ABSTRACT
Background
Children with nephrotic syndrome (NS) are at risk for the development of acute kidney injury (AKI) through a variety of mechanisms.The frequency of NS hospitalizations complicated by AKI has almost doubled in the last decade. Children with AKI have longer hospital length of stay and increased need for intensive care unit admission. The main objectives of this study were to determine the incidence, clinical characteristics, risk factors and short-term outcome of AKI in children hospitalized with NS.
Methods
In this retrospective study, 355 children ≤18 years of age with a clinical diagnosis of NS admitted in the Department of Nephrology, Gauhati Medical College and Hospital from January 2012 to December 2015 were reviewed.
Results
The incidence of AKI in children with NS was found to be 23.66%, 11.24%, 7.95% and 4.48% of children entered Pediatric Risk, Injury, Failure, Loss, End-Stage Renal Disease (pRIFLE) Stages R, I and F, respectively. Infection {odds ratio [OR] 2.53 [95% confidence interval (CI) 1.52–4.22]} and nephrotoxic medication exposure [OR 7.8 (95% CI 4.06–15.01)] were common factors associated with AKI. Children with steroid-dependent NS (SDNS) and steroid-resistant NS (SRNS) were more likely to develop AKI compared with children with steroid-sensitive NS (SSNS). The mean time to recovery for groups pRIFLE Stages R, I and F were 15 ± 2 , 22 ± 3 and 28 ± 5 days, respectively. Children with NS who were hypertensive, had higher urinary protein excretion and low serum albumin were more prone to develop AKI.
Conclusions
AKI is not uncommon in children with NS. Infection and exposure to nephrotoxic drugs are common factors associated with AKI. AKI is more frequent in SDNS and SRNS compared with SSNS. The mean time to recovery is prolonged with more severe AKI.
Keywords: acute kidney injury, nephrotic syndrome, pRIFLE, steroid-dependent nephrotic syndrome, steroid-sensitive nephrotic syndrome
INTRODUCTION
Nephrotic syndrome (NS) is caused by renal diseases that increase permeability across the glomerular filtration barrier. Complications in children result from abnormalities related to NS and secondarily from therapy used for its treatment. The five major complications directly related to the underlying NS in children are infection, thromboembolism, renal insufficiency, anasarca and hypovolemia.
Acute kidney injury (AKI) is an alarming complication of idiopathic NS. Possible causes of AKI include bilateral renal vein thrombosis, interstitial edema, tubular obstruction, allergic interstitial nephritis, acute pyelonephritis, rapid progression of the original glomerular disease and acute tubular necrosis secondary to sepsis or hypovolemia [1, 2]. The incidence of AKI varies from 0.8 to 58.6% in different studies [3–5]. AKI is associated with increased morbidity in children with NS, including longer hospital length of stay and intensive care unit care [3].
However, there is a paucity of Indian studies on AKI in children with NS. Thus this retrospective study was undertaken in a tertiary care center in north-eastern India to determine the incidence, clinical characteristics, risk factors and short-term outcome in children hospitalized with NS.
MATERIALS AND METHODS
This is a single-center retrospective study that was conducted in the Department of Nephrology, Gauhati Medical College and Hospital, Guwahati, India. All hospitalizations for children ≤18 years of age between January 2012 and December 2015 with a discharge diagnosis of NS were reviewed. NS, or nephrosis, is defined by the presence of nephrotic-range proteinuria, edema, hyperlipidemia and hypoalbuminemia. While nephrotic-range proteinuria in adults is characterized by protein excretion of ≥3.5 g/d, in children it is defined as protein excretion of >40 mg/m2/h or a first-morning urine protein:creatinine ratio of 2–3 mg/mg creatinine or greater.
Steroid-resistant NS (SRNS) was defined if there was persistent proteinuria of >40 mg/m2/h after 4 weeks of prednisolone treatment. Steroid-dependent NS (SDNS) was defined as two relapses during steroid treatment or within 2 weeks of completing the treatment. Frequently relapsing NS was defined if there were two or more relapses in 6 months or four or more relapses in 12 months. Hypertension on admission is defined as the average systolic blood pressure and/or diastolic blood pressure that is ≥95th percentile for sex, age and height on three or more occasions.
Children with NS who at admission had CKD Stage ≥III, known secondary NS (e.g. systemic lupus erythematosus, Henoch–Schönlein purpura, etc.) and history and investigations suggestive of rapidly progressive glomerulonephritis were excluded from the study.
The creatinine value was determined using the Jaffe method and estimated glomerular filtration rate (eGFR) was calculated with the original Schwartz formula with a constant of 0.45 for children <1 year, 0.55 for female and male patients ≤12 years and 0.7 for male patients ≥13 years of age. The original Schwartz formula is .
The baseline creatinine value was defined as the most recent creatinine value before admission obtained within the prior 6 months. If no prior creatinine value was available, then the lowest creatinine value obtained during the hospitalization was defined as the baseline value.
AKI was defined according to the Pediatric Risk, Injury, Failure, Loss, End-Stage Renal Disease (pRIFLE) score through use of glomerular filtration rate (GFR) criteria only (Stage R, eGFR decreased by 25%; Stage I, eGFR decreased by 50%; Stage F, eGFR decreased by 75% or eGFR <35 mL/min/1.73 m2). Infection was categorized according to symptoms pertaining to the system involved. Pneumonia, peritonitis, gastrointestinal sepsis and urinary tract infection were confirmed as per the culture and sensitivity reports. Cellulitis was diagnosed by pain over the affected area and local signs of inflammation. Prerenal causes have been included as a cause of AKI when other causes have been excluded and physical signs of dehydration were present, such as sunken eyes, dry tongue, decrease in urine output and return to baseline creatinine within 48 h after volume expansion with crystalloids.
Blood sampling was done at admission and then every day or alternate day until return to baseline creatinine. Time to recovery is defined as a return to the baseline value or to the nadir of creatinine if no preadmission baseline was available.
Nephrotoxic medication exposure was defined as the administration of medication prior to admission, 1 week at the maximum, following which the child developed a decrease in urine output and an increase in serum creatinine. Nephrotoxic medications received before admission were gentamicin, amikacin, diclofenac and aceclofenac. Even a single dose was enough to be considered nephrotoxic exposure.
Statistical analyses
Descriptive statistics are shown as frequency and percentage or as mean ± SD as appropriate. Multivariate logistic regression was applied to find the association between AKI and its risk factors. P-value was determined using the chi-square test. All analyses were performed using SPSS, version 21.0 (IBM, Armonk, NY, USA). P-values are two sided and a P-value ≤0.05 is considered to represent a statistically significant difference.
RESULTS AND OBSERVATIONS
This study included 355 children with a diagnosis of NS among whom 23.66% had AKI at admission (Figure 1).
FIGURE 1:
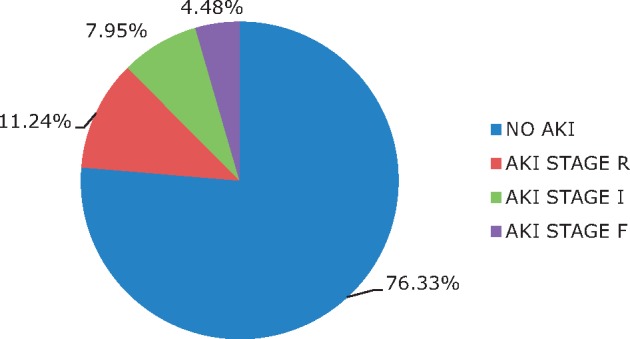
Stages of AKI according to pRIFLE criteria.
AKI demographics
The risk for AKI did not significantly differ by age and sex.
Disease severity
Of all patients, 23.66% of children were complicated by any pRIFLE stage of AKI; 11.24% of children met Stage R criteria, 7.95% met Stage I criteria and 4.48% met Stage F criteria (Figure 1).
Factors associated with AKI
Factors associated with AKI are shown in Table 1. Infection was a common factor associated with AKI {odds ratio [OR] 2.53 [95% confidence interval (CI) 1.52–4.22]}. Cellulitis, pneumonia, peritonitis, gastrointestinal sepsis and urinary tract infection were the common infections in descending order of frequency. Nephrotoxic medication exposure [OR 7.8 (95% CI, 4.06–15.01)] was another factor found to be associated with AKI in NS. The risk for AKI in NS did not differ significantly by ingestion of indigenous medicines or the presence of prerenal causes.
Table 1.
Factors associated with AKI in children with NS
| Associated factor | AKI, any stage | No AKI | OR (95% CI) | P-value |
|---|---|---|---|---|
| (n = 84) | (n = 271) | |||
| Infection, n | 2.53 (1.52–4.22) | <0.0001 | ||
| Yes | 55 | 116 | ||
| No | 29 | 155 | ||
| Nephrotoxic medication exposure, n | 7.8 (4.06–15.01) | <0.0001 | ||
| Yes | 30 | 18 | ||
| No | 54 | 253 | ||
| Indigeneous medicines, n | 2.2 (0.60–8.01) | 0.218 | ||
| Yes | 4 | 6 | ||
| No | 80 | 265 | ||
| Prerenal, n | 1.07 (0.33–3.43) | 0.897 | ||
| Yes | 4 | 12 | ||
| No | 80 | 259 |
Follow-up and outcome
The mean time to recovery for groups AKI-R, AKI-I and AKI-F were 15 ± 2, 22 ± 3 and 28 ± 5 days, respectively. None of the children required renal replacement therapy.
Characteristics of children with and without AKI
Children with SDNS and SRNS were more likely to develop AKI compared with children with SSNS. Moreover, children with AKI were more likely to be hypertensive and had higher urinary protein excretion and lower serum albumin compared with those without AKI (Table 2).
Table 2.
Characteristics of children with and without AKI
| Characteristic | AKI (n=84) | Non-AKI (n=271) | P-value |
|---|---|---|---|
| Age at admission (years), n | |||
| 0–3 | 11 | 43 | 0.638 |
| 4–7 | 13 | 45 | |
| 8–11 | 25 | 70 | |
| 12–15 | 23 | 76 | |
| 16–18 | 12 | 37 | |
| Clinical pattern of NS | |||
| SSNS, infrequently relapsing | 21 | 162 | <0.0001 |
| SDNS | 34 | 78 | |
| SRNS | 29 | 31 | |
| Gender (male), % | 65.47 | 64.94 | 0.929 |
| Hypertension, % | 69.04 | 32.84 | <0.0001 |
| Serum albumin (g/dL), mean ± SD | 1.68±0.48 | 2.06±0.03 | <0.0001 |
| Proteinuria (g/day), mean ± SD | 11.89±2.70 | 10.17±1.82 | <0.0001 |
Limitations of the study
The study is retrospective in nature. Another limitation is the lack of a temporal relationship between AKI and factors associated with it. It was not possible to determine if a diagnosis of sepsis and other causes occurred before or after a diagnosis of AKI and it was not possible to control for additional risk factors in patients with AKI. Prerenal causes included in AKI causality could not be confirmed, as the fractional excretion of sodium and change in body weight over the first 24 h of admission were not recorded to allow us to determine whether prerenal azotemia was a possible cause of AKI on admission. Cyclosporine, tacrolimus, angiotensin-converting enzyme (ACE) inhibitors/angiotensin II receptor blockers (ARBs) were excluded as nephrotoxins because most of the children with SDNS and SRNS were on calcineurin inhibitors and ACE inhibitors/ARBs and causality could not be established.
DISCUSSION
This retrospective study gives us an overview of AKI in children with NS. The incidence of AKI according to the pRIFLE definition of AKI has been found to be 23.66%. A recent study that also used the pRIFLE definition of AKI found a much higher incidence of AKI (58.6%) in children hospitalized with NS [3]. However, this study included children who had AKI at admission and also developed it during the entire hospital stay. In contrast, our study included children who had AKI only at admission. In earlier studies that used the Healthcare Cost and Utilization Project (HCUP)-Kids' Inpatient Database (KID), the AKI rates were reported to be 8.5–9.1% and the definition of AKI was not standardized [5–7]. A Polish study noticed acute renal failure (ARF) in only 0.8% of children with idiopathic NS [4].
Sutherland et al. [7] found the highest AKI incidence was among 15–18-year-old hospitalized children in general. However, this study found that the risk of AKI in children with NS did not differ significantly by age at admission and sex, as also seen by Rheault et al. [3].
The present study found that infection was a common factor associated with AKI. Kiliś-Pstrusińska et al. [4] also found the presence of infection as one of the common risk factors for ARF appearance in the course of idiopathic nephrotic syndrome. Sutherland et al. [7] also found that shock [OR 2.15 (95% CI 1.95–2.36)] and septicemia [OR 1.37 (95% CI 1.32–1.47)] were associated with AKI in children. Rheault et al. [3] found that children with infection were twice as likely to develop AKI as children without infection [OR 2.20 (95% CI 1.44–3.36)]. Nephrotoxic medication exposure was another common cause leading to AKI in children with NS. The administration of intravenous aminoglycosides in primary care or local clinics is not unusual due to price and widespread availability, in contrast to the USA and Europe where these drugs would be used almost exclusively in hospitals. The pharmacokinetics of nephrotoxic agents such as gentamicin are altered in nephrotic children, which may increase their drug exposure [8]. Rheault et al. [3] also found that nephrotoxic medication exposure, which most commonly included ACE inhibitors, calcineurin inhibitors and antibiotics, was strongly associated with the risk of AKI. SDNS and SRNS were more likely to be associated with AKI compared with SSNS. Rheault et al. [3] found that children with SRNS were more likely to develop AKI than children with SSNS.
The mean time to recovery was prolonged with more severe AKI. Rheault et al. [3] found that children with more severe stages of AKI had longer hospitalizations. Sutherland et al. [7] found that AKI in hospitalized children was associated with a prolonged length of stay.
The incidence of AKI was found to be higher in hypertensive children, children with lower serum albumin and children with higher urinary protein excretion. Waldman et al. [9] found that ARF occurred in 24 adult patients with minimal change disease; they tended to be older and hypertensive with lower serum albumin and higher urinary protein excretion than those without ARF. Evidence suggests that exposure of proximal tubular cells to albumin overload can lead to endoplasmic reticulum stress [10], induction of apoptosis [11], tubular chemokine and cytokine expression and activation of the complement cascade, resulting in inflammation [12–14] and interstitial fibrosis [15]. The nephrotoxicity of protein in the tubules could explain the higher incidence of AKI in children with higher urinary protein excretion. Also, the presence of low serum albumin as a consequence of an increase in proteinuria leads to more edema and ascites, which can predispose children to infectious complications that again increase the risk of AKI. Consequently, SDNS and SRNS patients had a higher risk of developing AKI compared with SSNS.
CONCLUSION
This study found that AKI occurred in around one-fourth of children hospitalized with NS. Infection and exposure to nephrotoxic medications were important factors associated with the risk of AKI. AKI was more frequent in children with SDNS and SRNS compared with SSNS. The mean time to recovery was prolonged with more severe AKI. Children with NS who were hypertensive, had higher urinary protein excretion and had low serum albumin were more prone to develop AKI.
However, larger prospective studies will be required to further validate the clinical characteristics and outcomes of children with NS in developing AKI.
CONFLICT OF INTEREST STATEMENT
None declared. The results presented in this article have not been published previously in whole or in part except in abstract form.
REFERENCES
- 1. Agarwal N, Phadke KD, Garg I. et al. Acute renal failure in children with idiopathic nephrotic syndrome. Pediatr Nephrol 2003; 18: 1289–1292 [DOI] [PubMed] [Google Scholar]
- 2. Sakarcan A, Timmons C, Seikaly MG.. Reversible idiopathic acute renal failure in children with primary nephritic syndrome. J Pediatr 1994; 125: 723–727 [DOI] [PubMed] [Google Scholar]
- 3. Rheault MN, Zhang L, Selewski DT. et al. AKI in children hospitalized with nephrotic syndrome. Clin J Am Soc Nephrol 2015; 10: 2110–2118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kiliś-Pstrusińska K, Zwolińska D, Musiał K.. Acute renal failure in children with idiopathic nephrotic syndrome. Pol Merkur Lekarski 2000; 8: 462–464 [PubMed] [Google Scholar]
- 5. HCUP Kids’ Inpatient Database (KID): Healthcare Cost and Utilization Project (HCUP). 1997, 2000, 2003, 2006. Rockville, MD: Agency for Healthcare Research and Quality. www.hcup-us.ahrq.gov/kidoverview.jsp (2 March 2010, date last accessed)
- 6. Rheault MN, Wei CC, Hains DS. et al. Increasing frequency of acute kidney injury amongst childrenhospitalized with nephrotic syndrome. Pediatr Nephrol 2014; 29: 139–147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sutherland SM, Ji J, Sheikhi FH. et al. AKI in hospitalized children: epidemiology and clinicalassociations in a national cohort. Clin J Am Soc Nephrol 2013; 8: 1661–1669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. González-Martin G, Bravo I, Vargas H. Arancibia a: pharmacokinetics of gentamicin in children with nephrotic syndrome. Int J Clin Pharmacol Ther Toxicol 1986; 24: 555–558 [PubMed] [Google Scholar]
- 9. Waldman M, Crew RJ, Valeri A. et al. Adult minimal-change disease: clinical characteristics, treatment, and outcomes. Clin J Am Soc Nephrol 2007; 2: 445–453 [DOI] [PubMed] [Google Scholar]
- 10. Ohse T, Inagi R, Tanaka T. et al. Albumin inducesendoplasmic reticulum stress and apoptosis in renal proximal tubular cells. Kidney Int 2006; 70: 1447–1455 [DOI] [PubMed] [Google Scholar]
- 11. Thomas ME, Brunskill NJ, Harris KP. et al. Proteinuria induces tubular cell turnover: a potential mechanism for tubular atrophy. Kidney Int 1999; 55: 890–898 [DOI] [PubMed] [Google Scholar]
- 12. Wang Y, Rangan GK, Tay YC. et al. Induction of monocyte chemoattractant protein-1 by albumin is mediated by nuclear factor kappaB in proximal tubule cells. J Am Soc Nephrol 1999; 10: 1204–1213 [DOI] [PubMed] [Google Scholar]
- 13. van Timmeren MM, Bakker SJ, Vaidya VS. et al. Tubular kidney injury molecule-1 in protein-overload nephropathy. Am J Physiol Renal Physiol 2006; 291: F456–F464 [DOI] [PubMed] [Google Scholar]
- 14. Burton CJ, Combe C, Walls J. et al. Secretion of chemokines and cytokines by human tubular epithelial cells in response to proteins. Nephrol Dial Transplant 1999; 14: 2628–2633 [DOI] [PubMed] [Google Scholar]
- 15. Zoja C, Benigni A, Remuzzi G.. Cellular responses to protein overload: key event in renal disease progression. Curr Opin Nephrol Hypertens 2004; 13: 31–37 [DOI] [PubMed] [Google Scholar]


