ABSTRACT
Introduction
Nearly 220000 patients are diagnosed with end-stage renal disease (ESRD) every year, which calls for an additional demand of 34 million dialysis sessions in India. The government of India has announced a National Dialysis Programme to provide for free dialysis in public hospitals. In this article we estimate the overall cost of performing hemodialysis (HD) in a tertiary care hospital. Second, we assess the catastrophic impact of out-of-pocket expenditures (OOPEs) for HD on households and its determinants.
Methods
The economic health system cost of HD was estimated using bottom-up costing methods. All resources, capital and recurrent, utilized for service delivery from April 2015 to March 2016 were identified, measured and valued. Capital costs were annualized after accounting for their useful life and discounting at 3% for future years. Sensitivity analyses were undertaken to determine the effect of variation in the input prices and other assumptions on the annual health system cost. OOPEs were assessed by interviewing 108 patients undergoing HD in the study hospital to account for costs from the patient’s perspective. The prevalence of catastrophic health expenditures (CHEs) was computed per threshold of 40% of non-food expenditures.
Results
The overall average cost incurred by the health system per HD session was INR 4148 (US$64). Adjusting for capacity utilization, the health system incurred INR 3025 (US$47) per HD at 100% bed occupancy. The mean OOPE per patient per session was INR 2838 (US$44; 95% confidence interval US$34–55). The major components of this OOPE were medicines and consumables (64.1%). The prevalence of a CHE per HD session was 11.1%.
Conclusion
Our study findings would be useful in the context of planning for dialysis services, setting provider payment rates for dialysis under various publicly sponsored health insurance schemes and undertaking future cost-effectiveness analysis to guide resource allocation decisions.
Keywords: catastrophic expenditure, cost analysis, dialysis, economic evaluation, hemodialysis, out-of-pocket expenditure
Introduction
The burden of end-stage renal disease (ESRD) is rising dramatically in India, with the proportion of deaths due to kidney failure increasing from 2.1% in 2001–3 to 2.9% in 2010–13 [1]. The age-adjusted incidence of ESRD in India is 226 per million population [2]. It is estimated that ∼220 000 new ESRD patients are added to the pool every year. Dialysis is a life-sustaining treatment modality for these patients. The shortage of nephrologists, late referral of patients, inadequate health awareness about preventive measures and a lack of more cost-effective alternatives like renal transplantation or peritoneal dialysis (PD) are important issues in the provision of care to ESRD patients [3, 4]. Unequal distribution of nephrologists, with a concentration in large cities and in the private sector are major barriers to equitable provision of dialysis to all sections of the society [5, 6]. Inadequate insurance coverage further aggravates the situation [6, 7]. Furthermore, ∼70% of those who start dialysis in India eventually give up dialysis due to financial constraints or death [8, 9]. Thus only 10–20% of dialysis patients in India continue long-term treatment. This high need for care is particularly relevant given the way health care is financed in India. Most of the health expenditures in India are borne by households as direct out-of-pocket expenditures (OOPEs). This poses significant barriers to accessing services. About 60 million households are pushed below the poverty line every year in India as a result of OOPEs [10].
Although access to dialysis, particularly hemodialysis (HD), has increased in recent years, only a minority of patients are able to continue long-term HD, mostly because of the high OOPEs. Making dialysis available to all who can benefit from the therapy will create an additional demand for 34 million dialysis sessions in India [11]. Taking into account the financial pressures on the affected households, the government of India recently announced a National Dialysis Service Programme (now referred as the Pradhan Mantri National Dialysis Programme) to provide free dialysis services to the poor in public sector hospitals in its Union Budget 2016–17 [12]. Implementation of this ambitious programme will involve major augmentation of existing service delivery infrastructure. Alternatively, the government may consider purchasing dialysis services from the private sector. Presently the National Dialysis Programme is in its nascent stages in India. The proposed programme aims to deliver dialysis services to the poor through a public–private partnership mode. In this programme the private partner provides for medical human resources, dialysis machines, water treatment infrastructure, dialyzer and consumables. The state government provides space, power and water within district hospitals so as to provide dialysis care [13]. It is important to note that dialysis is not the final curative treatment for those with ESRD. The management of ESRD needs to be considered on a holistic basis, which implies adequate attention on prevention of ESRD through better primary and secondary prevention strategies. There is also a need to develop capacity and infrastructure for provision of renal transplantation. PD, found to be cost-containing in the long term, should be strongly considered in the low- and middle-income country context [7]. While the more expensive HD is the dominant dialysis modality in the health benefit plans in Malaysia, Taiwan and the UK, PD is preferred in Thailand and Hong Kong [14].
Whatever the service provisioning model, it first calls for estimating the economic implications of such a programme. Second, given that it will entail a significant cost [14], it becomes imperative to assess the cost-effectiveness of various service delivery models. A recent systematic review of economic evaluation points to a gross lack of data on the cost of health services in India [15]. In order to bridge this gap in evidence, we undertook this study to assess the cost of HD in a tertiary care public sector hospital. We report our estimates from both a health system and societal perspective. Second, we assess the economic impact of OOPEs for HD on households and its determinants.
Materials and methods
Study setting
The study hospital is a large tertiary care multispecialty public sector hospital in North India with 1950 beds. It has a large referral base and receives patients from adjoining states such as Haryana, Punjab, Himachal Pradesh, Uttarakhand, Jammu and Kashmir as well as Uttar Pradesh, Bihar and Jharkhand. The study hospital has an exclusive 24-station HD unit that operates three shifts, with each HD session lasting 4 h. More details on infrastructure are provided in the supplementary material (Table S1). An independent water treatment plant provides high-quality water for this HD unit. The institute also has facilities for emergency dialysis and continuous ambulatory PD as well as kidney transplantation.
Costing methodology and data collection
In this study, the economic health system cost of HD was estimated using bottom-up costing methods [16, 17]. All resources, capital and recurrent, utilized for service delivery from April 2015 to March 2016 were identified, measured and valued from health system and societal perspectives. First, all cost centers were identified, along with the nature of the service being provided. Further, all the resources used in providing services were identified and measured. The total cost was estimated by adding the individual costs of each cost center. Unit cost value was arrived at by dividing this total cost by the number of units of services rendered. In addition, capacity utilization of the unit was estimated from a provider perspective. OOPEs incurred on HD were assessed by interviewing 108 HD patients in the study hospital to account for the cost from the patient’s perspective.
A primary costing survey was undertaken in the study hospital, as per standard norms of costing [16, 17], and with the tool used elsewhere in costing studies to capture the health system costs [18–20]. As mentioned previously, the cost centers involved in the provision of HD were identified. The cost center that was directly rendering the service for treating the patient, that is, the dialysis unit, was deemed as primary. Cost centers not directly involved in treating the patient, like laundry and administration, were considered as secondary cost centers. After identifying service centers, the resources used during the reference period (April 2015–March 2016) were assessed from the hospital records, stock registers, etc. (Table 1) and were categorized into capital and recurrent based on their time use. Buildings, medical and non-medical equipment and any other item lasting for >1 year were considered as capital resources. In contrast, resources such as salaries of staff, consumables and drugs were considered as recurrent.
Table 1.
Overview of study methodology for HD health system costing
| Type of resource | Source of data | Data collection method | Apportioning statistic |
|---|---|---|---|
| Capital | |||
| Building/space used | Observation of unit, records (maps) | Estimated floor (in square feet) and monthly rental price | Utility of each room ascertained by direct observation and hospital records |
| Equipment (medical and non-medical) | Stock register, direct observation | Purchase price with annual maintenance costs taken and annualized. Average life taken by expert opinion and records | Those involved in provision of HD included |
| Recurrent | |||
| Drugs and consumables | Stock registers, vouchers, indent records | Amount consumed annually; price data also taken | Amount indented to HD cost center |
| Human resource | Interview, direct observation, record review, salary slips | Gross salary multiplied by proportion of time spent annually in HD unit | Apportioning done based on the time spent allocated to HD cost center. Most of human resource stationed was dedicated fully to HD unit |
| Other consumables (like stationary) | Record review, stock register, indent slips | Annual amount consumed, price from hospital procurement department | Amount taken for HD cost center |
| Overheads such as electricity, water | Record review of monthly bills | Annual consumption in HD unit | Apportioning done based on floor area |
For capital resources such as buildings, the utility of each room, whether related to HD or not, was assessed. The data on the area of rooms, including the waiting rooms and any other space used for HD patients, were elicited by direct observation and records obtained from the hospital engineering department. All medical and non-medical equipment and furniture utilized for the HD unit were determined from stock registers, supplemented by direct observation. Assumptions regarding the life of the equipment were made after discussion with experts. Prices for equipment, consumables and medicines were obtained from department records or hospital procurement section. In case of non-availability of a price for any item, market prices were used.
Information on recurrent resources like salaries of the medical and non-medical staff involved with HD was drawn from their salary slips. In case a staff member was involved in more than one activity, their time spent on different activities was estimated using the standard interview schedules [19]. This was further validated by direct observation during the period of data collection. The capacity utilization rate of the HD unit was assessed based on the bed occupancy rate in the unit. The clean-up time for preparing an HD bed for the next patient as well as the annual time spent on infection control measures was also taken into account.
A total of 108 patients who underwent HD at the study hospital during the data collection period were recruited consecutively and interviewed to elicit data on OOPEs on various components such as medicines, diagnostics, travel, food, etc. These patients or their accompanying caregivers (when the latter knew more about expenses) were interviewed with their prior written informed consent. Besides OOPEs, patients were interviewed to record their demographic details, including age, gender, education, area of residence and socio-economic profile in terms of occupation, income and monthly consumption expenditure.
Data analysis
Health system costs
All capital costs were annualized after accounting for their useful life and discounting for future years. The original price of equipment was adjusted with the consumer price index to arrive at replacement costs. In order to estimate the opportunity cost of building/space being used for provision of HD, the estimated floor area was multiplied by the rental price value of a similar space. As for the cost of recurrent resources such as drugs, the quantities of resources were multiplied by their unit price to arrive at their overall cost. All costs were discounted at 3% [21].
Joint or shared costs, be it for capital or recurrent resources, were apportioned by suitable statistics. For example, if the space was being jointly used for more than one activity, then it was apportioned by the proportion of person-time hours for which the space was used for dialysis services. Similarly, for recurrent costs of human resources, cost apportioning was done based on the proportion of time spent in HD care. Overhead costs such as sanitation, electricity, etc. were apportioned as per proportional floor area [22]. These costs were estimated in Indian National Rupees (INR) and converted into US dollars. As per the average conversion rate in the year 2015–16 [23], US$1 was equivalent to INR 65. Since the majority of patients using HD did so during daytime shifts, the health system cost was calculated specifically during daytime shifts. We assessed the standardized unit cost for an HD session at 90% and 100% bed occupancy. In order to do so, we assumed human resources, equipment and capital as fixed while consumables and overhead costs were taken as variable.
Sensitivity analysis
A univariate sensitivity analysis was performed to determine the effect of any variation in the inputs on the annual costs. In order to do so, the base values of capital resources such as building, medical and non-medical equipment and salaries of the staff were varied by 25% on both the sides of the base value. Since the study hospital was a government-funded institution, a wide variation in the prices of consumables and drugs outside the hospital was assumed. Thus the prices of the latter were varied by 50% on either side of the base value in the sensitivity analysis. The assumptions on variations in prices are consistent with what has been reported in other recent costing studies from India [22].
Financial risk protection
The mean OOPE with 95% confidence interval (CI) was computed. Wealth quintiles were estimated based on the annual household consumption expenditure per capita. The prevalence of catastrophic health expenditures (CHEs) was computed as per the threshold of 40% of capacity to pay (CTP) or non-food expenditure [24]. As per the methodology for CHE by Xu [24], the CHE variable was calculated with the help of a dummy variable with a value of 1 indicating the presence of CHE and 0 for absence of CHE:
CHE is present (equivalent to 1) if OOPE/non-food consumption expenditure ≥0.4
CHE is absent (equivalent to 0) if OOPE/non-food consumption expenditure <0.4 [24]
Logistic regression was used to examine the association of CHE with independent covariates including gender, age, area of living (rural/urban), education, occupation and wealth quintiles. Before using multivariate analysis, a bivariate correlation matrix was ascertained to determine if there was any significant interaction (>0.4) between the independent factors and to check for multi-collinearity. Based on the initial assessment, education was excluded from the final regression model. In this logistic regression technique, the effect of each independent variable was assessed by keeping the first or the last category as a reference and all the independent variables were entered in the model. The odds ratio (OR) along with its 95% CI and corresponding P-value was reported.
Ethical considerations
Ethical approval was obtained from the Institute Ethics Committee of the Postgraduate Institute of Medical Education and Research, Chandigarh. Written informed consent was sought from all study participants.
Results
Sample characteristics
Of the 108 patients interviewed concerning OOPEs, 56.5% were males, predominantly married, literate (80.6%) and employed (51%). Close to two-thirds of the respondents lived in rural areas and 50% had visited a private doctor/hospital for HD before coming to the study hospital.
Health system costs
Overall the health system incurred a cost of INR 4148 (US$64) per HD session. The details of annual health system costs are shown in Table 2. Among the various cost heads identified, time cost of human resources was the major component (45%), followed by building (29%) and medical equipment (27%) (Figure 1). The cost of dialysis during the daytime shifts was INR 3621 (US$56), which was at a capacity utilization rate of 83%. As the capacity utilization increases to 90–100%, the cost per HD session decreased to INR 3348 (US$52) and INR 3025 (US$47), respectively. Uncertainty in the cost of human resources had the major effect on the cost of HD (supplementary material Figure S1)
Table 2.
Annual cost of dialysis care in a tertiary care hospital of India
| Cost type | Annual health system cost, INR (US$)a |
|---|---|
| Human resource | 23 904 865 (367 767) |
| Building/space | 15 277 896 (235 045) |
| Medical equipment | 16 541 265 (254 481) |
| Non-medical equipment | 1 73 540 (2770) |
| Drugs and consumables | 14 09 839 (21 690) |
| Overhead | 1 34 600 (2070) |
| Total cost | 55 826 805 (858 873) |
US$1 is equivalent to INR 65.
Fig. 1.
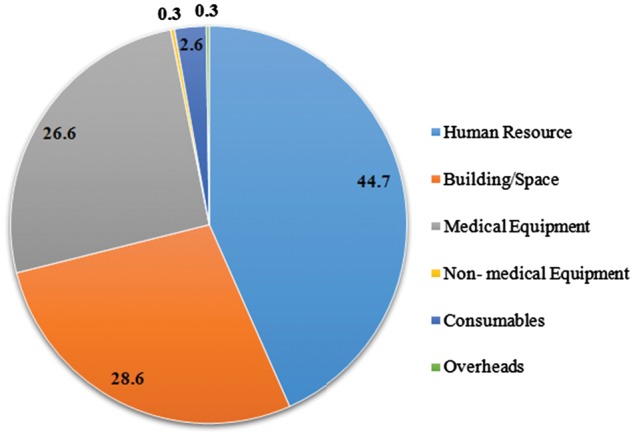
Distribution of health system costs for HD in a tertiary care hospital of India.
OOPEs
The mean OOPE per patient per HD session was INR 2838 (US$44; 95% CI US$34–55). The majority of the OOPE was on medicines and consumables (64.1%), followed by travel (18.4%), boarding/food (7.9%) and diagnostics (5.2%) (Figure 2). Males reported a higher mean OOPE [INR 3029 (US$47)] than females [INR 2592 (US$40)] (Table 3). Similarly, those patients residing in rural areas reported higher OOPEs [INR 3128 (US$49)] than those from urban areas [INR 2049 (US$32)]. Patients in the poorest quintile experienced almost double the OOPEs of those belonging to the richest quintile.
Fig. 2.
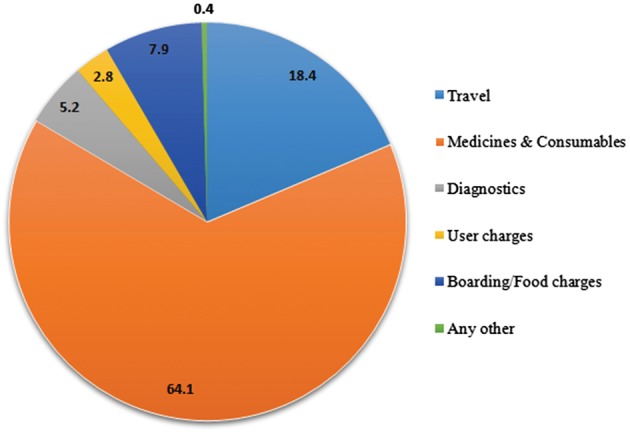
Distribution of components (%) of OOPEs for HD in a tertiary care hospital of India.
Table 3.
OOPEs for HD in a tertiary care hospital in India
| Characteristic | Mean OOPE, INR (US$) | SE, INR (US$)a | 95% CI (in INR) |
|---|---|---|---|
| Gender | |||
| Male (n = 61) | 3029 (47) | 433 (6.7) | 2211–3875 |
| Female (n = 47) | 2592 (40) | 548 (8.4) | 1609–3816 |
| Total | 2838 (44) | 339 (5.2) | 2203–3546 |
| Age | |||
| <47 years (n = 55) | 2845 (44) | 491 (7.6) | 2002–3773 |
| >47 years (n = 53) | 2832 (44) | 477 (7.3) | 1937–3797 |
| Total | 2838 (44) | 339 (5.2) | 2203–3546 |
| Religion | |||
| Hindu (n = 82) | 2621 (40) | 373 (5.7) | 1976–3395 |
| Muslim (n = 5) | 6004 (92) | 2453 (37.7) | 1156–11 710 |
| Sikh (n = 21) | 2932 (45) | 749 (11.5) | 1550–4543 |
| Total | 2838 (44) | 339 (5.2) | 2203–3546 |
| Caste | |||
| SC (n = 23) | 2067 (32) | 759 (11.7) | 906–3899 |
| ST (n = 2) | 1025 (16) | 245 (3.8) | 700–1350 |
| OBC (n = 21) | 3092 (48) | 703 (10.8) | 1806–4596 |
| Gen (n = 62) | 3098 (47) | 462 (7.1) | 2220–4077 |
| Total | 2838 (44) | 339 (5.2) | 2203–3546 |
| Area | |||
| Rural (n = 79) | 3128 (48) | 432 (6.6) | 2304–4063 |
| Urban (n = 29) | 2049 (32) | 466 (7.2) | 1233–3072 |
| Total | 2838 (44) | 339 (5.2) | 2203–3546 |
| Marital status | |||
| Unmarried (n = 84) | 1843 (28) | 482 (7.4) | 1051–2984 |
| Married (n = 18) | 3083 (47) | 414 (6.4) | 2308–3928 |
| Widow/widower (n = 6) | 2405 (37) | 800 (12.3) | 1010–4188 |
| Total | 2838 (44) | 339 (5.2) | 2203–3546 |
| Education | |||
| Illiterate (n = 21) | 2357 (36) | 659 (10) | 1156–3742 |
| Literate (n = 87) | 2955 (45) | 396 (6.1) | 2250–3799 |
| Total | 2838 (44) | 339 (5.2) | 2203–3546 |
| Occupation | |||
| Unemployed (n = 48) | 2564 (39) | 479 (7.4) | 1695–3622 |
| Employed (n = 60) | 3059 (47) | 467 (7.2) | 2187–4045 |
| Total | 2838 (44) | 339 (5.2) | 2203–3546 |
| Wealth quintiles | |||
| Poorest (n = 21) | 3967 (61) | 943 (14.5) | 2129–5788 |
| Poor (n = 22) | 2369 (37) | 789 (12.2) | 1074–4164 |
| Moderate rich (n = 22) | 2605 (40) | 671 (10.3) | 1447–4085 |
| Richer (n = 22) | 3321 (51) | 786 (12.1) | 1866–5041 |
| Richest (n = 21) | 1943 (30) | 522 (8) | 1026–3026 |
| Total | 2838 (44) | 339 (5.2) | 2203–3546 |
US$1 is equivalent to INR 65. Gen, general; OBC, other backward class; SC, Scheduled Caste; ST, Scheduled Tribe.
Financial risk protection
The prevalence of CHE per HD session was 11.1%. With an assumption of twice-weekly HD sessions, the prevalence of CHE among those undergoing HD was 38.1%. With thrice-weekly HD sessions, it rose to 51.9%. Of all the patients interviewed about their sources of finance for these OOPEs on HD, 59.3% confirmed borrowing from friends/relatives, 35.2% confirmed using their salary/savings and 5.6% sold assets.
Multiple logistic regression analysis showed that wealth status was the only significant predictor of incurring CHE as a result of OOPEs on HD. The odds of CHE at two and three HD sessions per week were 21 times and 45 times higher, respectively, in the poorest versus the richest quintile (P < 0.001) (Table 4).
Table 4.
Determinants of CHEs in HD in the study hospital
| Characteristic | CHE (38%) |
CHE (51.9%) |
||||
|---|---|---|---|---|---|---|
| OR | P-value | 95% CI | OR | P-value | 95% CI | |
| Age | ||||||
| <47 years | 0.8 | 0.63 | 0.31–2.05 | 0.53 | 0.2 | 0.2–1.40 |
| >47 years | Ref. | |||||
| Gender | ||||||
| Male | Ref. | |||||
| Female | 0.4 | 0.13 | 0.11–1.34 | 0.44 | 0.2 | 0.12–1.6 |
| Area | ||||||
| Urban | 0.61 | 0.35 | 0.21–1.72 | 0.8 | 0.7 | 0.3–2.23 |
| Rural | Ref. | |||||
| Occupation | ||||||
| Unemployed | 1.5 | 0.50 | 0.5–5.20 | 0.9 | 0.8 | 0.23–3.13 |
| Employed | Ref. | |||||
| Wealth quintiles | ||||||
| Poorest | 21.89 | <0.001 | 4.25–112.85 | 45.4 | <0.001 | 7.43–277.07 |
| Poor | 2.9 | 0.2 | 0.60–13.83 | 14.64 | 0.001 | 2.94–72.80 |
| Moderate | 3.3 | 0.14 | 0.68–16.08 | 5.73 | 0.02 | 1.2–27.53 |
| Richer | 3.4 | 0.12 | 0.72–15.8 | 5.8 | 0.02 | 1.23–28.12 |
| Richest | Ref. | |||||
| Adjusted R2 | 0.257 | 0.341 | ||||
| Unadjusted R2 | 0.187 | 0.255 | ||||
Discussion
Overall, we found the health system incurred INR 4148 (US$64) per HD session, while the patients spent INR 2838 (US$44) per HD session. Assuming two dialysis sessions in a week, the patient experiences 38.1% catastrophic spending, which increases to 52% with thrice-weekly HD. Our findings showed that the odds of CHE at two and three HD sessions per week were 21 times and 45 times higher, respectively, in the poorest than the richest quintiles.
Comparison of findings
Our study is the most comprehensive economic analysis of HD cost in India to date. Previous studies from India have analyzed OOPEs on dialysis from patient perspective. In a public sector tertiary hospital [25], the mean OOPE on dialysis was estimated to be INR 2230 (US$34). Another study reported median direct costs for dialysis [INR 2628 (US$43)] [26]. In a private tertiary care hospital in South India, the cost per HD session borne by the patient was found to be INR 4428 (US$68) [6]. Most studies involving HD documented the expenditures on medicines and consumables to be responsible for a substantial proportion of OOPEs, as noted in our study as well [26–28].
The calculation of health system costs and OOPEs in our analysis is entirely mutually exclusive. Typically, in public health care facilities in India, resources are spent by the government on the provision of health services, such as personnel salaries, capital infrastructure (building and equipment) and basic medical supplies. However, the supply of medicines and consumables generally does not cover all the required items for treatment, including dialyzer and tubing. Moreover, there are frequent stock-outs of medicines. A survey of health facilities in North Indian states showed that only about half of the essential drugs are available [29]. Similar findings reflecting a lack of availability of all essential medicines in public facilities are reported in the recent National Sample Survey 71st Round Report [30, 31]. Therefore most medicines and consumables need to be purchased by patients, thereby incurring OOPEs. Expenditures on medicines constitute the largest share of OOPEs on health care in public facilities in India [30–34]. The cost of dialyzer reuse and erythropoiesis-stimulating agents were borne by the patient and hence were elicited under the OOPE in our study.
Second, we would like to clarify that differences in absolute OOPEs among the population subgroups based on wealth status was not statistically significant. However, it is important to note that while there is no difference in the mean OOPE, CHEs were was significantly greater among the poorer quintiles as compared to the richest. This finding is similar to other studies, which showed that the CHEs were regressively skewed at a higher rate among the poorer sections [31–34]. None of the patients in our study sample had any insurance. However, the findings from a recent systematic review of impact evaluations for publicly financed insurance schemes in India showed the absence of any reduction in OOPEs or CHEs [35]. In view of this, it is important to strengthen the public sector delivery of services through adequate availability of medicines and supplies, such that OOPEs are minimized, which is likely to result in adequate financial risk protection.
Findings in terms of the distribution of health system costs (with the time cost of human resources as the predominant component) are worth highlighting. Appropriate dialysis delivery requires contributions from doctors, nurses, nutritionists, social workers, technicians and other staff [36]. Our findings also suggest that HD costs vary at different points of capacity utilization. There is a relative reduction in the health system unit cost at 100% bed occupancy. This suggests there is room for making service delivery in the public sector more efficient. One way to address this could be by utilizing the late shifts or nighttime to cater to patients, especially those who are willing to be treated at that time—be it an inpatient case or outpatient. The marginal cost of such an increase in service delivery is likely to be less than the average cost and increase overall efficiency.
Existing insurance packages covering dialysis
It is important to compare our findings with the dialysis service packages being offered by existing health insurance schemes in India [37–40]. The Chief Minister’s Comprehensive Health Insurance Scheme in Tamil Nadu offers INR 8000 (US$123) per month for maintenance HD [37]. The Aarogyasri scheme in Telangana [39] and Mahatma Jyotiba Phule Jan Arogya Yojana in Maharashtra (earlier known as Rajiv Gandhi Jeevandayee Arogya Yojana) [41] pay INR 10 000 (US$154) every month for dialysis (eight sessions) and supportive care, and the Rajiv Aarogyasri Community Health Insurance Scheme in Andhra Pradesh pays INR 12 500 (US$192) (for a minimum of 10 dialysis sessions) [38]. Further, in the central government health scheme, the entitlement for an HD patient is around INR 2900–3335 (US$45–51) per dialysis session [42].
In a nephrologist-owned unit, the average cost of an HD session was reported as INR 700 (US$11) [43], which is significantly lower than the cost calculated in this study. In that study, the authors included only recurrent operational costs and did not consider capital costs. Variation in HD practice based on the trained capacity, capital infrastructure as well as the level of health care setting also explains the difference [44]. Many options such as frequent dialyzer reuse, philanthropist contributions and limiting the visits of nephrologists are some of the mechanisms of cutting costs in the non-government sector [5, 43]. Procedures like vascular access creation for HD are generally charged for in the private sector and are directly borne by the patient, compared with free or highly subsidized in the government or charitable sector [45]. A free-standing (minimal care) HD unit [5] or a charitable HD unit often has a free choice in the selection of stable patients eligible for day care dialysis [43]. On the contrary, acutely ill patients or those in advanced stages of kidney disease get referred to public sector hospitals by the private sector [5, 25, 46]. In our study hospital, the majority of patients had reported in the late stages of kidney disease [5, 25].
The recent National Sample Survey on health found that only 13% of the rural population and 12% of the urban population are covered under any insurance [30]. Without financial risk protection, nearly one-fourth of the households resort to either borrowing or selling of assets to finance their health expenditures [30]. A recent systematic review [35] also documented an increase in service utilization but noted a lack of evidence that shows any reduction in OOPEs due to these publicly financed health insurance schemes. These prohibitive costs compel patients to forgo care or end up with suboptimal dialysis [5, 47].
Our study once again highlights the high OOPEs incurred by patients on HD and extends this by highlighting the inequities. The economically worse off are much more likely to suffer catastrophic OOPEs, which will deepen the rich–poor gap. Similar to our findings, another study reported that 63% of those who underwent HD had borrowed money from an employer/friends, 20% opted for loans and 30% resorted to selling assets to finance for dialysis treatment [48]. Previous evidence also suggests that dwindling finances act as a barrier in accessing dialysis treatment for the majority of patients [5, 49].
Our study has several strengths. This is the first study to undertake a comprehensive economic analysis of dialysis care and incorporated assessment of health system costs as well as patient OOPEs. Standard methods were used for data collection. Generally, there is the possibility of recall bias when OOPEs are recalled by the patient over long reference periods. We interviewed patients for OOPEs incurred for the HD session on the day of the survey, which was verified by written bills. Hence the possibility of any recall bias is weak. One of the limitations of our study is that we did not collect detailed information on clinical profiles, which could have influenced variations in OOPEs due to the stage of kidney failure. Second, we did not consider lost productivity of the patient/caregiver due to dialysis in our economic analyses. Other studies have shown that these indirect costs can be substantial [50, 51].
Policy implications
The dialysis market in India is estimated to be growing at the rate of 31% per annum [4]. The National Dialysis Service Programme envisages free dialysis services delivered in a public–private partnership mode [11]. While the operational guidelines are being outlined for the programme, economic implications of such a programme are likely to be substantial [52]. The government needs to decide on realistic reimbursement rates. Our data suggest that rates currently fixed in the state reimbursement programmes are unlikely to support a sustainable dialysis delivery model. In a system with weak supervision, unrealistically low reimbursement will lead to compromises on quality, such as poor-quality infrastructure, hiring cheap inadequately trained staff, the use of low-quality disposables and compromises in the medical management of dialysis patients. Concomitantly, poor quality may predispose HD patients to infections, leading to exorbitant expenditures due to associated morbidities.
Apart from these economic challenges, the delivery of dialysis involves ethical dilemmas, in particular ensuring equity and care for disadvantaged groups [53]. The proposed National Dialysis Service does not include PD, which has been widely documented to cost less to health care systems around the world. Because of fewer infrastructure requirements, PD is more likely to promote equity in dialysis delivery. Most countries that deliver dialysis through a public delivery mechanism use PD as the first choice [54]. Currently only ∼10% of all dialysis patients are on PD in India. Further, economic research and cost-effectiveness analyses of different modalities are needed to find an answer to these important policy concerns.
Conclusion
Our study findings may be useful in the context of planning to set up or expand health services for dialysis and to set reimbursement rates/provider payments for dialysis under various publicly sponsored health insurance schemes. Further, our findings on cost could be used by researchers for undertaking cost-effectiveness analyses to guide resource allocation decisions.
Conflict of interest statement
None declared.
Supplementary Material
References
- 1. Dare AJ, Fu SH, Patra J. et al. Renal failure deaths and their risk factors in India 2001-13: nationally representative estimates from the Million Death Study. Lancet Glob Health 2017; 5: e89–e95 [DOI] [PubMed] [Google Scholar]
- 2. Modi GK, Jha V.. The incidence of end-stage renal disease in India: a population-based study. Kidney Int 2006; 70: 2131–2133 [DOI] [PubMed] [Google Scholar]
- 3. Veerappan I, Abraham G. Chronic kidney disease: current status, challenges and management in India. In Medicine Update. Mumbai: Association of Physicians of India, 2013, 593–597. http://www.apiindia.org/medicine_update_2013/chap130.pdf
- 4. Jha V, John O, Joshi R. et al. Dialysis outcomes in India: a pilot study. Nephrology (Carlton) 2015; 20: 329–334 [DOI] [PubMed] [Google Scholar]
- 5. Jha V, Chugh K.. Dialysis in developing countries: priorities and obstacles. Nephrology 1996; 996: 65–72 [Google Scholar]
- 6. Suja A, Anju R, Anju V. et al. Economic evaluation of end stage renal disease patients undergoing hemodialysis. J Pharm Bioallied Sci 2012; 4: 107–111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Vanholder R, Lameire N, Annemans L. et al. Cost of renal replacement: how to help as many as possible while keeping expenses reasonable? Nephrol Dial Transplant 2016; 31: 1251–1261 [DOI] [PubMed] [Google Scholar]
- 8. Kher V. End-stage renal disease in developing countries. Kidney Int 2002; 62: 350–362 [DOI] [PubMed] [Google Scholar]
- 9. Sakhuja V, Sud K.. End-stage renal disease in India and Pakistan: burden of disease and management issues. Kidney Int Suppl 2003; 63(Suppl 83): S115–S118 [DOI] [PubMed] [Google Scholar]
- 10. Balarajan Y, Selvaraj S, Subramanian SV.. Health care and equity in India. Lancet 2011; 377: 505–515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. MInistry of Health and Family Welfare. National Dialysis Program Under National Health Mission. New Delhi: Government of India, 2016 [Google Scholar]
- 12. National Dialysis Services Programme to be launched [press release]. Ministry of Finance DSM/NB/GB/UD/2016-17/ 2016. (02 March 2017, date last accessed)
- 13.National Dialysis Services Programme [press release]. Ministry of Health and Family Welfare 2017. [cited 5 April 2017]
- 14. Teerawattananon Y, Luz A, Pilasant S. et al. How to meet the demand for good quality renal dialysis as part of universal health coverage in resource-limited settings? Health Res Policy Syst 2016; 14: 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Prinja S, Chauhan AS, Angell B. et al. A systematic review of the state of economic evaluation for health care in India. Appl Health Econ Health Policy 2015; 13: 595–613 [DOI] [PubMed] [Google Scholar]
- 16. Janowitz B, Bratt JH.. Methods for Costing Family Planning Services. New York: United Nations Population Fund and Family Health International, 1994 [Google Scholar]
- 17. Beck EJ, Harling G, Gerbase S. et al. The cost of treatment and care for people living with HIV infection: implications of published studies, 1999-2008. Curr Opin in HIV AIDS 2010; 5: 215–224 [DOI] [PubMed] [Google Scholar]
- 18. Prinja S, Mazumder S, Taneja S. et al. Cost of delivering child health care through community level health workers: how much extra does IMNCI program cost? J Trop Pediatr 2013; 59: 489–495 [DOI] [PubMed] [Google Scholar]
- 19. Prinja S, Manchanda N, Mohan P. et al. Cost of neonatal intensive care delivered through district level public hospitals in India. Indian Pediatr 2013; 50: 839–846 [DOI] [PubMed] [Google Scholar]
- 20. Prinja S, Gupta A, Verma R. et al. Cost of delivering health care services in public sector primary and community health centres in North India. PLoS One 2016; 11: e0160986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wilkinson T, Sculpher MJ, Claxton K. et al. The international decision support initiative reference case for economic evaluation: an aid to thought. Value Health 2016; 19: 921–928 [DOI] [PubMed] [Google Scholar]
- 22. Sangwan A, Prinja S, Aggarwal S. et al. Cost of trauma care in secondary- and tertiary-care public sector hospitals in North India. Appl Health Econ Health Policy 2017; 15: 681–692 [DOI] [PubMed] [Google Scholar]
- 23. Economic Times Forex Rates 2015. http://economictimes.indiatimes.com/markets/forex (12 September 2016, date last accessed)
- 24. Xu K. Distribution of Health Payments and Catastrophic Expenditures Methodology. Geneva: Department of Health System Financing and Cluster Evidence and Information for Policy, World Health Organization, 2005 [Google Scholar]
- 25. Ramachandran R, Jha V.. Kidney transplantation is associated with catastrophic out of pocket expenditure in India. PLoS One 2013; 8: e67812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mateti UV, Nagappa AN, Vooradi S. et al. Pharmacoeconomic evaluation of hospitalised pre-dialysis and dialysis patients: a comparative study. Australas Med J 2015; 8: 132–138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kaitelidou D, Ziroyanis PN, Maniadakis N. et al. Economic evaluation of hemodialysis: implications for technology assessment in Greece. Int J Technol Assess Health Care 2005; 21: 40–46 [DOI] [PubMed] [Google Scholar]
- 28. Ranasinghe P, Perera YS, Makarim MF. et al. The costs in provision of haemodialysis in a developing country: a multi-centered study. BMC Nephrol 2011; 12: 42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Prinja S, Bahuguna P, Tripathy JP. et al. Availability of medicines in public sector health facilities of two North Indian states. BMC Pharmacol Toxicol 2015; 16: 43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. National Sample Survey Office. Key Indicators of Social Consumption in India Health. New Delhi: Ministry of Statistics and Programme Implementation, 2015 [Google Scholar]
- 31. Prinja S, Aggarwal AK, Kumar R. et al. User charges in health care: evidence of effect on service utilization and equity from north India. Indian J Med Res 2012; 136: 868–876 [PMC free article] [PubMed] [Google Scholar]
- 32. Prinja S, Kanavos P, Kumar R.. Health care inequities in north India: role of public sector in universalizing health care. Indian J Med Res 2012; 136: 421–431 [PMC free article] [PubMed] [Google Scholar]
- 33. Balasubramanian D, Prinja S, Aggarwal AK.. Effect of user charges on secondary level surgical care utilization and out-of-pocket expenditures in Haryana state, India. PLoS One 2015; 10: e0125202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Prinja S, Gupta R, Bahuguna P. et al. A composite indicator to measure universal health care coverage in India: way forward for post-2015 health system performance monitoring framework. Health Policy Plan 2017; 32: 43–56 [DOI] [PubMed] [Google Scholar]
- 35. Prinja S, Chauhan AS, Karan A. et al. Impact of publicly financed health insurance schemes on healthcare utilization and financial risk protection in India: a systematic review. PLoS One 2017; 12: e0170996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Karopadi AN, Mason G, Rettore E. et al. The role of economies of scale in the cost of dialysis across the world: a macroeconomic perspective. Nephrology, dialysis, transplantation: official publication of the European. Nephrol Dial Transplant 2014; 29: 885–892 [DOI] [PubMed] [Google Scholar]
- 37.Chief Minister’s Comprehensive Health Insurance Scheme [cited 21 April 2017]. http://www.cmchistn.com/
- 38.Rajiv Aarogyasri Health Insurance Scheme. http://www.aponline.gov.in/apportal/HomePageLinks/aarogyasri.html (21 April 2017, date last accessed)
- 39.Aarogyasri Scheme [cited 20 April 2017]. http://aarogyasri.telangana.gov.in/web/guest/aarogyasri-scheme
- 40.Rashtriya Swasthya Bima Yojana. http://www.rsby.gov.in/ (21 April 2017, date last accessed)
- 41.Mahatma Jyotiba Phule Jan Arogya Yojana in Maharashtra. https://www.jeevandayee.gov.in/ (20 April 2017, date last accessed)
- 42.Procedure/Investigation List. http://cghs.gov.in/ (21 April 2017, date last accessed)
- 43. Khanna U. The economics of dialysis in India. Indian J Nephrol 2009; 19: 1–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Rao M, Juneja R, Shirly RB. et al. Haemodialysis for end-stage renal disease in Southern India—a perspective from a tertiary referral care centre. Nephrol Dial Transplant 1998; 13: 2494–2500 [DOI] [PubMed] [Google Scholar]
- 45. Abraham G, Jayaseelan T, Matthew M. et al. Resource settings have a major influence on the outcome of maintenance hemodialysis patients in South India. Hemodial Int 2010; 14: 211–217 [DOI] [PubMed] [Google Scholar]
- 46. Parameswaran S, Geda SB, Rathi M. et al. Referral pattern of patients with end-stage renal disease at a public sector hospital and its impact on outcome. Natl Med J India 2011; 24: 208–213 [PubMed] [Google Scholar]
- 47. Karopadi AN, Mason G, Rettore E. et al. Cost of peritoneal dialysis and haemodialysis across the world. Nephrol Dial Transplant 2013; 28: 2553–2569 [DOI] [PubMed] [Google Scholar]
- 48. Mani MK. The management of end-stage renal disease in India. Artif Organs 1998; 22: 182–186 [DOI] [PubMed] [Google Scholar]
- 49. Prasad N, Jha V.. Hemodialysis in Asia. Kidney Dis 2015; 1: 165–177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Begley CE, Famulari M, Annegers JF. et al. The cost of epilepsy in the United States: an estimate from population-based clinical and survey data. Epilepsia 2000; 41: 342–351 [DOI] [PubMed] [Google Scholar]
- 51. Economic costs associated with mental retardation, cerebral palsy, hearing loss, and vision impairment—United States, 2003. MMWR Morb Mortal Wkly Rep 2004; 53: 57–59 [PubMed] [Google Scholar]
- 52. Jha V. Current status of end-stage renal disease care in South Asia. Ethn Dis 2009; 19(1 Suppl 1): 27–32 [PubMed] [Google Scholar]
- 53. Jha V, Martin DE, Bargman JE. et al. Ethical issues in dialysis therapy. Lancet 2017; 389: 1851–1856 [DOI] [PubMed] [Google Scholar]
- 54. Abraham G. The challenges of renal replacement therapy in Asia. Nat Clin Pract Nephrol 2008; 4: 643. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


