Chronic lymphocytic leukemia (CLL) is the most common leukemia among adults in Western countries, with a median age at diagnosis of 70 years.1 Until 2014, chemoimmunotherapy combinations were the mainstay treatment, particularly for physically fit patients.2,3 However, older individuals or those with comorbid conditions may be less likely to tolerate standard CLL chemoimmunotherapy combinations. It is important to establish a baseline understanding of real-world CLL treatments and outcomes prior to the introduction of novel agents, as this would allow for a better understanding of unmet needs, particularly among older patients in the pre-novel therapy era, and also inform future comparative effectiveness studies.4 Current literature provides limited data about real-world treatment patterns and survival among older CLL patients. To address these gaps in the literature, we utilized comprehensive prescription and medical insurance claims linked with cancer registry data to analyze both first-line and second-line treatment patterns as well as survival outcomes in older adults newly diagnosed with CLL, in the time period (2007-2013) that immediately predated FDA approval of novel agents. We also stratified outcomes by age (66-74 “younger seniors” vs. ≥75 years “older seniors”).
Our retrospective cohort study used 2007-2013 Surveillance, Epidemiology, and End Results (SEER) data linked with Medicare files to compare treatment patterns, time-to-treatment initiation, and overall and CLL-specific survival from first-line and second-line treatments among Medicare beneficiaries aged >65 years who were newly diagnosed with CLL between 2007 and 2011. The SEER registries capture newly diagnosed cancer patients, covering about 28% of the U.S. population.5 The SEER-Medicare files utilized in our study included all SEER patients from 2007 through 2011 who had fee-for-service Medicare claims (inpatient, outpatient, physician, skilled nursing facility care, home health, hospice, pharmacy [Part D] claims) linked from 2007 through 2013.
Details of the sample selection process may be found in Online Supplementary Figure S1. Our final study sample consisted of 3214 newly diagnosed CLL patients. Initial treatment pattern outcomes consisted of the percentage of patients receiving a CLL treatment, mean time to first-line treatment initiation, and the type of treatment regimen initiated. A full list of CLL treatments may be found in Online Supplementary Table S1. Time to first-line treatment initiation was defined as the time elapsed between the index (diagnosis) date and the date of the first claim for a CLL treatment. Type of treatment regimen was categorized as chlorambucil monotherapy, rituximab monotherapy, rituximab-containing chemoimmunotherapy combination, or other chemotherapy. A therapy was considered part of the first-line treatment regimen if it was received within 60 days of the initial drug claim.6,7
We also examined second-line treatments, defined as re-initiation of at least one or all of the agents in the first-line treatment regimen following a treatment-free interval of >180 days, or the addition of a new treatment that was not part of the first-line treatment regimen. The second-line treatment start date was defined as the date on which the re-initiated therapy was administered or filled or the date on which the new treatment was added. Time to second-line treatment initiation was defined as the time elapsed between the index (diagnosis) date and the start of the second-line treatment date.
Survival outcomes included overall survival (OS) and CLL-specific survival from first-line and second-line CLL treatment. We also examined 1-year and 2-year OS rates. CLL-specific survival was defined as the net survival measure representing cancer survival in the absence of other causes of death. Kaplan-Meier estimates were used to examine OS from first-line and second-line treatment dates among patients receiving these treatments.8 Cox regression models examined factors associated with OS and CLL-specific survival from the first treatment date and second-line treatment date among treated patients.9
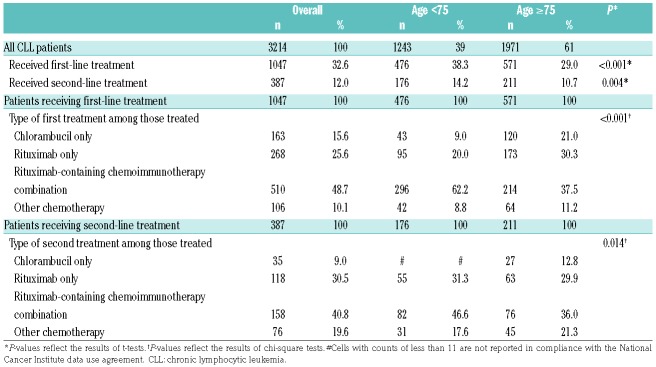
Online Supplementary Tables S2 and S3 provide sociodemographic and clinical characteristics, by age group and treatment status, respectively, for our final sample. Median follow up was 36.1 months from CLL diagnosis. As shown in Table 1, 1047 (32.6%) of the overall patient sample received first-line treatment, with lower rates of treatment in the older seniors group (29.0% vs. 38.3%; P<0.001). However, the older seniors group had a shorter median time to first treatment (4.4 vs. 6.8 months; P<0.001), (data not shown). Rituximab-containing chemoimmunotherapy combinations were the most common treatment approaches overall (utilized in nearly half of patients receiving first-line treatment), yet significant differences were observed in the distribution of treatment approaches between the two age groups (P<0.001). For example, a higher proportion of the older seniors group (vs. younger group) received monotherapy with either chlorambucil (21.0% vs. 9.0%) or rituximab (30.3% vs. 20.0%).
Table 1.
Treatment patterns of elderly Medicare patients newly diagnosed with chronic lymphocytic leukemia, by age group.
Twelve percent of patients received second-line treatment; again, rates were lower in the older seniors group (10.7% vs. 14.2%; P=0.004). Among patients receiving second-line treatment, the median time from CLL diagnosis to initiation of the second-line treatment was 21.8 months (data not shown). Similar to the findings for first-line treatment, rituximab-containing chemoimmunotherapy combinations were the most common second-line treatments (40.8%).
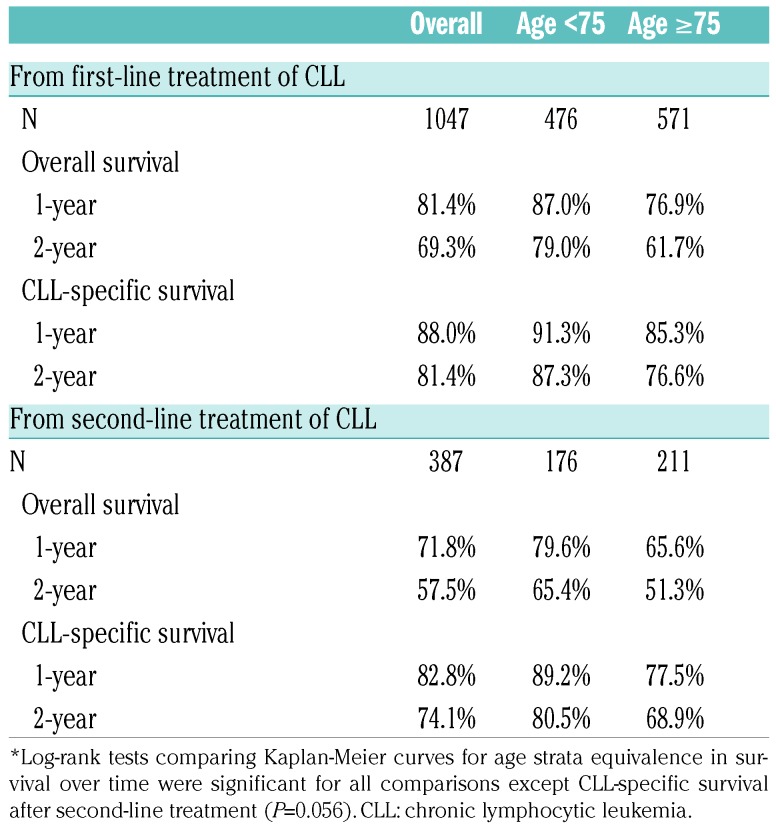
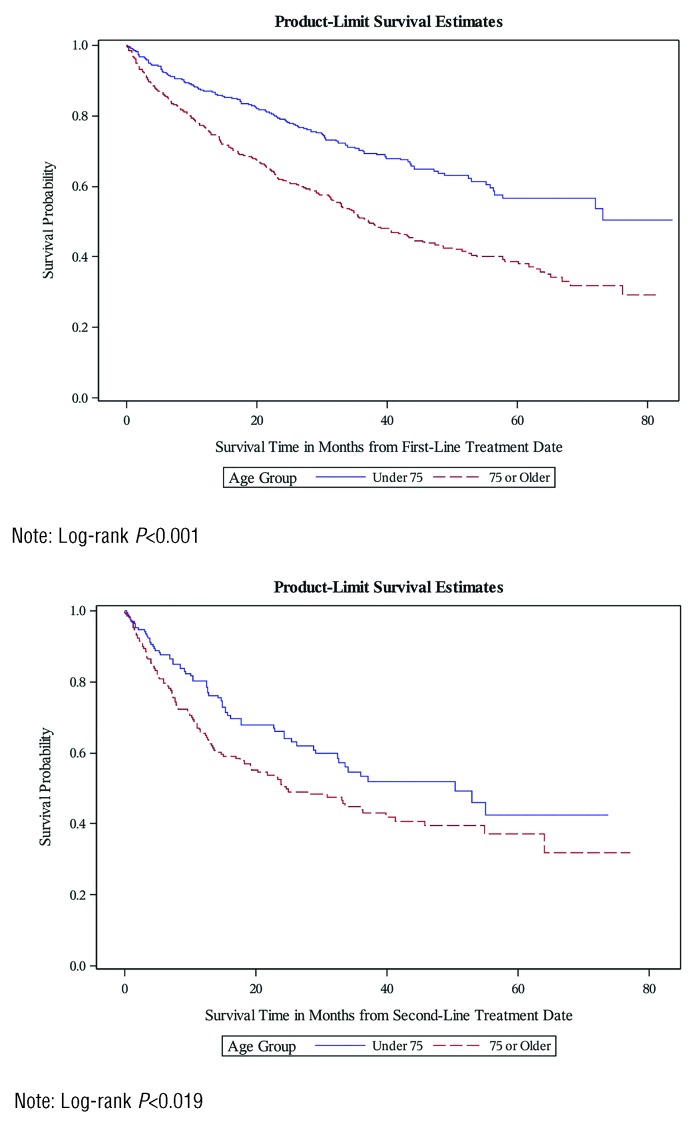
The median OS from first-line treatment initiation and second-line treatment initiation was 52.4 months and 33.7 months, respectively (data not shown). Estimated 1-year and 2-year OS rates after first-line treatment initiation were 81.4% and 69.3%, respectively (Table 2). OS rates after second-line treatment initiation were 71.8% at 1 year and 57.5% at 2 years. Patients in the older seniors group had a 1-year OS rate of 66.7% and 2-year OS rate of 61.7%, whereas those in the younger seniors group had a 1-year OS rate of 87.0% and 2-year OS rate of 79.0%. Similar patterns were observed for OS rates from the time of second-line treatment. Unadjusted Kaplan-Meier curves for OS from first- and second-line treatments are shown in Figure 1.
Table 2.
Survival rates among elderly Medicare patients newly diagnosed with chronic lymphocytic leukemia, by age group.*
Figure 1.
Overall survival from first-line treatment date and second-line treatment date, by age group.
Regression results are displayed in Online Supplementary Table S4. Among patients receiving first-line treatment, older age was associated with worse OS (hazard ratio [HR],1.81; 95% confidence interval [CI],1.46 to 2.24). Additionally, male sex, a Northeast location relative to the West, receiving Medicare Part D low-income subsidies, higher National Cancer Institute (NCI) comorbidity index score,10 and presence of disability were associated with worse OS. Receipt of rituximab monotherapy (HR, 0.69; 95% CI, 0.51 to 0.91) or rituximab-containing chemoimmunotherapy combinations (HR, 0.64; 95% CI, 0.49 to 0.84), compared to chlorambucil monotherapy, were associated with better OS after first-line treatment. As with first-line treatment, older age was associated with worse OS among patients receiving second-line treatment.
Among patients receiving first-line treatment, older age (HR, 1.81; 95% CI, 1.34 to 2.44), male sex, receiving Medicare Part D low-income subsidies, and higher NCI comorbidity index score were associated with worse CLL-specific survival outcomes. Receipt of rituximab or rituximab-containing chemoimmunotherapy combinations compared to chlorambucil monotherapy was associated with better CLL-specific survival outcomes. Similarly, older age was associated with worse CLL-specific survival among patients receiving second-line treatment (HR, 1.86; 95% CI, 1.15 to 2.99).
The study herein provides detailed observational data regarding treatment patterns and outcomes of CLL patients treated in the pre-novel therapy era with both first-line and second-line treatments. Such data will be an important baseline for future evaluations of novel therapies. We found that approximately one-third of newly diagnosed elderly CLL patients received first-line treatment, over a median follow-up of 3 years. Among those who received CLL treatment, approximately 37% progressed to a relapse/refractory phase, with a median time to second-line treatment of 22 months. Rituximab combination therapies were common for both first-line and second-line treatment. In our study 21% of older seniors received chlorambucil as a first-line treatment.
Of note, patients aged ≥75 years were less likely to initiate treatment compared to younger seniors. When they did initiate treatment, they were more likely to receive monotherapy with chlorambucil or rituximab. These findings may be related to data from clinical trials suggesting a minimal benefit of adding rituximab to chlorambucil monotherapy or to fludarabine and cyclophosphamide in patients older than 65 years.11,12 Given that novel therapies, including ibrutinib13 and the combination of the glycoengineered anti-CD20 antibody obinutuzumab and chlorambucil14 have shown superior outcomes as compared to chlorambucil monotherapy in clinical trials,13,14 these agents may represent promising new therapies for current and future patients similar to those included in our study.
In addition to having lower rates of treatment, treated patients aged ≥75 years had significantly lower OS as compared to younger seniors, and this discrepancy persisted even after controlling for socioeconomic, clinical, and treatment characteristics. This older population is prone to a greater incidence and severity of comorbidities, as well as disease- and infection-related death, all of which may lead to delays in therapy initiation and poorer outcomes.15–18 OS rates among all treated patients were modest (median 52.4 months for first-line therapy and 33.7 months for second-line therapy). Although our study does not permit causal inferences, we observed that rituximab monotherapy and rituximab-containing chemoimmunotherapy combinations were associated with favorable survival outcomes when compared to chlorambucil monotherapy in the front-line setting.
Our study has some limitations. First, we utilized registry and claims data and did not have access to all relevant prognostic factors, such as β2-microglobulin, lactate dehydrogenase, white cell count, or molecular and genetic abnormalities (e.g., presence of Del(17p)/TP53 mutation), which could have influenced treatment choice or survival outcomes. Additionally, the SEER-Medicare registry does not contain staging information for leukemia patients and our markers for disease severity (e.g., patients with claims-based diagnoses of anemia and thrombocytopenia) may not have been an adequate proxy of severity for all the patients in our study. If these unmeasured confounders correlated with the variables (e.g., age group, treatment group) included in our multi-variable survival model, our estimates would be biased.
In this population-based study of Medicare beneficiaries diagnosed with CLL in the years prior to the novel therapy era, we observed modest overall and CLL-specific survival among patients treated in both first-line and relapse-refractory contexts. Older seniors had lower rates of treatment, were more commonly treated with monotherapies, and had poorer disease-specific and OS outcomes compared to younger patients. As longitudinal data on novel therapies continue to accumulate, future studies should examine CLL outcomes to investigate how results obtained in recent landmark clinical trials translate into clinical practice.
Supplementary Material
Acknowledgments
This study used the linked SEER-Medicare database. The interpretation and reporting of these data are the sole responsibility of the authors. The authors acknowledge the efforts of the National Cancer Institute; the Office of Research, Development and Information, CMS; Information Management Services (IMS), Inc.; and the Surveillance, Epidemiology, and End Results (SEER) Program tumor registries in the creation of the SEER-Medicare database. The authors wish to thank Amy R. Pettit, PhD, consultant and Adjunct Fellow, University of Pennsylvania Center for Public Health Initiatives, for her feedback on the manuscript and assistance with editing. The authors would also like to thank Molly Fanning, BA, Marian Bergkamp, BA, and Manuela Casuriaga, BA from the University of Pennsylvania for assistance with formatting the manuscript
Footnotes
Funding: this study was funded by research funding from Janssen Inc. to JAD.
Information on authorship, contributions, and financial & other disclosures was provided by the authors and is available with the online version of this article at www.haematologica.org.
References
- 1.National Cancer Institute. Chronic lymphocytic leukemia/small lymphocytic lymphoma. Available from: https://seer.cancer.gov/stat-facts/html/clyl.html Last accessed: 31 July 2018.
- 2.Eichhorst B, Fink A, Busch R, et al. First-line chemoimmunotherapy with bendamustine and rituximab versus fludarabine, cyclophosphamide, and rituximab in patients with advanced chronic lymphocytic leukaemia (CLL10): an international, open-label, randomised, phase 3, non-inferiority trial. Lancet Oncol. 2016;17(7):928–942. [DOI] [PubMed] [Google Scholar]
- 3.Keating MJ, O’Brien S, Albitar M, et al. Early results of a chemoimmunotherapy regimen of fludarabine, cyclophosphamide, and rituximab as initial therapy for chronic lymphocytic leukemia. J Clin Oncol. 2005;23(18):4079–4088. [DOI] [PubMed] [Google Scholar]
- 4.Nabhan C, Mato AR. Economic modeling of the cost of chronic lymphocytic leukemia therapy: it is about the model. J Clin Oncol. 2017;35(16):1863–1864. [DOI] [PubMed] [Google Scholar]
- 5.National Cancer Institute. Overview of the SEER program. Available from: https://seer.cancer.gov/about/overview.html Last accessed: 27 October 2017.
- 6.Danese MD, Griffiths RI, Gleeson M, et al. An observational study of outcomes after initial infused therapy in Medicare patients diagnosed with chronic lymphocytic leukemia. Blood. 2011;117(13):3505–3513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Satram-Hoang S, Reyes C, Hoang KQ, Momin F, Skettino S. Treatment practice in the elderly patient with chronic lymphocytic leukemia-analysis of the combined SEER and Medicare database. Ann Hematol. 2014;93(8):1335–1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bland JM, Altman DG. Survival probabilities (the Kaplan-Meier method). BMJ. 1998;317(7172):1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Andersen PK, Gill RD. Cox’s regression model for counting processes: a large sample study. The Annals of Statistics. 1982;10(4):1110–1120. [Google Scholar]
- 10.National Cancer Institute. Chronic lymphocytic leukemia/small lymphocytic lymphoma. Available from: https://healthcaredelivery.cancer.gov/seermedicare/considerations/comorbidity.html Last accessed: 31 July 2018.
- 11.Hallek M, Fischer K, Fingerle-Rowson G, et al. Addition of rituximab to fludarabine and cyclophosphamide in patients with chronic lymphocytic leukaemia: A randomised, open-label, phase 3 trial. Lancet. 2010;376(9747):1164–1174. [DOI] [PubMed] [Google Scholar]
- 12.Eichhorst BF, Busch R, Stilgenbauer S, et al. First-line therapy with fludarabine compared with chlorambucil does not result in a major benefit for elderly patients with advanced chronic lymphocytic leukemia. Blood. 2009;114(16):3382–3391. [DOI] [PubMed] [Google Scholar]
- 13.Burger JA, Tedeschi A, Barr PM, et al. Ibrutinib as initial therapy for patients with chronic lymphocytic leukemia. N Engl J Med. 2015;373(25):2425–2437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goede V, Fischer K, Busch R, et al. Obinutuzumab plus chlorambucil in patients with CLL and coexisting conditions. N Engl J Med. 2014; 370(12):1101–1110. [DOI] [PubMed] [Google Scholar]
- 15.Brenner H, Gondos A, Pulte D. Trends in long-term survival of patients with chronic lymphocytic leukemia from the 1980s to the early 21st century. Blood. 2008;111(10):4916–4921. [DOI] [PubMed] [Google Scholar]
- 16.Pflug N, Bahlo J, Shanafelt TD, et al. Development of a comprehensive prognostic index for patients with chronic lymphocytic leukemia. Blood. 2014;124(1):49–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nabhan C, Mato A, Flowers CR, et al. Characterizing and prognosticating chronic lymphocytic leukemia in the elderly: prospective evaluation on 455 patients treated in the United States. BMC Cancer. 2017;17(1):198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stauder R, Eichhorst B, Hamaker ME, et al. Management of chronic lymphocytic leukemia (CLL) in the elderly: a position paper from an international Society of Geriatric Oncology (SIOG) task force. Ann Oncol. 2017;28(2):218–227. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.