Abstract
Purpose
To evaluate the role of adjuvant radiotherapy after narrow-margin (<1.0 cm) resection in patients with intrahepatic cholangiocarcinoma (ICC) adherent to major vessels.
Patients and methods
This retrospective study included 70 ICC patients. Forty-nine patients received narrow-margin (<1.0 cm) hepatectomy and 21 patients underwent wide-margin (≥1.0 cm) hepatectomy (Group C). Twenty-six of 49 were treated with postoperative radiotherapy (Group A), while the remaining 23 did not receive radiotherapy (Group B). Clinical outcomes were compared in the 3 groups. Toxicities of radiotherapy were evaluated.
Results
With a median follow-up time of 42 months, the 3-year overall survival (OS) and disease-free survival rates were 55% and 44% for Group A, 20% and 10% for Group B, and 65% and 33% for Group C, respectively. The OS and disease-free survival in Groups A and C were comparable and improved compared to Group B (Group A vs B, P=0.011 and P=0.031; and Group C vs B, P=0.031 and P=0.105). Multivariate analysis showed that receiving narrow-margin resection only (adjusted hazard ratio: 3.73; 95% CI: 1.36–10.25; P=0.001) was a significant poor prognostic risk factor of OS. Group B experienced more intrahepatic recurrence and extrahepatic recurrence than Groups A and C. For Groups A and B, the 3-year intrahepatic recurrence rates were 36% vs 67% (P=0.133) and extrahepatic recurrence rates were 43% vs 65% (P=0.007). Only 2 patients in Group A suffered from grade 3 toxicities. No patient developed classic or nonclassic radiation-induced liver disease.
Conclusion
Postoperative radiotherapy following narrow-margin hepatectomy seems to be efficacious and well-tolerated in patients with ICC adjacent to major vessels.
Keywords: intrahepatic cholangiocarcinoma, surgical margin, hepatectomy, surgery, postoperative radiotherapy, prognosis
Plain language summary
The prognosis of intrahepatic cholangiocarcinoma patients is poor. Surgery is the only curative treatment for these patients. But survival after surgery is still not satisfying. Margin status significantly affected survival after surgery. Radiotherapy is a local treatment modality. It might improve survival in patients with inadequate margin. To investigate the role of radiotherapy in postoperative patients, we divided 70 patients who received surgery into 3 groups according to the width of the resection margin and whether the patient received radiotherapy or not. The results showed that the outcome of patients who received radiotherapy after narrow-margin resection were comparable to patients who received wide-margin resection. The toxicities of radiotherapy were acceptable. It means that postoperative radiotherapy seems to be efficacious and well-tolerated in patients with narrow-margin resection.
Introduction
Cholangiocarcinoma (CCA) is the second most common primary liver tumor and accounts for approximately 3% of all gastrointestinal tumors.1,2 It is categorized into intrahepatic cholangiocarcinoma (ICC) and extrahepatic cholangiocarcinoma (ECC), including hilar cholangiocarcinoma. ICC arises from the small bile ducts in the liver.3,4 The incidence of ICC continues to rise worldwide.5–7
Operative resection remains the only potentially curative option for patients with ICC.2,8 However, most patients present with advanced disease and radical operation may not be a feasible treatment. In America, only 12% of patients with ICC received radical resection.9 Unfortunately, for the minority who received surgical resection, the results still need to be improved. In different series, the 3- and 5-year overall survival (OS) ranged from 36% to 53% and 25% to 40%, respectively, in patients who received resection. Among these operations, R0 resection rate varied from 69% to 88%.10–16 Margin status significantly affected survival, where a positive margin (R1 resection) remained a negative prognostic factor for ICC patients.17,18 Moreover, several studies suggested a survival advantage for negative margins of 1.0 cm or more (wide margin) compared to negative margins of <1.0 cm (narrow margin).19–21
The degree of margin resection is restricted by the location of the tumor. Centrally located liver tumors, which involve Couinaud’s segments IV, V, and VIII ± I, are usually adjacent to major vessels such as the inferior vena cava, hepatic vein, portal vein, and hepatic artery. Extended hemihepatectomy and mesohepatectomy are 2 conventional surgical procedures in the management of centrally located liver tumors.22,23 Surgeons sometimes have to carefully dissect and peel the tumor away from the vascular surface, which may result in a null-margin (no real resection margin) resection.24 Thus, obtaining a safe margin remains a challenge. Adjuvant therapies vary considerably, without a consensus in what postoperative treatments can improve survival in narrow-margin (<1.0 cm) resections.2,25,26 Therefore, it is urgent to seek an effective adjuvant therapy for those who received narrow-margin resection.
In recent years, radiotherapy has proved to be a safe and efficacious treatment in patients with unresectable ICC.27,28 However, the role of postoperative radiotherapy in patients who have undergone narrow-margin resection is still unclear. Thus, we retrospectively reviewed ICC patients who have undergone liver resection in our hospital and aim to investigate whether postoperative radiotherapy can benefit survival in patients with narrow-margin resections.
Patients and methods
Patient selection
A retrospective cohort study was conducted using data from the National Cancer Center/National Clinical Research Center for Cancer/Cancer Hospital, Chinese Academy of Medical Sciences, and Peking Union Medical College. The eligibility criteria for the study were as follows: 1) patients with ICC who had undergone liver resection in our hospital from 2007 to 2016; 2) histological confirmation of ICC; 3) macroscopically removal of tumor and an absence of gross tumors confirmed by intraoperative ultrasonography; 4) absence of ICC-related treatments prior to surgery; 5) Eastern Cooperative Oncology Group Performance Status ≤1; 6) Child–Pugh Class A. Written informed consent in accordance with the Declaration of Helsinki was acquired from every patient. The study was approved by the Independent Ethics Committee of Cancer Institute and Hospital, Chinese Academy of Medical Sciences.
From 2007 to 2016, 70 ICC patients who had undergone liver resection were recruited. Forty-nine patients underwent narrow-margin (<1.0 cm) hepatectomy, where the tumors were adjacent to major vessels. Among them, patients who underwent postoperative radiotherapy were categorized as Group A and patients who did not receive postoperative radiotherapy were Group B. Twenty-one patients with tumors distant from major vessels received wide-margin (≥1.0 cm) resections. Treatment for every patient was conducted by the same surgical team and radiotherapy team in order to standardize the quality of this study.
Surgery
The extent of tumor resection was determined according to tumor diameter, location, presence or absence of cirrhosis, and estimated volume of the liver remnant. Nonanatomical hepatectomy was performed in 37 patients. Anatomical hepatectomy based on Couinaud’s segments, sectors, and hemilivers, was performed in 33 patients. Regional lymph node dissection was performed in 47 patients who had suspicious lymph node metastasis based on preoperative imaging and/or intraoperative findings. Intraoperative ultrasonography was used to evaluate the size of the tumor and its relationship with the major vascular structures. Patients in Groups A and B, which had a resection margin (RM) of <1.0 cm, underwent selective and dynamic region-specific vascular occlusion. Patients with tumors adjacent to major vessels received null-margin resections, where surgeons carefully peeled the tumor away from the vascular surface with a Cavitron Ultrasonic Surgical Aspirator to protect the vessels. After removal of the tumor, 4–6 silver markers were stitched into the tumor bed for more accurate postoperative radiotherapy. Group C consisted of patients whose lesions were away from major vascular structure and in whom an at least 1.0 cm RM could be obtained.
Postoperative radiotherapy
Patients with narrow-margin hepatectomy were recommended to receive postoperative radiotherapy by the Multidisciplinary Liver Cancer Team in our institute. The recommendations were based on physicians’ judgment of margin status according to operative and pathological reports and the judgment of patients’ tolerance for further radiotherapy. Meanwhile, we objectively explained the potential benefits, risks, and the lack of evidence of adjuvant radiotherapy for ICC to these patients. The enrollment was based on both the patients’ willingness and the physicians’ judgment.
Radiotherapy was delivered 4–6 weeks after surgical resection via intensity-modulated radiotherapy (IMRT) technique. There was 1 case where a VIII segment lesion was treated by volumetric modulated arc therapy (VMAT) for its superior dose distribution and normal tissue protection.
All patients underwent computed tomography (CT) scan (Brilliance 16, Philips Medical Systems, Cleveland, OH, USA) in supine position with thermoplastic mask immobilization to restrain liver motion and reduce setup error. Preoperative magnetic resonance image (MRI) scans were used to optimize target and normal structure delineation using the Pinnacle3 9.0 treatment planning systems (Philips Healthcare, Andover, MA, USA).
The location of tumor beds was based on review of preoperative scans, postoperative scans, markers placed by the surgeon, and surgery summary notes. The clinical target volume (CTV) was defined as the tumor bed plus a 1.0 cm margin, or 1.5 cm margin in regions where the tumor adhered to major vascular structures. When regional lymph nodes were involved, CTV also included the adjacent lymph node drainage stations in the porta hepatis, celiac axis, and pancreaticoduodenal ligament. To compensate for respiratory liver motion and setup variations in 4-dimensional CT, we defined the planning target volume by expanding the CTV by 0.5 cm in the anterior–posterior and left–right directions and by 1.0 cm in the cranial–caudal direction. IMRT or VMAT planning was designed to ensure that the PTV was covered by the 95% isodose envelope while minimizing normal tissue injuries.
Normal tissue dose-volume constraints were as follows: 1) dose to normal liver (total liver volume minus tumor bed volume) was limited to ≤30 Gy; 2) maximum allowable point dose to the stomach and duodenum was set to ≤54 Gy; 3) Maximum point dose of cord was set to ≤45 Gy; 4)<50% of kidney volume received a dose over 20 Gy (V20 <50%).
The prescription dose ranged from 50 to 60 Gy in 25–30 fractions of 2 Gy according to individual conditions. Pretreatment online repositioning was accomplished by Cone beam CT.
Follow-up and definition
Patients were assessed every 3 months during the first 2 years and every 6 months thereafter (or more frequently if clinically indicated). Follow-up included liver function tests, routine blood and coagulation tests, chest radiography, and CT and/or MRI of the abdomen.
Location of recurrence was categorized as 1) marginal, if the tumor reappeared <2 cm from the tumor bed; 2) nodular, if a single lesion was located >2 cm from the transection plane; and 3) diffuse, if the recurrence consisted of >1 nodule scattered throughout the remaining liver.
Toxicity was graded using the National Cancer Institute Common Toxicity Criteria for Adverse Events version 3.0. Acute toxicity was evaluated weekly during treatment and the first month after radiotherapy. Morbidity occurring >1 month after the completion of radiotherapy was defined as late toxicity.
Radiation-induced liver disease (RILD) was evaluated 4 months after radiotherapy for all the patients in Group A. RILD was defined as either anicteric elevation of alkaline phosphatase level of ≥ 2-fold and nonmalignant ascites (classic RILD), or elevated transaminases of ≥ 5-fold the upper limit of normal or of pretreatment level (nonclassic RILD), in the setting of disease nonprogress.29,30
Statistical analysis
OS was defined as the time between surgical resection and death of any cause. Disease-free survival (DFS) was calculated as the time from surgical resection to intrahepatic or extrahepatic recurrence of ICC. Kaplan–Meier analysis was performed to report survival outcomes. Univariate and multivariate Cox regression analyses were performed to explore associations of factors with OS. Hazard ratios (HRs) and 95% confidence intervals (CI) were calculated. In the multivariate analysis, HRs were adjusted for age, sex, group, American Journal of Critical Care pathologic stage, and preoperative carcinoembryonic antigen level. The χ2 test was used for categorical variables. The Wilcoxon rank sum test was used for continuous variable of skewness distribution. All statistical analyses were performed using SPSS software, version 24 (IBM Corporation, Armonk, NY, USA). GraphPad Prism Version 6.0 .c software (GraphPad Software, Inc., La Jolla, CA, USA) was used for graph making.
Results
Patient characteristics
The 3 groups in this study shared similar demographic and clinicopathologic features (Table 1). The median age was 57 years (range, 45–76 years), 55 years (range, 36–72 years), and 51 years (range, 33–75 years) for Groups A, B, and C, respectively. About 34.6% of patients in Group A were over 60 years old. Patients in Group A tend to have received more nonanatomical resection compared to Group B (61.5%vs 34.8% P=0.062), with 15% more null-margin resections (P=0.303). There were more cases of intraoperative blood transfusion in Groups A and B compared to Group C (P=0.089), likely due to the difficulty of a null-margin resection. One patient in Group B was receiving radiotherapy in our hospital because of recurrence at the end of follow-up. Overall, there were no statistically significant differences between the 3 groups with regards to tumor stage, tumor distribution, or treatment modalities after recurrence. Radiotherapy was given to Group A with a total dose of 50 to 60 Gy in 2 Gy/fx (median, 56 Gy in 28 fx).
Table 1.
Patient and treatment characteristics
| Clinical characteristic | Group A, (n=26), N (%) |
Group B, (n=23), N (%) |
Group C, (n=21), N (%) |
P-value |
|---|---|---|---|---|
| Age (years) | ||||
| Median (range) | 57.23 (45–76) | 55.56 (36–72) | 51.43 (33–75) | 0.141 |
| ≤60 | 17 (65.4) | 17 (73.9) | 19 (90.5) | |
| >60 | 9 (34.6) | 6 (26.1) | 2 (9.5) | |
| Gender | ||||
| Male | 16 (61.5) | 15 (65.2) | 14 (66.7) | 0.930 |
| Female | 10 (38.5) | 8 (34.8) | 7 (33.3) | |
| CEA (ng/mL) | ||||
| ≤5.0 | 20 (76.9) | 15 (65.2) | 17 (81.0) | 0.465 |
| >5.0 | 3 (11.5) | 5 (21.7) | 4 (19.0) | |
| Tumor distribution | ||||
| Solitary | 22 (84.6) | 18 (78.3) | 18 (85.7) | 0.787 |
| Multifocal | 4 (15.4) | 5 (21.7) | 3 (14.3) | |
| Tumor size | ||||
| ≤5 cm | 12 (46.2) | 9 (39.1) | 9 (42.9) | 0.884 |
| >5 cm | 14 (53.8) | 14 (60.9) | 12 (57.1) | |
| Mean margin distance (mm) (SD) | 1.37 mm (2.75) | 1.52 mm (1.84) | – | 0.271 |
| Null-margin resection | 14 (53.8) | 9 (39.1) | – | 0.303 |
| Positive lymph node | 4 (15.4) | 6 (26.1) | 3 (14.3) | 0.449 |
| Stage (AJCC, seventh edition) | ||||
| Stage I | 5 (19.2) | 4 (17.4) | 9 (42.9) | 0.276 |
| Stage II | 9 (34.6) | 7 (30.4) | 4 (19.0) | |
| Stage III | 8 (30.8) | 6 (26.1) | 5 (23.8) | |
| Stage IVA | 4 (15.4) | 6 (26.1) | 3 (14.3) | |
| Histological grading (WHO) | ||||
| Well | 2 (7.7) | 0 (0.0) | 1 (4.8) | 0.320 |
| Moderate | 13 (50.0) | 12 (52.2) | 5 (23.8) | |
| Poor | 10 (38.5) | 10 (43.5) | 11 (52.4) | |
| Unclear | 1 (3.8) | 1 (4.3) | 4 (19.0) | |
| Resection type | ||||
| Anatomical resection | 10 (38.5) | 15 (65.2) | 8 (38.1) | 0.106 |
| Nonanatomical resection | 16 (61.5) | 8 (34.8) | 13 (61.9) | |
| Liver capsule invasion | 17 (65.4) | 17 (73.9) | 17 (81) | 0.499 |
| Intraoperative blood transfusion | 4 (15.4) | 7 (30.4) | 1 (4.8) | 0.089 |
| Treatment modalities after recurrence | ||||
| Chemotherapy | 2 (7.7) | 2 (8.7) | 2 (9.5) | 0.484 |
| Radiofrequency ablation or transarterial interventional therapy | 3 (11.5) | 3 (13.0) | 5 (23.8) | |
| Surgery | 0 (0) | 0 (0) | 1 (4.8) | |
| Radiotherapy | 0 (0) | 1 (4.3) | 0 (0) | |
| Othersa | 9 (34.6) | 8 (34.8) | 3 (14.3) |
Notes:
Including patients received supportive care or traditional Chinese medicine as palliative treatment. Group A, narrow-margin hepatectomy plus postoperative radiotherapy; Group B, narrow-margin hepatectomy alone; Group C, wide-margin hepatectomy alone.
Abbreviations: CEA, carcinoembryonic antigen; SD, standard deviation; AJCC, American Joint Committee on Cancer; WHO, World Health Organization.
Survival
The median follow-up time for all patients was 42 months (range, 5–96 months), and the 3-year OS and DFS rates were 46.6% and 33%, respectively. Thirty-three patients (47%) died at the end of follow-up, predominantly due to tumor recurrence. One patient died of hematemesis in the setting of hepatocirrhosis.
The 3-year OS and DFS rates for Group A, Group B, and Group C were 55% and 44%, 20% and 10%, and 65% and 33%, respectively. Kaplan–Meier analysis showed superior OS (P=0.011) and DFS (P=0.031) in Group A compared to Group B, and improved OS (P=0.031) and DFS (P=0.105) in Group C compared to Group B. There was no statistical difference in OS (P=0.685) and DFS (P=0.583) between Group A and Group C (Figure 1A and B). Univariate Cox regression analysis indicated that preoperative carcinoembryonic antigen >5.0 ng/mL and receiving narrow-margin resection without adjuvant radiotherapy were significantly poor prognostic factors of OS (Table 2). After multivariate analysis, receiving narrow-margin resection only (adjusted HR: 3.73; 95% CI: 1.36–10.25; P=0.001) was a significant poor prognostic risk factor of OS (Table 2).
Figure 1.
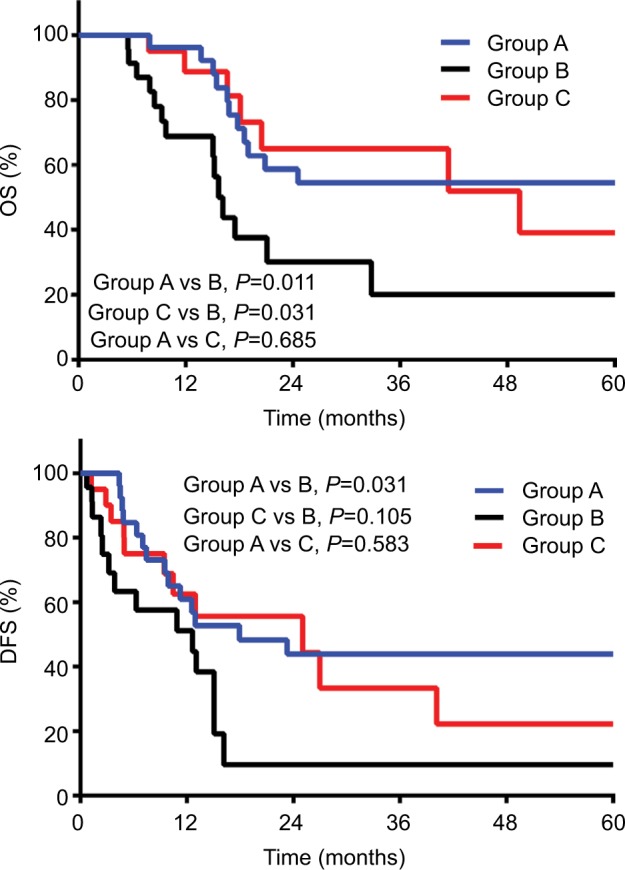
Kaplan–Meier curves of OS and DFS in 3 groups.
Notes: OS (A) and DFS (B) of patients in 3 groups. Group A, narrow-margin hepatectomy plus postoperative radiotherapy; Group B, narrow-margin hepatectomy alone; Group C, wide-margin hepatectomy alone.
Abbreviations: OS, overall survival; DFS, disease-free survival.
Table 2.
Cox regression analysis of mortality risk for all patients
| Variable | Univariate analysis (n =70)
|
Multivariate analysis (n =70)
|
||||
|---|---|---|---|---|---|---|
| HR | P-value | 95% CI | aHR | P-value | 95% CI | |
| Group | ||||||
| Group A (Ref.) | 1.00 | – | – | 1.00 | – | – |
| Group B | 2.94 | 0.009 | (1.32–6.57) | 3.73 | 0.011 | (1.36–10.25) |
| Group C | 1.17 | 0.737 | (0.47–2.91) | 1.67 | 0.356 | (0.57–4.86) |
| Sex | ||||||
| Male (Ref.) | 1.00 | – | – | 1.00 | – | – |
| Female | 0.86 | 0.685 | (0.42–1.76) | 0.64 | 0.299 | (0.27–1.50) |
| Age (years) | ||||||
| ≤60 (Ref.) | 1.00 | – | – | 1.00 | – | – |
| >60 | 1.00 | 0.997 | (0.45–2.23) | 1.09 | 0.866 | (0.40–2.95) |
| Stage | ||||||
| Stage I (Ref.) | 1.00 | – | – | 1.00 | – | – |
| Stage II | 1.28 | 0.657 | (0.43–3.82) | 1.56 | 0.510 | (0.42–5.84) |
| Stage III | 1.67 | 0.334 | (0.59–4.70) | 1.91 | 0.272 | (0.60–6.06) |
| Stage IVA | 5.56 | 0.001 | (2.01–15.40) | 7.73 | 0.002 | (2.17–27.51) |
| CEA (ng/mL) | ||||||
| ≤5.0 (Ref.) | 1.00 | – | – | 1.00 | – | – |
| >5.0 | 3.08 | 0.016 | (1.23–7.71) | 1.874 | 0.252 | (0.64–5.49) |
Notes: All variables were used in multivariate analysis. Group A, narrow-margin hepatectomy plus postoperative radiotherapy; Group B, narrow-margin hepatectomy alone; Group C, wide-margin hepatectomy alone.
Abbreviations: aHR, adjusted hazard ratio; HR, hazard ratio; CI, confidence interval; CEA, carcinoembryonic antigen; Ref, reference group.
Patterns of recurrence
Recurrence was recorded in 39 patients (55.7%) at the end of follow-up. Incidence and pattern of ICC recurrence are detailed in Table 3. Group B experienced a greater number of intrahepatic and extrahepatic recurrences than Groups A and C. Most recurrences (34.8%) in Group B were diffuse (≥2 lesions) type while Group A and C consisted of less diffuse recurrence type. There was 1 case of marginal failure in a Group B patient. As for cumulative incidence of intrahepatic recurrence and extrahepatic recurrence, the difference between Groups B and C was minute. For Groups A and B, the 3 years intrahepatic recurrence rates were 36%vs 67% (P=0.133) and extrahepatic recurrence rates were 43%vs 65% (P=0.007) (Figure 2A and B).
Table 3.
Incidence and pattern of ICC recurrence in the 3 groups
| Pattern of recurrence | Number of patients (%)
|
||
|---|---|---|---|
| Group A | Group B | Group C | |
| (n=26) | (n=23) | (n=21) | |
| Total recurrencea | 14 (53.8) | 14 (60.9) | 11 (52.4) |
| Intrahepatic recurrence | 9 (34.6) | 10 (43.5) | 9 (42.9) |
| Marginal | 0 (0.0) | 1 (4.3) | 0 (0.0) |
| Nodular | 2 (7.7) | 0 (0.0) | 5 (23.8) |
| Diffuse | 7 (26.9) | 8 (34.8) | 4 (19.0) |
| Unclear | 0 (0.0) | 1 (4.3) | 0 (0.0) |
| Extrahepatic recurrence | 10 (38.5) | 10 (43.5) | 9 (42.9) |
Notes:
If patients were found to have both intrahepatic recurrence and extrahepatic recurrence simultaneously at follow-up, they were counted into both groups. Group A, narrow-margin hepatectomy plus postoperative radiotherapy; Group B, narrow-margin hepatectomy alone; Group C, wide-margin hepatectomy alone.
Abbreviation: ICC, intrahepatic cholangiocarcinoma.
Figure 2.
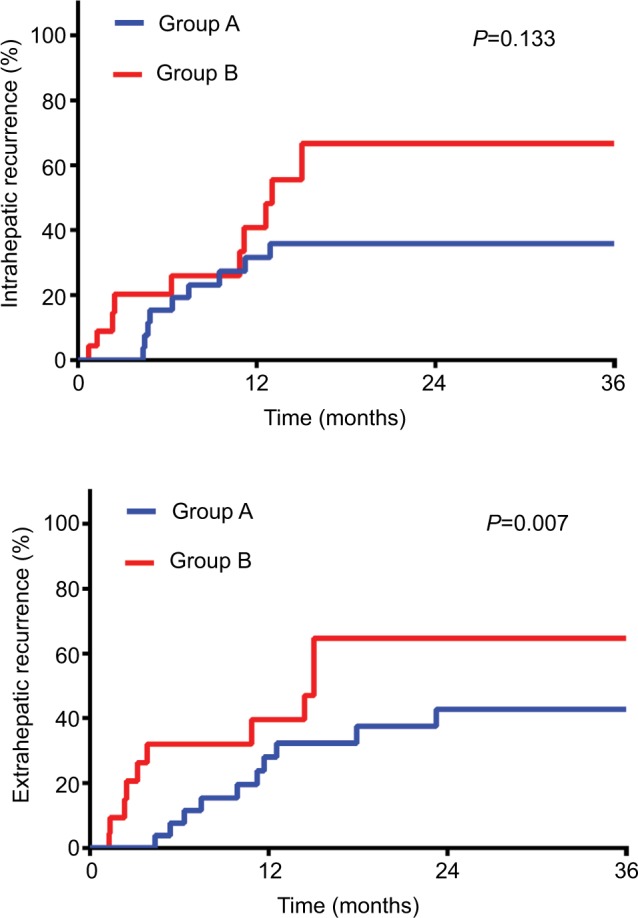
Cumulative incidence of intrahepatic recurrence and extrahepatic recurrence of Groups A and B.
Notes: Intrahepatic recurrence and extrahepatic recurrence of patients in Groups A and B. Group A, narrow-margin hepatectomy plus postoperative radiotherapy; Group B, narrow-margin hepatectomy alone.
Toxicity
The toxicities caused by postoperative radiotherapy were moderate (Table 4). Six patients (23.1%) experienced grade 2 toxicities. Two patients (7.7%) suffered from grade 3 toxicities, 1 patient with myeloid suppression and the other with liver dysfunction. There was no toxicity over grade 4 observed in our series and no incidence of classic or nonclassic RILD. All patients recovered from acute toxicities within 3 weeks after treatment.
Table 4.
Incidence of acute radiotherapy-related toxicities in patients receiving postoperative radiotherapy
| Toxicity | Number of patients (%)
|
|||
|---|---|---|---|---|
| Grade 0 | Grade 1 | Grade 2 | Grade 3 | |
| Nausea | 20 (76.9) | 6 (23.1) | 0 (0.0) | 0 (0.0) |
| Vomiting | 26 (100.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Anorexia | 20 (76.9) | 6 (23.1) | 0 (0.0) | 0 (0.0) |
| Abdominal pain | 24 (92.3) | 2 (7.7) | 0 (0.0) | 0 (0.0) |
| Fatigue | 22 (84.6) | 4 (15.4) | 0 (0.0) | 0 (0.0) |
| Dermatitis | 22 (84.6) | 3 (11.5) | 1 (3.8) | 0 (0.0) |
| Myeloid suppression | 9 (34.6) | 11 (42.3) | 5 (19.2) | 1 (3.8) |
| Leukocytes | 11 (42.3) | 9 (34.6) | 5 (19.2) | 1 (3.8) |
| Platelets | 19 (73.1) | 7 (26.9) | 0 (0.0) | 0 (0.0) |
| Hemoglobin | 23 (88.5) | 2 (7.7) | 1 (3.8) | 0 (0.0) |
| Liver dysfunction | 7 (26.9) | 14 (53.8) | 4 (15.4) | 1 (3.8) |
| Alanine aminotransferase | 19 (73.1) | 7 (7.7) | 0 (0.0) | 0 (0.0) |
| Aspartate aminotransferase | 19 (73.1) | 5 (19.2) | 1 (3.8) | 1 (3.8) |
| Alkaline phosphatase | 20 (76.9) | 6 (23.1) | 0 (0.0) | 0 (0.0) |
| γ-Glutamyl transpeptidase | 14 (53.8) | 7 (26.9) | 4 (15.4) | 1 (3.8) |
| Total bilirubin | 19 (73.1) | 7 (26.9) | 0 (0.0) | 0 (0.0) |
| Albumin | 26 (100.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Creatinine | 25 (96.2) | 1 (3.8) | 0 (0.0) | 0 (0.0) |
Discussion
In the scope of the information we have, this is the largest retrospective study to evaluate the role of postoperative radiotherapy after narrow-margin (<1.0 cm) resection in patients with ICC that adhere to the major vessels. The present study includes 70 patients with a median follow-up time of 42 months. We compared narrow-margin resection plus radiotherapy with narrow-margin resection alone and wide-margin resection in this study. In our cohort, radiotherapy following narrow-margin resection produced similar survival outcomes as wide-margin resections and was associated with longer OS and DFS than narrow-margin resection alone. Given the highly conformal and precise technique of IMRT and VMAT, only 2 patients (7.6%) developed grade 3 myeloid suppression or liver dysfunction, with no incidence of RILD.
While surgery remains the first-line treatment and presents the best chance of cure for ICC patients, survival remains poor in the absence of adjuvant treatments, especially in cases of positive or narrow margin.2,8,10–21,31 In a retrospective study carried out by Cho et al,32 patients were analyzed based on the width of the margin after surgical resection. Median survival time was 23 months for patients (n=23) with a RM of ≥10 mm (n=23) compared to 18 months for patients (n=40) with a RM <10 mm (P=0.049). The decreased survival with a narrower margin was attributed to postresection high recurrence rate of ICC. Recurrence rates after surgery have been reported to range between 60%–75%.33–36 Shimada et al37 found that, while not statistically significant, patients with narrow-margin resection tended to experience more intrahepatic recurrence. However, there remains a lack of established adjuvant treatments in patients with resected ICC.
Data supporting radiotherapy as a treatment option for ICC patients are sparse. The largest study to date is a retrospective report by Shinohara et al38 that analyzed 3,839 ICC patients from 1973 to 2003 based on the Surveillance, Epidemiology, and End Results database. Those who received adjuvant radiotherapy following surgery had a median OS of 11 months, while those receiving surgery alone only had 6 months (P=0.014). However, more details in terms of radiation dose, target definition, radiation technique, or toxicities were not mentioned. More recently, Jiang et al39 reviewed 90 patients with resected ICC and concurrent regional lymph node metastases. Twenty-four patients received radiotherapy with a median total dose of 50 Gy (range 34–60 Gy) in fractions of 2 Gy. Among them, traditional 2D radiotherapy was applied in 11 patients, while 3D conformal radiation therapy was applied in the other 13 patients. Radiotherapy was found to shrink metastatic lymph nodes (CR in 9 patients and PR in 9 patients) and prolong median survival time (19.1 months in the radiotherapy group vs 9.5 months in the nonradiotherapy group [P=0.011]). While reported toxicities were mild, traditional 2D radiotherapy (total dose <50 Gy) was discontinued in 3 patients for intolerable gastrointestinal side effects or increased levels of liver enzymes. Due to limited studies, the latest National Comprehensive Cancer Network (NCCN) guidelines recommend postoperative radiotherapy only in R1 resection case. Our study demonstrates that a subgroup with narrow margins after surgery might also benefit from adjuvant radiotherapy.
Chemoradiation is another option in the treatment of cholangiocarcinoma. Several retrospective studies have shown the benefit of chemoradiation in ICC. Kim et al27 retrospectively reviewed 92 patients with unresectable advanced-stage ICC, where 25 patients received concurrent chemoradiotherapy and 67 patients received chemotherapy only. Concurrent radiotherapy was delivered in single fractions of 2.0–3.0 Gy once a day and 5 times a week, with a mean total radiotherapy dose of 44.7 Gy (range 25.0–60.0 Gy). At a median follow-up of 5.3 months, concurrent radiotherapy had better PFS (4.3 vs 1.9 months, P=0.001) and OS (9.3 vs 6.2 months, P=0.048). Lin et al40 studied 599 patients with resectable ICC who received surgery without distant metastasis. Of them, 174 received adjuvant concurrent chemoradiotherapy, 146 received adjuvant sequential chemotherapy and radiotherapy, and 279 received adjuvant chemotherapy alone. IMRT was delivered with a total dose ≥45 Gy. Fluoropyrimidine- or gemcitabine-based CT regimen was given to all patients. A stratified Cox proportional hazard model was built to assess the risk of death and the associated adjuvant treatment modalities by considering both pathologic stage and margin status. This showed that adjuvant concurrent chemoradiotherapy was found to improve OS for patients at early stages with a positive margin and those at advanced stages with either a positive or negative margin. Although it was analyzed using a larger patient cohort, the purpose and conclusion were quite different from our study. Our study aimed to evaluate the role of adjuvant radiotherapy after narrow-margin (<1.0 cm) resection in patients with ICC adherent to major vessels. So, our result may provide reference for the decision of adjuvant radiotherapy after narrow-margin resection. In our series, concurrent or sequential chemotherapy was not included. However, as more and more evidences support the efficacy of chemoradiation, it is reasonable to investigate its role in ICC with further studies.
The results of this study demonstrate a survival benefit associated with adjuvant radiotherapy in patients with narrow or positive margins after surgery. However, as this was a retrospective study, patients were not randomized to the type of treatment. For instance, patients’ physical performance after surgery might influence the physician’s suggestion to the patients, which might favor the patients who receive radiation to some extent. The low incidence of ICC resulted in a small sample size. Thus, these results need to be further validated in a larger and prospective study.
Conclusion
Our result showed that postoperative radiotherapy following narrow-margin hepatectomy seems to be efficacious and well tolerated in patients with ICC adjacent to major vessels. Moreover, the OS and DFS were comparable to those who received wide-margin resection. This result provides support for us to carry out larger prospective study to further validate the role of postoperative radiotherapy in patients with ICC abutting the major vascular structures.
Acknowledgments
The authors are thankful to Shu-Lian Wang, MD, Jing Jin, MD, Yong-Wen Song, MD, Hui Fang, MD, Yue-Ping Liu, MD, Xin-Fan Liu, MD, and Zi-Hao Yu, MD, for participating in patient treatment and manuscript revision. This work was supported by the National Key Projects of Research and Development of China (grant number 2016YFC0904600) and the CAMS Innovation Fund for Medical Sciences (grant numbers 2016-I2M-1-001, 2017-I2M-3-005). The funding agency has no involvement with the design of the study or the collection, analysis, and interpretation of data, as well as writing of the manuscript.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Bergquist A, von Seth E. Epidemiology of cholangiocarcinoma. Best Pract Res Clin Gastroenterol. 2015;29(2):221–232. doi: 10.1016/j.bpg.2015.02.003. [DOI] [PubMed] [Google Scholar]
- 2.Guro H, Kim JW, Choi Y, Cho JY, Yoon YS, Han HS. Multidisciplinary management of intrahepatic cholangiocarcinoma: Current approaches. Surg Oncol. 2017;26(2):146–152. doi: 10.1016/j.suronc.2017.03.001. [DOI] [PubMed] [Google Scholar]
- 3.Sia D, Tovar V, Moeini A, Llovet JM. Intrahepatic cholangiocarcinoma: pathogenesis and rationale for molecular therapies. Oncogene. 2013;32(41):4861–4870. doi: 10.1038/onc.2012.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sempoux C, Jibara G, Ward SC, et al. Intrahepatic cholangiocarcinoma: new insights in pathology. Semin Liver Dis. 2011;31(1):49–60. doi: 10.1055/s-0031-1272839. [DOI] [PubMed] [Google Scholar]
- 5.Endo I, Gonen M, Yopp AC, et al. Intrahepatic cholangiocarcinoma: rising frequency, improved survival, and determinants of outcome after resection. Ann Surg. 2008;248(1):84–96. doi: 10.1097/SLA.0b013e318176c4d3. [DOI] [PubMed] [Google Scholar]
- 6.Patel T. Increasing incidence and mortality of primary intrahepatic cholangiocarcinoma in the United States. Hepatology. 2001;33(6):1353–1357. doi: 10.1053/jhep.2001.25087. [DOI] [PubMed] [Google Scholar]
- 7.Khan SA, Taylor-Robinson SD, Toledano MB, Beck A, Elliott P, Thomas HC. Changing international trends in mortality rates for liver, biliary and pancreatic tumours. J Hepatol. 2002;37(6):806–813. doi: 10.1016/s0168-8278(02)00297-0. [DOI] [PubMed] [Google Scholar]
- 8.Maithel SK, Gamblin TC, Kamel I, Corona-Villalobos CP, Thomas M, Pawlik TM. Multidisciplinary approaches to intrahepatic cholangiocarcinoma. Cancer. 2013;119(22):3929–3942. doi: 10.1002/cncr.28312. [DOI] [PubMed] [Google Scholar]
- 9.Tan JC, Coburn NG, Baxter NN, Kiss A, Law CH. Surgical management of intrahepatic cholangiocarcinoma – a population-based study. Ann Surg Oncol. 2008;15(2):600–608. doi: 10.1245/s10434-007-9627-x. [DOI] [PubMed] [Google Scholar]
- 10.Inoue K, Makuuchi M, Takayama T, et al. Long-term survival and prognostic factors in the surgical treatment of mass-forming type cholangiocarcinoma. Surgery. 2000;127(5):498–505. doi: 10.1067/msy.2000.104673. [DOI] [PubMed] [Google Scholar]
- 11.Weber SM, Jarnagin WR, Klimstra D, Dematteo RP, Fong Y, Blumgart LH. Intrahepatic cholangiocarcinoma: resectability, recurrence pattern, and outcomes. J Am Coll Surg. 2001;193(4):384–391. doi: 10.1016/s1072-7515(01)01016-x. [DOI] [PubMed] [Google Scholar]
- 12.Konstadoulakis MM, Roayaie S, Gomatos IP, et al. Fifteen-year, single-center experience with the surgical management of intrahepatic cholangiocarcinoma: operative results and long-term outcome. Surgery. 2008;143(3):366–374. doi: 10.1016/j.surg.2007.10.010. [DOI] [PubMed] [Google Scholar]
- 13.Nakagohri T, Kinoshita T, Konishi M, Takahashi S, Gotohda N. Surgical outcome and prognostic factors in intrahepatic cholangiocarcinoma. World J Surg. 2008;32(12):2675–2680. doi: 10.1007/s00268-008-9778-3. [DOI] [PubMed] [Google Scholar]
- 14.Choi SB, Kim KS, Choi JY, et al. The prognosis and survival outcome of intrahepatic cholangiocarcinoma following surgical resection: association of lymph node metastasis and lymph node dissection with survival. Ann Surg Oncol. 2009;16(11):3048–3056. doi: 10.1245/s10434-009-0631-1. [DOI] [PubMed] [Google Scholar]
- 15.de Jong MC, Nathan H, Sotiropoulos GC, et al. Intrahepatic cholangiocarcinoma: an international multi-institutional analysis of prognostic factors and lymph node assessment. J Clin Oncol. 2011;29(23):3140–3145. doi: 10.1200/JCO.2011.35.6519. [DOI] [PubMed] [Google Scholar]
- 16.Fisher SB, Patel SH, Kooby DA, et al. Lymphovascular and perineural invasion as selection criteria for adjuvant therapy in intrahepatic cholangiocarcinoma: a multi-institution analysis. HPB. 2012;14(8):514–522. doi: 10.1111/j.1477-2574.2012.00489.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Uenishi T, Kubo S, Yamazaki O, et al. Indications for surgical treatment of intrahepatic cholangiocarcinoma with lymph node metastases. J Hepatobiliary Pancreat Surg. 2008;15(4):417–422. doi: 10.1007/s00534-007-1315-5. [DOI] [PubMed] [Google Scholar]
- 18.Wang Y, Duan B, Yan L, et al. Long-term outcome after surgical resection for cholangiocarcinoma and prognostic index value. Surgeon. 2016;14(1):38–43. doi: 10.1016/j.surge.2014.05.003. [DOI] [PubMed] [Google Scholar]
- 19.Tang H, Lu W, Li B, Meng X, Dong J. Influence of surgical margins on overall survival after resection of intrahepatic cholangiocarcinoma: A meta-analysis. Medicine. 2016;95(35):e4621. doi: 10.1097/MD.0000000000004621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cho SY, Park S-J, Kim SH, et al. Survival Analysis of Intrahepatic Cholangiocarcinoma After Resection. Ann Surg Oncol. 2010;17(7):1823–1830. doi: 10.1245/s10434-010-0938-y. [DOI] [PubMed] [Google Scholar]
- 21.Spolverato G, Yakoob MY, Kim Y, et al. The Impact of Surgical Margin Status on Long-Term Outcome After Resection for Intrahepatic Cholangiocarcinoma. Ann Surg Oncol. 2015;22(12):4020–4028. doi: 10.1245/s10434-015-4472-9. [DOI] [PubMed] [Google Scholar]
- 22.Li J, Wang C, Song J, et al. Mesohepatectomy Versus Extended Hemihepatectomies for Centrally Located Liver Tumors: A Meta-Analysis. Sci Rep. 2017;7(1):9329. doi: 10.1038/s41598-017-09535-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Qiu J, Chen S, Wu H, Du C. The prognostic value of a classification system for centrally located liver tumors in the setting of hepatocellular carcinoma after mesohepatectomy. Surg Oncol. 2016;25(4):441–447. doi: 10.1016/j.suronc.2016.03.001. [DOI] [PubMed] [Google Scholar]
- 24.Miao XY, Hu JX, Dai WD, Zhong DW, Xiong SZ. Null-margin mesohepatectomy for centrally located hepatocellular carcinoma in cirrhotic patients. Hepatogastroenterology. 2011;58(106):575–582. [PubMed] [Google Scholar]
- 25.Doherty B, Nambudiri VE, Palmer WC. Update on the Diagnosis and Treatment of Cholangiocarcinoma. Curr Gastroenterol Rep. 2017;19(1):2. doi: 10.1007/s11894-017-0542-4. [DOI] [PubMed] [Google Scholar]
- 26.Howell M, Valle JW. The role of adjuvant chemotherapy and radiotherapy for cholangiocarcinoma. Best Pract Res Clin Gastroenterol. 2015;29(2):333–343. doi: 10.1016/j.bpg.2015.03.001. [DOI] [PubMed] [Google Scholar]
- 27.Kim YI, Park JW, Kim BH, et al. Outcomes of concurrent chemoradiotherapy versus chemotherapy alone for advanced-stage unresectable intrahepatic cholangiocarcinoma. Radiat Oncol. 2013;8:292. doi: 10.1186/1748-717X-8-292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen YX, Zeng ZC, Tang ZY, et al. Determining the role of external beam radiotherapy in unresectable intrahepatic cholangiocarcinoma: a retrospective analysis of 84 patients. BMC Cancer. 2010;10:492. doi: 10.1186/1471-2407-10-492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Munoz-Schuffenegger P, Ng S, Dawson LA. Radiation-Induced Liver Toxicity. Semin Radiat Oncol. 2017;27(4):350–357. doi: 10.1016/j.semradonc.2017.04.002. [DOI] [PubMed] [Google Scholar]
- 30.Guha C, Kavanagh BD. Hepatic radiation toxicity: avoidance and amelioration. Semin Radiat Oncol. 2011;21(4):256–263. doi: 10.1016/j.semradonc.2011.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li MX, Bi XY, Li ZY, et al. Impaction of surgical margin status on the survival outcome after surgical resection of intrahepatic cholangiocarcinoma: a systematic review and meta-analysis. J Surg Res. 2016;203(1):163–173. doi: 10.1016/j.jss.2016.02.012. [DOI] [PubMed] [Google Scholar]
- 32.Cho SY, Park SJ, Kim SH, et al. Survival analysis of intrahepatic cholangiocarcinoma after resection. Ann Surg Oncol. 2010;17(7):1823–1830. doi: 10.1245/s10434-010-0938-y. [DOI] [PubMed] [Google Scholar]
- 33.Yedibela S, Demir R, Zhang W, Meyer T, Hohenberger W, Schönleben F. Surgical treatment of mass-forming intrahepatic cholangiocarcinoma: an 11-year Western single-center experience in 107 patients. Ann Surg Oncol. 2009;16(2):404–412. doi: 10.1245/s10434-008-0227-1. [DOI] [PubMed] [Google Scholar]
- 34.Saiura A, Yamamoto J, Kokudo N, et al. Intrahepatic cholangiocarcinoma: analysis of 44 consecutive resected cases including 5 cases with repeat resections. Am J Surg. 2011;201(2):203–208. doi: 10.1016/j.amjsurg.2008.12.035. [DOI] [PubMed] [Google Scholar]
- 35.Ohtsuka M, Kimura F, Shimizu H, et al. Significance of repeated resection for recurrent intrahepatic cholangiocarcinoma. Hepatogastroenterology. 2009;56(89):1–5. [PubMed] [Google Scholar]
- 36.Ercolani G, Vetrone G, Grazi GL, et al. Intrahepatic cholangiocarcinoma: primary liver resection and aggressive multimodal treatment of recurrence significantly prolong survival. Ann Surg. 2010;252(1):107–114. doi: 10.1097/SLA.0b013e3181e462e6. [DOI] [PubMed] [Google Scholar]
- 37.Shimada K, Sano T, Sakamoto Y, Esaki M, Kosuge T, Ojima H. Clinical impact of the surgical margin status in hepatectomy for solitary mass-forming type intrahepatic cholangiocarcinoma without lymph node metastases. J Surg Oncol. 2007;96(2):160–165. doi: 10.1002/jso.20792. [DOI] [PubMed] [Google Scholar]
- 38.Shinohara ET, Mitra N, Guo M, Metz JM. Radiation therapy is associated with improved survival in the adjuvant and definitive treatment of intrahepatic cholangiocarcinoma. Int J Radiat Oncol Biol Phys. 2008;72(5):1495–1501. doi: 10.1016/j.ijrobp.2008.03.018. [DOI] [PubMed] [Google Scholar]
- 39.Jiang W, Zeng ZC, Tang ZY, et al. Benefit of radiotherapy for 90 patients with resected intrahepatic cholangiocarcinoma and concurrent lymph node metastases. J Cancer Res Clin Oncol. 2010;136(9):1323–1331. doi: 10.1007/s00432-010-0783-1. [DOI] [PubMed] [Google Scholar]
- 40.Lin Y-K, Hsieh M-C, Wang W-W, et al. Outcomes of adjuvant treatments for resectable intrahepatic cholangiocarcinoma: Chemotherapy alone, sequential chemoradiotherapy, or concurrent chemoradiotherapy. Radiother Oncol. 2018 doi: 10.1016/j.radonc.2018.05.011. [DOI] [PubMed] [Google Scholar]


