atrial wall 5. Pulmonary valve 6. Cross‐section of the superior vena cava 7. Transverse aortic arch |
| Diagnostic planes |
9 standard fetal echocardiography views automatically displayed by FINE in a single template: 1. 4 chamber 2. 5 chamber 3. Left ventricular outflow tract 4. Short‐axis view of great vessels/right ventricular outflow tract 5. 3 vessels and trachea 6. Abdomen/stomach 7. Ductal arch 8. Aortic arch 9. Superior and inferior vena cava |
| Automatic labeling |
• Fetal echocardiographic views, anatomic structures (atrial and ventricular chambers, great vessels, venae cavae, and stomach), left and right side of fetus, and cranial and caudal ends • Labeling stays with the corresponding anatomical structure(s), even as the image is increased or decreased in size |
| VIS‐Assistance |
Operator‐independent sonographic navigation and exploration of surrounding structures in a cardiac diagnostic plane of interest (“virtual” sonographer) • Double VIS‐Assistance: technique in which VIS‐Assistance is applied twice to a diagnostic plane of interest, allowing expanded navigational movements and further exploration • Triple VIS‐Assistance: technique in which VIS‐Assistance is applied 3 times to a diagnostic plane of interest, allowing expanded navigational movements and further exploration |
| Intelligent alerts |
• Captions and/or a movie notifying the user about potential issues with the STIC volume data set. The movies depict reference examples to guide and teach the operator • If intelligent alerts appear, this will occur during the marking process • 3 types: (1) breech alert: notifies user that the fetus appears to be in a breech presentation; (2) possible drifting spine alert: notifies user that there may be a “drifting” fetal spine in the volume data set (ie, when the spine location migrates on the screen); and (3) spine location alert: notifies user that the fetal spine appears to be located at a position different from what is recommended (ie, between 5 and 7 o'clock). Once a spine location alert has appeared, 3 marking alerts will appear next in sequence. ○ A spine location alert will appear only if the fetal spine is located at these “times” (o'clock): 12, 1, 2, 3, 4, between 4 and 5, between 7 and 8, 8, 9, 10, 11 |
| Marking alerts |
• Captions/movies notifying the user that fetal anatomic structures for marking (eg, pulmonary valve) may be in a different location than what is expected. The movies depict reference examples to guide and teach the operator. • Marking alerts occur during the marking process and will appear only if the spine location alert has already appeared • 3 types: (1) pulmonary valve alert; (2) superior vena cava alert; and (3) transverse aortic arch alert |
Evaluation of STIC Volume Data Sets by FINE
After all identifying information was removed from the 150 STIC volume data sets, they were randomly distributed to the trained investigator for analysis using the FINE method. Such investigator did not acquire any of the STIC volumes and was blinded to the fetal cardiac diagnosis. To perform the analysis, the STIC volumes were imported into a software system (5D Heart/FINE, version 2015.09.30; Samsung Healthcare, Seoul, Korea) that was installed onto a laptop computer.
The investigator used all of the features of FINE (eg, automatic labeling) when interrogating each STIC volume data set to assist in determining whether the fetal heart was normal or abnormal and to make a specific diagnosis. When using the Anatomic Box tool, if marking any of the anatomic structures of the fetal heart seemed difficult, the structure was absent (eg, crux of the heart), or the structure was not in its usual location, the investigator used her best judgment and estimated the location for marking.
For each STIC volume, VIS‐Assistance video clips were observed in their entirety for all 9 fetal echocardiographic views because this feature of FINE75, 76 (1) improves the success of obtaining the fetal echocardiographic view of interest, (2) allows operator‐independent sonographic navigation and exploration of surrounding structures in the diagnostic plane (ie, a “virtual” sonographer), and (3) can show the appropriate azimuth. Written callouts (eg, “move cephalad”) appear at the top of each VIS‐Assistance video clip to inform the operator of the purpose or action of the navigational movements that are occurring automatically. This brings organization to the video clip and allows operators to use the callouts to target their navigation.
In 4 cardiac VIS‐Assistance views (3 vessels and trachea, left ventricular outflow tract, short‐axis view of great vessels/right ventricular outflow tract, and abdomen/stomach) for normal hearts, we prespecified that certain anatomic structures should also be visualized to consider the VIS‐Assistance as being successful in depicting the echocardiographic view; this has been reported elsewhere.76, 77
Using the FINE method, the investigator initially determined whether the fetal heart was normal or abnormal and then recorded the following information, respectively:
Normal heart: (1) fulfillment of all 9 STICLoop criteria; (2) appearance of intelligent and/or marking alerts; and (3) success rate of generating each of the 9 echocardiographic views using diagnostic planes and/or VIS‐Assistance.
Abnormal heart (CHD): (1) fulfillment of all 9 STICLoop criteria; (2) appearance of intelligent and/or marking alerts; (3) frequency of abnormal echocardiographic views (out of 9); (4) which echocardiographic views were abnormal and a description of the findings (eg, ventricular septal defect); (5) whether diagnostic planes and/or VIS‐Assistance views were abnormal; (6) use of the double or triple VIS‐Assistance technique; (7) correct or incorrect labeling of anatomic structures; and (8) summary of individual findings (eg, overriding aorta) and the final presumptive diagnosis (eg, tetralogy of Fallot).
The sensitivity and specificity of FINE for the prenatal detection of CHD, as well as positive and negative likelihood ratios, were determined. Finally, among cases with confirmed CHD, the degree of concordance with the diagnosis made on the basis of the FINE method was categorized as follows: (1) category A, complete agreement; (2) category B, minor discrepancies in the diagnosis; and (3) category C, major discrepancies in the diagnosis.
Results
A total of 150 STIC volume data sets (100 controls and 50 cases) were evaluated using the FINE method. The median gestational age at the time of STIC volume acquisition was 25 (interquartile range, 22–29) weeks. However, STIC volumes were obtained from 16 to 37 weeks' gestation, with 36% (54 of 150) of fetuses being in the third trimester.
Normal Fetal Hearts Evaluated by FINE
STICLoop Criteria
All 100 STIC volumes of normal hearts were initially evaluated by STICLoop criteria (Table 1). Seven of the 9 criteria were met 95% to 99% of the time. For example, in 99% (99 of 100) of STIC volumes, the upper mediastinum and stomach were included within the volume and clearly visible. However, approximately 20% of the STIC volumes of normal hearts did not meet the following STICLoop criteria: (1) minimal or absent shadowing (21%; n = 21); and (2) minimal or no motion artifacts observed in the STICLoop (23%; n = 23). Therefore, the investigator recorded that 23% of such STIC volumes contained motion artifacts.
Intelligent and Marking Alerts
While marking anatomic structures of the normal fetal heart within the STIC volume (using the feature Anatomic Box), an intelligent alert automatically appeared in 27% (27 of 100): (1) breech alert in 18% (n = 18); (2) possible drifting spine alert in 5% (n = 5); and (3) spine location alert in 4% (n = 4). Therefore, 18% of the fetuses were in an original breech presentation so that the cardiac apex was originally pointing to the right side of the monitor screen. The spine location alert appeared in only 4% of the volumes, and such alert was automatically activated during the marking process because the fetal spine in the STIC volume was located in a position other than between 5 and 7 o'clock: (1) 7 to 8 o'clock in 75% (n = 3); and (2) 8 o'clock in 25% (n = 1). Therefore, 96 STIC volumes had a fetal spine located at 5, 6, or 7 o'clock. Each time the spine location alert was activated, 3 marking alerts (pulmonary valve, superior vena cava, and transverse aortic arch) appeared next in sequence. These marking alerts “informed” the investigator (through captions and a movie) that fetal anatomic structures for marking (eg, superior vena cava) could be in a different location than expected because the fetal spine was not located between the 5‐ and 7‐o'clock positions.
Success Rates of Generating Fetal Echocardiographic Views
For the 100 normal hearts, the FINE method was able to successfully generate 9 fetal echocardiographic views using diagnostic planes in 94% to 100% of cases, VIS‐Assistance in 100% of cases, and a combination of diagnostic planes and/or VIS‐Assistance in 100% of cases (Table 2). Figure 1 and Video 1 show an example of 9 normal cardiac diagnostic planes in a single template with the additional feature of automatic labeling through intelligent navigation.
Table 2.
Fetal Intelligent Navigation Echocardiography: Success Rates of Obtaining 9 Fetal Echocardiographic Views After Applying Intelligent Navigation to 100 Normal STIC Volume Data Sets Using Diagnostic Planes and/or VIS‐Assistance
| Diagnostic Plane (n = 100) | VIS‐Assistance (n = 100) | Diagnostic Plane and/or VIS‐Assistance (n = 100) | ||||
|---|---|---|---|---|---|---|
| Fetal Echocardiographic View | n (%) | 95% CIa | n (%) | 95% CIa | n (%) | 95% CIa |
| 1. 4‐chamber | 97 (97) | 91–99 | 100 (100) | 96–100 | 100 (100) | 96–100 |
| 2. 5‐chamber | 99 (99) | 94–>99.9 | 100 (100) | 96–100 | 100 (100) | 96–100 |
| 3. LVOT | 99 (99) | 94–>99.9 | 100 (100) | 96–100 | 100 (100) | 96–100 |
| 4. Short axis view of great vessels/RVOT | 97 (97) | 91–99 | 100 (100) | 96–100 | 100 (100) | 96–100 |
| 5. 3VT | 99 (99) | 94–>99.9 | 100 (100) | 96–100 | 100 (100) | 96–100 |
| 6. Abdomen/stomach | 100 (100)b | 96–100 | 100 (100)c | 96–100 | 100 (100) | 96–100 |
| 7. Ductal arch | 95 (95) | 95–100 | 100 (100) | 96–100 | 100 (100) | 96–100 |
| 8. Aortic arch | 98 (98) | 95–100 | 100 (100) | 96–100 | 100 (100) | 96–100 |
| 9. SVC/IVC | 94 (94) | 95–100 | 100 (100) | 96–100 | 100 (100) | 96–100 |
| • SVC | 97 (97) | 91–99 | ||||
| • IVC | 97 (97) | 91–99 | ||||
CI indicates confidence interval; IVC, inferior vena cava; LVOT, left ventricular outflow tract; RVOT, right ventricular outflow tract; SVC, superior vena cava; and 3VT, 3 vessels and trachea.
The Wald method was used to calculate 2‐sided CIs for proportions expressed in the table; as the true proportion cannot exceed 100%, upper confidence limits are truncated at 100%.
Defined as visualization of the stomach in the diagnostic plane.
Defined as visualization of both the stomach and 4‐chamber view in VIS‐Assistance (to determine situs).
Figure 1.
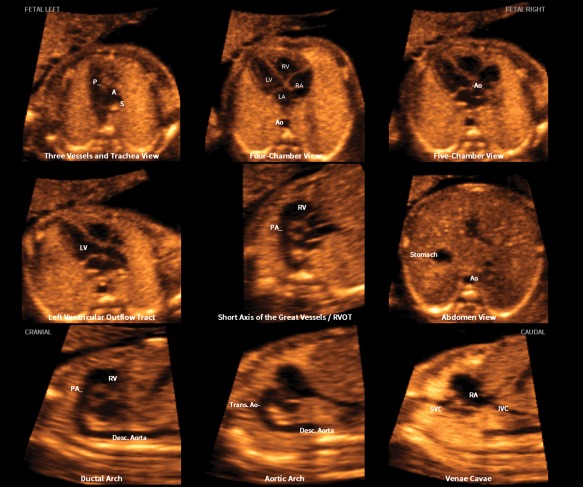
Application of the FINE method to a fetus with a normal heart. Nine normal cardiac diagnostic planes in a single template are shown with the unique feature of automatic labeling (through intelligent navigation) of each plane, anatomic structures, fetal left and right sides, and cranial and caudal ends (also see Video 1). The labeling is distinctive because it stays with the corresponding anatomical structure(s), even as the image is increased or decreased in size. A indicates transverse aortic arch; Ao, aorta; Desc., descending; IVC, inferior vena cava; LA, left atrium; LV, left ventricle; P, pulmonary artery; PA, pulmonary artery; RA, right atrium; RV, right ventricle; RVOT, right ventricular outflow tract; S, superior vena cava; SVC, superior vena cava; and Trans., transverse
Congenital Heart Disease Cases Evaluated by FINE
Of the 50 cases, the main categories of CHD included (Table 3): (1) conotruncal anomalies (42%; n = 21); (2) left heart anomalies (20%; n = 10); (3) right heart anomalies (16%; n = 8); (4) complex cardiac defects (12%; n = 6); and (5) septal defects (10%; n = 5).
Table 3.
Main Categories of Congenital Heart Defects Evaluated by the FINE Method
| Congenital Heart Defect | n (%) |
|---|---|
| Conotruncal anomalies | 21 (42) |
| Tetralogy of Fallota | 9 (18) |
| DORV | 5 (10) |
| Complete TGV | 4 (8) |
| Pulmonary atresia with VSD | 2 (4) |
| Truncus arteriosus | 1 (2) |
| Left heart anomalies | 10 (20) |
| Coarctation of aorta | 5 (10) |
| Hypoplastic left heart | 4 (8) |
| Interrupted aortic arch | 1 (2) |
| Right heart anomalies | 8 (16) |
| Pulmonary atresia with intact ventricular septum | 3 (6) |
| Tricuspid atresia with VSD | 2 (4) |
| Pulmonary stenosis | 2 (4) |
| Ebstein anomaly | 1 (2) |
| Complex cardiac defects | 6 (12) |
| Small RV, VSD, small pulmonary artery, large pericardial effusion | 1 (2) |
| Hypoplastic left heart, DORV, TGV, stomach on fetal right side | 1 (2) |
| Double inlet left ventricle, TGV, pulmonary stenosis, ASD, left SVC | 1 (2) |
| Double inlet right ventricle, DORV, coarctation of aorta | 1 (2) |
| Interrupted IVC with azygos vein continuation; stomach on right side | 1 (2) |
| LV noncompaction, dilated cardiomyopathy, left SVC, secundum ASD, VSD, hypoplastic tricuspid valve | 1 (2) |
| Septal defects | 5 (10) |
| Atrioventricular septal defect | 3 (6) |
| VSD | 2 (4) |
| Total | 50 |
ASD indicates atrial septal defect; DORV, double‐outlet right ventricle; IVC, inferior vena cava; LV, left ventricle; RV, right ventricle; SVC, superior vena cava; TGV, transposition of the great vessels; and VSD, ventricular septal defect.
Includes the following types (n = 3): (1) tetralogy of Fallot with right aortic arch; (2) tetralogy of Fallot with absent pulmonary valve; and (3) tetralogy of Fallot with absent pulmonary valve, right aortic arch, vascular ring.
STICLoop Criteria
All STIC volumes of CHD were initially evaluated by STICLoop criteria (Table 1). Two of the 9 criteria were met 98% of the time. For example, 98% (98 of 100) of the STIC volumes included the upper mediastinum and stomach, which were clearly visible. However, 50% (25 of 50) of the STIC volumes of CHD contained motion artifacts. Moreover, the investigator recorded that 20% (10 of 50) and 26% (13 of 50) of CHD volumes were characterized by inadequate image quality and shadowing, respectively.
Intelligent and Marking Alerts
For CHD, while marking anatomic structures of the fetal heart within the STIC volume (using the feature Anatomic Box), an intelligent alert automatically appeared in 42% (21 of 50): (1) spine location alert in 20% (n = 10); (2) breech alert in 12% (n = 6); and (3) possible drifting spine alert in 10% (n = 5). Therefore, 12% of the fetuses were in an original breech presentation so that the cardiac apex was originally pointing to the right side of the monitor screen. The spine location alert was automatically activated during the marking process because the fetal spine in the STIC volume was located in a position other than between 5 and 7 o'clock: (1) 7 to 8 o'clock in 50% (n = 5); (2) 4 to 5 o'clock in 30% (n = 3); and (3) 4 o'clock in 20% (n = 2). For each spine location alert activated, 3 marking alerts (pulmonary valve, superior vena cava, and transverse aortic arch) appeared next in sequence.
Abnormal Fetal Echocardiographic Views Generated by FINE
Eighty percent (40 of 50) of the STIC volumes of CHD had 6 to 9 abnormal fetal echocardiographic views (diagnostic planes and/or VIS‐Assistance; Figures 2 and 3 and Videos 2 and 3): (1) 9 abnormal views (8%; n = 4); (2) 8 abnormal views (42%; n = 21); (3) 7 abnormal views (16%; n = 8); and (4) 6 abnormal views (14%; n = 7). Therefore, of all the CHD cases, approximately half had 8 abnormal fetal echocardiographic views generated by FINE, making it highly likely that an operator would recognize the heart to be abnormal.
Figure 2.
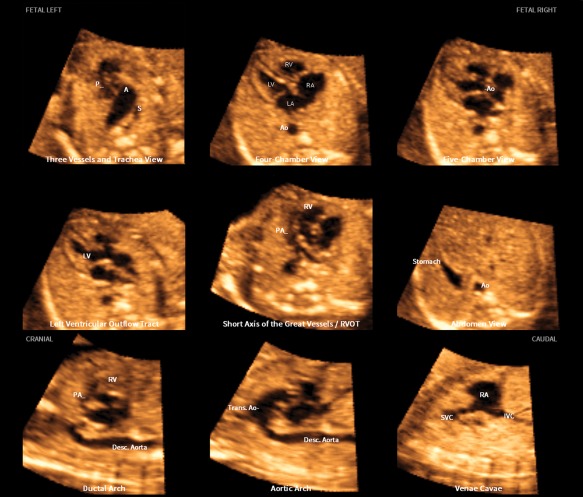
Application of the FINE method to a fetus with tetralogy of Fallot at 23 weeks' gestation (diagnostic planes or VIS‐Assistance with automatic labeling are shown; also see Video 2). Six echocardiographic views were abnormal and demonstrate the typical features of this cardiac defect. The 3‐vessels and trachea view shows a narrow pulmonary artery caused by stenosis, while the transverse aortic arch is prominent. There is a “Y‐shaped” appearance of the great vessels. As is commonly noted in conotruncal abnormalities, the 4‐chamber view appeared normal in the diagnostic plane; however, VIS‐Assistance demonstrates a large ventricular septal defect (not shown here). The 5‐chamber view shows a ventricular septal defect. The left ventricular outflow tract view shows an overriding aorta, dilated aortic root, and ventricular septal defect. In the short‐axis view of great vessels/right ventricular outflow tract (obtained via VIS‐Assistance), the pulmonary artery is narrow with a tortuous ductus arteriosus. There is difficulty in visualizing a normal ductal arch. In the aortic arch view, the aortic root is dilated and there is a prominent ascending aorta. A indicates transverse aortic arch; Ao, aorta; Desc., descending; IVC, inferior vena cava; LA, left atrium; LV, left ventricle; P, pulmonary artery; PA, pulmonary artery; RA, right atrium; RV, right ventricle; RVOT, right ventricular outflow tract; S, superior vena cava; SVC, superior vena cava; and Trans., transverse.
Figure 3.
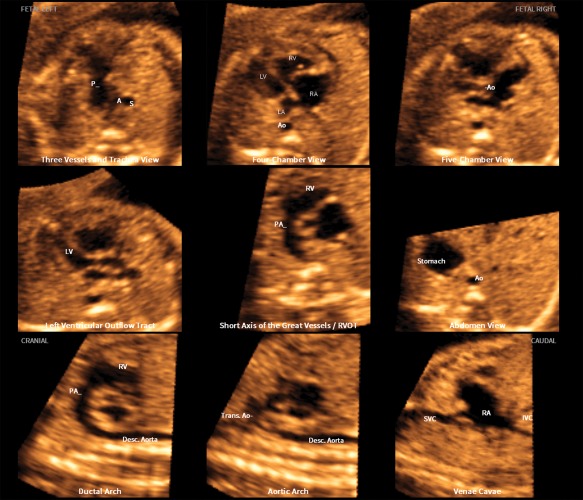
Application of the FINE method to a fetus with coarctation of the aorta at 24 weeks' gestation (diagnostic planes or VIS‐Assistance with automatic labeling are shown; also see Video 3). Seven echocardiographic views are abnormal. The 3‐vessels and trachea view shows a narrow transverse aortic arch. In the 4‐chamber view, the left side of the heart is smaller than the right side; however, the left ventricle is apex forming. The right side of the heart appears enlarged, with the right ventricle being moderately dilated and hypertrophied. The 5‐chamber view shows similar findings to that of the 4‐chamber view. In addition, there is a narrow aortic root. The left ventricular outflow tract view shows a narrow aorta (obtained via VIS‐Assistance). In the short‐axis view of great vessels/right ventricular outflow tract, the cross‐section of the aorta is small compared to the pulmonary artery. The enlarged right atrium is apparent. The ductal arch view demonstrates that the cross‐section of the aorta is small compared to the pulmonary artery. In the aortic arch view, the coarctation is demonstrated as hypoplasia and narrowing of the transverse aortic arch as well as in the isthmus region. A indicates transverse aortic arch; Ao, aorta; Desc., descending; IVC, inferior vena cava; LA, left atrium; LV, left ventricle; P, pulmonary artery; PA, pulmonary artery; RA, right atrium; RV, right ventricle; RVOT, right ventricular outflow tract; S, superior vena cava; SVC, superior vena cava; and Trans., transverse.
Of all the cases, 96% (48 of 50) and 94% (47 of 50) had an abnormal 5‐chamber view and left ventricular outflow tract view via FINE, respectively, making these the most frequent abnormal fetal echocardiographic views in our study. In contrast, only 10% (5 of 50) of CHD cases had an abnormal vena cava view.
VIS‐Assistance and Automatic Labeling
When implementing VIS‐Assistance for the CHD cases, the following advantages were noted (Table 4): (1) depicted cardiac abnormalities when the diagnostic plane appeared normal; (2) provided further diagnostic information even when the diagnostic plane was abnormal (Figure 4 and Video 4); (3) changed a suspected abnormal fetal echocardiographic view to one that was interpreted as normal; and (4) improved the success of obtaining the fetal echocardiographic view of interest when this was not initially obtained via the diagnostic plane.
Table 4.
Characteristics of FINE Fundamental in Determining Whether the Fetal Heart Is Abnormal and in Determining the Specific Cardiac Diagnosis
| Characteristic | FINE |
|---|---|
| General | Automatic realignment of STIC volume and reorientation and standardization of the anatomic position so that the fetus and cardiac diagnostic planes are consistently displayed in the same manner each time |
| No manual navigation |
• Manual standardization or manipulation of the STIC volume data set and reference planes is not required (eg, alignment or rotation) • Reduced operator dependency • Examination of the fetal heart is standardized and simplified |
| STICLoop | While the 2‐dimensional cine loop is automatically scrolling in a continuous fashion, abnormal cardiac anatomy is often displayed |
| Marking of anatomic structures within the STIC volume | During the marking process, sometimes it is evident that: (1) anatomic structures are absent; (2) anatomic structures are not in their usual location; or (3) marking anatomic structures is difficult. One explanation is the presence of CHD |
| Cardiac diagnostic planes |
• All 9 planes (including transverse and sagittal) are displayed simultaneously in a single template • Various features of the CHD can be visualized and compared side‐by‐side (eg, overriding aorta, ventricular septal defect, pulmonary stenosis in tetralogy of Fallot) • The same specific abnormality can be confirmed in multiple echocardiographic views at the same time (eg, hypoplasia of the pulmonary artery seen in the 3VT view, short‐axis view of great vessels/right ventricular outflow tract, and ductal arch view) |
| VIS‐Assistance |
• Depiction of cardiac abnormalities when the diagnostic plane appears normal • Provides further diagnostic information even when the diagnostic plane is abnormal • Changes a suspected abnormal fetal echocardiographic view to one that is interpreted as normal • Improves the success of obtaining the fetal echocardiographic view of interest when this is not initially obtained via the diagnostic plane • Double, triple VIS‐Assistance techniques |
| Automatic labeling | Assists in identification of fetal echocardiographic views, anatomic structures (atrial and ventricular chambers, great vessels, venae cavae and stomach), left and right side of fetus, and cranial and caudal ends |
| Color Doppler FINE | Depicts abnormal fetal cardiac anatomy and/or hemodynamic flow characteristics |
3VT indicates 3 vessels and trachea.
Figure 4.
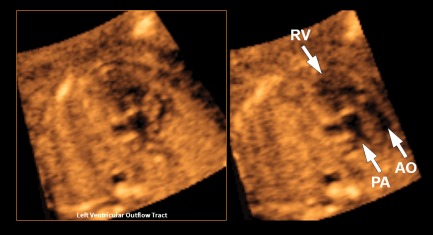
VIS‐Assistance of the left ventricular outflow tract view in a 19‐week fetus with hypoplastic left heart, double outlet right ventricle, transposition of the great vessels, and fetal stomach on the right side (also see Video 4). The abnormal diagnostic plane (left panel) demonstrates a single vessel arising from the right ventricle that is consistent with the pulmonary artery due to its bifurcation. A second vessel could not be clearly identified. However, when VIS‐Assistance is activated (right panel), automatic navigational movements now demonstrate a second vessel (aorta) that is rightward and anterior and exiting the right ventricle. These 2 vessels are parallel and side by side, consistent with transposition. AO indicates aorta; PA, pulmonary artery; and RV, right ventricle.
To gain further assistance in determining the CHD diagnosis and obtaining more information, the investigator employed double VIS‐Assistance (Table 1) in 56% (28 of 50) of the STIC volumes for 1 to 3 fetal echocardiographic views. On the other hand, triple VIS‐Assistance (Table 1) was activated for only 10% (5 of 50) of the STIC volumes for 1 to 2 fetal echocardiographic views.
It is noteworthy that although the fetal cardiac anatomy was abnormal for all 50 cases, the FINE method was able to automatically generate the correct labeling of anatomic structures (atrial and ventricular chambers, great vessels, venae cavae, and stomach) for all 9 fetal echocardiographic views in 74% (37 of 50) of cases.
Diagnostic Performance of FINE
The overall sensitivity, specificity, and positive and negative likelihood ratios of FINE for the prenatal detection of CHD were 98% (49 of 50), 93% (93 of 100), 14, and 0.02, respectively. The 7 false‐positive diagnoses included: (1) 6 fetuses suspected to have ventricular septal defects (gestational ages ranged from 19 to 31 weeks); and (2) 1 fetus with a suspected cardiac tumor in the right ventricle (29 weeks' gestation). The single false‐negative case was a fetus examined by FINE at 26 weeks' gestation who was later diagnosed with pulmonary artery branch stenosis and a small secundum atrial septal defect in the neonatal period.
Complete concordance (category A) or a minor discrepancy (category B) between the diagnosis made on the basis of FINE and the final CHD diagnosis occurred in 86% (43 of 50) of cases: (1) complete concordance (54%; n = 27); and (2) minor discrepancy (32%; n = 16). All of the discordant diagnoses in category B were related to: (1) secundum atrial septal defect (75%; n = 12); (2) ventricular septal defect (19%; n = 3); (3) left superior vena cava (19%; n = 3); and (4) primum atrial septal defect (6%; n = 1). Major differences (category C) between the FINE and final CHD diagnoses occurred in only 14% (n = 7) of cases (Table 5).
Table 5.
Details of CHD Cases With Major Discordancy Between the FINE Diagnosis Compared to Final Diagnosis
| GA, wk | FINE Diagnosis | Final Diagnosis |
|---|---|---|
| 19 | Complete atrioventricular septal defect, univentricle, large VSD, pulmonary stenosis, DORV, TGV, stomach on fetal right side | Hypoplastic left heart, DORV, TGV, stomach on fetal right side |
| 21 | Tetralogy of Fallot | Pulmonary atresia, VSD |
| 22 | Left ventricle smaller than right ventricle, dilated left atrium, mitral dysplasia, aortic stenosis, VSD, DORV, left SVC, small pulmonary artery | Variant of hypoplastic left heart, mitral dysplasia, aortic atresia, VSD |
| 26 | Normal | Pulmonary artery branch stenosis, small secundum ASD |
| 30 | Tetralogy of Fallot | Pulmonary atresia, VSD, supravalvular aortic stenosis |
| 35 | LV noncompaction, dilated cardiomyopathy, left SVC, VSD, aortic stenosis, small pulmonary artery and ductus arteriosus, hypoplastic and dysplastic tricuspid valve | LV noncompaction, dilated cardiomyopathy, left SVC, VSD, secundum ASD, hypoplastic tricuspid valve |
| 37 | Tetralogy of Fallot | DORV, VSD, pulmonary stenosis, left SVC |
ASD indicates atrial septal defect; DORV, double‐outlet right ventricle; GA, gestational age; LV, left ventricle; SVC, superior vena cava; TGV, transposition of great vessels; and VSD, ventricular septal defect.
It is noteworthy that when we excluded from category B cases in which the only discrepancy between the FINE diagnosis and the final CHD diagnosis was a secundum atrial septal defect, then 10 cases would be reclassified into category A with the following new classification results: (1) complete concordance (74%; n = 37); (2) minor discrepancy (12%; n = 6); and (3) major discrepancy (14%; n = 7). Such reclassification is justified because the diagnosis of a secundum atrial septal defect is almost nonexistent in the prenatal period.81
Discussion
Principal Findings of the Study
After applying the FINE method to STIC volume data sets acquired with grayscale information in second‐ and third‐trimester fetuses with normal and abnormal hearts: (1) the sensitivity, specificity, and positive and negative likelihood ratios of FINE for the prenatal detection of CHD were 98%, 93%, 14, and 0.02, respectively; (2) among cases with confirmed CHD, the diagnosis made on the basis of FINE completely matched the final diagnosis in 74%; minor discrepancies were seen in 12%; and major discrepancies were seen in 14%; (3) 80% of the CHD cases had 6 to 9 abnormal fetal echocardiographic views out of 9, making it highly likely that the heart would be recognized as abnormal; (4) automatic labeling of anatomic structures in all 9 fetal echocardiographic views was correct in 74% of CHD cases; and (5) 9 fetal echocardiographic views were generated successfully in normal hearts using diagnostic planes in 94% to 100% of cases, VIS‐Assistance in 100% of cases, and a combination of diagnostic planes and/or VIS‐Assistance in 100% of cases.
Performance of 4D Sonography With STIC in the Prenatal Detection of Congenital Heart Disease
At the time of its original conception and development, we proposed using the FINE method as an aid to examine the fetal heart in the population at large, rather than to diagnose specific CHD.76 Indeed, we had speculated that for some types of CHD, the FINE method may not be useful or applicable (eg, hypoplastic left heart and atrioventricular canal defect).76 However, since that time we have gained considerable experience in the application of FINE and color Doppler FINE to a broad spectrum of CHD types65, 76, 79 and concluded that such technology is both successful and informative. For example, in a case of fetal dextrocardia and situs solitus with complex CHD, FINE was invaluable in diagnosing multiple abnormalities and defining complex anatomic relationships, which had been very difficult to do using real‐time echocardiography.79
However, for a test to be clinically useful, it should demonstrate both high specificity and sensitivity. As a result, we set out to determine the diagnostic performance of FINE and report herein for the first time that there is 98% sensitivity and 93% specificity for the prenatal detection of a broad spectrum of CHD. This outcome was obtained despite the fact that approximately 20% of STIC volumes of normal hearts had shadowing present or contained motion artifacts. Moreover, STIC volumes of CHD were characterized by inadequate image quality, shadowing, or motion artifacts in 20%, 26%, and 50% of cases, respectively. Therefore, not all the volumes analyzed in this study were ideal or optimal. Recently, an electronic matrix 4D probe has been developed that allows a marked reduction in the STIC volume acquisition time (which could lead to fewer motion artifacts), as well as improved image resolution in the B plane.54, 82 Such electronic STIC volumes have been shown to be of more significant optimal diagnostic quality compared to conventional STIC volumes.83 Therefore, this new STIC technology could be advantageous for the FINE method.
Likelihood ratios summarize how many times more (or less) likely patients with the disease are to have that particular result than are patients without the disease.84 The further likelihood ratios are from 1, the stronger the evidence for the presence or absence of disease. Indeed, likelihood ratios greater than 10 and less than 0.1 are considered to provide strong evidence to rule in or rule out diagnoses, respectively, in most circumstances.84, 85 Thus, in this study, the positive (14) and negative (0.02) likelihood ratios indicate that results of the FINE method have a large effect on increasing or decreasing, respectively, the probability of the presence of CHD.
Prior investigators have examined the diagnostic performance of 4D sonography with STIC by using a display modality86 or manual navigation (retrieval and display of relevant cardiac diagnostic planes by interrogating volume data sets).69, 71, 72, 73 Bennasar et al71 used the multiplanar view to analyze STIC volumes of normal and abnormal fetal hearts and reported an overall sensitivity and specificity of 94.9% (166 of 175) and 88.1% (141 of 160), respectively. STIC volumes had been acquired with both grayscale and color Doppler information. In a multicenter study of 7 centers with expertise in 4D sonography of the fetal heart, 90 STIC volume data sets of normal (n = 45) or abnormal fetal hearts (n = 45) acquired at 18 to 26 weeks' gestation using B‐mode or color Doppler sonography were blindly evaluated by all centers.72 The approach used to analyze the volumes was left to the discretion of each center. Overall, the median (range) sensitivity and specificity for the identification of fetuses with CHD were 93% (77%–100%) and 96% (84%–100%).72 Years ago, our team used tomographic ultrasound imaging in combination with B‐mode STIC volume data sets to diagnose CHD and reported a sensitivity and specificity of 85.7% (12 of 14) and 100% (101 of 101), respectively.86 However, such study performance was based on only 14 cases with CHD. Paladini et al69 investigated whether 14 sonographers could detect major abnormalities of the outflow tracts by reviewing the A plane of 16 normal and 10 abnormal STIC volume data sets. The sensitivity and specificity were reported as 83% and 87%, respectively. The individual diagnostic accuracy ranged from 66% to 100% (median, 85.5%).69 In contrast, Adriaanse et al73 evaluated the clinical accuracy of 4D ultrasound with STIC in a telemedicine setting. Ten STIC volumes from the second trimester were analyzed by 3 different observers using any desired postprocessing modality (eg, sectional planes, rendering, or tomographic ultrasound imaging). The authors concluded that while STIC by telemedicine is promising, it was not accurate enough for exclusive use in clinical decision making regarding treatment or prognosis.73
Taken together, while most of these studies reported high sensitivity and specificity in the detection of CHD, STIC volume analysis required manual standardization or manipulation of the data set and reference planes by the operator, or was dependent on the tomographic ultrasound imaging display. Yet operator dependency is reported to be the main problem when STIC volumes are analyzed.66 On the other hand, the FINE method substantially decreases the number of steps required to obtain echocardiographic views, making this method less operator dependent and considerably simplifying the fetal cardiac examination.76 The sonologist is only required to mark anatomic structures of the fetal heart within the STIC volume data set, triggering intelligent navigation technology and resulting in the successful generation of 9 echocardiographic views automatically (this occurred in 100% of normal hearts via a combination of diagnostic planes and/or VIS‐Assistance for the study herein). For CHD cases analyzed using FINE, abnormal cardiac anatomy was evident in multiple echocardiographic views simultaneously. A summary of the characteristics of the FINE method that are fundamental in determining whether the fetal heart is abnormal and in determining the type of CHD, are described in Table 4.
Comparisons Between FINE and Final Diagnoses
Seven false‐positive diagnoses of ventricular septal defects were made on the basis of FINE, which would not have had a major impact on the clinical outcome. Similarly, Bennasar et al71 reported that 52% (10 of 19) of their false‐positive diagnoses from STIC echocardiography were ventricular septal defects. The accurate prenatal detection of ventricular septal defects can be influenced by both the location and size of the defect, as well as dropout artifacts. It is possible that if STIC volumes had been obtained with color or power Doppler information and analyzed by FINE (ie, color Doppler FINE) 65 in this study, the number of false‐positive diagnoses would have been reduced.
Among cases with confirmed CHD, the diagnosis made on the basis of FINE completely matched the final diagnosis in 74%; minor discrepancies were seen in 12%; and major discrepancies were seen in 14%. Such results are very similar to a different study in which the authors examined the concordance of prenatal and postnatal echocardiographic examinations performed by specialized pediatric cardiologists in a large referral center.87 Overall, the prenatal diagnosis precisely matched the postnatal diagnosis in 69.8%; minor discrepancies were seen in 14.2%; and major differences were seen in 16% of cases. Similarly, among cases with confirmed CHD, Bennasar et al71 reported that with use of STIC echocardiography, there was absolute concordance with the final diagnosis in 74.3%.
Of the 7 cases with major differences between the FINE diagnosis and final CHD diagnosis (category C), 43% (n = 3) were conotruncal abnormalities (Table 5). Prior studies have shown that conotruncal anomalies are the CHD in which discrepancies with the postnatal diagnosis are the most common.88, 89, 90, 91
Clinical Implications of the Study
On the basis of the study herein, there are several clinical implications regarding the FINE method: (1) it may be used to assess fetuses with normal hearts and a broad spectrum of CHD with a high sensitivity and specificity; (2) it can be considered both a cardiac screening and diagnostic tool in the clinical setting; (3) because STIC volume data sets, diagnostic planes, and VIS‐Assistance video clips can be transmitted by telemedicine for expert consultation, the outreach of fetal cardiac imaging is expanded, which could favorably impact the current sensitivity of CHD; and (4) it can be used for educational and training purposes.
Limitations
In cases of CHD, only a single STIC volume per fetus was examined using FINE. It is possible that examining multiple STIC volume data sets at a given gestational age or examining STIC volumes over time would have led to improved and/or different diagnostic information (eg, some CHDs are progressive). The FINE method is not meant to replace real‐time fetal echocardiography because the latter allows evaluation of cardiac rate or rhythm disturbances, the performance of pulsed wave Doppler velocimetry, and so on. Therefore, when fetal CHD is suspected, such patients should undergo a real‐time sonographic examination by an appropriate sonologist (eg, pediatric cardiologist). Finally, studies are required to determine the learning curve for FINE among operators with varying levels of experience.
Conclusions
We present herein for the first time the sensitivity and specificity of the FINE method in fetuses with normal hearts and CHD in the second to third trimesters. Because FINE identifies a broad spectrum of CHD with 98% sensitivity, this method could be used prenatally to screen for and diagnose CHD.
Supporting information
Videos online at http://jultrasoundmed.org/journal/jum
Video 1. Nine normal cardiac diagnostic planes in a single template with the unique feature of automatic labeling (through intelligent navigation) of each plane, anatomic structures, fetal left and right sides, and cranial and caudal ends (also see Figure 1). The labeling is distinctive because it stays with the corresponding anatomical structure(s), even as the image is increased or decreased in size. A indicates transverse aortic arch; Ao, aorta; Desc., descending; IVC, inferior vena cava; LA, left atrium; LV, left ventricle; P, pulmonary artery; PA, pulmonary artery; RA, right atrium; RV, right ventricle; RVOT, right ventricular outflow tract; S, superior vena cava; SVC, superior vena cava; and Trans., transverse.
Video 2. Application of the FINE method to a fetus with tetralogy of Fallot at 23 weeks' gestation (diagnostic planes or VIS‐Assistance with automatic labeling are shown; also see Figure 2). Six echocardiographic views were abnormal and demonstrate the typical features of this cardiac defect. The 3‐vessel and trachea view shows a narrow pulmonary artery caused by stenosis, while the transverse aortic arch is prominent. There is a “Y‐shaped” appearance of the great vessels. As is commonly noted in conotruncal abnormalities, the 4‐chamber view appeared normal in the diagnostic plane; however, VIS‐Assistance demonstrates a large ventricular septal defect (not shown here). The 5‐chamber view shows a ventricular septal defect. The left ventricular outflow tract view shows an overriding aorta, dilated aortic root, and ventricular septal defect. In the short‐axis view of great vessels/right ventricular outflow tract (obtained via VIS‐Assistance), the pulmonary artery is narrow with a tortuous ductus arteriosus. There is difficulty in visualizing a normal ductal arch. In the aortic arch view, the aortic root is dilated and there is a prominent ascending aorta. A indicates transverse aortic arch; Ao, aorta; Desc., descending; IVC, inferior vena cava; LA, left atrium; LV, left ventricle; P, pulmonary artery; PA, pulmonary artery; RA, right atrium; RV, right ventricle; RVOT, right ventricular outflow tract; S, superior vena cava; SVC, superior vena cava; and Trans., transverse.
Video 3. Application of the FINE method to a fetus with coarctation of the aorta at 24 weeks' gestation (diagnostic planes or VIS‐Assistance with automatic labeling are shown; also see Figure 3). Seven echocardiographic views are abnormal. The 3‐vessel and trachea view shows a narrow transverse aortic arch. In the 4‐chamber view, the left side of the heart is smaller than the right side; however, the left ventricle is apex forming and there is good movement of the mitral valve. The right side of the heart appears enlarged, with the right ventricle being moderately dilated and hypertrophied. The 5‐chamber view shows similar findings to that of the 4‐chamber view. In addition, there is a narrow aortic root. The left ventricular outflow tract view shows a narrow aorta (obtained via VIS‐Assistance). In the short‐axis view of great vessels/right ventricular outflow tract, the cross‐section of the aorta is small compared to the pulmonary artery. The enlarged right atrium is apparent. The ductal arch view demonstrates that the cross‐section of the aorta is small compared to the pulmonary artery. In the aortic arch view, the coarctation is demonstrated as hypoplasia and narrowing of the transverse aortic arch as well as in the isthmus region. A indicates transverse aortic arch; Ao, aorta; Desc., descending; IVC, inferior vena cava; LA, left atrium; LV, left ventricle; P, pulmonary artery; PA, pulmonary artery; RA, right atrium; RV, right ventricle; RVOT, right ventricular outflow tract; S, superior vena cava; SVC, superior vena cava; and Trans., transverse.
Video 4. VIS‐Assistance of the left ventricular outflow tract view in a 19‐week fetus with a hypoplastic left heart, double‐outlet right ventricle, transposition of the great vessels, and fetal stomach on the right side (also see Figure 4). The abnormal diagnostic plane (left panel) demonstrates a single vessel arising from the right ventricle that is consistent with the pulmonary artery due to its bifurcation. A second vessel could not be clearly identified. However, when VIS‐Assistance is activated (right panel), automatic navigational movements now demonstrate a second vessel (aorta) that is rightward and anterior and exiting the right ventricle. These 2 vessels (indicated by white arrows) are parallel and side by side, consistent with transposition.
An application for a patent (“Apparatus and Method for Fetal Intelligent Navigation Echocardiography”) has been filed with the US Patent and Trademark Office, and the patent is pending. Dr Lami Yeo and Dr Roberto Romero are coinventors, along with Mr Gustavo Abella and Mr Ricardo Gayoso. The rights of Drs Yeo and Romero have been assigned to Wayne State University and the National Institute of Child Health and Human Development/National Institutes of Health (NICHD/NIH), respectively.
The work of Dr Romero was supported by the Perinatology Research Branch, Division of Intramural Research, NICHD, NIH, Department of Health and Human Services (DHHS). Dr Romero has contributed to this work as part of his official duties as an employee of the US federal government. Drs Lami Yeo and Suchaya Luewan were funded by Wayne State University through a service contract in support of the Perinatology Research Branch.
This research was supported, in part, by the Perinatology Research Branch, Division of Intramural Research, NICHD, NIH, DHHS; and, in part, with federal funds from NICHD, NIH, under contract No. HHSN275201300006C.
Contributor Information
Lami Yeo, Email: lyeo@med.wayne.edu.
Roberto Romero, Email: prbchiefstaff@med.wayne.edu.
References
- 1. Simeone RM, Feldkamp ML, Reefhuis J, et al. CDC Grand Rounds: understanding the causes of major birth defects: steps to prevention. MMWR Morb Mortal Wkly Rep 2015; 64:1104–1107. [DOI] [PubMed] [Google Scholar]
- 2. Yang Q, Chen H, Correa A, Devine O, Mathews TJ, Honein MA. Racial differences in infant mortality attributable to birth defects in the United States, 1989–2002. Birth Defects Res A Clin Mol Teratol 2006; 76:706–713. [DOI] [PubMed] [Google Scholar]
- 3. Allan L. Antenatal diagnosis of heart disease. Heart 2000; 83:367–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Friedberg MK, Silverman NH, Moon‐Grady AJ, et al. Prenatal detection of congenital heart disease. J Pediatr 2009; 155:26–31. [DOI] [PubMed] [Google Scholar]
- 5. Jaeggi ET, Sholler GF, Jones OD, Cooper SG. Comparative analysis of pattern, management and outcome of pre‐ versus postnatally diagnosed major congenital heart disease: a population‐based study. Ultrasound Obstet Gynecol 2001; 17:380–385. [DOI] [PubMed] [Google Scholar]
- 6. Acherman RJ, Evans WN, Luna CF, et al. Prenatal detection of congenital heart disease in southern Nevada: the need for universal fetal cardiac evaluation. J Ultrasound Med 2007; 26:1715–1719. [DOI] [PubMed] [Google Scholar]
- 7. Garne E, Stoll C, Clementi M. Evaluation of prenatal diagnosis of congenital heart diseases by ultrasound: experience from 20 European registries. Ultrasound Obstet Gynecol 2001; 17:386–391. [DOI] [PubMed] [Google Scholar]
- 8. Westin M, Saltvedt S, Bergman G, et al. Routine ultrasound examination at 12 or 18 gestational weeks for prenatal detection of major congenital heart malformations? A randomized controlled trial comprising 36,299 fetuses. BJOG 2006; 113:675–682. [DOI] [PubMed] [Google Scholar]
- 9. Nikkila A, Bjorkhem G, Kallen B. Prenatal diagnosis of congenital heart defects: a population based study. Acta Paediatr 2007; 96:49–52. [DOI] [PubMed] [Google Scholar]
- 10. Pinto NM, Keenan HT, Minich LL, Puchalski MD, Heywood M, Botto LD. Barriers to prenatal detection of congenital heart disease: a population based study. Ultrasound Obstet Gynecol 2012; 40:418–425. [DOI] [PubMed] [Google Scholar]
- 11. McBrien A, Sands A, Craig B, Dornan J, Casey F. Major congenital heart disease: antenatal detection, patient characteristics and outcomes. J Matern Fetal Neonatal Med 2009; 22:101–105. [DOI] [PubMed] [Google Scholar]
- 12. Sklansky MS, Berman DP, Pruetz JD, Chang RK. Prenatal screening for major congenital heart disease: superiority of outflow tracts over the 4‐chamber view. J Ultrasound Med 2009; 28:889–899. [DOI] [PubMed] [Google Scholar]
- 13. Allan L, Benacerraf B, Copel JA, et al. Isolated major congenital heart disease. Ultrasound Obstet Gynecol 2001; 17:370–379. [DOI] [PubMed] [Google Scholar]
- 14. Carvalho JS, Mavrides E, Shinebourne EA, Campbell S, Thilaganathan B. Improving the effectiveness of routine prenatal screening for major congenital heart defects. Heart 2002; 88:387–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hunter S, Heads A, Wyllie J, Robson S. Prenatal diagnosis of congenital heart disease in the northern region of England: benefits of a training programme for obstetric ultrasonographers. Heart 2000; 84:294–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Rustico MA, Benettoni A, D'Ottavio G, et al. Early screening for fetal cardiac anomalies by transvaginal echocardiography in an unselected population: the role of operator experience. Ultrasound Obstet Gynecol 2000; 16:614–619. [DOI] [PubMed] [Google Scholar]
- 17. Tegnander E, Eik‐Nes SH. The examiner's ultrasound experience has a significant impact on the detection rate of congenital heart defects at the second‐trimester fetal examination. Ultrasound Obstet Gynecol 2006; 28:8–14. [DOI] [PubMed] [Google Scholar]
- 18. Wong SF, Chan FY, Cincotta RB, Lee‐Tannock A, Ward C. Factors influencing the prenatal detection of structural congenital heart diseases. Ultrasound Obstet Gynecol 2003; 21:19–25. [DOI] [PubMed] [Google Scholar]
- 19. Franklin O, Burch M, Manning N, Sleeman K, Gould S, Archer N. Prenatal diagnosis of coarctation of the aorta improves survival and reduces morbidity. Heart 2002; 87:67–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Tibballs J, Cantwell‐Barti A. Outcomes of management decisions by parents for their infants with hypoplastic left heart syndrome born with and without a prenatal diagnosis. J Paediatr Child Health 2008; 44:321–324. [DOI] [PubMed] [Google Scholar]
- 21. Daubeney PE, Sharland GK, Cook AC, Keeton BR, Anderson RH, Webber SA. Pulmonary atresia with intact ventricular septum: impact of fetal echocardiography on incidence at birth and postnatal outcome. UK and Eire Collaborative Study of Pulmonary Atresia with Intact Ventricular Septum. Circulation 1998; 98:562–566. [DOI] [PubMed] [Google Scholar]
- 22. Tworetzky W, McElhinney DB, Reddy VM, Brook MM, Hanley FL, Silverman NH. Improved surgical outcome after fetal diagnosis of hypoplastic left heart syndrome. Circulation 2001; 103:1269–1273. [DOI] [PubMed] [Google Scholar]
- 23. Kumar RK, Newburger JW, Gauvreau K, Kamenir SA, Hornberger LK. Comparison of outcome when hypoplastic left heart syndrome and transposition of the great arteries are diagnosed prenatally versus when diagnosis of these two conditions is made only postnatally. Am J Cardiol 1999; 83:1649–1653. [DOI] [PubMed] [Google Scholar]
- 24. Verheijen PM, Lisowski LA, Stoutenbeek P, Hitchcock JF, Bennink GK, Meijboom EJ. Lactacidosis in the neonate is minimized by prenatal detection of congenital heart disease. Ultrasound Obstet Gynecol 2002; 19:552–555. [DOI] [PubMed] [Google Scholar]
- 25. Schultz AH, Localio AR, Clark BJ, Ravishankar C, Videon N, Kimmel SE. Epidemiologic features of the presentation of critical congenital heart disease: implications for screening. Pediatrics 2008; 121:751–757. [DOI] [PubMed] [Google Scholar]
- 26. Brown KL, Ridout DA, Hoskote A, Verhulst L, Ricci M, Bull C. Delayed diagnosis of congenital heart disease worsens preoperative condition and outcome of surgery in neonates. Heart 2006; 92:1298–1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. van Velzen CL, Haak MC, Reijnders G, et al. Prenatal detection of transposition of the great arteries reduces mortality and morbidity. Ultrasound Obstet Gynecol 2015; 45:320–325. [DOI] [PubMed] [Google Scholar]
- 28. Holland BJ, Myers JA, Woods CR Jr. Prenatal diagnosis of critical congenital heart disease reduces risk of death from cardiovascular compromise prior to planned neonatal cardiac surgery: a meta‐analysis. Ultrasound Obstet Gynecol 2015; 45:631–638. [DOI] [PubMed] [Google Scholar]
- 29. Grinenco S, Meller C, Marantz P, Izbizky G. Prenatal diagnosis of congenital heart disease: improving survival. Ultrasound Obstet Gynecol 2015; 46:633. [DOI] [PubMed] [Google Scholar]
- 30. Bonnet D, Coltri A, Butera G, et al. Detection of transposition of the great arteries in fetuses reduces neonatal morbidity and mortality. Circulation 1999; 99:916–918. [DOI] [PubMed] [Google Scholar]
- 31. Khoshnood B, De Vigan C, Vodovar V, et al. Trends in prenatal diagnosis, pregnancy termination, and perinatal mortality of newborns with congenital heart disease in France, 1983–2000: a population‐based evaluation. Pediatrics 2005; 115:95–101. [DOI] [PubMed] [Google Scholar]
- 32. Kipps AK, Feuille C, Azakie A, et al. Prenatal diagnosis of hypoplastic left heart syndrome in current era. Am J Cardiol 2011; 108:421–427. [DOI] [PubMed] [Google Scholar]
- 33. Mahle WT, Clancy RR, McGaurn SP, Goin JE, Clark BJ. Impact of prenatal diagnosis on survival and early neurologic morbidity in neonates with the hypoplastic left heart syndrome. Pediatrics 2001; 107:1277–1282. [DOI] [PubMed] [Google Scholar]
- 34. DeVore GR, Falkensammer P, Sklansky MS, Platt LD. Spatiotemporal image correlation (STIC): new technology for evaluation of the fetal heart. Ultrasound Obstet Gynecol 2003; 22:380–387. [DOI] [PubMed] [Google Scholar]
- 35. Goncalves LF, Lee W, Chaiworapongsa T, et al. Four‐dimensional ultrasonography of the fetal heart with spatiotemporal image correlation. Am J Obstet Gynecol 2003; 189:1792–1802. [DOI] [PubMed] [Google Scholar]
- 36. Chaoui R, Hoffmann J, Heling KS. Three‐dimensional (3D) and 4D color Doppler fetal echocardiography using spatio‐temporal image correlation (STIC). Ultrasound Obstet Gynecol 2004; 23:535–545. [DOI] [PubMed] [Google Scholar]
- 37. Molina FS, Faro C, Sotiriadis A, Dagklis T, Nicolaides KH. Heart stroke volume and cardiac output by four‐dimensional ultrasound in normal fetuses. Ultrasound Obstet Gynecol 2008; 32:181–187. [DOI] [PubMed] [Google Scholar]
- 38. Tutschek B, Sahn DJ. Semi‐automatic segmentation of fetal cardiac cavities: progress towards an automated fetal echocardiogram. Ultrasound Obstet Gynecol 2008; 32:176–180. [DOI] [PubMed] [Google Scholar]
- 39. Zhao L, Wu Y, Chen S, et al. Feasibility study on prenatal cardiac screening using four‐dimensional ultrasound with spatiotemporal image correlation: a multicenter study. PLoS One 2016; 11:e0157477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Avnet H, Mazaaki E, Shen O, Cohen S, Yagel S. Evaluating spatiotemporal image correlation technology as a tool for training nonexpert sonographers to perform examinations of the fetal heart. J Ultrasound Med 2016; 35:111–119. [DOI] [PubMed] [Google Scholar]
- 41. Bennasar M, Martínez JM, Olivella A, et al. Feasibility and accuracy of fetal echocardiography using four‐dimensional spatiotemporal image correlation technology before 16 weeks' gestation. Ultrasound Obstet Gynecol 2009; 33:645–651. [DOI] [PubMed] [Google Scholar]
- 42. Yeo L, Romero R. How to acquire cardiac volumes for sonographic examination of the fetal heart: part 1. J Ultrasound Med 2016; 35:1021–1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Novaes JY, Zamith MM, Araujo Junior E, Barreto EQ, Barros FS, Moron AF. Screening of congenital heart diseases by three‐dimensional ultrasound using spatiotemporal image correlation: influence of professional experience. Echocardiography 2016; 33: 99–104. [DOI] [PubMed] [Google Scholar]
- 44. Jantarasaengaram S, Vairojanavong K. Eleven fetal echocardiographic planes using 4‐dimensional ultrasound with spatio‐temporal image correlation (STIC): a logical approach to fetal heart volume analysis. Cardiovasc Ultrasound 2010; 8:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Crispi F, Gratacós E. Fetal cardiac function: technical considerations and potential research and clinical applications. Fetal Diagn Ther 2012; 32:47–64. [DOI] [PubMed] [Google Scholar]
- 46. Cohen L, Mangers K, Grobman WA, Platt LD. Satisfactory visualization rates of standard cardiac views at 18 to 22 weeks' gestation using spatiotemporal image correlation. J Ultrasound Med 2009; 28:1645–1650. [DOI] [PubMed] [Google Scholar]
- 47. Luewan S, Yanase Y, Tongprasert F, Srisupundit K, Tongsong T. Fetal cardiac dimensions at 14–40 weeks' gestation obtained using cardio‐STIC‐M. Ultrasound Obstet Gynecol 2011; 37:416–422. [DOI] [PubMed] [Google Scholar]
- 48. Uittenbogaard LB, Haak MC, Spreeuwenberg MD, van Vugt JM. Fetal cardiac function assessed with four‐dimensional ultrasound imaging using spatiotemporal image correlation. Ultrasound Obstet Gynecol 2009; 33:272–281. [DOI] [PubMed] [Google Scholar]
- 49. Yeo L, Romero R, Jodicke C, et al. Simple targeted arterial rendering (STAR) technique: a novel and simple method to visualize the fetal cardiac outflow tracts. Ultrasound Obstet Gynecol 2011; 37:549–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Espinoza J, Kusanovic JP, Goncalves LF, et al. A novel algorithm for comprehensive fetal echocardiography using 4‐dimensional ultrasonography and tomographic imaging. J Ultrasound Med 2006; 25:947–956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Araujo Junior E, Rolo LC, Rocha LA, Nardozza LM, Moron AF. The value of 3D and 4D assessments of the fetal heart. Int J Womens Health 2014; 6:501–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Goncalves LF, Romero R, Espinoza J, et al. Four‐dimensional ultrasonography of the fetal heart using color Doppler spatiotemporal image correlation. J Ultrasound Med 2004; 23:473–481. [DOI] [PubMed] [Google Scholar]
- 53. Turan S, Turan OM, Ty‐Torredes K, Harman CR, Baschat AA. Standardization of the first‐trimester fetal cardiac examination using spatiotemporal image correlation with tomographic ultrasound and color Doppler imaging. Ultrasound Obstet Gynecol 2009; 33:652–656. [DOI] [PubMed] [Google Scholar]
- 54. Yeo L, Romero R. How to acquire cardiac volumes for sonographic examination of the fetal heart: part 2. J Ultrasound Med 2016; 35:1043–1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Yagel S, Cohen SM, Shapiro I, Valsky DV. 3D and 4D ultrasound in fetal cardiac scanning: a new look at the fetal heart. Ultrasound Obstet Gynecol 2007; 29:81–95. [DOI] [PubMed] [Google Scholar]
- 56. Rizzo G, Capponi A, Muscatello A, Cavicchioni O, Vendola M, Arduini D. Examination of the fetal heart by four‐dimensional ultrasound with spatiotemporal image correlation during routine second‐trimester examination: the “three‐steps technique.” Fetal Diagn Ther 2008; 24:126–131. [DOI] [PubMed] [Google Scholar]
- 57. Hamill N, Yeo L, Romero R, et al. Fetal cardiac ventricular volume, cardiac output, and ejection fraction determined with 4‐dimensional ultrasound using spatiotemporal image correlation and virtual organ computer‐aided analysis. Am J Obstet Gynecol 2011; 205:76.e1–e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Barros FS, Moron AF, Rolo LC, et al. Fetal myocardial wall area: constructing a reference range by means of spatiotemporal image correlation in the rendering mode. Fetal Diagn Ther 2015; 37:44–50. [DOI] [PubMed] [Google Scholar]
- 59. DeVore GR, Polanco B, Sklansky MS, Platt LD. The “spin” technique: a new method for examination of the fetal outflow tracts using three‐dimensional ultrasound. Ultrasound Obstet Gynecol 2004; 24:72–82. [DOI] [PubMed] [Google Scholar]
- 60. Yeo L, Romero R, Jodicke C, et al. Four‐chamber view and “swing technique” (FAST) echo: a novel and simple algorithm to visualize standard fetal echocardiographic planes. Ultrasound Obstet Gynecol 2011; 37:423–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Nardozza LM, Rolo LC, Araujo E Jr, et al. Reference range for fetal interventricular septum area by means of four‐dimensional ultrasonography using spatiotemporal image correlation. Fetal Diagn Ther 2013; 33:110–115. [DOI] [PubMed] [Google Scholar]
- 62. Viñals F. Current experience and prospect of Internet consultation in fetal cardiac ultrasound. Fetal Diagn Ther 2011; 30:83–87. [DOI] [PubMed] [Google Scholar]
- 63. Godfrey ME, Messing B, Valsky DV, Cohen SM, Yagel S. Fetal cardiac function: M‐mode and 4D spatiotemporal image correlation. Fetal Diagn Ther 2012; 32:17–21. [DOI] [PubMed] [Google Scholar]
- 64. Abuhamad A, Chaoui R. Three‐dimensional fetal echocardiography: basic and advanced applications In: Abuhamad A, Chaoui R. (eds). A Practical Guide to Fetal Echocardiography: Normal and Abnormal Hearts. Philadelphia, PA: Lippincott Williams & Wilkins; 2010:110–127. [Google Scholar]
- 65. Yeo L, Romero R. Color and power Doppler combined with fetal intelligent navigation echocardiography (FINE) to evaluate the fetal heart. Ultrasound Obstet Gynecol 2017; 50:476–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Adriaanse BM, van Vugt JM, Haak MC. Three‐ and four‐dimensional ultrasound in fetal echocardiography: an up‐to‐date overview. J Perinatol 2016; 36:685–693. [DOI] [PubMed] [Google Scholar]
- 67. Espinoza J. Contemporary clinical applications of spatio‐temporal image correlation in prenatal diagnosis. Curr Opin Obstet Gynecol 2011; 23:94–102. [DOI] [PubMed] [Google Scholar]
- 68. Goncalves LF, Espinoza J, Romero R, et al. A systematic approach to prenatal diagnosis of transposition of the great arteries using 4‐dimensional ultrasonography with spatiotemporal image correlation. J Ultrasound Med 2004; 23:1225–1231. [DOI] [PubMed] [Google Scholar]
- 69. Paladini D, Sglavo G, Greco E, Nappi C. Cardiac screening by STIC: can sonologists performing the 20‐week anomaly scan pick up outflow tract abnormalities by scrolling the A‐plane of STIC volumes? Ultrasound Obstet Gynecol 2008; 32:865–870. [DOI] [PubMed] [Google Scholar]
- 70. Gindes L, Hegesh J, Weisz B, Gilboa Y, Achiron R. Three and four dimensional ultrasound: a novel method for evaluating fetal cardiac anomalies. Prenat Diagn 2009; 29:645–653. [DOI] [PubMed] [Google Scholar]
- 71. Bennasar M, Martínez JM, Gómez O, et al. Accuracy of four‐dimensional spatiotemporal image correlation echocardiography in the prenatal diagnosis of congenital heart defects. Ultrasound Obstet Gynecol 2010; 36:458–464. [DOI] [PubMed] [Google Scholar]
- 72. Espinoza J, Lee W, Comstock C, et al. Collaborative study on 4‐dimensional echocardiography for the diagnosis of fetal heart defects: the COFEHD study. J Ultrasound Med 2010; 29:1573–1580. [DOI] [PubMed] [Google Scholar]
- 73. Adriaanse BM, Tromp CH, Simpson JM, et al. Interobserver agreement in detailed prenatal diagnosis of congenital heart disease by telemedicine using four‐dimensional ultrasound with spatiotemporal image correlation. Ultrasound Obstet Gynecol 2012; 39:203–209. [DOI] [PubMed] [Google Scholar]
- 74. Gómez O, Soveral I, Bennasar M, et al. Accuracy of fetal echocardiography in the differential diagnosis between truncus arteriosus and pulmonary atresia with ventricular septal defect. Fetal Diagn Ther 2016; 39:90–99. [DOI] [PubMed] [Google Scholar]
- 75. Yeo L, Romero R. Intelligent navigation to improve obstetrical sonography. Ultrasound Obstet Gynecol 2016; 47: 403–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Yeo L, Romero R. Fetal Intelligent Navigation Echocardiography (FINE): a novel method for rapid, simple, and automatic examination of the fetal heart. Ultrasound Obstet Gynecol 2013; 42:268–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Garcia M, Yeo L, Romero R, et al. Prospective evaluation of the fetal heart using fetal intelligent navigation echocardiography (FINE). Ultrasound Obstet Gynecol 2016; 47:450–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Veronese P, Bogana G, Cerutti A, Yeo L, Romero R, Gervasi MT. A prospective study of the use of fetal intelligent navigation echocardiography (FINE) to obtain standard fetal echocardiography views. Fetal Diagn Ther 2017; 41:89–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Yeo L, Luewan S, Markush D, Gill N, Romero R. Prenatal diagnosis of dextrocardia with complex congenital heart disease using fetal intelligent navigation echocardiography (FINE) and a literature review [published online ahead of print June 23, 2017]. Fetal Diagn Ther. doi: http://10.1159/000468929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Evans MI, Galen RS, Britt DW. Principles of screening. Semin Perinatol 2005; 29:364–366. [DOI] [PubMed] [Google Scholar]
- 81. Allan LD, Sharland GK, Milburn A, et al. Prospective diagnosis of 1006 consecutive cases of congenital heart disease in the fetus. J Am Coll Cardiol 1994; 23:1452–1458. [DOI] [PubMed] [Google Scholar]
- 82. Angiulli A, Tai A, Easterbrook S. Voluson: Electronic 4D imaging—Designed for Women's Health. Chicago, IL: GE Healthcare; 2014. [Google Scholar]
- 83. Guasina F, Bellussi F, Morganelli G, Salsi G, Pilu G, Simonazzi G. Electronic STIC improves four‐dimensional fetal echocardiography [published online ahead of print March 24, 2017]. Ultrasound Obstet Gynecol. doi: http://10.1002/uog.17474. [DOI] [PubMed] [Google Scholar]
- 84. Deeks JJ, Altman DG. Diagnostic tests 4: likelihood ratios. BMJ 2004; 329:168–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Jaeschke R, Guyatt G, Lijmer J. Diagnostic tests In: Guyatt G, Rennie D. (eds). Users' Guides to the Medical Literature. Chicago, IL: AMA Press; 2002:121–140. [Google Scholar]
- 86. Gonçalves LF, Espinoza J, Romero R, et al. Four‐dimensional ultrasonography of the fetal heart using a novel tomographic ultrasound imaging display. J Perinat Med 2006; 34:39–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Aguilera M, Dummer K. Concordance of fetal echocardiography in the diagnosis of congenital cardiac disease utilizing updated guidelines [published online ahead of print March 12, 2017]. J Matern Fetal Neonatal Med. doi: http://10.1080/14767058.2017.1297791. [DOI] [PubMed] [Google Scholar]
- 88. Galindo A, Mendoza A, Arbues J, Grañeras A, Escribano D, Nieto O. Conotruncal anomalies in fetal life: accuracy of diagnosis, associated defects and outcome. Eur J Obstet Gynecol Reprod Biol 2009; 146:55–60. [DOI] [PubMed] [Google Scholar]
- 89. Paladini D, Rustico M, Todros T, et al. Conotruncal anomalies in prenatal life. Ultrasound Obstet Gynecol 1996; 8:241–246. [DOI] [PubMed] [Google Scholar]
- 90. Tometzki AJP, Suda K, Khol T, Kovalchin JP, Silverman NH. Accuracy of prenatal echocardiographic diagnosis and prognosis of fetuses with conotruncal anomalies. J Am Coll Cardiol 1999; 33:1696–1701. [DOI] [PubMed] [Google Scholar]
- 91. Sivanandam S, Glickstein JS, Printz BF, et al. Prenatal diagnosis of conotruncal malformations: diagnostic accuracy, outcome, chromosomal abnormalities and extracardiac anomalies. Am J Perinatol 2006; 23:241–245. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Videos online at http://jultrasoundmed.org/journal/jum
Video 1. Nine normal cardiac diagnostic planes in a single template with the unique feature of automatic labeling (through intelligent navigation) of each plane, anatomic structures, fetal left and right sides, and cranial and caudal ends (also see Figure 1). The labeling is distinctive because it stays with the corresponding anatomical structure(s), even as the image is increased or decreased in size. A indicates transverse aortic arch; Ao, aorta; Desc., descending; IVC, inferior vena cava; LA, left atrium; LV, left ventricle; P, pulmonary artery; PA, pulmonary artery; RA, right atrium; RV, right ventricle; RVOT, right ventricular outflow tract; S, superior vena cava; SVC, superior vena cava; and Trans., transverse.
Video 2. Application of the FINE method to a fetus with tetralogy of Fallot at 23 weeks' gestation (diagnostic planes or VIS‐Assistance with automatic labeling are shown; also see Figure 2). Six echocardiographic views were abnormal and demonstrate the typical features of this cardiac defect. The 3‐vessel and trachea view shows a narrow pulmonary artery caused by stenosis, while the transverse aortic arch is prominent. There is a “Y‐shaped” appearance of the great vessels. As is commonly noted in conotruncal abnormalities, the 4‐chamber view appeared normal in the diagnostic plane; however, VIS‐Assistance demonstrates a large ventricular septal defect (not shown here). The 5‐chamber view shows a ventricular septal defect. The left ventricular outflow tract view shows an overriding aorta, dilated aortic root, and ventricular septal defect. In the short‐axis view of great vessels/right ventricular outflow tract (obtained via VIS‐Assistance), the pulmonary artery is narrow with a tortuous ductus arteriosus. There is difficulty in visualizing a normal ductal arch. In the aortic arch view, the aortic root is dilated and there is a prominent ascending aorta. A indicates transverse aortic arch; Ao, aorta; Desc., descending; IVC, inferior vena cava; LA, left atrium; LV, left ventricle; P, pulmonary artery; PA, pulmonary artery; RA, right atrium; RV, right ventricle; RVOT, right ventricular outflow tract; S, superior vena cava; SVC, superior vena cava; and Trans., transverse.
Video 3. Application of the FINE method to a fetus with coarctation of the aorta at 24 weeks' gestation (diagnostic planes or VIS‐Assistance with automatic labeling are shown; also see Figure 3). Seven echocardiographic views are abnormal. The 3‐vessel and trachea view shows a narrow transverse aortic arch. In the 4‐chamber view, the left side of the heart is smaller than the right side; however, the left ventricle is apex forming and there is good movement of the mitral valve. The right side of the heart appears enlarged, with the right ventricle being moderately dilated and hypertrophied. The 5‐chamber view shows similar findings to that of the 4‐chamber view. In addition, there is a narrow aortic root. The left ventricular outflow tract view shows a narrow aorta (obtained via VIS‐Assistance). In the short‐axis view of great vessels/right ventricular outflow tract, the cross‐section of the aorta is small compared to the pulmonary artery. The enlarged right atrium is apparent. The ductal arch view demonstrates that the cross‐section of the aorta is small compared to the pulmonary artery. In the aortic arch view, the coarctation is demonstrated as hypoplasia and narrowing of the transverse aortic arch as well as in the isthmus region. A indicates transverse aortic arch; Ao, aorta; Desc., descending; IVC, inferior vena cava; LA, left atrium; LV, left ventricle; P, pulmonary artery; PA, pulmonary artery; RA, right atrium; RV, right ventricle; RVOT, right ventricular outflow tract; S, superior vena cava; SVC, superior vena cava; and Trans., transverse.
Video 4. VIS‐Assistance of the left ventricular outflow tract view in a 19‐week fetus with a hypoplastic left heart, double‐outlet right ventricle, transposition of the great vessels, and fetal stomach on the right side (also see Figure 4). The abnormal diagnostic plane (left panel) demonstrates a single vessel arising from the right ventricle that is consistent with the pulmonary artery due to its bifurcation. A second vessel could not be clearly identified. However, when VIS‐Assistance is activated (right panel), automatic navigational movements now demonstrate a second vessel (aorta) that is rightward and anterior and exiting the right ventricle. These 2 vessels (indicated by white arrows) are parallel and side by side, consistent with transposition.


