Abstract
Human IL12RB1 is an autosomal gene that is essential for mycobacterial disease resistance and T cell differentiation. Using primary human tissue and PBMCs, we demonstrate that lung and T cell IL12RB1 expression is allele-biased, and the extent to which cells express one IL12RB1 allele is unaffected by activation. Furthermore, following its expression the IL12RB1 pre-mRNA is processed into either IL12RB1 Isoform 1 (IL12Rβ1, a positive regulator of IL12-responsiveness) or IL12RB1 Isoform 2 (a protein of heretofore unknown function). T cells’ choice to process pre-mRNA into Isoform 1 or Isoform 2 is controlled by intragenic competition of IL12RB1 exon 9-10 splicing with IL12RB1 exon 9b splicing, as well as an IL12RB1 exon 9b-associated polyadenylation site. Heterogeneous nuclear ribonucleoprotein H (hnRNP H) binds near the regulated polyadenylation site, but is not required for exon 9b polyadenylation. Finally, microRNA-mediated knockdown experiments demonstrated that IL12RB1 Isoform 2 promotes T cell IL12 responses. Collectively, our data support a model wherein tissue expression of human IL12RB1 is allele-biased and produces an hnRNP H bound pre-mRNA, the processing of which generates a novel IL12 response regulator.
Keywords: Allele-bias, Monoallelic, hnRNP, IL12RB1, mycobacteria
INTRODUCTION
IL12RB1 is a Mendelian susceptibility to mycobacterial disease (MSMD) gene that promotes IFNγ immunity and mycobacterial disease resistance1. In the absence of a functional IL12RB1 allele, individuals are susceptible to lung infection with tuberculous and nontuberculous Mycobacteria species, and T cells are insensitive to the cytokines IL12 and IL232, 3. Aberrant splicing is among the most common causes of IL12RB1 deficiency4. In addition to mycobacterial disease, common IL12RB1 haplotypes also associate with measles vaccine efficacy5, 6, pediatric asthma susceptibility7, 8, and food allergy among breast-fed toddlers9. Given the impact of IL12RB1 on multiple diseases, it is important to identify and understand the factors governing IL12RB1 expression, splicing and function.
T cells express two major IL12RB1 isoforms as a result of alternative mRNA processing3. The first isoform (IL12Rβ1, or Isoform 1) is a cell surface receptor that binds the IL12p40 domain of IL12/IL2310–12, and cooperates with co-receptors IL12Rβ2 or IL23R to initiate intracellular STAT signaling3. An important consequence of IL12/IL23 signaling is increased secretion of IFNγ10, 13, which limits mycobacteria survival in multiple organs14, 15. The second isoform (Isoform 2) retains the IL12p40-binding domains of Isoform 1, but lacks the Isoform 1 transmembrane domain and localizes to an intracellular reticulum16. Based on these data we initially predicted that Isoform 2 is non-functional protein located distal to extracellular cytokine16; however, subsequent studies using IL12Rβ1ΔTM−/− mice (IL12Rβ1ΔTM is the mouse homolog of human Isoform 2) support a different model wherein Isoform 2 functions to promote IL12 responses and TH1 development during experimental tuberculosis17. The factors affecting T cells’ decision to transcribe and alternatively process IL12RB1 mRNA into Isoform 1 or Isoform 2 are unknown, nor has a role for human IL12RB1 Isoform 2 been empirically determined.
Among autosomal genes that regulate T cell function, the factors affecting mRNA processing include monoallelic or allele-biased expression18, and heterogeneous nuclear ribonucleoproteins19, 20. Allele-biased expression occurs when only one copy of a gene (either the maternal- or paternal-derived allele) is predominately transcribed, while the other allele is transcriptionally silent. Allele-biased expression is used by T cells to regulate transcription of genes that affect their thymic development21, 22, as well as the genes that affect their cytokine responses23–30. After T cells transcribe these and other genes, heterogeneous nuclear ribonucleoproteins (hnRNPs) physically associate with the pre-mRNA and participate in alternative splicing, polyadenylation, nuclear export and localization to ribosomes20. There are approximately twenty major hnRNPs in humans (hnRNP A1 – U) which vary in size, RNA recognition motif, and function31. Among the major hnRNPs that regulate alternative splicing in lymphocytes are hnRNPs A/B32, hnRNP F33, hnRNP H33, hnRNP L34–38, hnRNP LL38–41 and hnRNP U42.
Here we report the results of experiments which were designed to identify factors regulating IL12RB1 mRNA expression and processing, as well as determine Isoform 2′s function in the context of an IL12 response. Using primary tissues and T cell lines, we demonstrate that IL12RB1 expression is allele-biased, and T cells’ decision to produce either Isoform 1 or Isoform 2 is regulated by intragenic competition between IL12RB1 exon 9-10 splicing and IL12RB1 exon 9b splicing / polyadenylation. We also demonstrate that IL12-dependent IFNγ expression is attenuated in T cells that are transduced with Isoform 2 specific microRNAs. Collectively, these data support a model wherein human IL12RB1 pre-mRNAs are primarily transcribed from one allele, and that alternative processing of these pre-mRNAs is modulated an IL12RB1 exon 9b polyA site that is upstream of hnRNP H binding. A consequence of this alternative processing is the production of a novel IL12 response regulator.
RESULTS
Expression of IL12RB1 in human lung tissue is allele-biased
IL12RB1 expression in the lung is important for mycobacterial disease resistance1. To determine if IL12RB1 expression in the lungs is allele biased, we deep sequenced IL12RB1 gDNA and mRNA from human lung specimens, determined each donor genotype at eleven polymorphic positions in the IL12RB1 gene, and then used this information to educe the allelic origin of IL12RB1 mRNA in the same specimen. Deep sequencing was done using the Ion Torrent platform as previously described43; the average IL12RB1 gDNA and mRNA read depths across all samples were 2907× and 13222×, respectively (supplemental TABLE S2). To determine a donor’s genotype, we queried their gDNA sequence at eleven polymorphic sites in the IL12RB1 gene (chromosome 19 positions 18170384, 18170755, 18180413, 18180451, 18186575, 18186618, 18188408, 18191664, 18192977, 18194255 and 18197635), and whether they were heterozygous (i.e. each IL12RB1 allele had a different nucleotide at that position) or homozygous (i.e. both IL12RB1 alleles had the same nucleotide at that position). Donor gDNA that was heterozygous at a given site would have two nucleotides that each theoretically comprise 50% of all the reads, while a donor who was homozygous at a given site would have one nucleotide that theoretically comprises 100% of all reads. If a donor was heterozygous, we could determine if their IL12RB1 mRNAs represented both alleles equally (indicating biallelic expression) or predominantly represented one allele (indicating allele-biased transcription) by comparing the allele read frequency among gDNA and mRNA reads. The allele read frequency (ARF) is a numerical value that indicates the representation of a given nucleotide among all the reads of a specific position in the gDNA (ARFgDNA) or mRNA (ARFmRNA).
The results of our analyzing eight specimens in this manner are depicted in FIG 1. Four donors were homozygous at each polymorphic site, and thus could not be used to determine if IL12RB1 transcription was allele-biased (FIG 1A). Four other donors were heterozygous at one or more polymorphic sites in the IL12RB1 gene, and thus could be used to determine if mRNAs present in the same tissue were monoallelic or biallelic in origin (FIG 1B). Donor S3 was polymorphic at gDNA position 18170384; although the ARFgDNA values for both alleles were roughly equal (G, 48%; A, 52%), the ARFmRNA values were not (G, 100%; A, 0%). The same result was true for Donor N3. Donor N5 was polymorphic at six gDNA positions (18170384, 18180413, 18180451, 18186575, 18186618 and 18191664); the range of ARFgDNA values for these six positions was consistent with equal allelic representation (44%-56%), while the ARFmRNA values were biased towards one allele (82%-100%) at the expense of the other allele (0-18%). Donor S1 was polymorphic at seven gDNA positions (18170384, 18180413, 18180451, 18186575, 18186618, 18188408 and 18197635); as with Donor N5, the range of ARFgDNA values for these seven positions was consistent with equal allelic representation (41%-59%), while the ARFmRNA values were biased towards one allele (97%-100%) at the expense of the other allele (0-3%). When these ARFgDNA and ARFmRNA data from FIG 1B are pooled and plotted, there was a significant difference in the regression pattern of the two data sets (FIG 1C). Namely, while IL12RB1 ARFgDNA values displayed a Gaussian distribution (mean=50%), the IL12RB1 ARFmRNA values were non-Gaussian and bifurcated toward either 0% or 100% representation, which is consistent with monoallelic gene expression18.
FIGURE 1.
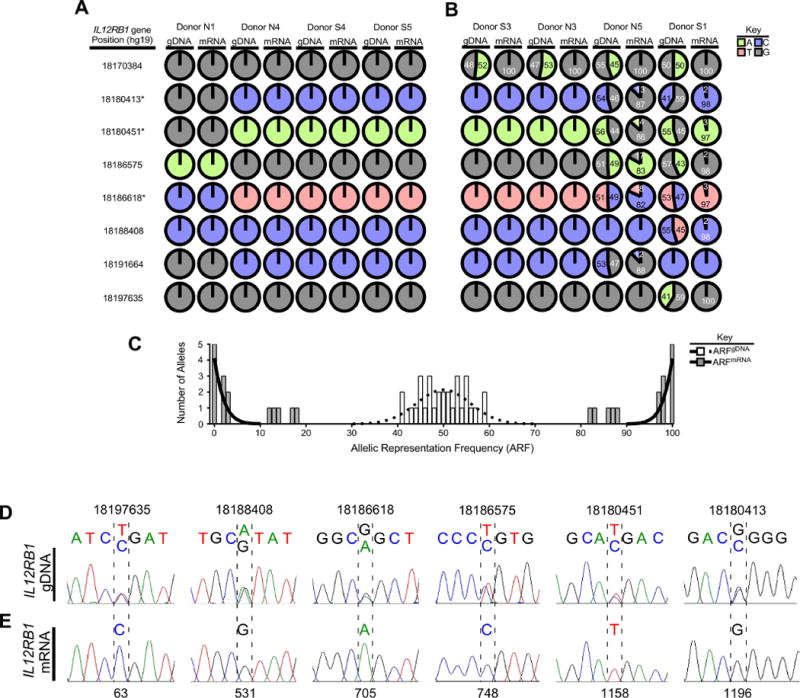
IL12RB1 expression in human lung tissue is allele-biased. (A-C) IL12RB1 gDNA and mRNA from 8 lung tissue donors (N1, N4, S4, S5, S3, N3, N5, S1) was sequenced via Ion Torrent. For each donor, the IL12RB1 gDNA sequence was queried at 11 polymorphic sites (18170384, 18170755, 18180413, 18180451, 18186575, 18186618, 18188408, 18191664, 18192977, 18194255 and 18197635) to determine if they were homozygous (i.e. only 1 nt was read at that site) or heterozygous (i.e. 2 nts were read at that site). The data from each donor are represented by pie charts indicating the nts detected at each site (G, gray; T, red; A, green; C, blue) and their relative representation among all reads of that site. We similarly queried and analyzed IL12RB1 mRNA sequence data from the same tissue specimens. Shown are (A) IL12RB1 gDNA and mRNA sequence data from four donors (N1, N4, S4 and S5) who were homozygous at all 11 polymorphic sites, as well as (B-C) IL12RB1 gDNA and mRNA sequence data from four donors (S3, N3, N5 and S1) who were heterozygous at ≥1 polymorphic sites. The numbers in each chart indicate the frequency with which the indicated allele was represented among all reads of that site (i.e. the allele read frequency, or ARF). The asterisks next to IL12RB1 gene positions 18186618/18180451/18180413 indicate the linked SNPs that comprise the “RTR haplotype” and “QMG haplotype”4, 46. (C) A histogram depicting the distribution of gDNA ARF values of each heterozygous allele (open bars), and the mRNA ARF values of the same alleles (closed bars). Nonlinear regression analysis was used to test the Gaussian distribution of the gDNA ARF values, and is depicted by a dotted regression line (r2=0.7, mean=50.0); mRNA ARF values were bifurcated and non-Gaussian (solid regression line). (D) Donor S1 lung gDNA was amplified with primers flanking 6 polymorphic sites in the IL12RB1 gene (18180413, 18180451, 18186575, 18186618, 18188408, and 18197635); amplicons were Sanger sequenced. Shown are representative Sanger traces demonstrating 2 nts at gDNA positions 18197635 (T/C), 18188408 (A/G), 18186618 (G/A), 18186575 (T/C), 18180451 (T/C), and 18180413 (G/C). (E) mRNA from the same specimen was used to make an IL12RB1 cDNA library, of which 10 clones were randomly chosen for Sanger sequencing. Shown are the Sanger traces of regions that correspond to nts transcribed from the gDNA alleles shown in (D). Indicated by hatched lines are the cDNA nt positions (63, 531, 705, 748, 1158 and 1196) that correspond to the above polymorphic gDNA positions. In contrast to the gDNA traces, there is only 1 nt present at each cDNA position, representing the gDNA allele from which that mRNA was originally transcribed. All 10 cDNA clones contained the same nt at positions 63 (C), 531 (G), 705 (A), 748 (C), 1158 (T) and 1196 (G).
To ensure that our deep sequencing results were reflected in individual mRNA clones, lung mRNA was used to generate an IL12RB1 cDNA library, from which individual clones were randomly selected for Sanger sequencing Donor S1 lung mRNA was chosen to generate this library because their gDNA was heterozygous for more IL12RB1 single nucleotide polymorphisms (SNPs) than any other donor (FIG 1B). Each clone within a library contained the entire length of the IL12RB1 cDNA (∼2,000 bp); ten individual clones were randomly selected and Sanger sequenced 2-3 times with overlapping primer sets. To educe each cDNAs clone’s allele-of-origin, its Sanger sequencing trace was visually inspected at six cDNA positions (63, 531, 705, 748, 1158 and 1196) that correspond to six gDNA positions that are polymorphic in Donor S1 gDNA (18197635, 18188408, 18186618, 18186575, 18180451 and 18180413). This analysis demonstrated that of ten IL12RB1 cDNA clones, all originated from the same IL12RB1 allele. Namely, each cDNA clone encoded the following SNPs: 18197635C, 18188408G, 18186618A, 18186575C, 18180451T and 18180413G (FIG 1D–E). This is the same allele that we concluded (based on Ion Torrent data) was preferentially expressed in Donor S1 (FIG 1B). Collectively, these data demonstrate that IL12RB1 expression in human lung tissue is allele biased, as determined by Ion Torrent and Sanger sequencing.
Expression of IL12RB1 in human T cells is allele-biased
Based on the above studies, we next wished to determine if IL12RB1 expression is allele biased in T cells, since T cell IL12RB1 expression promotes lung mycobacterial disease resistance44. IL12RB1 expression is required for CD4+ and CD8+ T cell differentiation in humans and mouse models1, 44, 45, and the two most common IL12RB1 haplotypes (“QMG” and “RTR”) encode receptors that differ in IL12-sensitivity4, 46. The three SNPs which comprise the QMG and RTR haplotype are located at gDNA positions 18186618, 18180451 and 18180413 (indicated by asterisks in FIG 1A). To determine if PBMC and T cell IL12RB1 expression is allele biased, PBMCs from nine healthy adult donors were isolated and cultured in the presence of PHA; CD4+ and CD8+ T cells were purified from PBMC preparations using an antibody-conjugated bead system, both before and after PHA-stimulation. The gDNA of each PBMC preparation was used for PCR amplification of IL12RB1 exon 10, which contains two constituent SNPs of the QMG allele (18180451T and 18180413G) or RTR allele (18180451C and 18180413C); exon 10 amplicons were Sanger sequenced to determine if the donor was heterozygous or homozygous for the QMG and/or RTR haplotype. The Sanger sequence trace of a heterozygous donor is shown in FIG 2A, demonstrating the detection of two nucleotides at each position in the QMG/RTR haplotype. The mRNA of each PBMC, CD4+ and CD8+ T cell preparation was used to generate and analyze individual IL12RB1 cDNA libraries, in the same manner as was done for lung tissue (FIG 1D–E).
FIGURE 2.
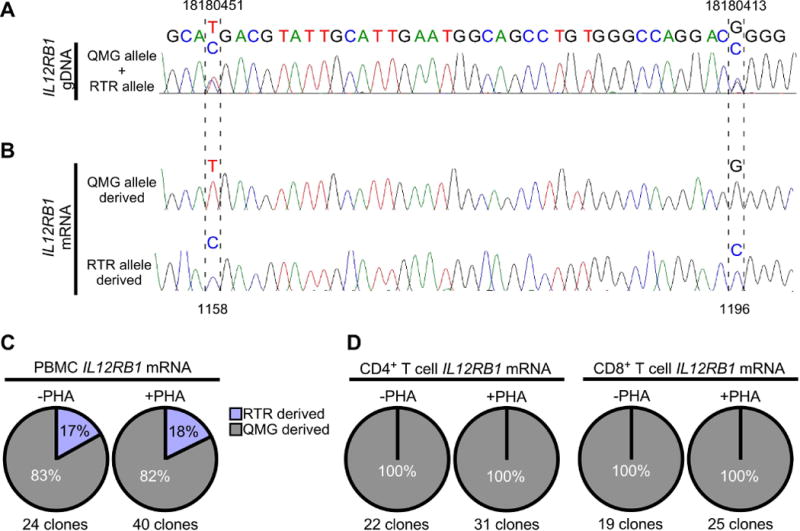
Allele-biased IL12RB1 expression in human PBMCs and T cells. PBMCs were isolated from 9 healthy adults; one PBMC portion was immediately lysed for gDNA/mRNA extraction, while the other portion was cultured with stimulant (PHA) prior to gDNA/mRNA extraction. PBMCs from 3 donors were further used to isolate CD4+ and CD8+ T cells, before (-PHA) and after PBMC stimulation (+PHA). PBMC gDNA was amplified with primers flanking 2 polymorphic sites in IL12RB1 exon 10 (18180451 and 18180413) that are constituents of the QMG allele (18180451T and 18180413G) or RTR allele (18180451C and 18180413C). Shown in (A) is the Sanger trace of a donor who was heterozygous for the QMG and RTR alleles, as indicated by 2 nts at gDNA positions 18180451 (T/C), and 18180413 (G/C). (B) mRNA from the same PBMC and T cell preparations (-PHA and +PHA) was used to generate donor- and cell-specific IL12RB1 cDNA libraries. From each library, multiple cDNA clones were randomly selected for Sanger sequencing. Shown are representative Sanger traces of clones transcribed from the QMG allele (top) and RTR allele (bottom); in contrast to the gDNA traces, there is only 1 nt present at each cDNA position, representing the gDNA allele from which that mRNA was originally transcribed. Indicated by hatched lines are the 2 cDNA positions (1158 and 1196) that correspond to nts transcribed from the gDNA SNPs above (18180451 and 18180413, respectively). (C-D) Pie charts depicting the relative representation QMG-derived cDNA clones (gray) and RTR-derived cDNA clones (blue) in (C) -PHA and +PHA PBMC preparations, and (D) -PHA and +PHA T cell preparations. Percentages are taken from the pooled Sanger sequencing data of all donors. The total number of clone sequences analyzed in this manner is indicated below each chart.
The results of our analyzing nine donors in this manner are shown in FIG 2. Three PBMC donors were homozygous for the QMG or RTR haplotype, and thus could not be used to determine if IL12RB1 transcription was allele-biased (data not shown). Six PBMC donors were heterozygous for the QMG and RTR haplotype, and thus could be used to determine the allelic origin of each IL12RB1 cDNA clone (FIG 2A–B). Among the IL12RB1 cDNA clones from unstimulated (-PHA) PBMCs, 83% were derived from the QMG allele; this frequency was virtually unaffected in stimulated (+PHA) PBMCs (FIG 2C). Among the IL12RB1 cDNA clones from CD4+ and CD8+ T cells, 100% were derived from the QMG allele, regardless of stimulation (FIG 2D). Collectively, these data demonstrate that IL12RB1 expression in human PBMCs and T cells is allele-biased.
IL12RB1 isoform production is due to intragenic competition between exons 9/10 splicing and exon 9b splicing/polyadenylation
After an IL12RB1 allele is expressed, T cells must choose whether to process the IL12RB1 pre-mRNA into Isoform 1 (the function of which is to promote IL12 responses) or Isoform 2 (the function of which is heretofore unknown). The elements which govern this processing choice are unknown. To identify elements within IL12RB1 that govern its alternative processing, and to determine the function of Isoform 2 in the context of IL12 responses, we used the Jurkat cell model of primary human T cells. Jurkat cells are a leukemic human T cell line that recapitulates many aspects of primary human T cells47, including allele-biased IL12RB1 expression (FIG 3A) and processing IL12RB1 pre-mRNA into Isoform 1 and Isoform 2 (FIG 3B).
FIGURE 3.
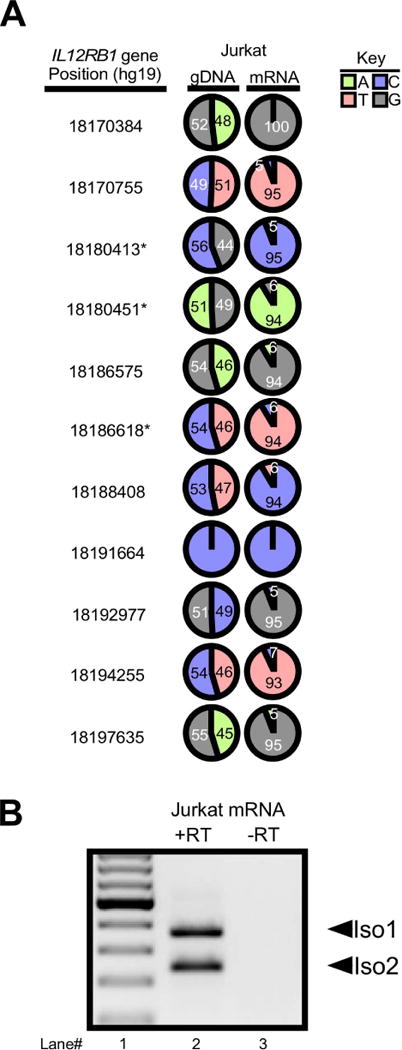
IL12RB1 expression in the Jurkat T cell line is allele-biased and results in the production of Isoform 1 and Isoform 2. (A) Jurkat cell IL12RB1 gDNA and mRNA were sequenced and analyzed in manner identical to that described in FIG 1A–C. The results demonstrate that Jurkat cells are polymorphic at 9 gDNA positions (18170384, 18170755, 18180413, 18180451, 18186575, 18186618, 18188408, 18194255 and 18197635); like primary human tissue, the range of ARFgDNA values for these 9 positions was consistent with equal allelic representation (44%-56%), while the ARFmRNA values were biased towards one allele (95%-100%) at the expense of the other allele (0-5%). (B) Jurkat mRNA was converted to cDNA, which was then used for Isoform 1 and Isoform 2 PCR amplification. Shown are the electrophoresis patterns of (lane 1) a ladder control and (lane 2) Jurkat T cell cDNA amplicons; (lane 3) a “no RT control” was also amplified to confirm cDNA specificity.
The production of Isoform 1 (Iso1) results from the splicing of IL12RB1 exons 1-17, with skipping of exon 9b (FIG 4A top), while production of Isoform 2 (Iso2) results from splicing IL12RB1 exons 1-9 and the alternate 3′ exon 9b (FIG 4A bottom) and subsequent use of a 9b-specific polyadenylation site16. To better understand the alternative splicing of IL12RB1 mRNA exon 9b, we generated a series of IL12RB1 minigenes that lacked regions with predicted regulatory sequences (FIG 4B), transfected each minigene into the Jurkat T cell line, and then measured the expression of minigene-derived Iso1 and Iso2 mRNA. To generate a wild type IL12RB1 minigene that recapitulated endogenous IL12RB1 mRNA processing, the 2804 bp sequence comprising IL12RB1 exons 9→9b→10 was cloned into vector p3XFLAG-CMV7.1 (pB19–10, FIG 4B). The rationale for this design was that most examples of alternative splicing involve cis-elements that are proximal to the affected exon, so we predicted that a minigene comprising IL12RB1 exons 9→9b→10 would harbor the cis elements necessary for alternative processing of IL12RB1 into Iso1 and Iso2. Iso2 uses the polyadenylation (polyA) site within exon 9b whereas Iso1 uses the polyA site provided by the vector, downstream of the IL12RB1 insert. In the event that sequences outside of IL12RB1 exons 9→9b→10 contribute to alternative splicing of Iso1 or Iso2, we also generated a minigene that included intron 10 and exon 11 (pB19–11, FIG 4B).
FIGURE 4.
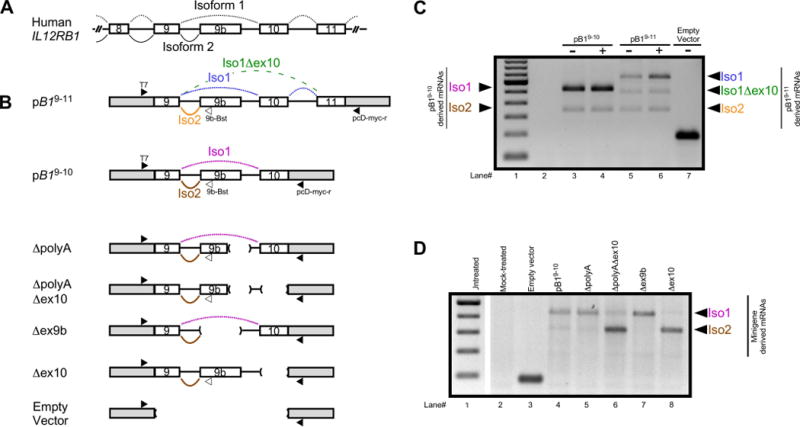
Minigene analysis of IL12RB1 alternative RNA processing. (A) Human IL12RB1 and the splicing patterns that result in expression of Isoform 1 (black dotted line) and Isoform 2 (black solid line). (B) The IL12RB1 minigenes that were used in our study, with the vector backbone represented by gray boxes at the 5′ and 3′ ends, the exons by open boxes, and the introns by solid lines between boxes. The minigene pB19–11 contained IL12RB1 exons 9, 9b, 10 and 11, as well as their intervening introns. Shown alongside the minigene are annealing sites of the 3 primers (T7, pcD-myc-r, and 9b-Bst) that were used for multiplex amplification of pB19–11-derived Iso1 mRNA (the splicing pattern of which is shown by the blue dotted line), pB19–11-derived Iso2 mRNA (orange solid line), and a pB19–11-derived minor variant (Iso1Δex10, green dashed line). The minigene pB19–10 contained IL12RB1 exons 9, 9b and 10, as well as their intervening introns. The sequences of pB19–10 and pB19–11 are identical, with the exception that exon 11 is absent from pB19–10; also depicted are the splicing patterns of pB19–10-derived Iso1 mRNA (pink dotted line) and pB19–10-derived Iso2 mRNA (brown solid line). Deletion mutant derivatives of pB19–10 were generated and include: ΔpolyA, which lacks 345 nt encompassing the IL12RB1 exon 9b polyadenylation site and a portion of the downstream intron; ΔpolyAΔex10, which eliminates the IL12RB1 exon 9b polyadenylation site region through IL12RB1 exon 10; Δex9b, which is a 991 nt deletion that removes IL12RB1 exon 9b and flanking intron sequences; Δex10, which lacks IL12RB1 exon 10 and upstream intron sequences; vector alone, which contains no IL12RB1 exons or introns. (C) Jurkat T cells were transfected with either pB19–10, pB19–11 or empty vector, and then cultured in the presence (+) or absence (-) of PHA; minigene-derived Iso1 and Iso2 expression was assessed 2 days later. Shown are the electrophoresis patterns of (lane 1) a ladder control, and amplicons from (lane 2) non-RT treated RNA, (lane 3-4) cells transfected with pB19–10, (lanes 5-6) cells transfected with pB19–11, and (lane 7) cells transfected with empty vector. Arrows on the left indicate which amplicons correspond to pB19–10-derived Iso1 and Iso2 mRNA; arrows on the right indicate which amplicons correspond to pB19–11-derived Iso1, Iso1Δex10 and Iso2 mRNA. (D) Jurkat cells were transfected with either pB19–10 or a pB19–10 mutant; minigene-derived Iso1 and Iso2 expression was assessed 2 days later. Shown are the electrophoresis patterns of (lane 1) a ladder control, and amplicons from (lane 2) mock-transfected cells, and cells transfected with either (lane 3) empty vector, (lane 4) pB19–10, (lane 5) ΔpolyA, (lane 6) ΔpolyAΔex10, (lane 7) Δex9b, and (lane 8) Δex10. The gel image was cropped between lane 1 and lane 2; arrows indicate which amplicons correspond to minigene-derived Iso1 and Iso2 mRNA.
The results of transfecting pB19–10 and pB19–11 into Jurkat T cells are shown in FIG 4C, and demonstrate that pB19–10 expressed two isoforms of IL12RB1 corresponding to Iso1 and Iso2, independent of PHA stimulation (FIG 4C, lanes 3–4). pB19–11 also recapitulated endogenous processing of IL12RB1 into Iso1 and Iso2, albeit this minigene also produced a minor Iso1 splice variant consistent with splicing from exon 9 to exon 11 (Iso1Δex10) that we have previously observed in primary T cells (FIG 4C, lanes 5–6)16. As anticipated, no isoforms were expressed from our empty vector control (FIG 4C lane 7), or cDNA prepared without RT (FIG 4C lane 2). The data in FIG 4C thus demonstrate that IL12RB1 minigenes recapitulate endogenous IL12RB1 mRNA processing. Based on alternative splicing literature48–50, we generated IL12RB1 minigene deletion mutants lacking the exon 9b polyA site (ΔpolyA, FIG 4B), exon 9b polyA site and exon 10 (ΔpolyAΔex10, FIG 4B), exon 9b (Δex9b, FIG 4B), and exon 10 (Δex10, FIG 4B). Each minigene deletion mutant was then transfected into Jurkat T cells, and the expression of minigene-derived Iso1 and Iso2 was assessed. The data from these experiments are shown in FIG 4D, and demonstrate that preferential expression of Iso1 is unaffected by exon 9b polyA deletion (compare FIG 4D lanes 4 & 5), nor is it affected by the deletion of exon 9b (compare FIG 4D lanes 4 & 7). However, deletion of exon 10 - either alone or in combination with the exon 9b polyA site – results in enhanced expression of Iso2 (compare FIG 4D lane 4 to lanes 6 & 8). Minigene-derived Iso1 and Iso2 mRNAs were not observed in mock-treated or minus-RT controls (FIG 4D, lanes 2–3). Collectively, these data show that i) the exon 9b polyA site is needed for splicing to the exon 9b 3′ splice site (ΔpA), ii) the exon 9b 3′ss is functional in the absence of competition with exon 10 (ΔpAex10), and iii) the exon 9b polyA site is functional in the absence of competition from exon 9 to 10 splicing (Δex10), and indicate that competition between splicing of exons 9 and 10 with exon 9b splicing / polyadenylation underlies isoform production.
hnRNP H binds to IL12RB1 pre-mRNAs via G-tracts that are downstream of the exon 9b polyA site
Heterogeneous nuclear ribonucleoprotein H (hnRNP H) is a ubiquitously expressed regulator of alternative splicing and polyadenylation that binds mRNA via poly-guanine sequences (G-tracts)51–59. Three consecutive guanines are sufficient for binding hnRNP H60, which in turn can influence spliceosome activity or polyadenylation. Since there are five G-tracts downstream of the regulated IL12RB1 poly(A) site that are putative hnRNP H binding sites (FIG 5A), we hypothesized that hnRNP H-bound G-tracts contribute to the alternative processing of IL12RB1. To test this hypothesis, we first determined if hnRNP H binds IL12RB1 transcripts via the G-tracts depicted in FIG 5A. Specifically, in vitro transcription (IVT) was used to produce 84 nt IL12RB1 RNAs that harbor each G tract, as well as RNA with mutations in the G-tracts that are known to abolish hnRNP H binding (i.e. GGG→GUG; mutated G-tracts depicted in FIG 5A). RNAs generated using radiolabeled32P-nucleotides were incubated with HeLa cell nuclear extract, UV-crosslinked to associated proteins, and immunoprecipitated using anti-hnRNP H or preimmune sera. Immunoprecipitated protein/RNA complexes were resolved via SDS-PAGE and visualized via32P-autoradiography. As a positive control for hnRNP binding we used the sense strand of a 118 nt Rous sarcoma virus RNA (RSVSense) that is known to bind hnRNP H52; the antisense strand of the same RSV RNA (RSVAS) served as a negative control for hnRNP H binding.
FIGURE 5.
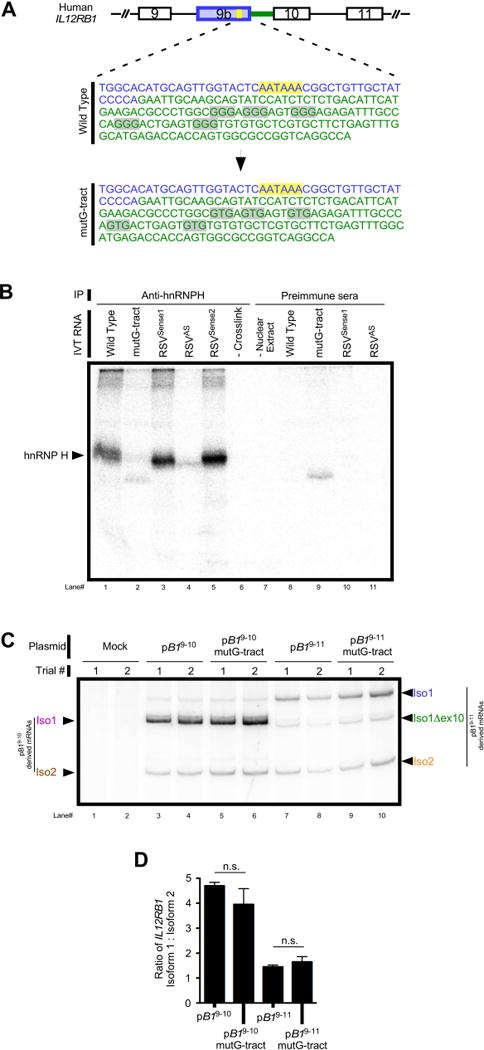
hnRNP H physically associates with IL12RB1 mRNA in a G-tract dependent manner. (A). Putative hnRNP H binding sites are located in the intron between exons 9b-10 (gray highlight) and comprises five GGG repeats (G-tracts). The exon 9b polyadenylation signal (yellow highlight) and 3' RNA terminus (blue) are shown for reference. Green denotes intron sequence. IL12RB1 minigenes were generated in which all five G-tracts were mutated from GGG (wild type) to GTG (mutG-tract). (B) To determine if hnRNP H physically associates with IL12RB1 pre-mRNAs at these sites, radiolabeled transcripts containing wild type (lane 1) or mutant (lane 2) IL12RB1 G tracts were incubated in HeLa nuclear extract, UV cross-linked, immunoprecipitated with anti-hnRNP H, and resolved using SDS-PAGE/autoradiography. RSVSense1 (lane 3) and RSVSense2 (lane 5) are positive control RNAs known to bind hnRNP H58; the antisense of RSVSense1 served a negative control (RSVAS, lane 4). Additional controls include wild type IL12RB1 RNA that was not crosslinked prior to immunoprecipitation (–Crosslink, lane 6), and a control reaction containing no HeLa nuclear extract (lane 7). Lanes 8-11 are control reactions immunoprecipitated using a nonspecific preimmune sera. (C) Jurkat cells were transfected with either pB19–10, pB19–10mutG-tract, pB19–11, or pB19–11mutG-tract (a derivative of pB19–11 harboring mutant G-tracts); minigene-derived Iso1 and Iso2 expression was assessed 2 days later by semi-quantitative PCR. Arrows on the left indicate which amplicons correspond to pB19–10-derived and pB19–10mutG-tract-derived Iso1 and Iso2 mRNA; arrows on the right indicate which amplicons correspond to pB19–11-derived and pB19–11mutG-tract-derived Iso1 and Iso2 mRNA. (D) Quantitation of the above semi-quantitative PCR.
The results of this analysis are shown in FIG 5B, and demonstrated that anti-hnRNP H immunoprecipitates hnRNP H cross-linked to RNA using wild type IL12RB1 RNA (compare FIG 5B lanes 1 & 8); the size of this band is similar to that immunoprecipitated from two independent positive controls from Rous sarcoma virus (RSVSense1 and RSVSense2, lanes 3 and 5) and is consistent with hnRNP H (compare FIG 5B lanes 1 &3), and is absent in negative controls including RSVAS (FIG 5B lanes 4, 6-11). Importantly, anti-hnRNP H fails to immunoprecipitate cross-linked hnRNP H using mutant G-tract IL12RB1 RNA (FIG 5B lane 2). Based on the data in FIG 5B, we conclude that hnRNP H physically associates with IL12RB1 RNAs via G-tracts that are downstream of its regulated poly(A) site. Since hnRNP H regulates pre-mRNA processing upon G-tract association56, we predicted that G-tract mutations would affect the relative levels of IL12RB1 Iso1 and Iso2. To test this hypothesis, we transfected Jurkat T cells with either pB19–10 or pB19–10mutG-tract, and then measured the expression of Iso1 and Iso2 mRNAs from each minigene. The results of this analysis are shown in FIG 5C–D and demonstrate that when the IL12RB1 G-tracts were mutated, T cells continued to preferentially express Iso1 over Iso2 (compare FIG 5C lanes 3–6); quantifying the ratio of plasmid-derived IL12RB1 Iso1:Iso2 levels also demonstrated no significant difference between pB19–10 or pB19–10mutG-tract (FIG 5D). Similar results were obtained from Jurkat T cells transfected with pB19–11 and pB19–11mutG-tract (pB19–11 with the G-tract mutations) (FIG 5C lanes 7–10, 5D). Collectively, these data demonstrate that hnRNP H binds to the G-tracts immediately downstream of IL12RB1 exon 9b, but that IL12RB1 pre-mRNA processing is G-tract independent.
Human IL12RB1 Isoform 2 positively regulates IL12-dependent IFNγ expression
The mouse homolog on human Iso2 (i.e. IL12Rβ1ΔTM) is a positive regulator of T cell responses to IL1217. Whether human Iso2 serves the same function has not yet been determined. Based on our previous studies of IL12Rβ1ΔTM knockout mice17, we hypothesized that human Iso2 positively regulates T cell responses to IL12. To test this hypothesis, we transduced Jurkat T cells with microRNAs (miRs) that we engineered to knockdown Iso2 mRNA without affecting Iso1 mRNA; transductants were then stimulated in the presence or absence of IL12, and their expression of IFNγ was used as readout of IL12-responsiveness. The five miRs we generated are depicted in FIG S1A; each miR was designed to target an mRNA sequence that is encoded by IL12RB1 Exon 9b and thus specific to Iso2 (FIG S1B). Of these five miRs (miRπ, miRϕ, miRσ, miRμ and miRε), validation studies demonstrated that only two (miRσ and miRμ) could knockdown Iso2 mRNA expression to ≤10% of control levels (FIG S1C–D). Based on these validation studies, lentiviral vectors were generated and used to transduce Jurkat T cells with a cDNA encoding GFP and miRσ, miRμ or miRNeg (a nonspecific control). Transductants were purified via FACS based on GFP expression, and stimulated for two days in media containing PHA alone (-IL12), PHA and 1 ng/mL IL12 (IL12Lo), or PHA and 10 ng/mL IL12 (IL12Hi). The cells and their supernatants were then collected and used for RT-PCR and ELISA analysis, respectively.
Relative to Jurkat cells that were either non-transduced or miRNeg-transduced, Iso2 mRNA expression was not observed in cells transduced with either miRσ or miRμ (FIG 6A, lower band), indicating efficient Iso2 knockdown. Importantly, Iso1 mRNA expression was intact in cells transduced with miRσ or miRμ, whether observed visually (FIG 6A, higher band) or measured quantitatively (FIG 6B). No significant differences in Iso1 expression existed between miRNeg, miRσ or miRμ groups (FIG 6B). Unexpectedly, all miR-transductants expressed higher levels of Iso1 relative to non-transduced cells (FIG 6B). We concluded from the data in FIG 6A–B that both miRσ and miRμ behaved as anticipated, and knocked down Iso2 mRNA without affecting Iso1 mRNA. We then moved forward and measured IFNγ mRNA and protein expression by the same cell preparations. The data from these measurements are shown in FIG 6C–D, and demonstrate that knockdown of Iso2 attenuates IL12-dependent IFNγ expression. Namely, miRNeg transductants responded to IL12 in a dose-dependent manner by increasing IFNγ mRNA transcription (albeit variable), whereas miRσ or miRμ transductants did not (FIG 6C). miRNeg transductants also (and less variably) responded to IL12 in a dose-dependent manner by increasing IFNγ secretion, whereas miRσ or miRμ transductants did not (FIG 6D). Collectively, these data demonstrate that IL12-elicited IFNγ transcription and secretion is attenuated in the absence of IL12RB1 Iso2.
FIGURE 6.
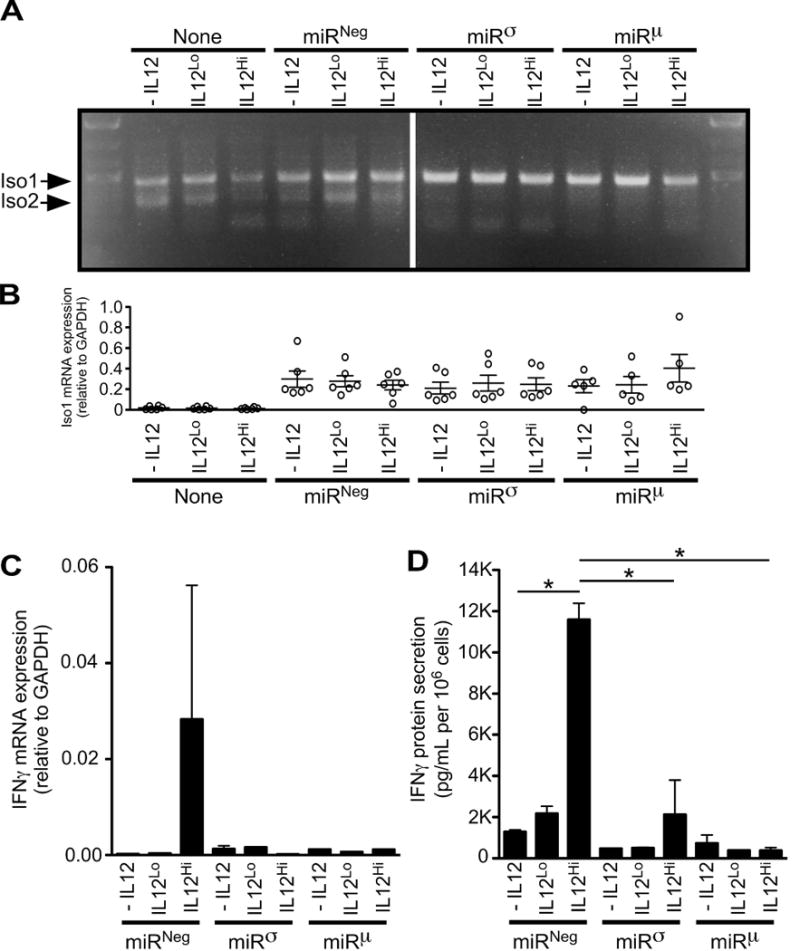
Human IL12RB1 Isoform 2 positively regulates IL12-dependent IFNγ expression. Jurkat T cells were transduced via lentivirus with one of two microRNAs (miRσ and miRμ) designed to knockdown IL12RB1 Isoform 2 expression, without affecting IL12RB1 Isoform 1. The design and validation studies of miRσ and miRμ are shown in FIG S1; the lentiviral vector used for miR transduction also encoded GFP. Control Jurkat T cells were either left non-transduced (none), or transduced with a microRNA that does not target any human gene (miRNeg). GFP+ transductants were sorted and cultured in the presence of PHA and 3 different IL12 concentrations (-IL12, IL12Lo and IL12Hi). The cells in each culture condition were collected 2 days later, counted and used for (A-C) mRNA expression studies; cell supernatants were collected at the same time and used for (D) IFNγ ELISA measurements. (A) Multiplex amplification of Isoform 1 (Iso1) and Isoform 2 (Iso2) mRNA from stimulated miR-transductants. Shown is an agarose gel image that was used to visually confirm the knockdown of Iso2 in miRσ- and miRμ -transduced cells, relative to both non- or miRNeg-transduced cells (B) qRT-PCR measurement of Isoform 1 mRNA expression, as normalized to the GAPDH expression in the same cell preparations. Each open circle represents the data from an individual experimental replicate (6 experimental replicates per condition). (C) qRT-PCR measurement of IFNγ mRNA expression, as normalized to the GAPDH expression in the same cell preparations. Shown are combined data from all 6 experimental replicates. (D) IFNγ levels in the supernatants of stimulated miR-transductants, as normalized to cell count. Shown are combined data from all 6 experimental replicates; *, p<0.05 as determined by ANOVA analysis.
DISCUSSION
The gene IL12RB1 regulates multiple aspects of human immunity, including resistance to tuberculous and nontuberculous mycobacterial pathogens2. Our current understanding of IL12RB1 is primarily derived from studies of IL12RB1-deficient children, who are otherwise healthy prior to mycobacteria exposure61. The propensity of IL12RB1-deficient children to develop mycobacterial disease is commonly interpreted to reflect the absence of Isoform 1 (i.e. IL12Rβ1), which is an integral membrane protein that associates with IL12Rβ2 and IL23R to form the IL12- and IL23-signaling complex, respectively3. IL12 and IL23 both promote IFNγ secretion10, 13, which in turn activates macrophages and limits mycobacteria survival14, 15. However, we and others have reported that a second IL12RB1 isoform (Isoform 2) exists in mice and humans as the result of alternative splicing16, 17, 62–64. Although the mouse homolog of Isoform 2 functions in mice to promote IL12-dependent IFNγ secretion17, its function in humans has heretofore been untested. There is also little to no information regarding the factors governing IL12RB1 expression in immunocompetent individuals. It was for these reasons that we undertook the studies described above, which we believe support the model depicted in FIGURE 7.
FIGURE 7.
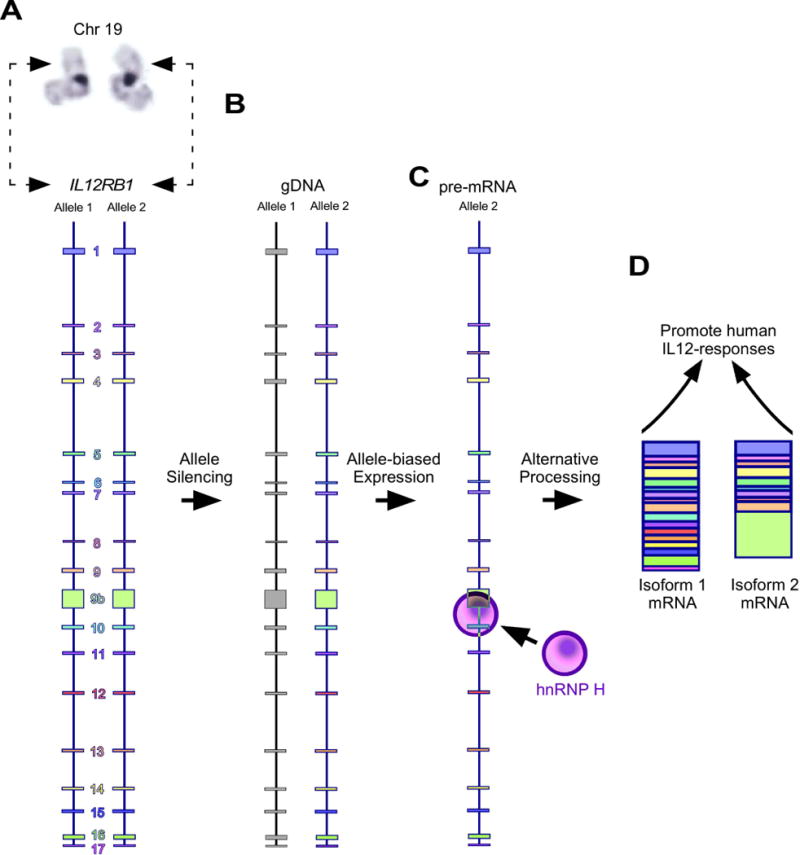
Model of IL12RB1 allele expression, processing and isoform function. (A) Human IL12RB1 is located on the p-arm of chromosome 19, and comprises exons 1-9, 9b, and 10-17. Shown is a karyotype image of chromosome 19, with the hatched arrows indicating the approximate position of IL12RB1. Depicted along the length of each IL12RB1 allele are the location and relative sizes of each exon and intron. (B) In primary human tissue, one IL12RB1 allele is silenced (represented by the gray exons of Allele 1) while and the other is transcriptionally active (represented by the colored exons of Allele 2). (C) The active allele is transcribed into a IL12RB1 pre-mRNA, which binds hnRNP H via G-tracts that are downstream of the Exon 9b poly(A) site. (D) The IL12RB1 pre-mRNA is the alternatively spliced into either Isoform 1 or Isoform 2, both of which promote IL12 responses.
Human IL12RB1 is located on chromosome 1965, and with rare exceptions each person inherits two functional IL12RB1 alleles (FIG 7A). We can say this with certainty given the rarity of Mendelian susceptibility to mycobacterial disease (MSMD), which is a primary immunodeficiency in one of nine genes that promote IFNγ-expression or -effectiveness (CYBB, IFNGR1, IFNGR2, IL12B, IL12RB1, IRF8, ISG15, NEMO and STAT1)1. Sequence data from the 1000 Genomes Project further confirm that most individuals inherit two functional IL12RB1 alleles66. However, the same sequence data demonstrate that IL12RB1 is polymorphic, with multiple SNPs in intronic and exonic regions. Several of these polymorphisms impact IL12RB1 function and associate with disease susceptibility3. For example, T cells complemented with an IL12RB1 allele containing a collection of linked SNPs termed the “RTR haplotype” (nucleotides C/G/G at chr19 positions 18186618/18180451/18180413) are less responsive to IL12 when compared to T cells complemented with the “QMG haplotype” (nucleotides T/A/C at the same chr19 positions)46. The SNPs that comprise the RTR haplotype are associated with tuberculosis susceptibility in residents of Kyushu Island, Japan67, cervical adenocarcinoma risk in residents of Seattle, USA68, breast cancer risk in African American residents of New York City and New Jersey, USA69, hidradenitis supperativa in Greek Caucasians70, and leptospirosis susceptibility among residents of Sao Miguel Island, Portugal71.
Prior to performing our allele-biased expression analysis, we assumed that both alleles of IL12RB1 were expressed in an immunocompetent individual. However, our data do not support this assumption and instead support the depiction in FIG 7B that, in human lung tissue and T cells, one IL12RB1 allele is silenced and the other transcriptionally active. This is important, as the allele chosen for expression will likely impact disease susceptibility. Again using the RTR and QMG haplotypes as an example, our sequencing data demonstrate that lung tissue donors N5 and S1 were heterozygotes who both inherited an RTR and QMG haplotype (FIG 1B, Donor N5 and S1 gDNA columns). However, the mRNAs produced in Donor N5 were primarily derived from RTR allele, while the mRNAs produced from Donor S1 were primarily derived from the QMG allele (FIG 1B, Donor N5 and S1 mRNA columns). Based on this information, we would predict the T cells from Donor N5 would respond to IL12 in a similar manner to T cells from someone who is homozygous for the RTR allele (e.g. Donor N1). Likewise, we would also predict that T cells from Donor S1 would respond to IL12 in a similar manner to T cells from someone who is homozygous for the QMG allele (e.g. Donor N4). Our future efforts will test this prediction, as well as determine if other MSMD-associated genes are regulated by monoallelic expression.
Monoallelic expression of autosomal genes is initiated and maintained by a variety of epigenetic mechanisms, including histone modifications, DNA methylation and nuclear organization18. The modification of histones can either promote or repress transcription of the associated allele, depending on the nature of the modification. For example, modifications of histone H3 that promote monoallelic expression include dimethylation and trimethylation of Lys4 (H3K4me2, H3K4me3), trimethylation of Lys36 (H3K36me3) and monoacetylation of Lys9 (H3K9ac)18. Modifications of histone H3 that repress expression include trimethylation of Lys9 (H3K9me3) and trimethylation of Lys27 (H3K27me3)18. Whereas histone modification affects proteins that are proximal to DNA, DNA methylation is a modification of DNA itself (i.e. cytosine) that represses transcription and is the basis for X-chromosome inactivation72. An additional epigenetic mechanism is the physical reorganization of all chromatin within the nucleus to permit interchromosomal interactions between an allele and enhancer elements73. MicroRNAs have also emerged as mechanism underlying allelic imbalance, as SNPs may create or disrupt microRNA target sites74–76. Future studies of IL12RB1 alleles with an ARFmRNA scores of 100 or 0 (e.g. ch19:18170384, FIG 1B,D) may demonstrate these SNPs to be microRNA binding sites.
Alternative RNA processing introduces variation in T cell responses to the “three signals” that govern T cell function20 (i.e. Signal 1, antigen recognition; Signal 2, co-stimulation; Signal 3, cytokine stimulation). Once an IL12RB1 allele is transcribed into a pre-mRNA, T cells must choose whether to alternatively process it (via alternative splicing and polyadenylation) into Isoform 1 or Isoform 2 (FIG 7B). The factors influencing cells’ alternative processing of IL12RB1 decision include activation status, as primary T cells express Isoform 2 more abundantly than Isoform 1 prior to activation16. Our results show that the exon 9b 3′ splice site and polyA site function efficiently in a minigene setting in the absence of exon 10, which suggests that inclusion of alternative exon 9b is out-competed by splicing from exon 9 to 10. The G-tracts downstream of the IL12RB1 exon 9b polyA site bind hnRNP H, which regulates pre-mRNA splicing and polyadenylation in a variety of systems77–79. hnRNP H is one member of the larger hnRNP family of proteins which facilitate mRNA processing80. Although eliminating hnRNP H binding via mutations did not impact T cells choice to express minigene-derived Isoform 1 or Isoform 2, it is possible that hnRNP H regulates the exon 9b polyA site in a manner that is not reflected with our minigenes. Importantly, our studies do not rule out hnRNP H binding as participating in regulation of the endogenous IL12RB1 gene, since minigene constructs pB19–11 and pB19–10 contained portions of IL12RB1, rather than the entire gene. Further work is needed to explore this possibility, including testing the hypothesis that hnRNP H knockdown alters T cell IL12RB1 Iso1:Iso2 ratios, and using hnRNP H RIP-Seq to test if endogenous IL12RB1 transcripts associate with hnRNP H in T cells (this approach was recently used to identify all hnRNP H bound transcripts in HeLa cells81). Additional hnRNPs that function in human T cells include hnRNP L which preferentially binds the pre-mRNA of genes that regulate T cell development34, 36, and hnRNP LL which facilitates expression of the CD45RO isoform via repression of PTPRC exon 438, 40, 41.
Should T cells choose to splice the IL12RB1 pre-mRNA into Isoform 1, the translated protein (IL12Rβ1) will be a type 1 transmembrane protein that localizes to the cell surface and positively regulates human IL12/23 sensitivity by binding the IL12p40-domain common to both cytokines3. Given that Isoform 2 lacks the transmembrane domain and has a localization pattern that is distinct from Isoform 116, 17, we initially predicted that Isoform 2 would either be non-functional or compete with Isoform 1 for access to IL12. However and unexpectedly, Isoform 2 potentiated IL12 responsiveness in mice and promoted their control of extrapulmonary TB17. It was these data that prompted our testing the hypothesis that Iso2 knockdown would attenuate IL12-dependent IFNγ secretion. Our results support this hypothesis and the model depicted in FIG 7C, wherein regardless of which way the IL12RB1 pre-mRNA is spliced (Isoform 1 or Isoform 2) the resulting protein will promote IL12 responsiveness. What will differ between these isoforms is their biochemical mechanism given Isoform 2′s distinct localization and altered C-terminal amino acid sequence.
In conclusion, we have demonstrated that in primary human lung tissue the IL12RB1 gene is preferentially expressed from one allele, and that the pre-mRNAs transcribed from IL12RB1 are processed into either Isoform 1 or Isoform 2 through competition between splicing and alternative polyadenylation of exon 9b. Should T cells choose to splice Isoform 2, our data demonstrate that the resulting protein will function to promote IL12-dependent IFNγ secretion. These results provide a rationale for future investigations of Isoform 2′s biochemical mechanism of action, as well as the epigenetic mechanisms responsible for establishing and sustaining allele-biased expression of IL12RB1.
MATERIALS AND METHODS
Human tissues and cell lines
Human lung tissue specimens were provided by the National Disease Research Interchange (NDRI, Philadelphia, PA). Between 1-5 hours postmortem, lung tissue specimens were collected and snap frozen for the NDRI repository. Upon receiving them in our laboratory, specimens were used for nucleic acid extraction and IL12RB1 amplification. Peripheral blood mononuclear cells (PBMCs) were purified from blood units that were donated by healthy adults, at the Blood Center of Wisconsin (Milwaukee, WI). Donors were excluded if they were taking (or had taken within 2 weeks before collection) any of the following categories of immunosuppressant medications: antineoplastic agent, antiviral agent, corticosteroid (either dermatological or nondermatological), a disease-modifying anti-rheumatic drug, or immunosuppressive mAb drugs. For purifying CD4+ and CD8+ T cells, PBMCs were suspended in a slurry of magnetic bead-Ab conjugates specific to CD4 (clone L200) or CD8 (clone SK1), according to the manufacturer’s protocols (BD Biosciences, San Diego, CA). After positive selection and two rounds of washing in PBS, subsets were counted and immediately lysed for RNA and DNA extraction. To determine the extent to which activation influenced allele bias, CD4+ and CD8+ T cells were purified from PBMCs before and after PBMC stimulation with PHA. The Jurkat T cell line was obtained from the American Type Culture Collection (ATCC, Manassas, VA) and passaged per ATCC protocols. BSC40Iso2 cells (a BSC40 cell line derivative that stably expresses Isoform 2 mRNA) have been previously described16. Cell lines were authenticated by PCR to be mycoplasma-free. All studies using human tissue and cells were approved by the Medical College of Wisconsin (MCW, Milwaukee, WI) Institutional Review Board. Informed consent was obtained from all subjects.
Monoallelic/allele-biased expression analysis
Genomic DNA (gDNA) and messenger RNA (mRNA) were extracted from human tissues and cell lines using the AllPrep DNA/RNA method (Qiagen, Germantown, MD). We performed PCR amplification and Ion Torrent sequencing of IL12RB1 gDNA/mRNA per our previously reported methods43. Single nucleotide polymorphisms (SNPs) in the donor IL12RB1 gDNA sequence were identified by comparing the donor gDNA sequence to the reference genome build Hg19. Among all the donor and cell line gDNA samples sequenced, we identified eleven positions that were polymorphic (chr19:18170384, 18170755, 18180413, 18180451, 18186575, 18186618, 18188408, 18191664, 18192977, 18194255 and 18197635). Ten of the positions had been previously described and assigned a reference SNP (rs) number (18170384, rs3746190; 18180413, rs401502; 18180451, rs375947; 18186575, rs17852635; 18186618, rs11575934; 18188408, rs11575926; 18191664, rs11086087; 18192977, rs11575925; 18197635, rs436857; 18194255, rs372258967). One of the positions we identified was Jurkat T cell line specific and did not have an assigned rs number (chr19:18170755). Whether a donor or cell line was homozygous or heterozygous for a given SNP was determined by querying the Ion Torrent data to see if only one nucleotide was detected at that site (indicating homozygosity) or two nucleotides were detected at that site (indicating heterozygosity). For donors who were genetically heterozygous at one or more polymorphic sites, the mRNA sequence data from the same tissue was queried to determine if both IL12RB1 alleles were equally represented in the mRNA (indicating biallelic expression), or if only one IL12RB1 allele was preferentially represented in the mRNA (indicating allele-biased expression). We used the allele read frequency (ARF) as a quantitative indicator of the extent to which a specific allele was represented in the gDNA or mRNA reads. The ARFgDNA value of a specific allele is determined by the equation , where indicates the number of Ion Torrent reads at gDNA position that were nucleotide (either an adenine [A], cytosine [C], guanine [G] or thymine [T]), and indicates the total number of Ion Torrent reads at position (all nucleotides). For example, a gDNA ARFA18170384 value of 50 indicates that 50% of Ion Torrent reads at gDNA position 18170384 were an A, whereas a gDNA ARFA18170384 value of 100 indicates that 100% of Ion Torrent reads at gDNA position 18170384 were an A. The same equation was used to determine the ARFmRNA value of a specific allele, with the exception that mRNA sequencing information was used for values and . After calculating each allele’s ARFgDNA and ARFmRNA values, we could then determine if IL12RB1 expression was biallelic (ARFmRNA ≅ ARFgDNA for both IL12RB1 alleles) or allele-biased (ARFmRNA≫ARFgDNA for one IL12RB1 allele). Per the criteria established by Gendral et al82, IL12RB1 expression was considered allele-biased if the predominantly expressed allele was expressed at ≥ 80% and the second allele at ≤ 20%. Our previously reported methods were used to generate and Sanger sequence IL12RB1 gDNA amplicons and IL12RB1 cDNA libraries43.
IL12RB1 minigene construction
Two IL12RB1 minigenes (pB19–10 and pB19–11) were constructed in anticipation that one or both would recapitulate IL12RB1 alternative RNA processing. Minigenes and their derivatives (i.e. deletion mutants) were generated using a commercial gene synthesis service (Invitrogen, Carlsbad, CA). pB19–10 was generated by cloning a 2804 bp insert encoding IL12RB1 exons 9→9b→10 (NCBI Reference Sequence: NG_007366.2, positions 26468-29271) into the HindIII/XbaI site of vector p3xFLAG-CMV7.1 (Sigma-Aldrich). pB19–11 was similarly generated, via cloning into the same site/vector a 3962 bp insert encoding IL12RB1 exons 9→9b→10→11 (NG_007366.2 positions 26468-30429). Restriction sites were engineered into the minigenes to facilitate generation of deletion mutants. Derivatives of pB19–10 that we generated and used for our study include the minigene ΔpolyA (removal of a 345 bp BsuI-BspEI fragment that encompasses the polyA signal and downstream intron sequence, including the G tracts: the polyadenylation signal in NG_007366.2 is at positions 28232-28237), ΔpolyAΔex10 (removal of a 1190 bp BsuI-HindIII fragment that encompasses the polyA region, downstream intron, and exon 10), Δex9b (removal of a 991 bp HpaI-BspEI fragment that harbors exon 9b, and upstream and downstream intron sequences, including the G tracts), Δex10 (deletion of a 845 bp BspEI-HindIII fragment that removes exon 10 and upstream introns sequences). G-tract mutations were generated by synthesizing a 359 bp BsuI-BspEI fragment (Invitrogen, Carlsbad, CA) spanning the G tract region that contained five GGG to GUG changes (see FIG 5A). Plasmids harboring G tract mutations (pB19–10mutG-tract and pB19–11mutG-tract were made by replacing the wild type fragment with the mutant. For UV cross-linking, an 84 bp fragment containing the wild type or mutant G tracts was cloned into the EcoRI-HindIII sites of pGEM-3Z (Promega) to generate p3Z-Gtract and p3Zmut.
Minigene transfection and expression analysis
IL12RB1 minigenes were transfected into Jurkat T cells using the Lipofectamine method (Invitrogen). Two days later, mRNA was extracted and used for RT-PCR analysis of minigene-derived Iso1 and Iso2 expression. Specifically, cDNA from minigene transfectants were added to a multiplex PCR reaction comprising three primers: T7 promoter, a forward primer that anneals to a vector-derived sequence upstream of the IL12RB1 insert (5′-TAATACGACTCACTATAGGG-3′); pcD-myc-r, an outside reverse primer that anneals to a vector-derived sequence downstream of the IL12RB1 insert (5′-GCTATTCAGATCCTCTTCTG-3′); 9b-Bst, a reverse primer that anneals to an IL12RB1 exon 9b-derived sequence (5′-CTGGAAGGCGGAGGTTACC-3′). The use of T7 and pcD-myc-r primers ensured that the Iso1 and Iso2 mRNAs we amplified were minigene-derived, and not the endogenous IL12RB1 isoforms expressed by Jurkat T cells. Amplicons were visualized using standard agarose gel electrophoresis methods.
Semiquantitative PCR
Jurkat cells were transfected with minigenes containing wild type or mutant G-tracts using Lipofectamine 2000 following manufacturer’s suggested conditions. Two days later total RNA was isolated using Qiagen RNeasy columns following manufacturer's suggested conditions, followed by reverse transcription. PCR reactions were done using primers described above. Reactions contained alpha-32P dCTP and went 25 cycles, after which products were separated on a 5% non-denaturing acrylamide gel, dried, and quantitated by phosphorimaging. Products were normalized for dCTP content.
UV cross-linking and hnRNP H immunoprecipitation
In vitro transcription (IVT) and hnRNP H immunoprecipitation were performed using previously reported methods52, 58. Briefly, 85 nt radiolabeled RNAs that encompass the G tracts were produced using32P-radionucleotides and incubated in HeLa cell nuclear cell extracts known to contain hnRNP H, and RNAs were UV-crosslinked to any associating protein. Cross-linked RNA/protein conjugates were then immunoprecipitated using either anti-hnRNP H antibody or preimmune sera, resolved via SDS-PAGE, and visualized by phosphorimaging.
Design and screen of microRNAs to knockdown Isoform 2 expression
To knockdown Isoform 2 mRNA expression, we designed and expressed five microRNAs targeting IL12RB1 exon 9b (an mRNA sequence specific to Isoform 2) using the BLOCK-iT Lentiviral Pol II miR RNAi expression system (Invitrogen). The five microRNAs we designed are referred to as miRπ, miRϕ, miRσ, miRμ and miRε. These five miRs and their sequences are depicted in supplemental FIG S1A; each miR’s corresponding target in the IL12RB1 exon 9b mRNA is depicted in supplemental FIG S1B. The cDNAs encoding each miR were commercially synthesized (Integrated DNA Technologies, Coralville, IA) (supplemental TABLE S1) and cloned into the GFP-miR expression cassette of the mammalian expression plasmid pcDNA 6.2-GW/EmGFP-miR. GFP-miR containing plasmid clones were identified by colony PCR, Sanger sequenced, and screened for their ability to knockdown Isoform 2 mRNA in BSC40Iso2 cells. Specifically, BSC40Iso2 cells were transfected with a miR-containing plasmid clone, cultured for two days, and FACS sorted based on GFP expression (supplemental FIG S1C); mRNA from GFP+ cells was then used to quantify Isoform 2 mRNA levels per our reported methods16. Control BSC40Iso2 cells were transfected with a miR that does not target any human gene (miRNeg, the cDNA sequence for which was provided by Invitrogen and is also reported in supplemental TABLE S1). Among the five miRs we designed and generated, only two miRs (miRσ and miRμ) caused a ≥90% reduction in Isoform 2 mRNA levels relative to miRNeg controls (supplemental FIG S1D); the three other miRs (miRπ, miRϕ, and miRε) were unable to knockdown Isoform 2 mRNA (data not shown). We then moved forward and cloned the GFP-miRσ, GFP-miRμ and GFP-miRNeg expression cassettes into lentiviral vector pLenti6. Lentivirus stocks were generated, titered and stored at -80°C.
Lentiviral Transduction
Jurkat cell cultures were maintained in complete RPMI (cRPMI) and transduced with GFP-miR containing pLenti6 lentivirus per the methods of Denning et al83. Transduced cells were then cultured for one week in selection media (cRPMI with 10 μg/mL blasticidin) and FACS sorted based on GFP expression. To reduce intra- and inter-experiment variation, separate cultures were transduced with each miR on the same day, and FACS sorted on the same day. In this manner, Jurkat cells expressing either miRσ, miRμ or miRNeg were purified.
IL12-stimulation assay
Purified Jurkat cells expressing miRσ, miRμ or miRNeg were stimulated in cRPMI containing either 1 μg/mL phytohemagglutinin (PHA alone), PHA and 1 ng/mL recombinant human IL12 (PHA+IL12Lo), or PHA and 10 ng/mL recombinant human IL12 (PHA+IL12Hi). Recombinant human IL12 was purchased from R&D Systems (Minneapolis, MN). Two days after stimulation, cells and their supernatants were collected and separated; the cells were counted and lysed for RT-PCR analysis, while the supernatants were frozen and later used for ELISA analysis.
RT-PCR analysis of transduced Jurkat cells
RNA from miR-transduced, stimulated Jurkat cells was collected and used for RT-PCR analysis of Isoform 1, Isoform 2, IFNγ and GAPDH mRNA levels. Isoform 1 and Isoform 2 mRNA levels were visualized using our previously reported multiplex PCR method16. The level of Isoform 1 mRNA in miR-transductants was also assessed quantitatively using our previously reported SYBR green PCR method16. Notably, the same SYBR green method could not reliably measure Isoform 2 mRNA levels in miR-transductants, and instead produced aberrant amplicons (data not shown) that were possibly due to interference from Iso2 specific miRs present in the RNA preparation. It was for this reason we used conventional PCR and agarose gel electrophoresis to visualize Isoform 2 mRNA levels in miR-transductants. The mRNA levels of IFNγ and GAPDH were determined using TaqMan Gene Expression Assays (Applied Biosystems, Foster City, CA), using the primer/probe sets Hs00989291_m1 (IFNγ) and Hs99999905_m1 (GAPDH), and the CT for each gene determined using an iQ5 Real-Time PCR System (BioRad, Hercules, CA). For each biological replicate, the ΔCT was determined (CTIFNγ- CTGAPDH) and the IFNγ mRNA level (x) calculated using the equation x =2−ΔCT.
ELISA analysis
Supernatants from miR-transduced, stimulated Jurkat cells were used for ELISA analysis of secreted IFNγ levels. The concentration of human IFNγ in each supernatant was determined using the OptEIA system (BD Biosciences, San Jose, CA). For graphing and statistical analysis, IFNγ concentrations were normalized to the number of Jurkat cells present in each well.
Graphing and statistical analysis
Graphs were prepared using Graph Pad Prism version 5.0. Statistical analyses were performed using the bundled software of Prism. Standard power formulas were used to calculate sample size and ensure adequate power to detect statistical differences. Statistical differences between two groups were determined using Student’s t-test, while differences between three or more groups were determined using one-way ANOVA; the variance was similar between groups that were statistically compared. Differences were considered significant if p < 0.05, and is graphically indicated by an asterisk.
Supplementary Material
Acknowledgments
We would like to thank and acknowledge the following individuals, whose work contributed to indicated Figures: Jill Waukau and Christine Bengtson (Figures 1 and 2), Mark McNally and Lisa McNally (Figures 4 and 5), Katrina Monson and Brady Brooks (Figure 6). We would also like to thank Abigail Robinson for the chromosome 19 karyotype image in Figure 7. This work was supported by the Medical College of Wisconsin (MCW) and National Institutes of Health grant R01 AI121212 (to R.T.R.).
Footnotes
CONFLICT OF INTEREST
The authors declare no competing financial interests in relation to the work described.
References
- 1.Bustamante J, Boisson-Dupuis S, Abel L, Casanova JL. Mendelian susceptibility to mycobacterial disease: genetic, immunological, and clinical features of inborn errors of IFN-gamma immunity. Semin Immunol. 2014;26(6):454–70. doi: 10.1016/j.smim.2014.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.de Beaucoudrey L, Samarina A, Bustamante J, Cobat A, Boisson-Dupuis S, Feinberg J, et al. Revisiting human IL-12Rbeta1 deficiency: a survey of 141 patients from 30 countries. Medicine (Baltimore) 2010;89(6):381–402. doi: 10.1097/MD.0b013e3181fdd832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Robinson RT. IL12Rbeta1: the cytokine receptor that we used to know. Cytokine. 2015;71(2):348–59. doi: 10.1016/j.cyto.2014.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.van de Vosse E, Haverkamp MH, Ramirez-Alejo N, Martinez-Gallo M, Blancas-Galicia L, Metin A, et al. IL-12Rbeta1 deficiency: mutation update and description of the IL12RB1 variation database. Hum Mutat. 2013;34(10):1329–39. doi: 10.1002/humu.22380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dhiman N, Ovsyannikova IG, Cunningham JM, Vierkant RA, Kennedy RB, Pankratz VS, et al. Associations between measles vaccine immunity and single-nucleotide polymorphisms in cytokine and cytokine receptor genes. J Infect Dis. 2007;195(1):21–9. doi: 10.1086/510596. [DOI] [PubMed] [Google Scholar]
- 6.White SJ, Haralambieva IH, Ovsyannikova IG, Vierkant RA, O’Byrne MM, Poland GA. Replication of associations between cytokine and cytokine receptor single nucleotide polymorphisms and measles-specific adaptive immunophenotypic extremes. Hum Immunol. 2012;73(6):636–40. doi: 10.1016/j.humimm.2012.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li X, Hawkins GA, Ampleford EJ, Moore WC, Li H, Hastie AT, et al. Genome-wide association study identifies TH1 pathway genes associated with lung function in asthmatic patients. J Allergy Clin Immunol. 2013;132(2):313–20 e15. doi: 10.1016/j.jaci.2013.01.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Takahashi N, Akahoshi M, Matsuda A, Ebe K, Inomata N, Obara K, et al. Association of the IL12RB1 promoter polymorphisms with increased risk of atopic dermatitis and other allergic phenotypes. Hum Mol Genet. 2005;14(21):3149–59. doi: 10.1093/hmg/ddi347. [DOI] [PubMed] [Google Scholar]
- 9.Hong X, Wang G, Liu X, Kumar R, Tsai HJ, Arguelles L, et al. Gene polymorphisms, breast-feeding, and development of food sensitization in early childhood. J Allergy Clin Immunol. 2011;128(2):374–81 e2. doi: 10.1016/j.jaci.2011.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Oppmann B, Lesley R, Blom B, Timans JC, Xu Y, Hunte B, et al. Novel p19 protein engages IL-12p40 to form a cytokine, IL-23, with biological activities similar as well as distinct from IL-12. Immunity. 2000;13(5):715–25. doi: 10.1016/s1074-7613(00)00070-4. [DOI] [PubMed] [Google Scholar]
- 11.Presky DH, Yang H, Minetti LJ, Chua AO, Nabavi N, Wu CY, et al. A functional interleukin 12 receptor complex is composed of two beta-type cytokine receptor subunits. Proc Natl Acad Sci U S A. 1996;93(24):14002–7. doi: 10.1073/pnas.93.24.14002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Parham C, Chirica M, Timans J, Vaisberg E, Travis M, Cheung J, et al. A receptor for the heterodimeric cytokine IL-23 is composed of IL-12Rbeta1 and a novel cytokine receptor subunit, IL-23R. J Immunol. 2002;168(11):5699–708. doi: 10.4049/jimmunol.168.11.5699. [DOI] [PubMed] [Google Scholar]
- 13.Khader SA, Pearl JE, Sakamoto K, Gilmartin L, Bell GK, Jelley-Gibbs DM, et al. IL-23 compensates for the absence of IL-12p70 and is essential for the IL-17 response during tuberculosis but is dispensable for protection and antigen-specific IFN-gamma responses if IL-12p70 is available. J Immunol. 2005;175(2):788–95. doi: 10.4049/jimmunol.175.2.788. [DOI] [PubMed] [Google Scholar]
- 14.Cooper AM, Dalton DK, Stewart TA, Griffin JP, Russell DG, Orme IM. Disseminated tuberculosis in interferon gamma gene-disrupted mice. J Exp Med. 1993;178(6):2243–7. doi: 10.1084/jem.178.6.2243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Flynn JL, Chan J, Triebold KJ, Dalton DK, Stewart TA, Bloom BR. An essential role for interferon gamma in resistance to Mycobacterium tuberculosis infection. J Exp Med. 1993;178(6):2249–54. doi: 10.1084/jem.178.6.2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ford NR, Miller HE, Reeme AE, Waukau J, Bengtson C, Routes JM, et al. Inflammatory signals direct expression of human IL12RB1 into multiple distinct isoforms. J Immunol. 2012;189(9):4684–94. doi: 10.4049/jimmunol.1200606. [DOI] [PubMed] [Google Scholar]
- 17.Ray AA, Fountain JJ, Miller HE, Cooper AM, Robinson RT. IL12Rbeta1DeltaTM is a secreted product of il12rb1 that promotes control of extrapulmonary tuberculosis. Infect Immun. 2015;83(2):560–71. doi: 10.1128/IAI.01230-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reinius B, Sandberg R. Random monoallelic expression of autosomal genes: stochastic transcription and allele-level regulation. Nat Rev Genet. 2015;16(11):653–64. doi: 10.1038/nrg3888. [DOI] [PubMed] [Google Scholar]
- 19.Chang X. RNA-binding protein hnRNPLL as a critical regulator of lymphocyte homeostasis and differentiation. Wiley Interdiscip Rev RNA. 2016;7(3):295–302. doi: 10.1002/wrna.1335. [DOI] [PubMed] [Google Scholar]
- 20.Martinez NM, Lynch KW. Control of alternative splicing in immune responses: many regulators, many predictions, much still to learn. Immunol Rev. 2013;253(1):216–36. doi: 10.1111/imr.12047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ku CJ, Lim KC, Kalantry S, Maillard I, Engel JD, Hosoya T. A monoallelic-to-biallelic T-cell transcriptional switch regulates GATA3 abundance. Genes Dev. 2015;29(18):1930–41. doi: 10.1101/gad.265025.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brady BL, Steinel NC, Bassing CH. Antigen receptor allelic exclusion: an update and reappraisal. J Immunol. 2010;185(7):3801–8. doi: 10.4049/jimmunol.1001158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bayley JP, van Rietschoten JG, Bakker AM, van Baarsen L, Kaijzel EL, Wierenga EA, et al. Allele-specific expression of the IL-1 alpha gene in human CD4+ T cell clones. J Immunol. 2003;171(5):2349–53. doi: 10.4049/jimmunol.171.5.2349. [DOI] [PubMed] [Google Scholar]
- 24.Bix M, Locksley RM. Independent and epigenetic regulation of the interleukin-4 alleles in CD4+ T cells. Science. 1998;281(5381):1352–4. doi: 10.1126/science.281.5381.1352. [DOI] [PubMed] [Google Scholar]
- 25.Calado DP, Paixao T, Holmberg D, Haury M. Stochastic monoallelic expression of IL-10 in T cells. J Immunol. 2006;177(8):5358–64. doi: 10.4049/jimmunol.177.8.5358. [DOI] [PubMed] [Google Scholar]
- 26.Hollander GA, Zuklys S, Morel C, Mizoguchi E, Mobisson K, Simpson S, et al. Monoallelic expression of the interleukin-2 locus. Science. 1998;279(5359):2118–21. doi: 10.1126/science.279.5359.2118. [DOI] [PubMed] [Google Scholar]
- 27.Kelly BL, Locksley RM. Coordinate regulation of the IL-4, IL-13, and IL-5 cytokine cluster in Th2 clones revealed by allelic expression patterns. J Immunol. 2000;165(6):2982–6. doi: 10.4049/jimmunol.165.6.2982. [DOI] [PubMed] [Google Scholar]
- 28.Rhoades KL, Singh N, Simon I, Glidden B, Cedar H, Chess A. Allele-specific expression patterns of interleukin-2 and Pax-5 revealed by a sensitive single-cell RT-PCR analysis. Curr Biol. 2000;10(13):789–92. doi: 10.1016/s0960-9822(00)00565-0. [DOI] [PubMed] [Google Scholar]
- 29.Riviere I, Sunshine MJ, Littman DR. Regulation of IL-4 expression by activation of individual alleles. Immunity. 1998;9(2):217–28. doi: 10.1016/s1074-7613(00)80604-4. [DOI] [PubMed] [Google Scholar]
- 30.van Rietschoten JG, Verzijlbergen KF, Gringhuis SI, van der Pouw Kraan TC, Bayley JP, Wierenga EA, et al. Differentially methylated alleles in a distinct region of the human interleukin-1alpha promoter are associated with allele-specific expression of IL-1alpha in CD4+ T cells. Blood. 2006;108(7):2143–9. doi: 10.1182/blood-2006-01-021147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chaudhury A, Chander P, Howe PH. Heterogeneous nuclear ribonucleoproteins (hnRNPs) in cellular processes: Focus on hnRNP E1's multifunctional regulatory roles. RNA. 2010;16(8):1449–62. doi: 10.1261/rna.2254110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yeh YM, Chen CY, Huang PR, Hsu CW, Wu CC, Wang TC. Proteomic analyses of genes regulated by heterogeneous nuclear ribonucleoproteins A/B in Jurkat cells. Proteomics. 2014;14(11):1357–66. doi: 10.1002/pmic.201300549. [DOI] [PubMed] [Google Scholar]
- 33.Veraldi KL, Arhin GK, Martincic K, Chung-Ganster LH, Wilusz J, Milcarek C. hnRNP F influences binding of a 64-kilodalton subunit of cleavage stimulation factor to mRNA precursors in mouse B cells. Mol Cell Biol. 2001;21(4):1228–38. doi: 10.1128/MCB.21.4.1228-1238.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gaudreau MC, Heyd F, Bastien R, Wilhelm B, Moroy T. Alternative splicing controlled by heterogeneous nuclear ribonucleoprotein L regulates development, proliferation, and migration of thymic pre-T cells. J Immunol. 2012;188(11):5377–88. doi: 10.4049/jimmunol.1103142. [DOI] [PubMed] [Google Scholar]
- 35.Rothrock CR, House AE, Lynch KW. HnRNP L represses exon splicing via a regulated exonic splicing silencer. EMBO J. 2005;24(15):2792–802. doi: 10.1038/sj.emboj.7600745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shankarling G, Cole BS, Mallory MJ, Lynch KW. Transcriptome-wide RNA interaction profiling reveals physical and functional targets of hnRNP L in human T cells. Mol Cell Biol. 2014;34(1):71–83. doi: 10.1128/MCB.00740-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tong A, Nguyen J, Lynch KW. Differential expression of CD45 isoforms is controlled by the combined activity of basal and inducible splicing-regulatory elements in each of the variable exons. J Biol Chem. 2005;280(46):38297–304. doi: 10.1074/jbc.M508123200. [DOI] [PubMed] [Google Scholar]
- 38.Topp JD, Jackson J, Melton AA, Lynch KW. A cell-based screen for splicing regulators identifies hnRNP LL as a distinct signal-induced repressor of CD45 variable exon 4. RNA. 2008;14(10):2038–49. doi: 10.1261/rna.1212008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chang X, Li B, Rao A. RNA-binding protein hnRNPLL regulates mRNA splicing and stability during B-cell to plasma-cell differentiation. Proc Natl Acad Sci U S A. 2015;112(15):E1888–97. doi: 10.1073/pnas.1422490112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Oberdoerffer S, Moita LF, Neems D, Freitas RP, Hacohen N, Rao A. Regulation of CD45 alternative splicing by heterogeneous ribonucleoprotein, hnRNPLL. Science. 2008;321(5889):686–91. doi: 10.1126/science.1157610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wu Z, Jia X, de la Cruz L, Su XC, Marzolf B, Troisch P, et al. Memory T cell RNA rearrangement programmed by heterogeneous nuclear ribonucleoprotein hnRNPLL. Immunity. 2008;29(6):863–75. doi: 10.1016/j.immuni.2008.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Meininger I, Griesbach RA, Hu D, Gehring T, Seeholzer T, Bertossi A, et al. Alternative splicing of MALT1 controls signalling and activation of CD4(+) T cells. Nat Commun. 2016;7:11292. doi: 10.1038/ncomms11292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Turner AJ, Aggarwal P, Miller HE, Waukau J, Routes JM, Broeckel U, et al. The introduction of RNA-DNA differences underlies interindividual variation in the human IL12RB1 mRNA repertoire. Proc Natl Acad Sci U S A. 2015;112(50):15414–9. doi: 10.1073/pnas.1515978112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Miller HE, Robinson RT. Early control of Mycobacterium tuberculosis infection requires il12rb1 expression by rag1-dependent lineages. Infect Immun. 2012;80(11):3828–41. doi: 10.1128/IAI.00426-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Xiao Z, Casey KA, Jameson SC, Curtsinger JM, Mescher MF. Programming for CD8 T cell memory development requires IL-12 or type I IFN. J Immunol. 2009;182(5):2786–94. doi: 10.4049/jimmunol.0803484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.van de Vosse E, de Paus RA, van Dissel JT, Ottenhoff TH. Molecular complementation of IL-12Rbeta1 deficiency reveals functional differences between IL-12Rbeta1 alleles including partial IL-12Rbeta1 deficiency. Hum Mol Genet. 2005;14(24):3847–55. doi: 10.1093/hmg/ddi409. [DOI] [PubMed] [Google Scholar]
- 47.Abraham RT, Weiss A. Jurkat T cells and development of the T-cell receptor signalling paradigm. Nat Rev Immunol. 2004;4(4):301–8. doi: 10.1038/nri1330. [DOI] [PubMed] [Google Scholar]
- 48.Lou H, Cote GJ, Gagel RF. The calcitonin exon and its flanking intronic sequences are sufficient for the regulation of human calcitonin/calcitonin gene-related peptide alternative RNA splicing. Mol Endocrinol. 1994;8(12):1618–26. doi: 10.1210/mend.8.12.7535892. [DOI] [PubMed] [Google Scholar]
- 49.Lou H, Gagel RF. Alternative RNA processing–its role in regulating expression of calcitonin/calcitonin gene-related peptide. J Endocrinol. 1998;156(3):401–5. doi: 10.1677/joe.0.1560401. [DOI] [PubMed] [Google Scholar]
- 50.Millevoi S, Vagner S. Molecular mechanisms of eukaryotic pre-mRNA 3' end processing regulation. Nucleic Acids Res. 2010;38(9):2757–74. doi: 10.1093/nar/gkp1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Caputi M, Zahler AM. Determination of the RNA binding specificity of the heterogeneous nuclear ribonucleoprotein (hnRNP) H/H'/F/2H9 family. J Biol Chem. 2001;276(47):43850–9. doi: 10.1074/jbc.M102861200. [DOI] [PubMed] [Google Scholar]
- 52.Fogel BL, McNally MT. A cellular protein, hnRNP H, binds to the negative regulator of splicing element from Rous sarcoma virus. J Biol Chem. 2000;275(41):32371–8. doi: 10.1074/jbc.M005000200. [DOI] [PubMed] [Google Scholar]
- 53.Hastings ML, Wilson CM, Munroe SH. A purine-rich intronic element enhances alternative splicing of thyroid hormone receptor mRNA. RNA. 2001;7(6):859–74. doi: 10.1017/s1355838201002084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kralovicova J, Vorechovsky I. Position-dependent repression and promotion of DQB1 intron 3 splicing by GGGG motifs. J Immunol. 2006;176(4):2381–8. doi: 10.4049/jimmunol.176.4.2381. [DOI] [PubMed] [Google Scholar]
- 55.Marcucci R, Baralle FE, Romano M. Complex splicing control of the human Thrombopoietin gene by intronic G runs. Nucleic Acids Res. 2007;35(1):132–42. doi: 10.1093/nar/gkl965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mauger DM, Lin C, Garcia-Blanco MA. hnRNP H and hnRNP F complex with Fox2 to silence fibroblast growth factor receptor 2 exon IIIc. Mol Cell Biol. 2008;28(17):5403–19. doi: 10.1128/MCB.00739-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.McCullough AJ, Berget SM. G triplets located throughout a class of small vertebrate introns enforce intron borders and regulate splice site selection. Mol Cell Biol. 1997;17(8):4562–71. doi: 10.1128/mcb.17.8.4562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.McNally LM, Yee L, McNally MT. Heterogeneous nuclear ribonucleoprotein H is required for optimal U11 small nuclear ribonucleoprotein binding to a retroviral RNA-processing control element: implications for U12-dependent RNA splicing. J Biol Chem. 2006;281(5):2478–88. doi: 10.1074/jbc.M511215200. [DOI] [PubMed] [Google Scholar]
- 59.Yeo G, Hoon S, Venkatesh B, Burge CB. Variation in sequence and organization of splicing regulatory elements in vertebrate genes. Proc Natl Acad Sci U S A. 2004;101(44):15700–5. doi: 10.1073/pnas.0404901101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Dominguez C, Allain FH. NMR structure of the three quasi RNA recognition motifs (qRRMs) of human hnRNP F and interaction studies with Bcl-x G-tract RNA: a novel mode of RNA recognition. Nucleic Acids Res. 2006;34(13):3634–45. doi: 10.1093/nar/gkl488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Fieschi C, Dupuis S, Catherinot E, Feinberg J, Bustamante J, Breiman A, et al. Low penetrance, broad resistance, and favorable outcome of interleukin 12 receptor beta1 deficiency: medical and immunological implications. J Exp Med. 2003;197(4):527–35. doi: 10.1084/jem.20021769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chua AO, Wilkinson VL, Presky DH, Gubler U. Cloning and characterization of a mouse IL-12 receptor-beta component. J Immunol. 1995;155(9):4286–94. [PubMed] [Google Scholar]
- 63.Robinson RT, Khader SA, Martino CA, Fountain JJ, Teixeira-Coelho M, Pearl JE, et al. Mycobacterium tuberculosis infection induces il12rb1 splicing to generate a novel IL-12Rbeta1 isoform that enhances DC migration. J Exp Med. 2010;207(3):591–605. doi: 10.1084/jem.20091085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Showe LC, Wysocka M, Wang B, Lineman-Williams D, Peritt D, Showe MK, et al. Structure of the mouse IL-12R beta 1 chain and regulation of its expression in BCG/LPS-treated mice. Ann N Y Acad Sci. 1996;795:413–5. doi: 10.1111/j.1749-6632.1996.tb52708.x. [DOI] [PubMed] [Google Scholar]
- 65.Yamamoto K, Kobayashi H, Miura O, Hirosawa S, Miyasaka N. Assignment of IL12RB1 and IL12RB2, interleukin-12 receptor beta 1 and beta 2 chains, to human chromosome 19 band p13.1 and chromosome 1 band p31.2, respectively, by in situ hybridization. Cytogenet Cell Genet. 1997;77(3–4):257–8. doi: 10.1159/000134589. [DOI] [PubMed] [Google Scholar]
- 66.Genomes Project C, Abecasis GR, Auton A, Brooks LD, DePristo MA, Durbin RM, et al. An integrated map of genetic variation from 1,092 human genomes. Nature. 2012;491(7422):56–65. doi: 10.1038/nature11632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Akahoshi M, Nakashima H, Miyake K, Inoue Y, Shimizu S, Tanaka Y, et al. Influence of interleukin-12 receptor beta1 polymorphisms on tuberculosis. Hum Genet. 2003;112(3):237–43. doi: 10.1007/s00439-002-0873-5. [DOI] [PubMed] [Google Scholar]
- 68.Hussain SK, Madeleine MM, Johnson LG, Du Q, Galloway DA, Daling JR, et al. Nucleotide variation in IL-10 and IL-12 and their receptors and cervical and vulvar cancer risk: a hybrid case-parent triad and case-control study. Int J Cancer. 2013;133(1):201–13. doi: 10.1002/ijc.28000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Quan L, Gong Z, Yao S, Bandera EV, Zirpoli G, Hwang H, et al. Cytokine and cytokine receptor genes of the adaptive immune response are differentially associated with breast cancer risk in American women of African and European ancestry. Int J Cancer. 2014;134(6):1408–21. doi: 10.1002/ijc.28458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Giatrakos S, Huse K, Kanni T, Tzanetakou V, Kramer M, Grech I, et al. Haplotypes of IL-12Rbeta1 impact on the clinical phenotype of hidradenitis suppurativa. Cytokine. 2013;62(2):297–301. doi: 10.1016/j.cyto.2013.03.008. [DOI] [PubMed] [Google Scholar]
- 71.Esteves LM, Bulhoes SM, Branco CC, Mota FM, Paiva C, Cabral R, et al. Human leptospirosis: seroreactivity and genetic susceptibility in the population of Sao Miguel Island (Azores, Portugal) PLoS One. 2014;9(9):e108534. doi: 10.1371/journal.pone.0108534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ruhrmann S, Stridh P, Kular L, Jagodic M. Genomic imprinting: A missing piece of the Multiple Sclerosis puzzle. Int J Biochem Cell Biol. 2015;67:49–57. doi: 10.1016/j.biocel.2015.05.010. [DOI] [PubMed] [Google Scholar]
- 73.Monahan K, Lomvardas S. Monoallelic expression of olfactory receptors. Annu Rev Cell Dev Biol. 2015;31:721–40. doi: 10.1146/annurev-cellbio-100814-125308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kim J, Bartel DP. Allelic imbalance sequencing reveals that single-nucleotide polymorphisms frequently alter microRNA-directed repression. Nat Biotechnol. 2009;27(5):472–7. doi: 10.1038/nbt.1540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Miller CL, Haas U, Diaz R, Leeper NJ, Kundu RK, Patlolla B, et al. Coronary heart disease-associated variation in TCF21 disrupts a miR-224 binding site and miRNA-mediated regulation. PLoS Genet. 2014;10(3):e1004263. doi: 10.1371/journal.pgen.1004263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Nicoloso MS, Sun H, Spizzo R, Kim H, Wickramasinghe P, Shimizu M, et al. Single-nucleotide polymorphisms inside microRNA target sites influence tumor susceptibility. Cancer Res. 2010;70(7):2789–98. doi: 10.1158/0008-5472.CAN-09-3541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gautrey H, Jackson C, Dittrich AL, Browell D, Lennard T, Tyson-Capper A. SRSF3 and hnRNP H1 regulate a splicing hotspot of HER2 in breast cancer cells. RNA Biol. 2015;12(10):1139–51. doi: 10.1080/15476286.2015.1076610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lee YB, Chen HJ, Peres JN, Gomez-Deza J, Attig J, Stalekar M, et al. Hexanucleotide repeats in ALS/FTD form length-dependent RNA foci, sequester RNA binding proteins, and are neurotoxic. Cell Rep. 2013;5(5):1178–86. doi: 10.1016/j.celrep.2013.10.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.McNally MT. RNA processing control in avian retroviruses. Front Biosci. 2008;13:3869–83. doi: 10.2741/2975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Geuens T, Bouhy D, Timmerman V. The hnRNP family: insights into their role in health and disease. Hum Genet. 2016;135(8):851–67. doi: 10.1007/s00439-016-1683-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Uren PJ, Bahrami-Samani E, de Araujo PR, Vogel C, Qiao M, Burns SC, et al. High-throughput analyses of hnRNP H1 dissects its multi-functional aspect. RNA Biol. 2016;13(4):400–11. doi: 10.1080/15476286.2015.1138030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Gendrel AV, Attia M, Chen CJ, Diabangouaya P, Servant N, Barillot E, et al. Developmental dynamics and disease potential of random monoallelic gene expression. Dev Cell. 2014;28(4):366–80. doi: 10.1016/j.devcel.2014.01.016. [DOI] [PubMed] [Google Scholar]
- 83.Denning W, Das S, Guo S, Xu J, Kappes JC, Hel Z. Optimization of the transductional efficiency of lentiviral vectors: effect of sera and polycations. Mol Biotechnol. 2013;53(3):308–14. doi: 10.1007/s12033-012-9528-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


