Abstract
Background
Anxiety disorders are associated with an overactive action monitoring system as indexed by a larger error-related negativity (ERN). This study tests whether ERN magnitude changes following treatment, predicts response to treatment, and varies by treatment type.
Methods
The sample included 130 youth (9–14 years): youth with an anxiety disorder (ANX; n=100) and healthy control (HC; n=30) youth with no lifetime DSM-IV disorders. ANX youth were randomized to either a manualized cognitive-behavior therapy (CBT) or a comparison child-centered therapy (CCT). The ERN was assessed before and after 16 sessions of treatment and within a comparable interval for HC. Subjective ratings about making errors on the task were obtained following each testing session. The ClinicalTrials.gov identifier is NCT00774150.
Results
The ERN was larger in ANX than HC youth but ERN magnitude did not significantly change following treatment in the ANX youth, regardless of treatment type, and baseline ERN did not predict treatment response. Post-task ratings revealed that ANX youth worried more about task performance feedback than HC. Like the ERN, mean ratings did not significantly change following treatment. However, these ratings were not correlated with ERN amplitude.
Conclusions
Findings of greater ERN in pediatric anxiety disorders are replicated in a larger sample. More importantly, findings from this randomized control trial show that a larger ERN and feeling worried about performance feedback remain unchanged following treatment and are unrelated to treatment response. Such findings suggest that action monitoring systems remain overactive in anxious youth treated with psychotherapy, suggesting the need for future investigation of whether novel complimentary cognitive and emotional training programs can modify these systems would be warranted.
Keywords: error-related negativity, pediatric anxiety disorders, cognitive-behavioral therapy, child-centered therapy, EEG
Pediatric anxiety disorders have their onset in childhood or early adolescence and frequently lead to other psychiatric disorders in adulthood, including anxiety disorders and depression (Copeland, Wolke, Shanahan, & Costello, 2015). A growing number of studies have reported that anxiety disorders are associated with an overactive action monitoring system as indexed by elevated error-related brain activity in anxiety disorders (Ladouceur, Dahl, Birmaher, Axelson, & Ryan, 2006; Weinberg, Olvet, & Hajcak, 2010) and obsessive-compulsive disorders (OCD) (Carrasco, Harbin, et al., 2013; Hajcak, Franklin, Foa, & Simons, 2008). Such findings were also reported in individuals with non-clinical symptoms of OCD (Santesso, Segalowitz, & Schmidt, 2006) and anxiety (Meyer, Weinberg, Klein, & Hajcak, 2012) as well as in children before the onset of anxiety disorders (Meyer, Proudfit, Torpey-Newman, Kujawa, & Klein, 2015). Such findings have led researchers to propose that elevated error-related brain activity could represent a potential biomarker for anxiety disorders (Meyer, 2016; Weinberg et al., 2016). Yet, few studies have investigated whether such brain activity could change with treatment or predict treatment response.
Error-related brain activity has been assessed using the error-related negativity (ERN), a negative deflection in the event-related potential (ERP) that occurs within 100 ms following the onset of a commission error. Studies using neuroimaging and dipole source localization suggest the ERN appears to be generated within a network of brain regions, including the anterior cingulate cortex (ACC) as well the supplementary motor area, dorsomedial prefrontal cortex and anterior insula (Bastin et al., 2017; Gehring, Liu, Orr, & Carp, 2012). It is thought to serve as a neural index of response monitoring and error detection processes (Gehring, Goss, Coles, Meyer, & Donchin, 1993) and has been shown to be stable across time and reliable across tasks (Meyer, Riesel, & Proudfit, 2013; Riesel, Weinberg, Endrass, Meyer, & Hajcak, 2013). While the ERN has been documented in younger children (Torpey, Hajcak, & Klein, 2009), ERN amplitude seems to increase with age (Grammer, Carrasco, Gehring, & Morrison, 2014) to reach adult levels by mid- to late adolescence (Davies, Segalowitz, & Gavin, 2004; Ladouceur, Dahl, & Carter, 2007). Such age-related changes are thought to reflect maturational changes in the ACC and its connection with other action monitoring (Tamnes, Walhovd, Torstveit, Sells, & Fjell, 2013) and fronto-striatal-limbic regions (Holroyd & Coles, 2002).
The functional role of the ERN remains a matter of intense debate. Some posit that the ERN reflects evaluative cognitive control sub-processes (Yeung, Botvinick, & Cohen, 2004) while others consider it to reflect dopamine learning signals in the ACC (Holroyd & Coles, 2002). However, relevant to anxiety, studies have shown that affective and motivational variables (e.g., losing points when making an error) influence the magnitude of the ERN (Riesel, Weinberg, Endrass, Kathmann, & Hajcak, 2012), which has led to the proposal that a larger ERN could index greater sensitivity to endogenous threat based on the perception of errors as catastrophic (Weinberg, et al., 2016). Findings suggest that ERN amplitude does not seem to be affected by state-related changes in anxiety symptoms in clinical samples (Ladouceur, et al., 2006; Moser, Hajcak, & Simons, 2005) and that more negative ERN in temperamentally inhibited children predicts adolescent onset of an anxiety disorder (McDermott et al., 2009). Taken together, these data suggest that greater ERN amplitude could represent a trait marker of anxiety disorders but it remains unclear whether the ERN changes with treatment.
Preliminary evidence suggests that ERN amplitude is not associated with treatment outcome in anxiety disorders (Carrasco, Hong, et al., 2013; Hajcak, et al., 2008; Kujawa et al., 2016; Riesel, Endrass, Auerbach, & Kathmann, 2015). For instance, the ERN was measured prior to and following treatment in a subset of 8–17 year-olds (n=23) diagnosed with OCD (n=18) seeking cognitive behavioral therapy (CBT) and a healthy comparison group (n=18) (Hajcak, et al., 2008). Results from this first study showed that despite changes in OCD symptom severity with treatment, there were no changes in ERN amplitude. However, the small sample size, the wide age range, the lack of randomization to treatment, and the potential confounding effects of medication precluded firm conclusions about the effects of treatment on the ERN. A more recent study also reported no change in ERN amplitude following treatment (i.e., CBT or selective serotonin reuptake (SSRI)) in 28 participants (8–26 years old) with an anxiety disorder and 35 healthy controls (Kujawa, et al., 2016). Results showed that greater ERN magnitude seemed to persist with symptom remission in patients with social (SocAD). ERN amplitude in patients with GAD did not significantly differ from controls before or after treatment. Here too, interpretations of the findings are limited by the relatively small heterogeneous sample and the combination of CBT and SSRI treatment.
The present study aimed to address these issues by employing a randomized controlled trial (RCT) design comparing CBT for child anxiety and Child-Centered Therapy (CCT) (Cohen, Deblinger, Mannarino, & Steer, 2004) and examining error-related brain activity prior to and following treatment in a large sample of young adolescents diagnosed with an anxiety disorder and a healthy age-matched comparison group. CBT has consistently been shown to be superior to wait-list control (Walkup et al., 2008) and focuses on improving anxious children’s ability to self-regulate their emotions and to habituate, through exposure, to the aversiveness of negative events, including making mistakes. In their research on trauma-focused CBT, Cohen and Mannarino developed Child-Centered Therapy (CCT), an active comparison intervention for children and adolescents that draws on principles from client-centered therapy, an approach that is widely used in the community (Cohen, et al., 2004). CCT has previously been implemented as an active comparison condition for two trials testing the efficacy of trauma-focused CBT for youth with Post-traumatic Stress Disorder (PTSD) (Cohen, et al., 2004; Cohen, Mannarino, & Iyengar, 2011; Cohen, Mannarino, & Knudsen, 2005). In these studies, children in both CBT and CCT improved from pre- to post-treatment, but CBT showed superiority over CCT in magnitude of treatment gains, rates of clinical remission, and treatment response at 1-year. It emphasizes the use of core non-specific therapeutic skills such as active listening, reflection, accurate empathy, and encouragement to talk about feelings, but does not include directive problem solving, psychoeducation about anxiety or coping skills, or exposure. In a recent study, we adapted CCT for use in GAD, SocAD, and SAD and reported that the majority of youth responded positively to both treatments but that youth treated with CBT were significantly more likely to reach full recovery of all targeted anxiety diagnoses and symptom normalization following acute treatment compared to youth treated with CCT (Silk et al., 2016). Compared to CCT, CBT focuses on self-regulatory processes such as reappraisal and employs exposure to anxiety-provoking situations. As such, we hypothesized that ERN magnitude would significantly reduce following CBT compared to CCT, and that it would predict reduction in symptoms following CBT but not CCT. We also examined subjective ratings regarding task performance and explored the influence of sex and pubertal status on these findings.
Methods
Participants
Participants (n=130; 9–14 years) included anxious (ANX; n=100) and healthy control youth (HC; n=30) with no lifetime DSM-IV disorders and good quality EEG data. They were a subset of participants recruited as part of a randomized clinical trial study examining the neurobehavioral mechanisms of individual treatment in anxious youth (see Silk et al., 2016 for a description of the larger sample, Figure 1 for the CONSORT Flow Diagram and Appendix S1 for CONSORT 2010 Checklist). Anxious youth were required to meet DSM-IV criteria for current GAD, SocAD, and/or separation anxiety disorder (SAD) (Table 1). Exclusion criteria included: IQ<70, use of psychoactive medications, presence of neurological impairments, current primary diagnosis of major depressive disorder and other current (e.g., PTSD) or lifetime (e.g., psychosis) Axis-I diagnoses.
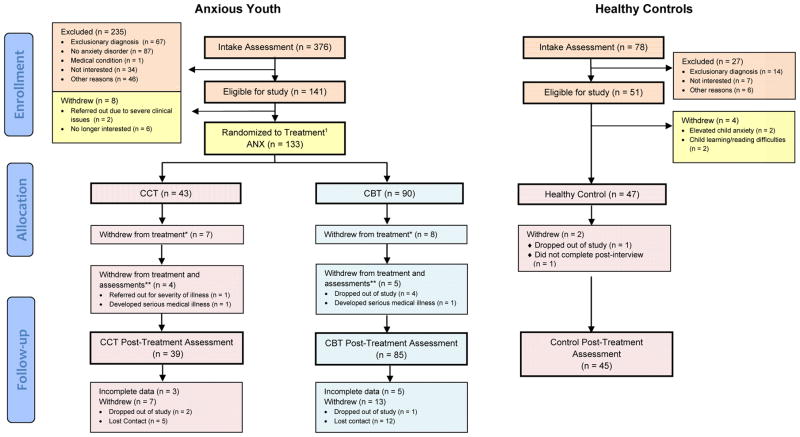
Figure 1.
CONSORT Flow Diagram
1Two participants were originally erroneously randomized to treatment, but did not meet inclusion/exclusion criteria for the study. These participants are included above as “ineligible”; *Subjects who withdrew from treatment continued to do follow-up assessments; **Subjects who withdrew from treatment and assessments did not do any follow-up assessments. Figure adapted from Silk, J. S., Tan, P. Z., Ladouceur, C. D., Meller, S., Siegle, G. J., McMakin, D. L., et al. (2016). A Randomized Clinical Trial Comparing Individual Cognitive Behavioral Therapy and Child-Centered Therapy for Child Anxiety Disorders. Journal of Clinical Child & Adolescent Psychology, 1–13.
Table 1.
Participant characteristics by group
| Variable | Anxious Youth (n = 100) | Healthy Controls (n = 30) |
|---|---|---|
| Age | 11.14 (1.46) | 11.50 (1.70) |
| Female, [n] | 54 | 15 |
| Caucasian, [n] | 88 | 22 |
| Current diagnosisa, [n] | ||
| Generalized Anxiety Disorder | 71 | – |
| Social Anxiety Disorder | 21 | – |
| Separation Anxiety Disorder | 23 | – |
| Specific Phobia | 12 | – |
| Major Depressive Disorder | 1 | – |
| ADHD (inattentive type) | 1 | – |
| ODD | 2 | |
| Tic Disorder | 4 | |
| PDS | 2.50 (0.99) | 2.54 (1.13) |
| PARS (6 items) | 17.76 (4.47) | 1.00 (1.88) |
| SCARED – parent | 37.04 (11.87) | 10.72 (7.64) |
| SCARED – child | 39.31 (12.48) | 3.46 (3.85) |
Note: Data presented as mean (SD) unless otherwise noted. ADHD: Attention Deficit Hyperactivity Disorder, ODD: Oppositional Defiant Disorder, PDS: Peterson Developmental Scale, PARS: Pediatric Anxiety Rating Scale 6-item score, SCARED: Screen for Childhood Anxiety and Related Disorders.
Diagnostic groups are partially overlapping due to inclusion of comorbid patients. Primary/principle diagnoses were not designated, meaning that percentages for the 3 diagnostic inclusion groups will not sum to 100.
Procedures
Study procedures, including obtaining written consent from the primary caregiver and written assent from the participant, were approved by the University of Pittsburgh Institutional Review Board. Following the intake assessment, participants completed an electroencephalography (EEG) assessment to assess error-related brain activity using event-related potentials (ERPs) at pre-treatment. EEG assessment was repeated following 16 sessions of individual therapy in the ANX group and within a comparable interval for HC (mean number of weeks following initial EEG assessment: ANX: 22.5 weeks, HC: 21.5 weeks; there were no group differences, t(92)=1.17, p=.24). Participants in the ANX group were randomized to either CBT or CCT treatment using restricted randomization procedures to balance participants across conditions by age and sex. The CBT treatment was delivered using the Coping Cat workbooks (Kendall & Hedtke, 2006) and the CCT treatment was delivered using a manualized supportive psychotherapy based on humanistic principles (CCT; Cohen, et al., 2004) (see Silk, et al., 2016 for more details). Interviews and rating scales were administered to the child and his/her primary caregiver before and after treatment by an independent evaluator unaware of treatment assignment condition. The ClinicalTrials.gov identifier is NCT00774150.
Clinical assessments
The Schedule for Affective Disorders and Schizophrenia in School-Age Children—Present and Lifetime version was administered to determine the presence of other Axis-I disorders. Anxiety severity was measured using the 6-item score (anxiety severity, frequency, distress, avoidance, and interference during the previous week (α=.76)) of the Pediatric Anxiety Rating Scale (PARS) (Group, 2002). Treatment response was defined as a 35% reduction in PARS from pre- to post-treatment. Child- and parent-report of anxiety symptoms were assessed using the Screen for Childhood Anxiety and Related Disorders (Birmaher et al., 1997).
Pubertal maturation
The Pubertal Development Scale (PDS) (Petersen, Crockett, Richards, & Boxer, 1988) assessed pubertal status based on child self-report about physical development, scored from 1 (no) to 4 (development seems complete).
Eriksen flanker task
An arrow version of the flanker task was administered using E-prime software (Eprime Inc., Pittsburgh, PA). As previously described (Ladouceur, et al., 2006), the task included presentation of five arrows, with 50% congruent trials (→→→→→ and ←←←←←) and 50% incongruent trials (←←→←← and →→←→→). Participants were asked to respond as fast and as accurately as possible on a button box using their right and left index fingers according to the direction of the central arrow. There were 3 blocks of 200 trials each, with 30 practice trials. All stimuli were presented for 200ms followed by an inter-trial interval that varied randomly from 500 and 1500ms during which a fixation cross was presented.
Post-task questionnaire
After completing the flanker task, participants rated how they felt about making errors on the task using a Likert scale (1–5) to indicate to what extent they: 1) worried about the feedback about their performance; 2) felt badly about making errors; 3) were certain, when making errors, that their response was incorrect.
EEG data acquisition and processing
Continuous EEG activity was recorded using an ActiveTwo head cap and the ActiveTwo 128-channel BioSemi system (BioSemi, Amsterdam, The Netherlands) sampled at 512Hz. An elastic lycra cap was placed on the child’s head and 128 Ag/AgCl-tipped electrodes were attached to the cap. Also, 7 flat electrodes were used to measure electrical activity generated by eye and muscle movements. Specifically, 2 electrodes were placed at supra and infra orbital sites of the right eye to monitor vertical eye movements and 2 on the outer canthi of the left and right eyes to monitor horizontal eye movements. In addition, 2 electrodes were placed on the mastoid (right and left) and 1 on the tip of the nose.
Offline, all data processing was performed using Brain Electrical Signal Analysis (BESA) software. EEG data were re-referenced to the nose and high-pass (.01Hz) and low-pass (30Hz) filtered. A semi-automated pre-processing procedure was used to reject bad channels and trials with significant signal artifact. After visual inspection to identify bad channels, segments were extracted from the continuous EEG, from 200 ms prior to correct and erroneous responses to 800ms following responses. ERP data were corrected for blinks and eye-movements using the method developed by Gratton et al. (Gratton, Coles, & Donchin, 1983). A semi-automatic procedure was used to detect and reject artifact according to the following criteria: a voltage step of more than 50 μV between data points, a voltage gradient of 150 μV within trials, a signal of less than 0.1 μV across the trial, or reaction times occurring outside of a 100–2000 ms window. Visual inspection of the data served to detect and reject any remaining artifacts. After pre-processing, ERN data was excluded from analyses if the EEG was contaminated by excessive artifact or the participant had fewer than 10 errors. As a result, of the 130 ANX and 47 HC youth who completed the baseline ERP assessment as part of their participation in the larger RCT (see Figure 1 for the CONSORT Flow Diagram), 30 ANX and 17 HC youth were excluded from the baseline group comparison analyses. Participants who had good quality ERN data at both pre- and post-treatment assessments were included in pre/post treatment-related analyses (ANX: n=67 and HC: n=27).
EEG data reduction and analyses
To quantify the response-locked ERN, averages were computed separately for correct and error trials for each group at pre- and post-treatment. Baseline correction was applied by subtracting from each data point the average activity in a −150 to −50 ms window prior to the response. The ERN and the negative deflection on correct trials (i.e., the correct response negativity, or CRN) were scored as the average activity on error and correct trials, respectively, from 0 to 90 ms window after response onset at scalp site FCz, where error-related brain activity was maximal. To minimize the number of tests, analyses focused on the difference between error and correct trials quantified using the ERN standardized residual score (ERNresid) (Meyer, Lerner, De Los Reyes, Laird, & Hajcak, 2017). The ERNresid is calculated by saving the variance leftover in a regression where CRN was the independent variable and ERN is the dependent variable.
Data Analysis
Mixed ANCOVA models were used to analyze, at baseline, behavioral performance and ERP measures, with group (ANX, HC) as between-subject variable, response type (correct, error) as within-subject variables. For behavioral data analyses, trial type (congruent, incongruent) was included as an additional factor.
To address questions regarding treatment-related changes, mixed ANCOVA models were computed with ERNresid as the dependent variable1, with group (ANX, HC) as between-subject variable and time (pre, post) as a within-subject factor (see Table 2). The effect of treatment type was evaluated in ANX only using treatment type (CBT, CCT) by time (pre, post) repeated measures analyses. Sub-analyses were performed examining whether changes correlated with anxiety symptom reduction. Pearson’s correlations were computed between change in the ERNresid and change in anxiety symptom severity (PARS 6-item and SCARED parent and child scores) from pre- to post- treatment. Hierarchical regression analyses and logistic regressions, covarying for age and pre-treatment anxiety symptoms, were performed to examine whether ERNresid predicted changes in anxiety symptom severity and treatment response, respectively.
Table 2.
Summary of behavioral performance, event-related potential (ERP) amplitudes, and symptom severity (means and standard deviations) by group and at pre-treatment and post-treatment in participants with useable ERP data at both time points
| Variables | Pre-treatment | Post-treatment | ||
|---|---|---|---|---|
|
| ||||
| Anxious Youth (n = 67) | Healthy Controls (n = 27) | Anxious Youth (n = 67) | Healthy Controls (n = 27) | |
| Reaction time | ||||
| Overall | 526.16 (100.14) | 507.18 (111.46) | 502.19 (86.08) | 484.34 (87.85) |
| Correct | 537.56 (99.04) | 518.92 (107.12) | 512.00 (85.19) | 496.31 (88.81) |
| Error | 426.47 (115.80) | 430.49 (134.46) | 428.33 (110.56) | 409.22 (94.56) |
| Congruent | 500.09 (95.13) | 482.35 (106.62) | 479.19 (83.99) | 464.88 (88.37) |
| Incongruent | 552.24 (107.25) | 532.01 (116.95) | 525.19 (89.53) | 503.81 (87.82) |
| Percentage of errors | ||||
| Overall | 8.37 (4.50) | 10.21 (4.99) | 8.06 (2.99) | 10.01 (4.37) |
| Congruent | 3.74 (3.51) | 4.27 (3.01) | 3.39 (2.05) | 4.04 (3.22) |
| Incongruent | 12.95 (6.30) | 16.15 (8.14) | 12.73 (5.07) | 15.97 (6.91) |
| ERP data (FCz) | ||||
| Correct trials | −1.36 (2.50) | −0.13 (1.38) | 1.75 (2.42) | 0.69 (2.65) |
| Error trials | −2.63 (3.70) | −1.45 (2.52) | −1.92 (3.86) | −1.98 (3.23) |
| ERNresid | −0.18 (1.10) | 0.38 (0.79) | −0.03 (1.07) | 0.09 (0.78) |
| Symptom severity | ||||
| PARS (6 items) | 17.41 (4.77) | 1.11 (1.95) | 9.08 (6.50) | 0.41 (1.58) |
| SCARED - parent | 36.95 (12.74) | 3.08 (3.66) | 22.02 (13.76) | 3.74 (3.66) |
| SCARED - child | 40.60 (12.06) | 9.81 (6.99) | 18.12 (15.56) | 5.44 (4.98) |
| Subjective ratings about task performance | ||||
| Worried about feedback | 2.35 (1.18) | 1.60 (0.71) | 2.03 (1.10) | 1.41 (0.69) |
| Felt badly | 3.32 (0.66) | 3.16 (0.62) | 3.33 (0.72) | 3.00 (0.73) |
| Felt certain | 3.53 (1.29) | 3.32 (1.38) | 3.48 (1.34) | 3.07 (1.59) |
Note: ERP: event-related potentials; PARS: Pediatric Anxiety Rating Scale 6-item score, SCARED: Screen for Childhood Anxiety and Related Disorders. ERNresid: Error-related negativity standardized score. a: Pretreatment: Anxious Youth: n=65, Healthy Controls: n=25; Post-treatment: Anxious Youth: n=66, Healthy Controls: n=27.
Statistical analyses were performed using SPSS 23. Greenhouse-Geisser correction was applied upon any violations of the assumption of sphericity. Post hoc and secondary analyses included t-tests and correlational analyses, with type-I error correction using Bonferroni and false discovery rate (FDR) as appropriate (Benjamini & Hochberg, 1995). Given evidence of age-related changes in ERN amplitude (Davies, et al., 2004; Ladouceur, et al., 2007), age was included as covariate. Secondary analyses examined differences according to sex and anxiety diagnosis as well as correlations between ERNresid and behavioral performance, PDS score, symptom severity, and subjective ratings of task performance.2
Results
Participant Characteristics
As shown in Table 1, ANX and HC groups did not differ on distribution of sex, mean age, and pubertal status (ps>0.25). Compared to HC, ANX had significantly higher scores on the 6-item PARS (t=19.97, df=126, p<.001), SCARED–parent (t=23.92, df=124, p<.001) and SCARED–child (t=15.03, df=124, p<.001). As shown in Table 2, anxious youth showed a reduction in symptom severity pre- to post-treatment (t=9.09, df=65, p<.001), SCARED–parent (t=9.57, df=65, p<.001), and SCARED–child (t=11.98, df=61, p<.001) rating scales. Furthermore, there were no significant differences in the number of anxious youth who responded to CBT (n=39 ANX; 62% responders) or CCT (n=27 ANX; 60% responders) treatment (χ2=0.35, p=.85).
Behavioral Data
There were no significant main effect of group or interactions for accuracy or reaction times (ps>.05). There were significant trial type and response type main effects, indicating that reaction times were slower for incongruent than congruent trials (F=4.79, df=1,124, p=.03, ηp2=.04) and faster for incorrect than correct responses (F=13.46, df=1,124, p<.001, ηp2=.10).
A shown in Table 2, there were no significant changes in accuracy or reaction times from the first to second assessment (ps>.10). However, in this subset of participants, ANX were more accurate than HC at both time points (F=5.61, df=1,92, p=.02, ηp2=.06). Regarding reaction times, there was no significant main effect of group or interactions (ps>.50). However, both groups were faster at the second compared to the first assessment (F=5.96, df=1,91, p=.02, ηp2=.06).
Event-related Potential Data: The Error-Related Negativity
Figure 2 presents baseline response-locked ERP data for correct and error trials at FCz for the ANX (n=100) and HC (n=30) groups. Amplitude was significantly more negative for error compared to correct trials (F=58.02, df=1,127, p<.001, ηp2=.32) and there was a significant group by response type interaction (F=13.11, df=1,127, p=.001, ηp2=.09). Independent t-tests performed on ERNresid indicated that ERN amplitude was greater for ANX than HC (t=−2.63, df=128, p=.009, d=.59).
Figure 2.
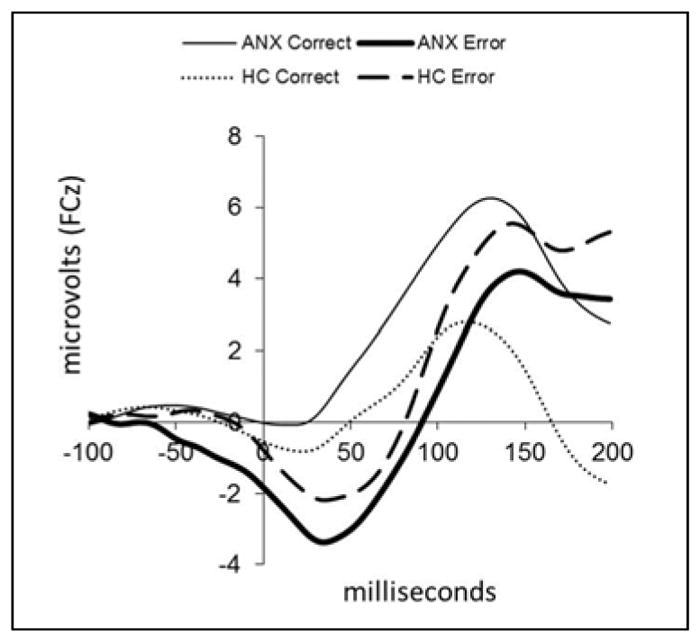
Baseline grand average event-related potential (ERP) waveforms are plotted at FCz following correct and error responses in anxious (ANX, n=100) and healthy youth (HC, n=30).
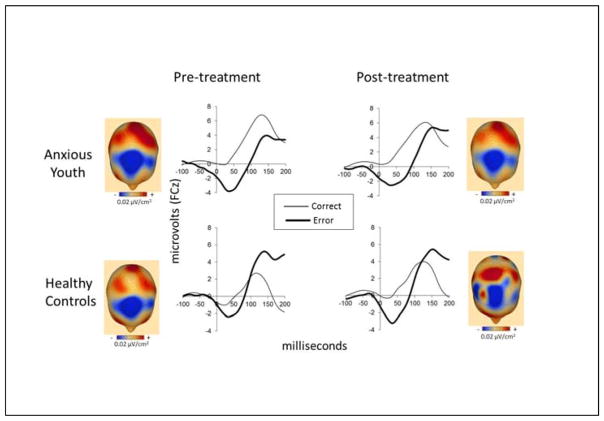
Figure 3 presents response-locked ERP data (at FCz) for pre-treatment (left) and post-treatment (right) assessments for participants with good quality data at both time points: ANX (n=67) (top) and HC (n=27) (bottom) groups. Results indicated that across groups, there was no significant change in ERNresid from pre- to post-treatment (F=.26, df=1,91, p=.61), and that there was no significant group by time interaction (F=3.03, df=1,91, p=.09). With regard to the effects of treatment type (CBT vs. CCT) on ERNresid in ANX youth, results indicated that treatment type did not have any significant effects on changes in ERNresid (F=1.21, df=1,64, p=.28). There were also no group by treatment type interaction when analyses were conducted only on ANX youth who responded to treatment (n=40) (F=0.46, df=1,37, p=.50). There were no significant correlations between change in ERNresid and change in symptom severity from pre- to post-treatment (PARS 6-item score: r=−.03, df=57, p=.81; SCARED parent: r=.23, df=57, p=.08; SCARED child: r=−.11, df=57, p=.42).
Figure 3.
Grand average event-related potential (ERP) waveforms are plotted at FCz following correct and error responses in anxious (n=67) and healthy youth (n=27) with useable ERP data at both pre- and post-treatment. Topographic current source density (CSD) maps display the projection of the currents on the scalp surface after onset of incorrect responses at their maximal peaks between 0–100 ms (note: blue = more negative; red = more positive; reference free, 0.02 μV/cm2/step).
Regression analyses in ANX youth showed that, after controlling for age and anxiety severity at baseline, baseline ERNresid (β=0.11, t=0.99, p=.32) did not significantly predict post-treatment anxiety severity (F=1.21, df=3,84 p=.31). Moreover, baseline ERNresid did not significantly reliably distinguish between treatment responders and non-responders (χ2=3.92, p=.27 with df=3). Also, there were no significant effects of age or anxiety severity (ps>.15).
Interestingly, results indicated that ANX youth were significantly more worried about feedback on their performance than HC youth (F=10.57, df=1,87, p=.002, ηp2=.11) at both pre- (t=2.99, df=88, p=.004, d=.78) and post-treatment (t=2.71, df=91, p=.008, d=.65). Also, mean ratings did not significantly change following treatment (F=.52, df=1,87, p=.47) and baseline ratings did not significantly distinguish between treatment responders and non-responders (χ2=1.47, p=.23 with df=1). ANX youths’ subjective ratings about how badly they felt about making errors and how certain they felt about their errors were not significantly different from HC youths’ ratings at both time points (ps>.05).
Findings from secondary analyses on behavioral performance and ERNresid indicated that there were no significant effects related to sex or significant correlations with behavioral performance, PDS, parent and child total SCARED scores, or subjective ratings in ANX or HC at pre- and post-treatment (pFDR>.05). However, when comparing HC and ANX youth at baseline based on anxiety disorder diagnosis (GAD only, SocAD and/or SAD only, GAD with SocAD and/or SAD), results indicated a trend for a main effect of group (F=2.61, df=3,126, p=.05). Independent t-tests revealed that, ERNresid was significantly greater, compared to HC, for GAD only (t=2.67, df=83, p=.009) or GAD with either SocAD and/or SAD (t=2.53, df=45, p=.015), but not for ANX youth with SocAD and/or SAD only (t=1.52, df=56, p=.13).
Discussion
Findings from this study suggest that despite changes in ratings of anxiety symptoms, ERN amplitude does not significantly change following treatment. This conclusion holds whether anxious youth were treated with a well-validated CBT program for anxious youth or a child-centered therapy. With this study, we also replicate, in a larger sample (n=100), previous findings (Ladouceur, et al., 2006; Meyer, et al., 2012; Weinberg, et al., 2010) showing that youth diagnosed with an anxiety disorder, particularly in those with GAD, exhibit a larger ERN compared to healthy youth. Such elevated neural response to errors in anxious individuals has led some researchers to suggest that elevated error-related brain activity could represent a potential diagnostic biomarker of anxiety disorders. Findings from the present study, together with evidence documenting that a larger ERN predicts future onset of anxiety disorders in at-risk youth (Meyer, et al., 2015), seem to support this idea.
Our finding of greater ERN amplitude in anxious youth that persists following treatment is consistent with previous treatment studies (Hajcak, et al., 2008). It is also consistent with findings in youth and adults with social anxiety disorders indicating that a larger ERN continues to persist following treatment with CBT or SSRI (Kujawa, et al., 2016) and with findings showing that ERN amplitude is independent of ongoing treatment (SSRI or CBT) in youth with OCD or other anxiety disorders (Carrasco, Hong, et al., 2013). By randomizing a sample of clinically anxious youth to CBT or CCT, we were able to address many of the limitations from previous treatment studies and demonstrate that greater error-related brain activity persists following individual psychotherapy. Furthermore, through the use of a post-task questionnaire, we discovered that ANX youth reported feeling more worried about performance feedback than their peers and that, like the ERN, such worries about feedback persisted following treatment. Other ratings about their performance on the task (i.e, how badly they felt or how certain they were about committing an error) were not significantly different than HC youth at both assessments, suggesting that concern about performance feedback seems to be an important factor. The lack of correlations between ERN amplitude and these subjective ratings as well as symptom severity is consistent with findings from other studies with clinical samples (Ladouceur, et al., 2006; Weinberg, Klein, & Hajcak, 2012), suggesting these may be parallel processes.
Our findings that larger ERN and feeling worried about performance feedback remain unchanged following treatment and do not predict treatment response suggest that anxiety disorders may implicate a stable overactive action monitoring system. Such an overactive system seems to cut across domains, including fast-occurring error processing brain activity as well as subjective self-monitoring processes, and suggest that it could represent a trait-like feature that appears to be independent of symptom severity or treatment effects (Carrasco, Harbin, et al., 2013; Endrass, Riesel, Kathmann, & Buhlmann, 2014; Hajcak, et al., 2008; Kujawa, et al., 2016). Nevertheless, it has yet to be determined the extent to which altered error-related brain activity contributes to subjective self-monitoring processes or vice versa. Our findings that participants reported significantly fewer anxiety symptoms following treatment without significant change in these action monitoring processes is intriguing. The relationship between elevated error-related brain activity and specific clusters of anxiety symptoms remains unclear. The dimension of action monitoring is not very well represented in self-report or clinical measures of anxiety disorders and as such, it is possible that it is an aspect that may not change with psychotherapy. Would targeting such an overactive monitoring system in anxious youth enhance treatment outcome or prevent relapse? Future research is needed to determine whether there is a need to develop complimentary cognitive and emotional training programs that can modify these processes. One strategy could be to employ a multipronged approach, for instance, through a combination of neurofeedback and targeted exposure sessions focused on habituation to making errors in the context of negative performance feedback from peers. If error-related brain activity in anxious individuals is a marker of elevated threat processing (Weinberg, et al., 2016), another strategy could be to target this brain activity through attention bias modification (ABM), which trains individuals to disengage attention from potential threat. Nelson et al. (2015) reported that ERN amplitude was less negative among undergraduates who completed a single session of ABM before relative to those who completed ABM after the ERN assessment using a flanker task (Nelson, Jackson, Amir, & Hajcak, 2015). Another study reported that ERN amplitude in OCD patients was significantly less negative (and comparable to healthy controls) when assessed during dual-task demands compared to the standard conditions with the flanker task (Klawohn, Endrass, Preuss, Riesel, & Kathmann, 2016). A more recent study showed that engaging in expressive writing compared to a control writing condition was associated with reductions in ERN amplitude in individuals with chronic worry (Schroder, Moran, & Moser, in press). The authors reasoned that expressive writing may serve to enhance cognitive control processes underlying action monitoring by reducing the distracting effects of worry. Together these findings suggest that focusing on how attentional resources are being deployed during task performance may be an important factor to consider. The question remains, however, would targeting such an overactive action monitoring system significantly improve clinical outcome? It is possible that anxiety symptom reduction occurs prior to changes in action monitoring systems and that further follow-up may be needed to detect such changes.
Analyses examining the effects of treatment on behavioral performance did not yield significant group differences or changes with treatment. These findings are consistent with previous findings in OCD (Hajcak, et al., 2008), but are inconsistent with the Kujawa et al. (2016) study, which reported correlations between ERN amplitude and accuracy at pre-treatment and ERN amplitude and reaction times at post-treatment in the combined sample. Furthermore, baseline comparisons across anxiety disorder diagnosis and HC suggest that ERN amplitude was greater than HC in anxious youth with GAD (i.e., with or without SocAD and/or SAD) but not in those without GAD (i.e., with SocAD and/or SAD). These findings are inconsistent with those in Kujawa et al. (2016), which reported no significant differences between individuals with GAD and HC. The discrepancy in findings could be due to the fact that our study focused on 9–14 year-old youth whereas the Kujawa et al. sample included children, adolescents, and adults (8–26 year-olds; mean age = 17.40, SD = 4.13) along with anxious participants with higher levels of depression symptoms. Nevertheless, further research is needed to elucidate how alterations in action monitoring systems may vary as a function of anxiety disorder symptom profile. Also, we did not find any main effects or interactions with puberty or sex. The potential moderating effects of sex on the relationship between anxiety and the ERN may emerge later (Moser, Moran, Kneip, Schroder, & Larson, 2016).
Findings from the present study include certain limitations. Data loss was higher in ANX than HC due to slightly higher levels of EEG artifact related to movement in ANX youth. Helping anxious youth remain still during EEG assessments may be an important factor to consider in future follow-up studies. Also, youth were primarily Caucasian and treatment was delivered in an academic medical setting with high quality conditions for treatment delivery, which are factors that could limit generalizability to more diverse samples in community settings. That said, there is little reason to think that in a more diverse or less optimally treated sample, the ERN would be more predictive, or would change more in treatment.
In conclusion, findings from this study provide evidence supporting the idea that error-related brain activity and subjective feelings of worry about performance feedback are elevated in anxious youth and persist regardless of the type of individual therapy in pediatric anxiety disorders. These findings along with those in OCD (Hajcak, et al., 2008) suggest that an overactive action monitoring system may represent a trait-like transdiagnostic marker underlying these disorders. Complimentary intervention strategies specifically targeting this system at multiple levels may be warranted to improve treatment outcome.
Supplementary Material
CONSORT 2010 Checklist.
Key Points.
Anxious youth exhibit a larger error-related negativity (ERN), which is thought to be a biomarker for anxiety disorders.
Few studies have investigated the effects of treatment on the ERN in pediatric anxiety disorders.
Findings show that ERN amplitude and subjective feelings of worry about performance feedback remain elevated in youth treated with CBT or CCT.
An overactive action monitoring system could represent a trait-like characteristic of anxiety disorders.
Studies examining the effects of intervention strategies targeting the action monitoring system in anxious youth may be warranted.
Acknowledgments
ClinicalTrials.gov identifier is NCT00774150. This research was supported by National Institute of Mental Health grant P50 MH080215 (Neal D. Ryan, P.I., Cecile Ladouceur, Project Co-Director). Support for research participant recruitment was also provided by the Clinical and Translational Science Institute at the University of Pittsburgh (NIH/NCRR/CTSA Grant UL1 RR024153). The authors are grateful to the participants and their families. They are also grateful to Kelly O’Neil and Jennifer Podell for their contributions to clinical supervision, and to Laura Trubnick, Jennifer Jacubcak, Marcus Min, Jessica Wilson, Marcie Walker, Greg Smith, Kara Colaizzi, Melissa Milbert, Matt George, Emily Yarrison, Rachel Kolko, Erika Joyce, Danielle Gilchrist, Jillian Rodgers, Katie Burkhouse, Diana Whalen, Stephanie Davis, Simona Graur, Sherri Karas, Abby Martin, Kristin Pracht, Grace Lee, for research assistance and data management. The authors have declared that they have no competing or potential conflicts of interest.
Footnotes
All analyses performed on the ERNresid were also performed on the mean amplitude of the difference waveform (ΔERN: ERN-CRN). The pattern of results was similar across the two sets of analyses.
Secondary analyses included: a) separate diagnostic group by sex ANCOVAs, with age as a covariate, on accuracy, reaction times, and ERNresid to examine group by sex interactions and main effects of sex at baseline, b) group (HC, GAD only (n=55), SocAD and/or SAD only (n=28), GAD with SocAD and/or SAD (n=17)) ANCOVA, with age as a covariate, on ERNresid to examine differences in ERN amplitude based on anxiety disorder diagnosis at baseline, c) mixed ANCOVA, with group (ANX, HC) and sex (male, female) as between-subject variables and time (pre, post) as a within-subject factor to examine whether there were sex differences in the effects of treatment on ERN amplitude, d) computation of Pearson correlations (pre- and post-treatment) between ERNresid and accuracy, reaction times, PDS score, symptom severity, and subjective ratings of task performance.
Conflict of interest statement: No conflicts declared.
Additional Supporting Information may be found in the online version of this article
References
- Bastin J, Deman P, David O, Gueguen M, Benis D, Minotti L, et al. Direct Recordings from Human Anterior Insula Reveal its Leading Role within the Error-Monitoring Network. Cerebral Cortex. 2017;27(2):1545–1557. doi: 10.1093/cercor/bhv352. [DOI] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. Journal of the Royal Statistical Society Series B (Methodological) 1995;57(1):289–300. [Google Scholar]
- Birmaher B, Khetarpal S, Brent D, Cully M, Balach L, Kaufman J, et al. The Screen for Child Anxiety Related Emotional Disorders (SCARED): Scale Construction and Psychometric Characteristics. Journal of the American Academy of Child and Adolescent Psychiatry. 1997;36(4):545–553. doi: 10.1097/00004583-199704000-00018. [DOI] [PubMed] [Google Scholar]
- Carrasco M, Harbin SM, Nienhuis JK, Fitzgerald KD, Gehring WJ, Hanna GL. Increased error-related brain activity in youth with obsessive-compulsive disorder and unaffected siblings. Depression and Anxiety. 2013;30(1):39–46. doi: 10.1002/da.22035. [DOI] [PubMed] [Google Scholar]
- Carrasco M, Hong C, Nienhuis JK, Harbin SM, Fitzgerald KD, Gehring WJ, et al. Increased error-related brain activity in youth with obsessive-compulsive disorder and other anxiety disorders. Neuroscience Letters. 2013;541(Supplement C):214–218. doi: 10.1016/j.neulet.2013.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen JA, Deblinger E, Mannarino AP, Steer RA. A multisite, randomized controlled trial for children with sexual abuse-related PTSD symptoms. Journal of the American Academy of Child & Adolescent Psychiatry. 2004;43(4):393–402. doi: 10.1097/00004583-200404000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen JA, Mannarino AP, Iyengar S. Community treatment of posttraumatic stress disorder for children exposed to intimate partner violence: a randomized controlled trial. Archives of pediatrics & adolescent medicine. 2011;165(1):16–21. doi: 10.1001/archpediatrics.2010.247. [DOI] [PubMed] [Google Scholar]
- Cohen JA, Mannarino AP, Knudsen K. Treating sexually abused children: 1 year follow-up of a randomized controlled trial. Child Abuse & Neglect. 2005;29(2):135–145. doi: 10.1016/j.chiabu.2004.12.005. [DOI] [PubMed] [Google Scholar]
- Copeland WE, Wolke D, Shanahan L, Costello E. Adult functional outcomes of common childhood psychiatric problems: A prospective, longitudinal study. JAMA Psychiatry. 2015;72(9):892–899. doi: 10.1001/jamapsychiatry.2015.0730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies PL, Segalowitz SJ, Gavin WJ. Development of response-monitoring ERPs in 7–25-year-olds. Developmental Neuropsychology. 2004;25:355–376. doi: 10.1207/s15326942dn2503_6. [DOI] [PubMed] [Google Scholar]
- Endrass T, Riesel A, Kathmann N, Buhlmann U. Performance monitoring in obsessive-compulsive disorder and social anxiety disorder. Journal of Abnormal Psychology. 2014;123(4):705–714. doi: 10.1037/abn0000012. [DOI] [PubMed] [Google Scholar]
- Gehring WJ, Goss B, Coles MGH, Meyer DE, Donchin E. A neural system for error detection and compensation. Psychological Science. 1993;4:385–390. [Google Scholar]
- Gehring WJ, Liu H, Orr J, Carp J. The error-related negativity (ERN/Ne) In: Luck SJ, Kappenman E, editors. Handbook of event-related potential components. New York: Oxford University Press; 2012. [Google Scholar]
- Grammer JK, Carrasco M, Gehring WJ, Morrison FJ. Age-related changes in error processing in young children: A school-based investigation. Developmental Cognitive Neuroscience. 2014;9:93–105. doi: 10.1016/j.dcn.2014.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gratton G, Coles MGH, Donchin E. A new method for offline removal of ocular artifacts. Electroencephalography and Clinical Neurophysiology. 1983;55:468–484. doi: 10.1016/0013-4694(83)90135-9. [DOI] [PubMed] [Google Scholar]
- Group RS. The Pediatric Anxiety Rating Scale (PARS): development and psychometric properties. Journal of the American Academy of Child & Adolescent Psychiatry. 2002;41:1061–1069. doi: 10.1097/00004583-200209000-00006. [DOI] [PubMed] [Google Scholar]
- Hajcak G, Franklin ME, Foa EB, Simons RF. Increased error-related brain activity in pediatric OCD before and after treatment. American Journal of Psychiatry. 2008;165:116–123. doi: 10.1176/appi.ajp.2007.07010143. [DOI] [PubMed] [Google Scholar]
- Holroyd CB, Coles MGH. The Neural Basis of Human Error Processing: Reinforcement Learning, Dopamine, and the Error-Related Negativity. Psychological Review. 2002;109:679–709. doi: 10.1037/0033-295X.109.4.679. [DOI] [PubMed] [Google Scholar]
- Kendall PC, Hedtke K. Cognitive-behavioral therapy for anxious children: Therapist manual. Ardmore, PA: Workbook Publishing; 2006. [Google Scholar]
- Klawohn J, Endrass T, Preuss J, Riesel A, Kathmann N. Modulation of hyperactive error signals in obsessive–compulsive disorder by dual-task demands. Journal of Abnormal Psychology. 2016;125(2):292–298. doi: 10.1037/abn0000134. [DOI] [PubMed] [Google Scholar]
- Kujawa A, Weinberg A, Bunford N, Fitzgerald KD, Hanna GL, Monk CS, et al. Error-related brain activity in youth and young adults before and after treatment for generalized or social anxiety disorder. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2016;71:162–168. doi: 10.1016/j.pnpbp.2016.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ladouceur CD, Dahl RE, Birmaher B, Axelson DA, Ryan ND. Increased error-related negativity in childhood anxiety disorders. Journal of Child Psychology and Psychiatry and Allied Disciplines. 2006;47(10):1073–1082. doi: 10.1111/j.1469-7610.2006.01654.x. [DOI] [PubMed] [Google Scholar]
- Ladouceur CD, Dahl RE, Carter CS. Development of action monitoring through adolescence into adulthood: ERP and source localization. Developmental Science. 2007;10(6):874–891. doi: 10.1111/j.1467-7687.2007.00639.x. [DOI] [PubMed] [Google Scholar]
- McDermott JM, Perez-Edgar K, Henderson HA, Chronis-Tuscano A, Pine DS, Fox NA. A history of childhood behavioral inhibition and enhanced response monitoring in adolescence are linked to clinical anxiety. Biological Psychiatry. 2009;65:445–448. doi: 10.1016/j.biopsych.2008.10.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer A. Developing psychiatric biomarkers: A review focusing on the error-related negativity as a biomarker for anxiety. Current Treatment Options in Psychiatry. 2016;3:1–9. [Google Scholar]
- Meyer A, Lerner MD, De Los Reyes A, Laird RD, Hajcak G. Considering ERP difference scores as individual difference measures: Issues with subtraction and alternative approaches. Psychophysiology. 2017;54(1):114–122. doi: 10.1111/psyp.12664. [DOI] [PubMed] [Google Scholar]
- Meyer A, Proudfit GH, Torpey-Newman DC, Kujawa A, Klein DN. Enhanced error-related brain activity in children predicts the onset of anxiety disorders between the ages of 6 and 9. Journal of abnormal psychology. 2015;124(2):266–274. doi: 10.1037/abn0000044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer A, Riesel A, Proudfit GH. Reliability of the ERN across multiple tasks as a function of increasing errors. Psychophysiology. 2013;50(12):1220–1225. doi: 10.1111/psyp.12132. [DOI] [PubMed] [Google Scholar]
- Meyer A, Weinberg A, Klein DN, Hajcak G. The development of the error-related negativity (ERN) and its relationship with anxiety: Evidence from 8 to 13 year-olds. Developmental Cognitive Neuroscience. 2012;2(1):152–161. doi: 10.1016/j.dcn.2011.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moser JS, Hajcak G, Simons RF. The effects of fear on performance monitoring and attentional allocation. Psychophysiology. 2005;42:261–268. doi: 10.1111/j.1469-8986.2005.00290.x. [DOI] [PubMed] [Google Scholar]
- Moser JS, Moran TP, Kneip C, Schroder HS, Larson MJ. Sex moderates the association between symptoms of anxiety, but not obsessive compulsive disorder, and error-monitoring brain activity: A meta-analytic review. Psychophysiology. 2016;53(1):21–29. doi: 10.1111/psyp.12509. [DOI] [PubMed] [Google Scholar]
- Nelson BD, Jackson F, Amir N, Hajcak G. Single-Session Attention Bias Modification and Error-Related Brain Activity. Cognitive, affective & behavioral neuroscience. 2015;15(4):776–786. doi: 10.3758/s13415-015-0365-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen A, Crockett L, Richards M, Boxer A. A self-report measure of pubertal status: Reliability, validity, and inital norms. Journal of Youth and Adolescence. 1988;17:117–133. doi: 10.1007/BF01537962. [DOI] [PubMed] [Google Scholar]
- Riesel A, Endrass T, Auerbach L, Kathmann N. Overactive Performance Monitoring as an Endophenotype for Obsessive-Compulsive Disorder: Evidence From a Treatment Study. American Journal of Psychiatry. 2015;172(7):665–673. doi: 10.1176/appi.ajp.2014.14070886. [DOI] [PubMed] [Google Scholar]
- Riesel A, Weinberg A, Endrass T, Kathmann N, Hajcak G. Punishment has a lasting impact on error-related brain activity. Psychophysiology. 2012;49(2):239–247. doi: 10.1111/j.1469-8986.2011.01298.x. [DOI] [PubMed] [Google Scholar]
- Riesel A, Weinberg A, Endrass T, Meyer A, Hajcak G. The ERN is the ERN is the ERN? Convergent validity of error-related brain activity across different tasks. Biological Psychology. 2013;93(3):377–385. doi: 10.1016/j.biopsycho.2013.04.007. [DOI] [PubMed] [Google Scholar]
- Santesso DL, Segalowitz SJ, Schmidt LA. Error-related electrocortical responses are enhanced in children with obsessive-compulsive behaviors. Developmental Neuropsychology. 2006;29(3):431–445. doi: 10.1207/s15326942dn2903_3. [DOI] [PubMed] [Google Scholar]
- Schroder HS, Moran TP, Moser JS. The effect of expressive writing on the error-related negativity among individuals with chronic worry. Psychophysiology. doi: 10.1111/psyp.12990. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silk JS, Tan PZ, Ladouceur CD, Meller S, Siegle GJ, McMakin DL, et al. A Randomized Clinical Trial Comparing Individual Cognitive Behavioral Therapy and Child-Centered Therapy for Child Anxiety Disorders. Journal of Clinical Child & Adolescent Psychology. 2016:1–13. doi: 10.1080/15374416.2016.1138408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamnes CK, Walhovd KB, Torstveit M, Sells VT, Fjell AM. Performance monitoring in children and adolescents: A review of developmental changes in the error-related negativity and brain maturation. Developmental Cognitive Neuroscience. 2013;6:1–13. doi: 10.1016/j.dcn.2013.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torpey DC, Hajcak G, Klein DN. An Examination of Error-Related Brain Activity and its Modulation by Error Value in Young Children. Developmental neuropsychology. 2009;34(6):749–761. doi: 10.1080/87565640903265103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walkup JT, Albano AM, Piacentini J, Birmaher B, Compton SN, Sherrill JT, et al. Cognitive behavioral therapy, sertraline, or a combination in childhood anxiety. New England Journal of Medicine. 2008;359(26):2753–2766. doi: 10.1056/NEJMoa0804633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg A, Klein DN, Hajcak G. Increased error-related brain activity distinguishes generalized anxiety disorder with and without comorbid major depressive disorder. Journal of abnormal psychology. 2012;121(4):885–896. doi: 10.1037/a0028270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg A, Meyer A, Hale-Rude E, Perlman G, Kotov R, Klein DN, et al. Error-related negativity (ERN) and sustained threat: Conceptual framework and empirical evaluation in an adolescent sample. Psychophysiology. 2016;53(5):372–385. doi: 10.1111/psyp.12538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg A, Olvet DM, Hajcak G. Increased error-related brain activity in generalized anxiety disorder. Biological Psychology. 2010;85(3):472–480. doi: 10.1016/j.biopsycho.2010.09.011. [DOI] [PubMed] [Google Scholar]
- Yeung N, Botvinick M, Cohen JD. The neural basis of error detection: Conflict monitoring and the error-related negativity. Psychological Review. 2004;111:931–959. doi: 10.1037/0033-295x.111.4.939. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
CONSORT 2010 Checklist.