Abstract
Succinate is an essential intermediate of the tricarboxylic acid cycle that exerts pleiotropic roles beyond metabolism in both physiological and pathological conditions. Recent evidence obtained in mouse models shows its essential role regulating blood cell function through various mechanisms that include pseudohypoxia responses by hypoxia-inducible factor-1α activation, post-translational modifications like succinylation, and communication mediated by succinate receptor 1. Hence, succinate links metabolism to processes like gene expression and intercellular communication. Interestingly, succinate plays key dual roles during inflammatory responses, leading to net inflammation or anti-inflammation depending on factors like the cellular context. Here, we further discuss current suggestions of the possible contribution of succinate to blood stem cell function and blood formation. Further study will be required in the future to better understand succinate biology in blood cells. This promising field may open new avenues to modulate inflammatory responses and to preserve blood cell homeostasis in the clinical setting.
Introduction: roles of succinate beyond metabolism
The metabolite succinate or succinic acid is at the hub of the tricarboxylic acid (TCA) cycle, and it is mainly produced by succinyl coenzyme A synthetase from succinyl coenzyme A, in a reversible reaction that generally occurs under aerobic conditions (Figure 1). Nonetheless, when cells rely on anaerobic glycolysis, like cancer cells and certain innate immune cells upon activation, other metabolic pathways sustain succinate levels, including glutamine-dependent anerplerosis to α-ketoglutarate, and eventually citrate by reductive carboxylation.1 Similarly, succinate may derive from the γ-aminobutyric acid shunt pathway that correlates with levels of expression of the γ-aminobutyric acid transporters solute carrier family 6 members 12 and 13 (SLC6A12, SLC6A13).2,3 Under physiological hypoxia, low oxygen levels lead to reduced activity of succinate dehydrogenase (SDH), which metabolizes succinate, and other oxygen-dependent enzymes in the electron transport chain, causing succinate accumulation.4,5 Succinate functions as a competitive inhibitor for prolyl hydroxylase domain (PHD) proteins that are central to degradation of hypoxia-inducible factor (HIF)-1α subunit.3–6 In fact, one of the first pieces of evidence for a role of succinate in cancer development was provided by the discovery of pseudohypoxia, which refers to activation of hypoxia signaling pathways under normal oxygen levels. Pseudohypoxia is a typical event in tumors with mutated SDH.7
Figure 1.
Succinate production and mechanisms of action. Succinate is an intermediate of several metabolic pathways, i.e. tricarboxylic acid (TCA) cycle under normoxic conditions (blue lines), and glutamine-dependent anerplerosis and γ-aminobutyric acid (GABA) shunt under anaerobic conditions (red lines). Accumulation of succinate associates with succinylation, i.e. addition of succinyl group to a lysine residue of a protein. Succinate inhibits action of prolyl hydroxylases (PHD) and thereby causes stabilization of hypoxia-inducible factor-1α (HIF-1α). Succinate further inhibits several dioxygenases involved in epigenetic regulation like ten-eleven translocation methylcytosine dioxygenase (TET) and jumonji C domain-containing histone lysine demethylases (JMJD3). Dicarboxylate carriers (DIC) and voltage-dependent anion channels (VDAC) control succinate release from mitochondria to cytosol. Succinate is released to the extracellular space through sodium-coupled citrate transporters (SLC13). GPR91 is a G protein–coupled cell surface receptor for extracellular succinate (Sucnr1). ACO: aconitase; IDH: isocitrate dehydrogenase; ODC: oxoglutarate dehydrogenase; SCS: α-succinyl-CoA synthetase; SDH: succinate dehydrogenase; FUM: fumarase; MDH: malate dehydrogenase; CSY: citrate synthase; GS: glutamine synthetase; GOGAT: glutamine oxoglutarate aminotransferase.
Hence, succinate functions may be classified as metabolic or non-metabolic. In mitochondria, succinate plays a crucial role in metabolism and operates in both anabolic and catabolic pathways.2,3 Mitochondria are the physiological source for succinate, but accumulated succinate may be transported to the cytosol through the dicarboxylic acid translocator in the mitochondrial inner membrane and the voltage-dependent anion channel in the outer membrane (Figure 1).6 In the cytosol, succinate plays regulatory roles beyond primary metabolism. Elevated cytosolic succinate levels may promote protein post-translational modifications by addition of succinyl groups to lysine residues.8,9 A remarkable effect of succinylati on is to alter the net charge of the protein by up to two charge units.8,9 Further, lysine succinylation is abundant and it induces significant structural changes in proteins,10 but its functional effects on protein and cellular functions have yet to be elucidated.
Interestingly, succinate connects intracellular metabolic status and intercellular communication, as it may be released to the extracellular space through plasma membrane transporters of the SLC13 family (Figure 1).11 Nevertheless, expression of these transporters on blood cells has not been well characterized. In the extracellular environment, succinate contributes to intercellular signaling by a receptor-mediated mechanism.12 Under steady-state conditions, circulating levels of succinate vary from 2 to 20 μM, and pro-inflammatory stimuli such as lipopolysaccharide (LPS), interleukin (IL)-8 and tumor necrosis factor (TNF)-α boost its concentration.13,14 In addition, activation of succinate receptor (Figure 1) was shown to be a critical mediator of inflammatory responses acting in synergy with toll-like receptors (TLR), thereby enhancing TNF-α and IL-1β expression in myeloid cells.12 Succinate further functions as chemoattractant, driving immune cell precursors from site of generation to place of maturation.12 In humans, circulating levels of succinate increase exponentially under certain circumstances like endurance exercise (93 μM)15 and pathological conditions such as type 2 diabetes (~47 to 125 μM),16,17 obesity (~80 μM),17 and ischemia-reperfusion injury following myocardial infarction.18 In addition, elevated levels of circulating succinate relate to development of solid tumors and poor prognosis in a variety of hematologic malignancies.6,19 Conversely, activation of succinate receptor on neural stem cells was recently shown to promote their anti-inflammatory activity in an experimental model of autoimmune encephalomyelitis.20
Here, we critically assess recent advances on the role of succinate, beyond its metabolic functions, at the intersection between inflammatory responses and blood cell activity with the aim of identifying gaps in the literature and proposing perspectives for further research.
Succinylation and its potential immunomodulatory effects
For some time now, succinate has been known to pro mote deoxyribonucleic acid methylation through inhibition of histone demethylases and the ten-eleven translocation family proteins (Figure 1), and thereby to play a role in the cellular epigenetic landscape.21,22 Recently, a novel post-translational modification was associated to succinate accumulation that results in lysine succinylation.3,9 Succinylation implies dramatic structural changes in proteins and confers a negative charge at physiological pH.9,10 Many succinylation sites on histones have been identified, which may result in alterations in chromatin structure and, consequently, gene expression.8,9 Mechanisms that trigger lysine succinylation are still not clear and little is known about its potential role in immune modulation. In Escherichia coli, where succinylation was discovered, accumulation of succinate promotes succinyl coenzyme A synthetase activity, leading to higher production of succinyl coenzyme A that acts, in turn, as succinyl donor.23 In mice, succinylation is reverted by sirtuin 5, a member of the nicotinamide adenine dinucleotide-dependent family of deacetylases that exhibits desuccinylase activity.23
Succinate dehydrogenase complex mutations seem to relate to hematopoietic malignancies, particularly lymphoid leukemias, in addition to endocrine cancers.19 Interestingly, human neutrophils isolated from patients with heterozygous germ line mutations in the enzyme SDHB, display enhanced cell survival and increased succinylation.24 However, the functional consequences of SDHB defects for the inflammatory phenotype were not identified, and neither was any causal mechanism found linking SDHB mutations and succinylation of proteins in neutrophils. Mouse models have been used to further understand these processes, but many questions remain. Macrophages from mice lacking SDHB accumulate succinate.25 This strategy together with LPS stimulation leads to decreased pro-inflammatory gene expression along with enhanced expression of a variety of anti-inflammatory soluble mediators, including IL-1 receptor antagonist and IL-10.25 Accordingly, inhibition of SDH with itaconate treatment, a metabolite structurally similar to succinate, abolishes inflammatory response of macrophages reducing myocardial infarct in both in vitro and in vivo models by limiting succinate oxidation.26 Thus, limiting the oxidation of succinate may have an anti-inflammatory effect. The prominent mechanisms mediating these effects seem to relate to mitochondrial metabolic reprogramming and inhibition of reactive oxygen species (ROS) production.25,26 Unfortunately, the potential role of succinylation in these processes has not been studied.
Conversely, macrophages from mice lacking sirtuin 5 become hypersensitive to LPS and display increased expression of IL-1β.27 One important limitation of this strategy is that it does not allow us to rule out possible deacetylation from desuccinylation activity of sirtuin 5. Nevertheless, the Authors elegantly showed a mechanism that at least partially explains these results.27 Succinylation is a modification that may occur to multiple proteins in addition to histones. This is the case of pyruvate kinase M2, whose succinylation at K311 promotes its translocation into the nucleus. Once in the nucleus, pyruvate kinase M2 forms a transcriptional complex with HIF-1α that directly binds to the IL-1β promoter gene and activates its transcription.27 Furthermore, pyruvate kinase M2 hyper-succinylation in sirtuin 5-deficient mice renders the animals more susceptible to experimental colitis through boosted IL-1β production.27
Thus, future study should examine the link between succinylation and inflammatory pathways, its regulation in different blood cell subsets, and its consequences in terms of gene expression and immune cell functions in both healthy and clinical conditions.
Succinate as intercellular communicator
Activation of succinate receptor 1 (SUCNR1), or G protein-coupled receptor 91 (GPR91), was recently recognized as a regulatory mediator on a variety of cell subsets, including cardiomyocytes, adipocytes, renal and blood cells.28–30 Half-maximal effective response for GPR91 is obtained with succinate concentrations of 28–56 μM, indicative of GPR91 activation at succinate concentrations higher than the steady-state levels.30 Thus, succinate-GPR91 axis may function as an early sensor of homeostasis perturbations. In this context, selection of appropriate experimental conditions is essential to prevent biased results. For example, use of carbon dioxide as a method of euthanasia in rodents should be avoided since it rapidly raises blood succinate levels.14
G protein-coupled receptors are typically classified based on the G protein subfamily that they activate, e.g. Gαi, Gαq, Gs or G12/13. Given the enriched expression of GPR91 in kidney, human embryonic kidney cells 293 are often used to examine its mechanisms of signal transduction. However, attempts to characterize downstream pathways have led to controversial results. Some studies show that GPR91 activates both Gαi and Gαq signaling, leading to inhibition of cyclic adenosine monophosphate production and deployment of calcium mobilization, respectively.30 In contrast, others have shown that Gαq is not required for succinate-induced activation of GPR91 and Gαi alone is sufficient to quench cyclic adenosine monophosphate and mobilize calcium.29 Despite these controversies, in vitro studies in kidney cell lines seem to agree that GPR91 signaling is mediated by mobilization of intracellular calcium stores rather than being dependent on influx of extracellular calcium.29 However, stimulation of platelets with succinate failed to induce intracellular calcium mobilization, indicating the potential cell type-specific signaling transduction pathways of GPR91.29 Clues as to the cell-wide plasticity of GPR91 can further be seen from mesenchymal cells in the liver, where GPR91 activation neither increases cytosolic calcium nor inhibits adenosine monophosphate production.31
Furthermore, distinct mechanisms are responsible for GPR91 switch off in a cell-type dependent manner. Unlike human embryonic kidney cells 293, which undergo internalization of GPR91 in presence of succinate,32 Madin-Darby canine kidney cells instead show a temporal desensitization of GPR91 following succinate binding.32 Thus, cell-type specific responses of GPR91 machinery and on/off dynamics may confer great plasticity to this regulatory pathway and may also underlie seemingly controversial results. Therefore, characterizing GPR91 signaling in cells of interest appears to be essential to assess the impact on functional activity and to identify specific drug targets upon pathogenesis.
GPR91 in inflammatory responses
Early observations on blood cells show that GPR91 activation boosts pro-inflammatory responses, but recent investigations have shown that this is not always the case and that the impact of GPR91 depends on the cellular context. For example, GPR91 may indirectly protect mice from low-grade systemic inflammation under high-fat-diet conditions, since GPR91-deficient mice show progressive hyperglycemia and reduced insulin secretion.33 This was related to a function of GPR91 in white adipose tissue, a tissue where GPR91 is highly expressed.33
Monocytes that egress from the bone marrow (BM) seem to lack expression of GPR91, yet the ability to respond to activation and differentiation signals is influenced by extracellular succinate.12 Unlike immature monocytes, both naive dendritic cells and macrophages express high levels of GPR91, and its activation promotes inflammation.12 In vitro, succinate failed to influence TNF-α and IL-1β expression by dendritic cells. However, higher expression of these cytokines is achieved by addition of succinate in combination with TLR-3 and TLR-7 ligands compared to use of TLR agonists alone.12 Macrophages respond to extracellular inflammatory signals like LPS in similar ways (Figure 2A), with GPR91-mediated signal transduction yielding a strong pro-inflammatory phenotype that results in increased production of IL-1β.2,34 Furthermore, LPS-stimulated macrophages release succinate into the cell culture medium.34 This effect seems to be independent of GPR91, as extracellular succinate is found more abundantly in cultures of GPR91 deficient macrophages than in corresponding wild-type (WT) cells.34 The underlying mechanism for this succinate transport still needs to be revealed. In this regard, future studies examining the activity and expression of the SLC13 transporters in blood cells will be of great interest.
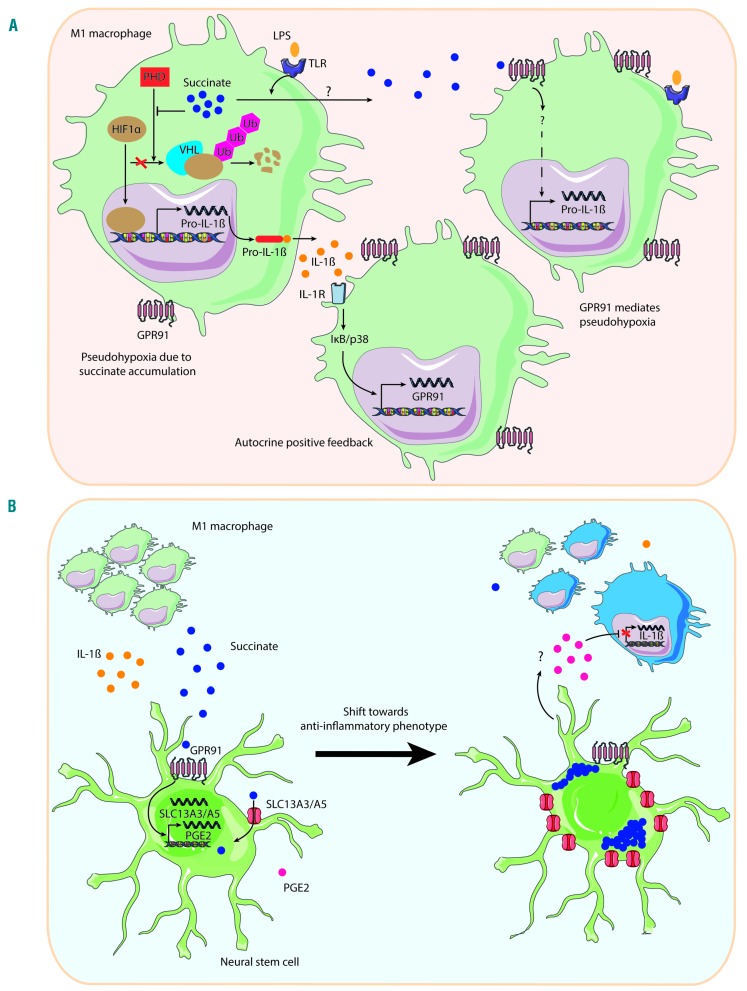
Figure 2.
Dual role for succinate as pro- and anti-inflammatory signal. (A) Succinate as pro-inflammatory signal. Toll-like receptor (TLR) activation in macrophages causes intracellular succinate accumulation and release. In the cytosol, succinate functions as competitive inhibitor for prolyl hydroxylase domain (PHD) proteins, promoting stabilization of hypoxia-inducible factor-1α (HIF-1α), that in turn leads to pro-inflammatory interleukin-1β (IL-1β) production.3 Activation of interleukin-1 receptor (IL-1R) increases G protein-coupled receptor 91 (GPR91) expression, and extracellular succinate interacts with GPR91. This results in pseudohypoxia and IL-1β production, independent of HIF.34 (B) Succinate as anti-inflammatory signal. M1 inflammatory macrophages release succinate, which activates GPR91 on neural stem cells increasing the expression in vitro of prostaglandin E2 (PGE2) and members of the sodium-coupled citrate (SLC) family of transporters 13 (i.e. SLC13A3 and SLC13A5). In vivo, the most prominent anti-inflammation mechanism is succinate scavenging by SLC13 transporters. This reduces IL-1β and succinate extracellular levels, and promotes a shift in macrophage polarity from pro-inflammatory (in green) to anti-inflammatory phenotype (in blue).20 LPS: lipopolysaccharide; VHL: von Hippel-Lindau protein; Ub: ubiquitin.
In addition, GPR91 seems to be required for dendritic cell activation and licensing in vivo. It leads dendritic cell migration to lymph nodes, as this function is impaired in GPR91-deficient mice. However, after maturation, GPR91 expression rapidly declines, making dendritic cells unresponsive to subsequent succinate stimulation after 24 hours of stimulation with LPS or the cytokine cocktail of TNF-α and IL-1β.12 Conversely, activated macrophages become more responsive to succinate stimulation.12
In both human cell lines and monocyte-derived dendritic cells, succinate activates the extracellular signal-regulated kinases Erk1 and Erk2,12,30 which are downstream signaling components of TLR pathways. In macrophages, HIF-1α is one key mediator of their response to succinate.3,25 Accumulation of succinate in the cytosol results in competitive inhibition of PHD enzymes and subsequent stabilization of HIF-1α, even in the presence of normal oxygen levels (Figure 2A).3,6 In turn, HIF-1α activation contributes to IL-1β expression.3,25 Later, succinate oxidation in mitochondria proved central to macrophage pro-inflammatory phenotype, given that competitive inhibition of SDH abrogates IL-1β and HIF-1α expression.25 In accordance with the IL-1β findings previously described, HIF-1α stability is similar in macrophages derived from both GPR91-deficient and WT mice in presence of extracellular succinate alone, whereas GPR91 promotes pseudohypoxia upon LPS stimulation (Figure 2A).34 Recently, this mechanism has been related to pathogenesis in an experimental model of antigen-induced arthritis, where GPR91-deficient mice display reduced macrophage activation and IL-1β production.34
Surprisingly, the inflammatory impact of GPR91 deficiency varies in different myeloid cell subsets. Animal models of allergic contact dermatitis revealed increased reactions in the absence of GPR91, and this was related to hyperactive mast cells in GPR91-deficient mice by means of increased TNF-α expression.35 This effect seems to be dependent on defective mast cell differentiation from BM precursors, as augmented mast cell responses could be recapitulated in WT mast cells differentiated in vitro in the absence of succinate.35 Unfortunately, no further efforts were made to explore the mechanisms involved.
Intriguingly, GPR91 may induce anti-inflammatory effects through non-cell-autonomous effects (Figure 2B). In an in vivo model of autoimmune encephalomyelitis, succinate produced by type I mononuclear phagocytes accumulates in the cerebrospinal fluid of the chronically inflamed central nervous system.20 Transplanted neural stem cells sense this extracellular succinate through GPR91. In vitro, GPR91 activation leads neural stem cells to secretion of prostaglandin E2, and upregulation of members of the SLC13 family of transporters (i.e. SLC13A3 and SLC13A5) that uptake and scavenge extracellular succinate. In vivo, succinate scavenging seems to be the main mediator of the anti-inflammatory effects of transplanted neural stem cells. As a result, the beneficial effects of this strategy include reduction of succinate levels in the cerebrospinal fluid, shifting from type 1 inflammatory mononuclear phagocytes to anti-inflammatory cells, reduced post mortem tissue pathology, and improvement of behavioral defects.20 However, GPR91 is essential for these effects in vivo, as GPR91-deficient neural stem cells show reduced ability to protect from chronic neuroinflammation upon transplantation.20 It remains to be seen how broadly applicable this succinate-mediated anti-inflammatory mechanism will be to additional regulatory cells and inflammatory disease systems. However, it is interesting to hypothesize that succinate may play dual roles in inflammation both as an early driver, through cell-autonomous mechanisms, and as a late terminator by non-cell autonomous processes. Future studies should clarify the validity of this exciting hypothesis.
In addition, given the contribution of GPR91 to tipping the inflammatory balance, studies are required to better understand how its modulation may provide a useful clinical tool for the control of inflammatory responses and maintenance of immune homeostasis. In this regard, recent development of GPR91-specific agonists, up to 20-fold more powerful compared to succinate,36 and antagonists,37 opens new avenues to explore the promising therapeutic value of GPR91 control with any accuracy.
New insights into hematopoiesis: what role does succinate play?
Early investigations into hematopoiesis have provided hints of a role for succinate signaling in blood stem cell function, i.e. blood formation or hematopoiesis. GPR91 is expressed in monocyte-derived cell subsets, as previously discussed, while it is absent in immature monocytes, and T cells and B cells,38 indicative of selective roles for GPR91 on different blood cell subsets. In contrast, others have found expression of GPR91 transcript in purified populations of blood CD14+ monocytes and platelets, whereas its absence was confirmed in CD4+ T cells, CD8+ T cells, CD16+ granulocytes and CD19+ B cells.39 Unexpectedly, western blot analysis of protein expression showed presence of GPR91 in all blood cells except granulocytes.39 Interestingly, human BM CD34+ progenitor cells have been reported to express GPR91 on their cell surface,38 suggesting a potential role for succinate signaling in hematopoietic stem cells (HSC). At present, the lack of commercially available mouse GPR91-specific antibody makes it difficult to further explore the significance of succinate-GPR91 signaling pathway in the biology of HSC and progenitor cells. In this regard, generation of reporter mouse lines will be of great value in the hematopoietic field.
In vitro, succinate stimulates the proliferation capacity of erythroid and megakaryocyte progenitors, and cell proliferation is significantly hampered in cells transfected with small interfering ribonucleic acid targeting GPR91.38 However, when megakaryocytes and erythroblasts are differentiated in vitro from human cord blood CD34+ cells, megakaryocytes show 7.2-fold higher GPR91 transcript expression as detected by cDNA microarrays.39 Of note, differentiation of CD34+ progenitors into megakaryocytes is performed in the presence of both thrombopoietin and IL-1β.39 As previously discussed, GPR91 signaling seems to be required for correct mast cell differentiation.35 GPR91-deficient mice show hypomorphic mast cells that display increased responses, and this can be recapitulated in vitro using WT BM cells differentiated in the absence of succinate.35
Following chemotherapy, succinate treatment in vivo stimulates multilineage blood cell recovery, including red blood cells, platelets and neutrophils.38 In addition, inducible deletion of the mitochondrial SDHD in mouse models leads to remarkable hematopoietic defects, including depletion of BM progenitors and differentiated cells, and apoptosis in the HSC compartment defined as LSK, i.e. lineage-negative, c-kit-positive, and sca-1-positive.40 However, the consequences of this strategy on succinate levels in LSK cells and their link to cell functional changes has not been studied. Furthermore, an inducible system was used to conditionally delete SDHD controlled in a temporal manner, using Cre mediated recombination that activates upon estrogen analog tamoxifen administration. However, this genetic approach does not allow the cell subset where the deletion occurs to be delimited, either within or outside the hematopoietic system. Given the key role of regulatory cells within the healthy HSC niche, that tightly controls the function, fate and numbers of HSC in the BM,41 it may well be that these effects are partially mediated by the stem cell niche. Future studies will be needed to validate this exciting hypothesis.
Interestingly, succinate is increased 24-fold in BM stromal cells derived from type 2 diabetes mellitus mice compared to normoglycemic mice.42 In this study, adherent cells were used after flushing out BM cells and culture of the adherent fraction for one week in vitro. Thus, levels of metabolites in this study may differ from those found in primary BM stromal cells in vivo. Furthermore, these adherent cells may consist of heterogeneous populations of stromal cells. Nevertheless, the Authors reveal an interesting mechanism that may contribute to bone dysregulation in metabolic disorders. In vitro, extracellular succinate binds to GPR91 on osteoclastic lineage cells and stimulates osteoclast differentiation; a process that may contribute to the bone resorption seen in vivo.42 Succinate may then have an indirect effect on hematopoiesis in diabetes, as osteoclasts function as negative regulators of HSC43 whereas osteoblasts support lymphoid progenitors.44,45 Future studies will be required to test these ideas.
Furthermore, HIF-1α contributes importantly to cell- cycle regulation in HSC,46 so identification of the potential link to succinate is of high relevance. Actually, HSC reside in hypoxic BM niches, which maintain their long-term self-renewal by mechanisms like limiting their production of ROS.47 This is performed through adaptation of their metabolism to maintain a high glycolysis rate by HIF-1α activation. These conditions change during the processes of proliferation and differentiation, with activated cells depending more on oxidative phosphorylation to meet the energy requirements.47 Besides this, HSC niches are closely related to the vasculature in the BM and have been defined as perivascular, with mainly endothelial cells and mesenchymal stromal cells secreting factors that promote HSC maintenance.48 In this regard, GPR91 has been suggested to link capillary function to metabolic needs in other tissues, like retina, where GPR91 is essential to establish a neovascular network in response to injury through production of numerous angiogenic factors including vascular endothelial growth factor by retinal ganglion neurons.49 Considering that SDH mutations, HIF-1α accumulation and elevated levels of circulating succinate relate to pathology, particularly in hematologic malignancies,6,7,16–19 characterization of succinate signaling network, both cell- and non-cell-autonomous, is pivotal to understand the link between metabolic alterations that affect HSC function and hematologic malignancies.
Conclusions
Succinate is an essential intermediate of the TCA cycle at the intersection among metabolism, gene expression and intercellular communication. Initially characterized as an inflammatory signal, recent data show succinate-mediated anti-inflammatory mechanisms depending on the cellular subsets. The great plasticity of succinate biology is exemplified by cell-type specific responses of GPR91 machinery and on/off dynamics that may contribute to seemingly controversial results. In this scenario, it is interesting to hypothesize that succinate may play dual roles both as early driver of inflammation, through cell-autonomous processes, and as late terminator by intercellular communication with regulatory cells. Future studies are required to demonstrate this idea. Furthermore, we are only starting to understand how succinylation may influence protein activation and epigenetic landscape, and thereby gene expression and cell function. Studies on both succinylation regulation and impact will be highly valuable for a complete picture of succinate biology in blood cells. Finally, early hints on hematopoiesis seem to involve succinate in the survival and differentiation of blood stem cells, through mechanisms that remain unclear. Future research should carefully explore the promising therapeutic value of succinate targeting in inflammatory diseases, including hematologic malignancies.50
Supplementary Material
Acknowledgments
Our work is supported by a joint meeting grant of the Northern Norway Regional Health Authority, the University Hospital of Northern Norway (UNN) and UiT The Arctic University of Norway (UiT) (2014/5668), Young Research Talent grants from the Research Council of Norway, (Stem Cell Program, 247596; FRIPRO Program, 250901), and grants from the Norwegian Cancer Society (6765150), the Northern Norway Regional Health Authority (HNF1338-17), and the Aakre-Stiftelsen Foundation (2016/9050) to LA.
Footnotes
Check the online version for the most updated information on this article, online supplements, and information on authorship & disclosures: www.haematologica.org/content/103/10/1586
References
- 1.Wise DR, Ward PS, Shay JES, et al. Hypoxia promotes isocitrate dehydrogenase-dependent carboxylation of -ketoglutarate to citrate to support cell growth and viability. Proc Natl Acad Sci USA. 2011; 10(49):19611–19616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kelly B, O’Neill LA. Metabolic reprogramming in macrophages and dendritic cells in innate immunity. Cell Res. 2015; 25(7):771–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tannahill GM, Curtis AM, Adamik J, et al. Succinate is an inflammatory signal that induces IL-1beta through HIF-1alpha. Nature. 2013; 496(7444):238–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Epstein AC, Gleadle JM, McNeill LA, et al. C. elegans EGL-9 and mammalian homologs define a family of dioxygenases that regulate HIF by prolyl hydroxylation. Cell. 2001; 107(1):43–54. [DOI] [PubMed] [Google Scholar]
- 5.Jaakkola P, Mole DR, Tian YM, et al. Targeting of HIF-alpha to the von Hippel-Lindau ubiquitylation complex by O2-regulated prolyl hydroxylation. Science. 2001; 292(5516):468–472. [DOI] [PubMed] [Google Scholar]
- 6.Selak MA, Armour SM, MacKenzie ED, et al. Succinate links TCA cycle dysfunction to oncogenesis by inhibiting HIF-alpha prolyl hydroxylase. Cancer Cell. 2005;7(1):77–85. [DOI] [PubMed] [Google Scholar]
- 7.Morin A, Letouze E, Gimenez-Roqueplo AP, Favier J. Oncometabolites-driven tumorigenesis: From genetics to targeted therapy. Int J Cancer. 2014; 135(10):2237–2248. [DOI] [PubMed] [Google Scholar]
- 8.Xie Z, Dai J, Dai L, et al. Lysine Succinylation and Lysine Malonylation in Histones. Mol Cell Proteomics. 2012; 11(5): 100–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Park J, Chen Y, Tishkoff DX, et al. SIRT5-mediated lysine desuccinylation impacts diverse metabolic pathways. Mol Cell. 2013; 50(6):919–930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang ZH, Tan MJ, Xie ZY, Dai LZ, Chen Y, Zhao YM. Identification of lysine succinylation as a new post-translational modification. Nat Chem Biol. 2011; 7(1):58–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Willmes DM, Birkenfeld AL. The Role of INDY in Metabolic Regulation. Comput Struct Biotechnol J. 2013; 6:e201303020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rubic T, Lametschwandtner G, Jost S, et al. Triggering the succinate receptor GPR91 on dendritic cells enhances immunity. Nat Immunol. 2008; 9(11):1261–1269. [DOI] [PubMed] [Google Scholar]
- 13.Kushnir MM, Komaromy-Hiller G, Shushan B, Urry FM, Roberts WL. Analysis of dicarboxylic acids by tandem mass spectrometry. High-throughput quantitative measurement of methylmalonic acid in serum, plasma, and urine. Clin Chem. 2001;47(11):1993–2002. [PubMed] [Google Scholar]
- 14.Sadagopan N, Li W, Roberds SL, et al. Circulating succinate is elevated in rodent models of hypertension and metabolic disease. Am J Hypertens. 2007; 20(11):1209–1215. [DOI] [PubMed] [Google Scholar]
- 15.Hochachka PW, Dressendorfer RH. Succinate accumulation in man during exercise. Eur J Appl Physiol Occup Physiol. 1976;35(4):235–242. [DOI] [PubMed] [Google Scholar]
- 16.van Diepen JA, Robben JH, Hooiveld GJ, et al. SUCNR1-mediated chemotaxis of macrophages aggravates obesity-induced inflammation and diabetes. Diabetologia. 2017; 60(7):1304–1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Serena C, Ceperuelo-Mallafré V, Keiran N, et al. Elevated circulating levels of succinate in human obesity are linked to specific gut microbiota. ISME J. 2018; 12(7):1642–1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kohlhauer M, Dawkins S, Costa ASH, et al. Metabolomic Profiling in Acute ST-Segment-Elevation Myocardial Infarction Identifies Succinate as an Early Marker of Human Ischemia-Reperfusion Injury. J Am Heart Assoc. 2018; 7(8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Renella R, Carnevale J, Schneider KA, Hornick JL, Rana HQ, Janeway KA. Exploring the association of succinate dehydrogenase complex mutations with lymphoid malignancies. Fam Cancer. 2014; 13(3):507–511. [DOI] [PubMed] [Google Scholar]
- 20.Peruzzotti-Jametti L, Bernstock JD, Vicario N, et al. Macrophage-Derived Extracellular Succinate Licenses Neural Stem Cells to Suppress Chronic Neuroinflammation. Cell Stem Cell. 2018; 22(3):355–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cervera AM, Bayley JP, Devilee P, McCreath KJ. Inhibition of succinate dehydrogenase dysregulates histone modification in mammalian cells. Mol Cancer. 2009; 8:89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xiao M, Yang H, Xu W, et al. Inhibition of alpha-KG-dependent histone and DNA demethylases by fumarate and succinate that are accumulated in mutations of FH and SDH tumor suppressors. Genes Dev. 2012; 26(12):1326–1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Du J, Zhou Y, Su X, et al. Sirt5 is a NAD-dependent protein lysine demalonylase and desuccinylase. Science. 2011; 334(6057):806–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jones R, McDonald KE, Willson JA, et al. Mutations in succinate dehydrogenase B (SDHB) enhance neutrophil survival independent of HIF-1alpha expression. Blood. 2016; 127(21):2641–2644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mills EL, Kelly B, Logan A, et al. Succinate Dehydrogenase Supports Metabolic Repurposing of Mitochondria to Drive Inflammatory Macrophages. Cell. 2016; 167(2):457–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lampropoulou V, Sergushichev A, Bambouskova M, et al. Itaconate Links Inhibition of Succinate Dehydrogenase with Macrophage Metabolic Remodeling and Regulation of Inflammation. Cell Metab. 2016; 24(1):158–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang F, Wang K, Xu W, et al. SIRT5 Desuccinylates and Activates Pyruvate Kinase M2 to Block Macrophage IL-1beta Production and to Prevent DSS-Induced Colitis in Mice. Cell Rep. 2017; 19(11):2331–2344. [DOI] [PubMed] [Google Scholar]
- 28.Hogberg C, Gidlof O, Tan C, et al. Succinate independently stimulates full platelet activation via cAMP and phosphoinositide 3-kinase-beta signaling. J Thromb Haemost. 2011; 9(2):361–372. [DOI] [PubMed] [Google Scholar]
- 29.Sundstrom L, Greasley PJ, Engberg S, Wallander M, Ryberg E. Succinate receptor GPR91, a Galpha(i) coupled receptor that increases intracellular calcium concentrations through PLCbeta. FEBS Lett. 2013;587(15):2399–2404. [DOI] [PubMed] [Google Scholar]
- 30.He W, Miao FJ, Lin DC, et al. Citric acid cycle intermediates as ligands for orphan G-protein-coupled receptors. Nature. 2004;429 (6988):188–193. [DOI] [PubMed] [Google Scholar]
- 31.Correa PR, Kruglov EA, Thompson M, Leite MF, Dranoff JA, Nathanson MH. Succinate is a paracrine signal for liver damage. J Hepatol. 2007; 47(2):262–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Robben JH, Fenton RA, Vargas SL, et al. Localization of the succinate receptor in the distal nephron and its signaling in polarized MDCK cells. Kidney Int. 2009; 76(12):1258–1267. [DOI] [PubMed] [Google Scholar]
- 33.McCreath KJ, Espada S, Gálvez BG, et al. Targeted disruption of the SUCNR1 metabolic receptor leads to dichotomous effects on obesity. Diabetes. 2015; 64(4):1154–1167. [DOI] [PubMed] [Google Scholar]
- 34.Littlewood-Evans A, Sarret S, Apfel V, et al. GPR91 senses extracellular succinate released from inflammatory macrophages and exacerbates rheumatoid arthritis. J Exp Med. 2016; 213(9):1655–1662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rubi -Schneider T, Carballido-Perrig N, Regairaz C, et al. GPR91 deficiency exacerbates allergic contact dermatitis while reducing arthritic disease in mice. Allergy. 2017; 72(3):444–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Geubelle P, Gilissen J, Dilly S, et al. Identification and pharmacological characterization of succinate receptor agonists. Br J Pharmacol. 2017; 174(9):796–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bhuniya D, Umrani D, Dave B, et al. Discovery of a potent and selective small molecule hGPR91 antagonist. Bioorg Med Chem Lett. 2011; 21(12):3596–3602. [DOI] [PubMed] [Google Scholar]
- 38.Hakak Y, Lehmann-Bruinsma K, Phillips S, et al. The role of the GPR91 ligand succinate in hematopoiesis. J Leukoc Biol. 2009;85 (5):837–843. [DOI] [PubMed] [Google Scholar]
- 39.Macaulay IC, Tijssen MR, Thijssen-Timmer DC, et al. Comparative gene expression profiling of in vitro differentiated megakaryocytes and erythroblasts identifies novel activatory and inhibitory platelet membrane proteins. Blood. 2007; 109(8):3260–3269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bejarano-Garcia JA, Millan-Ucles A, Rosado IV, et al. Sensitivity of hematopoietic stem cells to mitochondrial dysfunction by SdhD gene deletion. Cell Death Dis. 2016; 7(12):e2516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Morrison SJ, Scadden DT. The bone marrow niche for haematopoietic stem cells. Nature. 2014; 505(7483):327–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Guo Y, Xie C, Li X, et al. Succinate and its G-protein-coupled receptor stimulates osteo-clastogenesis. Nat Commun. 2017; 8:15621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Miyamoto K, Yoshida S, Kawasumi M, et al. Osteoclasts are dispensable for hematopoietic stem cell maintenance and mobilization. J Exp Med. 2011; 208(11):2175–2181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Visnjic D, Kalajzic Z, Rowe DW, Katavic V, Lorenzo J, Aguila HL. Hematopoiesis is severely altered in mice with an induced osteoblast deficiency. Blood. 2004; 103(9): 3258–3264. [DOI] [PubMed] [Google Scholar]
- 45.Zhu X, Gui J, Dohkan J, Cheng L, Barnes PF, Su DM. Lymphohematopoietic progenitors do not have a synchronized defect with age-related thymic involution. Aging Cell. 2007; 6(5):663–672. [DOI] [PubMed] [Google Scholar]
- 46.Takubo K, Goda N, Yamada W, et al. Regulation of the HIF-1 alpha Level Is Essential for Hematopoietic Stem Cells. Cell Stem Cell. 2010; 7(3):391–402. [DOI] [PubMed] [Google Scholar]
- 47.Suda T, Takubo K, Semenza GL. Metabolic regulation of hematopoietic stem cells in the hypoxic niche. Cell Stem Cell. 2011; 9(4): 298–310. [DOI] [PubMed] [Google Scholar]
- 48.Crane GM, Jeffery E, Morrison SJ. Adult haematopoietic stem cell niches. Nat Rev Immunol. 2017; 17(9):573–590. [DOI] [PubMed] [Google Scholar]
- 49.Sapieha P, Sirinyan M, Hamel D, Zaniolo K, Joyal JS, Cho JH. The succinate receptor GPR91 in neurons has a major role in retinal angiogenesis. Nat Med. 2008; 14(10):1067–1076. [DOI] [PubMed] [Google Scholar]
- 50.Arranz L, Arriero MDM, Villatoro A. Interleukin-1beta as emerging therapeutic target in hematological malignancies and potentially in their complications. Blood Rev. 2017; 31(5):306–317. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.