Abstract
The feasibility of ex vivo expansion allows us to consider the steady-state peripheral blood as an alternative source of hematopoietic stem progenitor cells for transplantation when growth factor-induced cell mobilization is contraindicated or inapplicable. Ex vivo expansion dramatically enhances the in vivo reconstituting cell population from steady-state blood. In order to investigate phenotype and the expression of homing molecules, the expression of CD34, CD133, CD90, CD45RA, CD26 and CD9 was determined on sorted CD34+ cells according to CXCR4 (“neg”, “low” “bright”) and CD133 expression before and after ex vivo expansion. Hematopoietic stem cell activity was determined in vivo on the basis of hematopoietic repopulation of primary and secondary recipients - NSG immuno-deficient mice. In vivo reconstituting cells in the steady-state blood CD34+ cell fraction before expansion belong to the CD133+ population and are CXCR4low or, to a lesser extent, CXCR4neg, while after ex vivo expansion they are contained only in the CD133+CXCR4low cells. The failure of the CXCR4bright population to engraft is probably due to the exclusive expression of CD26 by these cells. The limiting-dilution analysis showed that both repopulating cell number and individual proliferative capacity were enhanced by ex vivo expansion. Thus, steady-state peripheral blood cells exhibit a different phenotype compared to mobilized and cord blood cells, as well as to those issued from the bone marrow. These data represent the first phenotypic characterization of steady-state blood cells exhibiting short- and long-term hematopoietic reconstituting potential, which can be expanded ex vivo, a sine qua non for their subsequent use for transplantation.
Introduction
The introduction into clinical practice of “mobilization” from the bone marrow to peripheral blood, was an approach that resulted in an impressive increase of the number of hematopoietic progenitor cells (HPCs) and hematopoietic stem cells (HSCs) available for collection by cytapheresis. As such, this approach represented a revolutionary event in hematopoietic transplantation1 and, as a result, strategies involving steady-state peripheral blood (SS-PB)2 were abandoned. However, the procedure of mobilization of HPCs and HSCs, as well as their collection from the bone marrow, are not without risks.3 Such risks can also effectively pose a deterrent to the recruitment of voluntary donors. Besides, mobilization is contraindicated in some cases, leading to the exclusion of the potential donors. Thus, avoiding mobilization or bone marrow collection would be of great interest, especially in the context of allogeneic transplantation.
Ex vivo expansion procedures have evolved over the last few years and it is now possible to amplify committed HPCs to a great extent without losing the long-term reconstituting HSCs.4,5 Recently, we demonstrated the presence of both short- and long-term reconstituting HSCs in human SS-PB and also observed that the activity of these cells increases dramatically after ex vivo expansion.6,7 In this manner, we can safely source substantial numbers of SS-PB HPCs and HSCs, thus overcoming major obstacles to subsequent transplantation. In the light of this, SS-PB HPCs and HSCs should be reconsidered in the context of hematopoietic transplantation.
Based on previous literature regarding HSC activity,6 it was not possible to specify whether the increase in activity of HSCs capable of reconstituting in vivo hematopoiesis of severe combined immune-deficient mice (SCID) repopulating cells (SRCs) after ex vivo expansion is: (i) due to amplification of these cells during ex vivo culture; or (ii) corresponds to pre-existing SRCs before ex vivo expansion (at time 0), which during expansion (until day 7), gained the ability to engraft after transplantation; or (iii) a combination of the above.
In order to address this issue, we investigated both HSC functional capacity in in vivo assays and the expression of membrane markers known to be associated with cell adhesion and homing, such as CD9, CD26, CD49d, CD49e, CD49f and especially CXCR4, as well as markers enabling the enrichment of HSCs (CD133, CD90, CD45RA). The choice of the tetraspanin CD9 was based on the fact that it is regulated by the activity of stromal cell-derived factor-1 (SDF-1; the ligand of CXCR4 receptor)8 and CD26, since it is known to be an inhibitor of activity of the SDF-1/CXCR4 couple,9 which plays an essential role in HSC mobilization and homing.10–12 CD49d (VLA4), CD49e (VLA5), and CD49f (VLA6) are adhesion molecules of the integrin family associated with the anchorage and adhesion of cells in different situations and are considered essential for HSC homing.13 Furthermore CD49f, CD45RA and CD90 are used as markers of cord blood (CB) and/or bone marrow (BM) HSCs.14,15 Despite the fact that it largely overlaps with CD34, CD133 was chosen since it is not expressed on some subpopulations of committed progenitors and, hence, is more likely to include the HSCs.16–18
We found that HSC activity increases due to both amplification in their number and to enhancement of their individual proliferative capacity. Furthermore, in vivo reconstituting cells (both short- and long-term reconstituting cells i.e. ST-HSCs and LT-HSCs, respectively) in the fresh SS-PB CD34+ cell population belong to the subpopulation of CD133+ cells which are either CXCR4low or CXCR4neg, while after ex vivo expansion they are present only in the CD133+CXCR4low population.
Methods
Human steady-state peripheral blood cells
Leukocytes were recovered from leukodepletion filters (T2975, Fresenius Kabi, Louviers, France) by counterflow elution as described elsewhere6,19,20 with a slight modification, i.e. the cells were flushed directly into 50 mL tubes (Falcon, Dutscher, Brumath, France) (see Online Supplementary Methods).
Isolation and cryopreservation of CD34+ cells
CD34+ cells were isolated from the mononuclear cell fraction using Miltenyi’s (Miltenyi Biotec, Paris, France) “indirect” immunomagnetic technique19 (“LS” columns; Vario Macs Device). The CD34+ cell purity was 85–90% and the yield was 3–5×105 CD34+ cells per leukodepletion filter.
For each sample, 20 to 24 leukodepletion filters were processed and CD34+ cells were pooled before cryopreservation (4% human serum albumin solution, 10% dimethylsulfoxide; Wak-Chemie, Steinbach, France).21 Samples were thawed in cold 4% human serum albumin and washed in selection buffer. After thawing, the CD34+ cell purity was 90–95%.
Ex vivo expansion of CD34+ cells recovered from leukodepletion filters
All tests were performed on CD34+ cells after thawing, before expansion (day 0) and after expansion (day 7). Day-0 CD34+ cells were seeded at 2×104 cells/mL, and cultured in 75 cm2 flasks (NUNC, Roskilde, Denmark) for 7 days in liquid (clinical-grade serum-free medium Macopharma HP01) cultures supplemented with granulocyte colony-stimulating factor 100 ng/mL (Neuropen, Amgen SAS, Neuilly-sur-Seine, France), stem cell factor 100 ng/mL, thrombopoietin 20 ng/mL and interleukin-3 0.5 ng/mL (all from Peproteck, Rocky Hill, NJ, USA) (see Online Supplementary Methods).
CD34+ cell detection, immunophenotypic analysis and selection of cell subfractions
The CD34+ cell concentrations/purities were determined as previously described.19,22 Fluorescent monoclonal antibodies were used to analyze/isolate CXCR4neg, CXCR4low, CXCR4bright subfractions, and CXCR4negCD133−, CXCR4negCD133+, CXCR4lowCD133−, CXCR4lowCD133+, CXCR4brightCD133−, and CXCR4brightCD133+ subfractions. The details are provided in the Online Supplementary Methods.
Detection of stem cells by their in vivo repopulating capacity
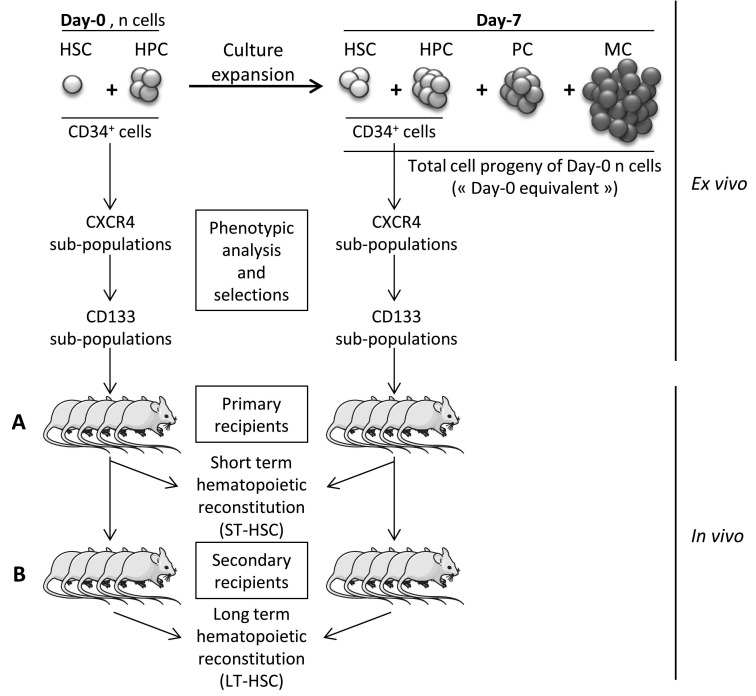
The only way to evaluate the activity of HSCs properly is to test their in vivo capacity of hematopoietic reconstitution.23 Hence, we employed the most widely used assay based on the repopulation, by human cells, of hematopoietic tissues of immune-deficient mice, thereby evaluating the cells usually called SRCs (Figure 1). This approach enables the detection of two SRC populations:
-
Short-term HSCs (ST-HSCs). ST-HSC activity was evaluated in vivo, following transplantation of different phenotypically defined fractions of human SS-PB CD34+ cells in immunodeficient [NOD/SCID/gamma-null (NSG)] mice. As described previously,24 the animal experiments were performed in compliance with French regulations (license n. 3306002) and with the approval of the Ethics Committee (n. 50120213-A). Either 1×105 CD34+ cells or 1×105 cells of sorted subfractions at day 0 were injected per mouse. After expansion, 2×105 of total day-7 cells or 2×105 cells of sorted subfractions were transplanted per mouse. In some experiments, the post-culture (day-7) equivalent of a defined number of day-0 cells i.e. the total day-7 progeny of a defined day-0 cell number, was injected per mouse (Figure 1). For all experiments, 10- to 12-week old female NSG mice (central animal-keeping facility of Bordeaux University) were conditioned by means of intra-peritoneal injections of 25 mg/kg busulfan (Busilvex, Pierre Fabre, Boulogne, France),25,26 After 8 weeks, the animals were sacrificed and their femoral mononuclear BM cells isolated and analyzed for human CD45, CD19 and CD33 (with anti-human antibodies coupled with, respectively, fluorescein isothiocyanate, phycoerythrin and allophycocyanin; BD Biosciences, Le Pont de Claix, France) by flow-cytometry (FACS Canto II; BD Biosciences, Le Pont de Claix, France). To avoid false- positive results due to control isotype, we used the non-injected mice to establish the “positivity threshold” for CD45, which was 0.1%.24,27 Furthermore, to avoid inhibition of CXCR4 activity due to fixation of clone 12G5 antibody on the same external loop as SDF-1α (CXCL12, CXCR4 specific ligand),28 we performed antibody elution by acid solution. For this, cells were incubated 20 min at 0°C in ACDA, pH 5 (Anticoagulant Citrate Dextrose solution formula A, Bioluz, Saint-Jean-de-Luz, France), then washed twice in RPMI medium before injection into mice. All cell suspensions were treated in an identical manner before injection.
To determine the SRC frequency, limiting dilution analysis29,30 was performed for the CD34+CXCR4lowCD133+ subpopulation. The details are given in the Online Supplementary Methods.
Long-term HSCs (LT-HSCs). For the detection of LT-HSCs, secondary recipient mice (Figure 1) were conditioned as primary recipients. The BM from both femora of primary recipients was flushed, resuspended and injected intrafemorally6,25 into the secondary recipient NSG mice (Figure 1), as described in detail in the Online Supplementary Methods. The mice were sacrificed 7 or 8 weeks later and analyzed as described above.
Figure 1.
Experimental design. Evaluation of human stem cells by employing a severe combined immunodeficiency repopulating cell assay before (day 0) and after (day 7) ex vivo expansion culture. (A) Human cell chimerism in primary mice recipients reflects the activity of short-term hematopoietic stem cells (ST-HSC) while (B) human cell chimerism in secondary mice recipients reflects the activity of long-term hematopoietic stem cells (LT-HSC). HPC: hematopoietic progenitor cell; PC: precursor cell; MC: mature cell.
Detection of colony-forming committed progenitors
Thawed CD34+ cells were selected for their CXCR4 and CD133 expression and sorted as CXCR4negCD133−, CXCR4negCD133+, CXCR4lowCD133− and CXCR4lowCD133+ subpopulations. Day-0 sorted subfractions were expanded separately ex vivo for 7 days. Day-0 and day-7 subpopulations were plated in methylcellulose cytokine-supplemented kits “Stemα-1D” (Saint Clement les Places, France) (1000 cells/mL for each cell population) and cul tured for 14 days (37°C, 20% O2, 5% CO2) in 35 mm Petri dishes (NUNC, Roskilde, Denmark) in duplicate. The colonies (>50 cells) were scored19 as burst-forming unit - erythroid (BFU-E), colony-forming unit - granulocyte and macrophage (CFU-GM) and multi-lineage colony-forming unit (CFU-mix).
Statistical analysis
The Mann-Whitney test for non-parametric values was applied. P values <0.05 were defined as statistically significant (*). P<0.01 (**) and P<0.001 (***) were highly significant values.
Results
Hematopoietic stem cells with short-term reconstituting capacity
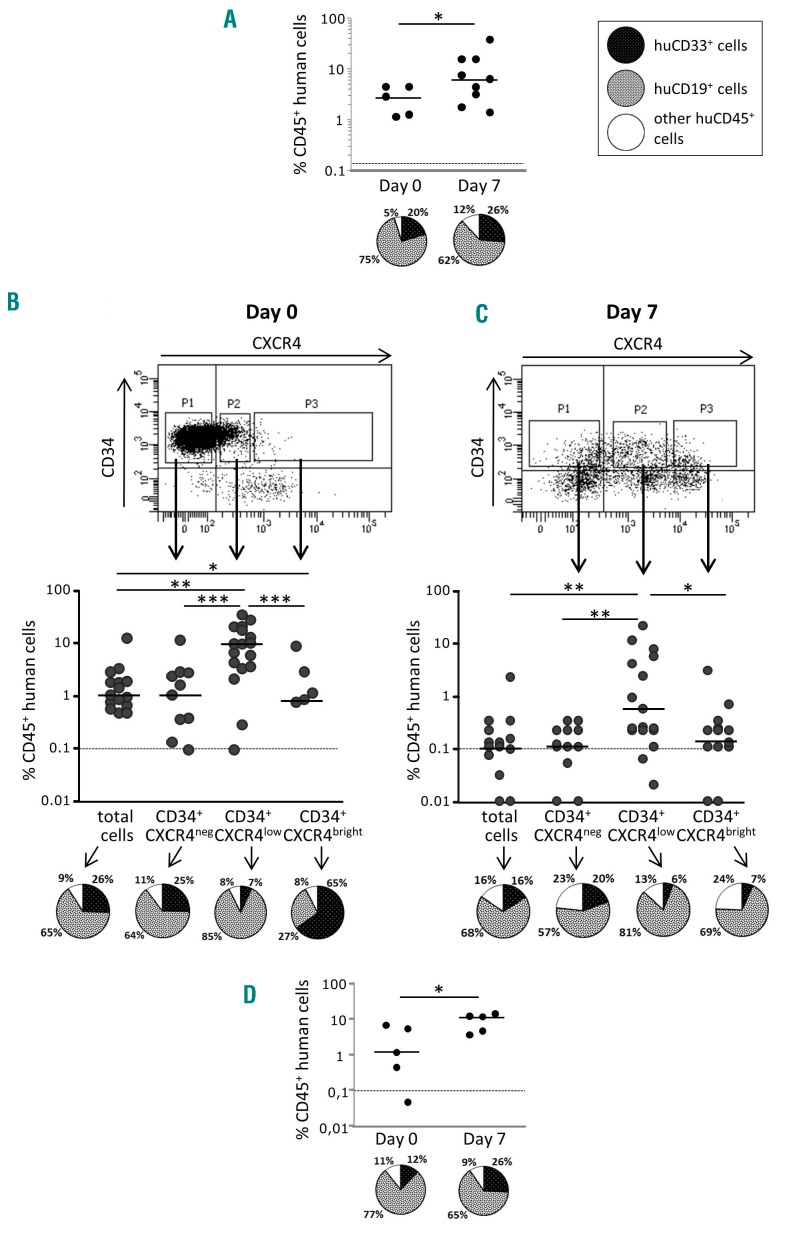
To estimate ST-HSC activity directly before and after ex vivo expansion, the mice were injected with 2×105 day-0 SS-PB CD34+ cells or with their total day-7 progeny, hereafter referred to as “day-0 equivalent”. These results from the NSG mice confirmed our previous findings obtained with NOD/SCID mice6 demonstrating that 7 days of culture greatly enhanced SRC activity (P<0.05) while it also maintained the differentiation potential, as judged on the basis of the proportion of lympho (CD19)-myeloid (CD33) chimerism (Figure 2A).
Figure 2.
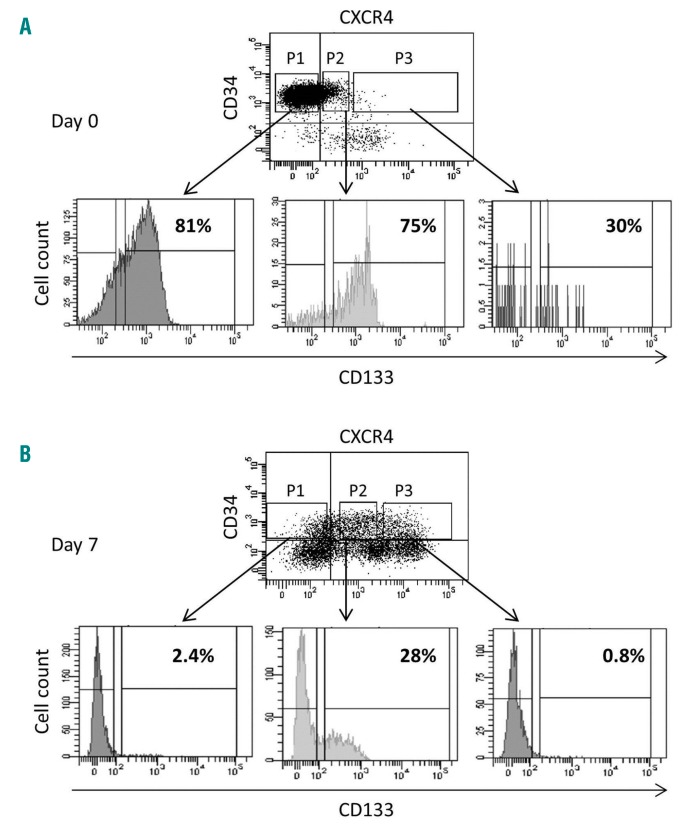
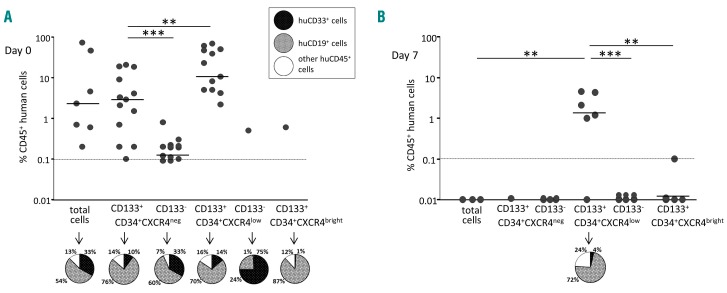
CXCR4 expression is related to the engraftment capacity of hematopoietic stem cells contained in the steady-state peripheral blood CD34+ cell population before and after ex vivo expansion. (A) The activity of severe combined immunodeficiency repopulating cells (SRCs) in CD34+ steady-state peripheral blood (SS-PB) cells is enhanced by 7 days of culture. Day 0: 2×105 SS-PB CD34+ cells were injected per mouse. Day 7: the total progeny of 2×105 day-0 SS-PB CD34+ cells was injected per mouse. (B-C) After culture day-0 (B) and day-7 (C) culture, three cell subpopulations were selected for injection into the recipient mice: P1, cells defined and sorted as the CD34+CXCR4neg subpopulation; P2, cells defined and sorted as the CD34+CXCR4low subpopulation; P3, cells defined and sorted as the CD34+CXCR4bright subpopulation. Day 0: 1×105 SS-PB total CD34+ cells or 1×105 cells from each sorted cell subpopulation were injected per mouse. Day 7: 2×105 of the total expanded cell population or 2×105 cells from each sorted cell subpopulation were injected per mouse. (D) Effect of ex vivo expansion on SRC activity in SS-PB CD34+CXCR4low cells: Day 0: 1×105 SS-PB CD34+CXCR4low cells were injected per mouse; day 7: the total progeny of 1×105 day-0 SS-PB CD34+CXCR4low cells were injected per mouse. (A-D) SRC activity was evaluated by short-term reconstitution (8 weeks) in NSG mice; each point represents the percentage of CD45+ human cells in one mouse bone marrow. For each condition (A-D) the “pie” graphs show the relative proportion of CD19+ and CD33+ cells of human origin within the huCD45+ population. Statistical significance: *P<0.05; **P<0.01; ***P<0.001.
Regarding the SS-PB CD34+ population, the most prominent changes in culture were related to the expression of CXCR4 between day 0 (~16% cells expressing CXCR4) and the end stage of the ex vivo culture (day 7) (~67% cells expressing CXCR4) (Online Supplementary Table S1). In all experiments, three distinct subpopulations of cells with respect to CXCR4 expression level were evidenced: CXCR4neg, CXCR4low and CXCR4bright (Online Supplementary Figure S1). Flow cytometry analysis after sorting showed that the cells belonged to only one of the subpopulations, categorized according to CXCR4 expression (Online Supplementary Figure S2). Most cells with in vivo repopulating capacity in the day-0 population were predominantly concentrated in the CXCR4low fraction, although some minor activity was found in the CXCR4neg and CXCR4bright populations (Figure 2B); these HSCs exhibit a lower lymphoid differentiation potential compared to CXCR4neg and, especially, CXCR4low repopulating HSCs.
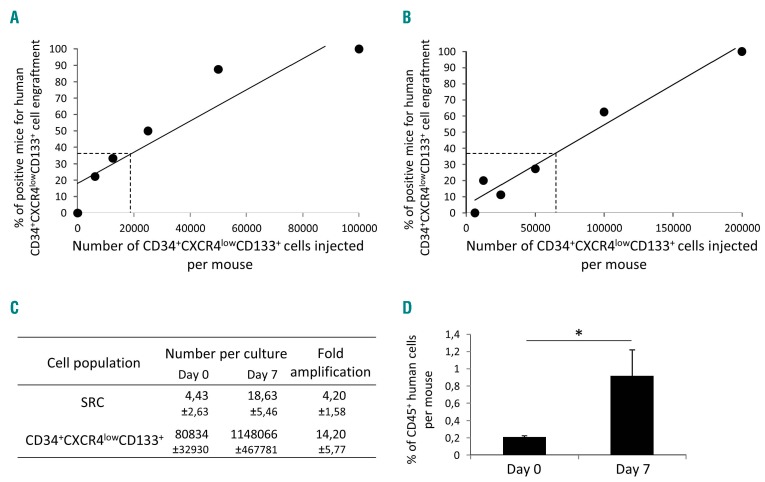
The engraftment of CXCR4neg cells prompted us to explore the hypothesis that at least some of the CXCR4neg cells can express CXCR4 once in an in vivo microenvironment of 37°C (i.e. after injection and transplantation). Thus, after an overnight incubation, 30% of the CD34+ cells that were initially CXCR4neg, became CXCR4low (Online Supplementary Figure S3). These data from the bulk CD34+ cultures were confirmed in the cultures initiated with the sorted CXCR4neg cells (Online Supplementary Figure S4). After ex vivo expansion, almost all cells with engraftment capacity (SRCs) were concentrated in the CXCR4low fraction (Figure 2C) and fully maintained their day-0 differentiation potential, although from these results it appears that SRC activity after ex vivo expansion (day-7) is lower than that of non-expanded cells at day 0. However, although injecting the same number of cells from each fraction into mice can show in which fraction the SRCs are concentrated, it cannot provide insight into changes of specific SRC activity during expansion culture. To obtain this information, we injected each mouse with the full day-7 progeny (equivalent) of 2×105 day-0 CD34+CXCR4low cells (Figure 2D) (the CD34+CXCR4low fraction was chosen since effectively all SRC activity is concentrated in this fraction). In this way, we obtained unequivocal proof that SRC activity was enhanced after the ex vivo expansion culture. To quantify these data, we performed a limiting dilution assay31 on the CD34+CXCRlowCD133+ population at the beginning and after culture (see further text for the CD133 issue), the results of which showed an ~4.2-fold expansion of SRCs after 7 days with respect to day 0 (Figure 3A-C). Furthermore, the mean individual SRC proliferative capacity was ~4-fold higher after 7 days of expansion culture compared to the capacity at day 0 (Figure 3D). In the same time period, a 14.2-fold expansion of the CD34+ CXCR4lowCD133+ fraction was found (Figure 3C; Online Supplementary Table S2).
Figure 3.
Frequencies and individual proliferative capacity of severe combined immunodeficiency repopulating cells within the CD34+CD133+CXCR4low cell population before and after ex vivo expansion. (A,B) Percentage ofmice “positive” for human CD45, 8 weeks after injection of CD34+CXCR4lowCD133+ cells, with respect to the cell dose, before expansion (A) and after expansion (B). (C) Absolute number of severe combined immunodeficiency repopulating cells (SRCs) estimated on the basis of the extreme limiting dilution assay (ELDA). (D) Mean chimerisms of the individual SRCs (only the doses giving less than 37% of positive mice were taken into consideration and only positive mice from these conditions were analyzed). The results presented were generated from the individual data given in Online Supplementary Table S2. Statistical significance: *P<0.05.
All (100%) SS-PB CD34+ cells, whatever their CXCR4 expression pattern, expressed all adhesion molecules analyzed (LFA-1, VLA-4, VLA-5, VLA-6) before and after ex vivo expansion (data not shown). Neither CD90 nor CD45RA was expressed by CD34+ SS-PB cells: all the sorted subpopulations were CD90− and CD45RA− (data not shown).
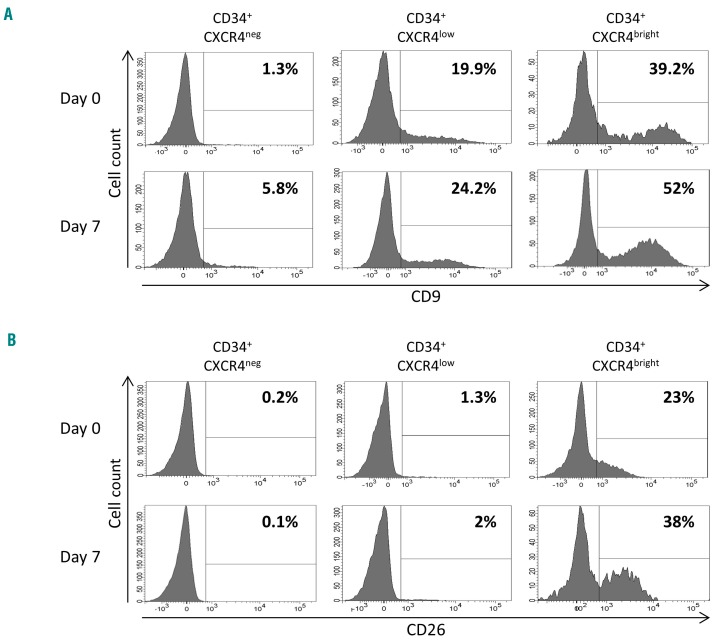
Before and after culture expansion, expression of the tetraspanin CD9 correlated closely with the expression of CXCR4 (Figure 4A). CD26 was not expressed on CXCR4neg or CXCR4low fractions of CD34+ cells at either day 0 or day 7, however 22% and 38% of CXCR4bright cells expressed CD26 on day 0 and day 7, respectively (Figure 4B). It is noteworthy that the expression of CD26 by CXCR4-expressing cells coincides with their loss of engraftment capacity (Figure 2).
Figure 4.
CD9 and CD26 cell expression among CXCR4 cell subpopulations. (A) CD9 expression at day 0 and after 7 days of culture. (B) CD26 expression at day 0 and after 7 days of culture. The same CD34+CXCR4neg, CD34+CXCR4low and CD34+CXCR4bright subpopulations were selected as those for the in vivo reconstitution experiments. Percentages of CD9+ or CD26+ cells are indicated for each subpopulation. Day 7: cell subpopulations were defined among the progeny of total day-0 CD34+ cells.
On day-0 CD34+ cells, CD133 was primarily expressed on CXCR4neg and CXCR4low CD34+ cell fractions (Figure 5A). In contrast, after expansion culture (day 7), the CD133+ cells were exclusively concentrated in the CXCR4low fraction of the cells remaining CD34+ (Figure 5B).
Figure 5.
CD133 expression by CD34+ cells selected on the basis of CXCR4 expression. Three gates were delimited: P1, CD34+CXCR4neg cell subpopulation; P2, CD34+CXCR4low cell subpopulation; P3, CD34+CXCR4bright cell subpopulation. Among these cell gates, CD133+ and CD133− fractions were defined and six cell subpopulations were sorted as CXCR4negCD133−, CXCR4negCD133+, CXCR4lowCD133−, CXCR4lowCD133+, CXCR4brightCD133−, and CXCR4brightCD133+ subfractions. For each of these subpopulations, the percent of CD133-expressing cells is indicated.
When day-0 cells from these fractions defined on the basis of CXCR4 and CD133 expression were injected into NSG mice, SRCs were evidenced only in CD133+ fractions, i.e. CXCR4negCD133+ and CXCR4lowCD133+ (Figure 6A). Furthermore, after expansion at day 7, the main SRC activity remained in the CXCR4lowCD133+ fraction: with the cell dose employed, all mice were “positive” and with high chimerism (Figure 6B). At day 0, we observed that SRCs were much more frequent in the CXCR4low fraction than in the CXCR4neg fraction (P<0.01) (Figure 4A). With regard to differentiation potential, a predominant “lymphoid” profile characterized the repopulating HSCs of CD133+ fractions, while the rare repopulating HSCs detected in CD133neg fractions showed much higher proportion of, or predominantly exhibited, a myeloid differentiation potential (Figure 6A). At day 7, only the CD34+CXCR4lowCD133+ fraction yielded HSCs capable of in vivo reconstitution. In this case, they displayed a predominant lymphoid differentiation potential (Figure 6B).
Figure 6.
CD133 determines hematopoietic severe combined immunodeficiency repopulating cell capacity of CXCR4-expressing CD34+ steady-state peripheral blood cells, before and after ex vivo expansion. Severe combined immunodeficiency repopulating cell (SRC) activity was evaluated by short-term engraftment (8 weeks) in NSG mice. Each point of the graphs represents the percentage of CD45+ human cells in one mouse bone marrow. (A) Day 0: 1×105 SS-PB total CD34+ cells or 1×105 cells from each sorted cell subpopulation were injected per mouse. (B) Day 7: 2×105 of the total expanded cell population or 2×105 cells from each sorted cell subpopulation were injected per mouse. The “pie” graphs in (A) and (B) show the relative proportions of CD19+ and CD33+ cells pf human origin within the huCD45+ population. Statistical significance: **P<0.01 and ***P<0.001.
Hematopoietic stem cells with long-term reconstituting ability
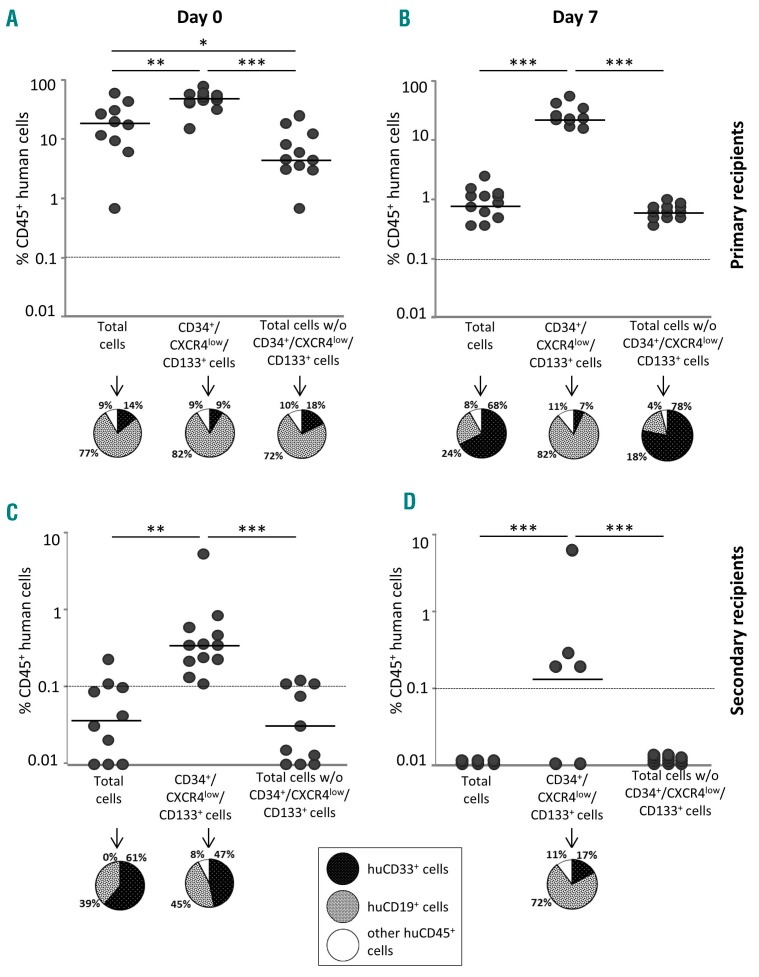
While the results presented above concern ST-HSCs, we also employed the primary/secondary recipient transplantation approach to detect the LT-HSC subpopulation in SS-PB CD34+ cells depending on their CD133 and CXCR4 expression pattern before and after ex vivo expansion.6,24,32 In fact, we tested the presence of LT-HSCs at day 0 and after expansion culture (day 7) in the total cell population, in the CD34+CXCR4lowCD133+ population (which, as described above and shown in Figure 7A,B, contains most of the ST-HSCs), and in the fraction containing all remaining cells after removal of the CD34+CXCR4lowCD133+ population (Figure 7). With the number of cells injected in our experiments, the LT-HSCs were practically undetectable both at day 0 (before expansion) and at day 7 (after expansion) (see CD45 chimerism in secondary recipients, Figure 7C,D), indicating that their frequency in the total CD34+ cell population is extremely low. However, once concen trated in the CD34+CXCR4lowCD133+ population, LT-HSCs become clearly detectable both before and after expansion (Figure 7C,D). Since we did not find “positive” secondary recipient mice after injection of BM from the primary recipient mice which had received the cell population composed of all other cells except CD34+CXCR4lowCD133+ ones, it can be concluded that the LT-HSCs are limited to the CD34+CXCR4lowCD133+ phenotype. In view of the fold expansion of the total cells (25.1 ± 9.9) (Online Supplementary Table S1) and the fact that the injected cell dose after expansion was only eight times higher than before expansion, it can be estimated that LT-HSCs were at minimum maintained during the culture. It is very interesting to note that the day-0 LT-HSCs (Figure 7C) showed a relatively lower lymphoid differentiation capacity compared to cultured (day-7) LT-HSCs (Figure 7D).
Figure 7.
Capacity of long-term hematopoietic reconstitution of NSG mice is restricted to the CD34+CXCR4lowCD133+ steady-state peripheral blood cell fraction. Before (day 0) (A, C) and after ex vivo expansion (day 7) (B, D), three cell populations were selected and sorted: total CD34+ steady-state peripheral blood (SS-PB) cells, the CD34+CXCR4lowCD133+ selected subpopulation, and total CD34+ cells without the CD34+CXCR4lowCD133+ subpopulation. (A) Day-0, short-term reconstitution in primary recipients, 2×105 cells of each subpopulation were injected intravenously per mouse. (B) Day-7, short-term reconstitution in primary recipients, 1.6×106 cells of each subpopulation were injected intravenously per mouse. Day-7 subpopulations were defined among the progeny of total day-0 CD34+ cells. (C) Day-0, long-term reconstitution in secondary recipients. Bone marrow cells from both femora of each primary recipient were injected into the bone marrow of the secondary recipient NSG mouse. (D) Day-7, long-term reconstitution in secondary recipients. Bone marrow cells from both femora of each primary recipient were injected into the bone marrow of the secondary recipient NSG mouse. For each condition (A-D) the “pie” graphs show the relative proportions of CD19+ and CD33+ cells of human origin within the huCD45+ population. Statistical significance: *P<0.05; **P<0.01; ***P<0.001.
Committed hematopoietic progenitors
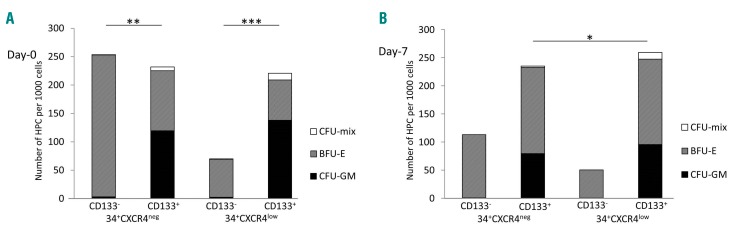
The content of committed progenitors in CD34+ cells belonging to the fractions defined by CXCR4 and CD133 expression is presented in Figure 8. Interestingly, the CD133− fractions before and after ex vivo expansion contained exclusively erythroid progenitors (BFU-E) irrespective of CXCR4 expression, while CD133+ cells always contained three classes of progenitors (CFU-GM, BFU-E and CFU-Mix). Furthermore, the committed progenitors were five times less concentrated in the CXCR4lowCD133-fraction than in the CXCR4lowCD133+ one.
Figure 8.
Hematopoietic committed progenitors in the CD34+ cell populations selected on day 0 on the basis of CXCR4 and CD133 expression. Day-0 (A) sorted subpopulations were expanded separately ex vivo and day-7 (B) clonogenic capacities of these subpopulations were analyzed. CFU-GM: colony-forming unit -granulocyte/monocyte; BFU-E: burst-forming unit – erythroid; CFU-mix: colony-forming unit - mixed. Statistical significance: *P<0.05; **P<0.01; ***P<0.001.
Discussion
The findings presented in this article clearly show that ST-HSCs and LT-HSCs present in SS-PB have particular phenotypic properties, which are different from those of HSCs in CB, BM or mobilized peripheral blood (M-PB). It is evident that the pattern of CXCR4 expression is related to the functional abilities of SS-PB ST-HSCs and LT-HSCs. This is not surprising since CXCR4 and its ligand SDF-1 have been demonstrated to have a major role in homing/mobilization of HPCs and HSCs.33,34
The presence of a small fraction (8%) of CXCR4-expressing CD34+ cells in SS-PB was first observed by Lataillade et al.35 Here, we found 16% of CXCR4+CD34+ cells in the mononuclear SS-PB fraction issued from leukodepletion filters. Only a very small fraction of M-PB CD34+ cells express CXCR4; these cells exhibit an in vitro capacity to migrate towards a SDF-1α gradient and result in high levels of multilineage engraftment upon injection into NOD/SCID mice.10 In CB, both CXCR4+ and CXCR4neg subsets were shown to be capable of engrafting NOD/SCID mice with similar frequencies36 suggesting that CXCR4 is not a suitable marker for purification of human CB HSCs before transplantation.
In addition, CXCR4 expression is rapidly regulated by environmental factors or induced ex vivo by cytokines such as granulocyte colony-stimulating factor (also used in our cultures) in CD34+ cells from all sources, including SS-PB CD34+ cells with primitive features.35 Furthermore, induction of CXCR4 expression on CB and M-PB CD34+ cells increased their capacity for in vivo engraftment in NOD/SCID mice.37 Apparently contradictory conclusions were reached in a study that found that the homing efficiency of CD34+ cells selected from BM or M-PB was not related to expression of either CXCR4 or adhesion molecules.38 However, blocking CXCR4 signaling on transplanted CB CD34+ cells prevented homing, whereas pretreatment of cells with cytokines led to up-regulation of CXCR4 expression and increased mice engraftment,39 which clearly highlights the crucial importance of CXCR4 expression for HSC engraftment.
This point is important because it helps to avoid erroneous conclusions due to artifacts induced by the technical procedure related to CXCR4 expression-based cell sorting; a low repopulating capacity of CD34+CXCR4+ cells (the authors did not discriminate between low and bright populations) from CB and BM could result from the neutralizing activity of the anti-CXCR4 monoclonal antibody that was used for cell sorting. This antibody (clone 12G5) binds the site that serves for the binding and signaling of the CXCR4 specific ligand SDF-1α28,39 and, depending on its concentration, can have either inhibitory or stimulatory effects.11 To avoid any artifacts in assaying CXCR4 activity and grafting capacity of CD34+ cells, we used antibody elution after cell subfraction sorting and before any engraftment assays.
In our hands, the engraftment capacity of the CD34+CXCR4neg subfraction in SS-PB was much lower than that of the CD34+CXCR4low population, as found for CB CD34+CXCR4neg versus CD34+CXCR4pos cells (including both “low” and “high” CXCR4 expression).12 However, a substantial number of CD34+CXCR4neg cells expresses CXCR4 (i.e. they become “low expressing”) after at least overnight ex vivo cytokine treatment, an effect which is even more pronounced after 4 days in culture (Online Supplementary Figure S3; confirmed also in the cultures initiated with the CD34+ cells sorted on the basis of CXCR4 expression: Online Supplementary Figure S4). This phenomenon could be proposed as the explanation for the ex vivo culture enhancing effect on the engrafting capacity of ST-HSCs and LT-HSCs. However, this is not the case, since practically all CXCR4neg cells which became CXCR4+ in culture lost their CD133 expression by day 4 of culture (only CD133+ cells exhibited engrafting capacity, see below) (Online Supplementary Figure S3). However, the CD133 expression was maintained for at least for 24 h in culture mimicking the in vivo situation after cell injection. This could explain some minor engraftment capacity of the CXCRneg cell population before expansion. In fact, these cells could become CXCR4low in vivo, during the first hours after injection. It should be emphasized that the highest engraftment capacity before (at day 0) or after (at day 7) cell culture is concentrated in the CXCR4low fraction and not in the CXCR4bright one, which seems to be surprising. Actually, the expression of CD26 (that, in our protocol, could be induced by granulocyte colony-stimulating factor, as shown for CD34+CD38− CB cells40), which is related only to CXCR4bright cells, can explain the decrease in CXCR4bright engrafting efficiency. It has been shown that CD26/dipeptidyl peptidase IV is a membrane-bound extracellular peptidase that cleaves polypeptides such as SDF-1, thus reducing CXCR4 activity. Furthermore, CD26 expression might be part of a mechanism regulating CXCR4 activity. The inhibition of CD26 expression on CB CD34+ cells enhances the in vitro migratory effect against the SDF-1 gradient9 and improves in vivo long-term engraftment in NOD/SCID mice.41,42 Furthermore, pretreatment of mice with a specific CD26 inhibitor (diprotin A) enhances engraftment of mouse BM cells in primary and secondary recipients.43 This is being considered among emerging strategies to improve homing and engraftment of HSCs in clinical transplants.44 A similar approach, allowing the CXCR4bright HSCs to engraft, might still enhance the engraftment efficiency of SS-PB after ex vivo expansion, although this remains to be confirmed. On the other hand, recent studies have shown that CD34+ cells also home to the BM in an SDF1-CXCR4 axis-independent manner and that “priming factors”45 as well as “mild heat treatment” facilitate incorporation of CXCR4 into functional lipid rafts.46 This might constitute another strategy to enhance engraftment of SS-PB cells.
Concerning our observation of a close relationship between the expression of CXCR4 and CD9, CD9 has been implicated in the regulation of various physiological processes, including cell motility and adhesion. Trafficking and homing is a multistep process, as demonstrated for lymphocytes and myeloma cells, in which CD9 has been proven essential for transendothelial invasion.47 In human CB, CD9 is expressed by CD34+ cells and is regulated by SDF-1. Anti-CD9 antibody alters migratory and adhesive functions of CB CD34+ cells in vitro and CD9 neutralization impairs homing of transplanted CD34+ cells in NOD/SCID mice.8 The functional relationship between CD9 and CD26 on CDCXCR4bright cells remains to be elucidated.
In our hands, all the sorted SS-PB CD34+ subpopulations were CD90− and CD45RA−. This phenotype is associated with committed progenitor cells in BM and CB CD34+ cells since HSCs seem to be CD90+48 and/or CD45RA+.14 However, our CD34+CXCR4lowCD133+CD90−CD45RA-SS-PB cells are enriched in true HSCs, as proven by efficient secondary recipient hematopoietic engraftment. CD49f, claimed to be a specific marker of CB repopulating HSCs,15 is expressed on all CD34+CD133+ SS-PB cells whatever their CXCR4 expression.
Perhaps the most interesting information emerging from our study is the fact that all SS-PB HSCs exhibiting in vivo repopulating capacity (both ST- and LT-HSCs) are found to be exclusively a CD133+ population of CD34+ cells, highly concentrated in the CXCR4low population. This particular phenotypic determinant does not change after ex vivo expansion. With respect to the committed progenitors in the CD34+ population, our results (Figure 8) clearly show that before and after ex vivo expansion, CFU-GM and CFU-Mix reside exclusively in the CD133+ population, whereas BFU-E are present in both the CD133+ and CD133− populations. This is in line with recent findings obtained with CB CD34+ cells.49–51 CD133 has long been considered a marker of stemness for CB, BM and M-PB cells although also expressed by most committed progenitor cells.16,18,49 Here, we show that CD133 could also be used for the enrichment of SS-PB HSCs. In CB, BM and M-PB cytokine-activated CD34+ cells, CD133 is concentrated in the uropod of the polarized migrating cells.52 A functional relationship has been observed between CD133/prominin-1 and CXCR4 in specific membrane micro-domains of magnupo-dia,17 suggesting a favored cell migration towards the in vivo hematopoietic niche and, hence, engraftment. Since LT-HSCs are present only in the CD133+ fraction of CD34+CXCR4low SS-PB cells before and after expansion, the loss of this particular phenotypically-defined population in the course of ex-vivo manipulation could be indicative of a loss of the long-term repopulating capacity of the graft. Clinical scale CD133+ selection is also considered among emerging strategies and alternative methods in clinical transplantation.53
BM mesenchymal stromal cell proliferation, but also fluctuation of the number of HSCs in peripheral blood are related to circadian oscillations.54 Since similar oscillations exist in humans,55 the circadian rhythm must be taken into consideration to optimize collection of SS-PB HSCs and HPCs.
Large, phenotypic and HPC analysis was performed on CD34+ cells isolated from SS-PB.56 Ex vivo culture of CD34+ SS-PB cells enhanced the total number of HSCs exhibiting in vivo repopulating capacity as well as their individual proliferative capacities, as shown by our limiting-dilution experiments. The maintenance of the lymphoid differentiation potential of repopulating HSCs after ex vivo culture is an additional important argument, since a shift towards predominant myeloid potential, as we detected in the rare CXCR4low and CD133− repopulating HSCs, has been found to occur during aging.57 In fact, our results suggest that reducing HSC differentiation capacity to the myeloid lineage represents a degree of HSC commitment. In this respect, aging is characterized by a higher proportion of more committed HSCs58 in a context of general “consumption” and is the first sign of imminent exhaustion of the system. This suggests that ex vivo expansion can provide an adequate tool to produce enough hematopoietic stem and progenitor cells to constitute a single hematopoietic graft from the contents of only one or two steady-state leukapheresis collections. The efficiency of the expansion procedure could, most likely, be further improved by using new approaches, for example the TAT-protein transduction peptide fused to regulatory factors or inhibition of HOXB4 degradation,59–62 which is the object of our ongoing work. Furthermore, the CD34neg fraction containing immuno-competent cells (T and B lymphocytes) can be preserved and an appropriate dose of these cells injected either during transplantation or later, depending on the need for an allogeneic immuno-effect. Furthermore, lymphocyte efficiency can be enhanced and specified by ex vivo engineering.
Taken together, the results presented here might help in the design of novel, advanced graft generation, which could simultaneously provide efficient immuno-hematopoietic reconstitution and a graft-versus-tumor/leukemia effect. Future work in our laboratory aims to explore this strategy.
Supplementary Material
Acknowledgments
The authors thank to Mr Santiago Gonzalez, Mrs Valérie De Luca, Mrs Anaëlle Stum and Mr Vincent Pitard from Flow-Cytometry Platform SFR Transbiomed, Bordeaux University, France, for their precious help with cell sorting experiments. The help in first-line English editing of Mrs Elisabeth Doutreloux is also gratefully acknowledged. This manuscript was funded by an EFS (French Blood Institute - Etablissement Français du Sang) grant (n. 2016-01-IVANOVIC-AQLI) and French Biomedical Agency (Agence de la Biomédecine) grant (AOR “Greffe” 2015). IS would like to acknowledge support from the Liaison Committee between the Central Norway Health Authority (RHA) and the Norwegian University of Science and Technology (NTNU).
Footnotes
Check the online version for the most updated information on this article, online supplements, and information on authorship & disclosures: www.haematologica.org/content/103/10/1604
References
- 1.Richman CM, Weiner RS, Yankee RA. Increase in circulating stem cells following chemotherapy in man. Blood. 1976;47(6):1031–1039. [PubMed] [Google Scholar]
- 2.Reiffers J, Faberes C, Boiron JM, et al. Peripheral blood progenitor cell transplantation in 118 patients with hematological malignancies: analysis of factors affecting the rate of engraftment. J Hematother. 1994;3(3):185–191. [DOI] [PubMed] [Google Scholar]
- 3.D’Souza A, Jaiyesimi I, Trainor L, Venuturumili P. Granulocyte colony-stimulating factor administration: adverse events. Transfus Med Rev. 2008;22(4):280–290. [DOI] [PubMed] [Google Scholar]
- 4.Bari S, Seah KK, Poon Z, et al. Expansion and homing of umbilical cord blood hematopoietic stem and progenitor cells for clinical transplantation. Biol Blood Marrow Transplant. 2015;21(6):1008–1019. [DOI] [PubMed] [Google Scholar]
- 5.Boiron JM, Dazey B, Cailliot C, et al. Large-scale expansion and transplantation of CD34(+) hematopoietic cells: in vitro and in vivo confirmation of neutropenia abrogation related to the expansion process without impairment of the long-term engraftment capacity. Transfusion. 2006;46(11):1934–1942. [DOI] [PubMed] [Google Scholar]
- 6.Brunet de la Grange P, Vlaski M, Duchez P, et al. Long-term repopulating hematopoietic stem cells and “side population” in human steady state peripheral blood. Stem Cell Res. 2013;11(1):625–633. [DOI] [PubMed] [Google Scholar]
- 7.Bourdieu A, Avalon M, Lapostolle V, et al. Steady state peripheral blood provides cells with functional and metabolic characteristics of real hematopoietic stem cells. J Cell Physiol. 2018;233(1):338–349. [DOI] [PubMed] [Google Scholar]
- 8.Leung KT, Chan KY, Ng PC, et al. The tetraspanin CD9 regulates migration, adhesion, and homing of human cord blood CD34+ hematopoietic stem and progenitor cells. Blood. 2011;117(6):1840–1850. [DOI] [PubMed] [Google Scholar]
- 9.Christopherson KW, 2nd, Hangoc G, Broxmeyer HE. Cell surface peptidase CD26/dipeptidylpeptidase IV regulates CXCL12/stromal cell-derived factor-1 alpha-mediated chemotaxis of human cord blood CD34+ progenitor cells. J Immunol. 2002;169(12):7000–7008. [DOI] [PubMed] [Google Scholar]
- 10.Peled A, Petit I, Kollet O, et al. Dependence of human stem cell engraftment and repopulation of NOD/SCID mice on CXCR4. Science. 1999;283(5403):845–848. [DOI] [PubMed] [Google Scholar]
- 11.Plett PA, Frankovitz SM, Wolber FM, Abonour R, Orschell-Traycoff CM. Treatment of circulating CD34(+) cells with SDF-1alpha or anti-CXCR4 antibody enhances migration and NOD/SCID repopulating potential. Exp Hematol. 2002;30(9): 1061–1069. [DOI] [PubMed] [Google Scholar]
- 12.Lapidot T, Kollet O. The essential roles of the chemokine SDF-1 and its receptor CXCR4 in human stem cell homing and repopulation of transplanted immune-deficient NOD/SCID and NOD/SCID/B2m (null) mice. Leukemia. 2002;16(10):1992–2003. [DOI] [PubMed] [Google Scholar]
- 13.Peled A, Kollet O, Ponomaryov T, et al. The chemokine SDF-1 activates the integrins LFA-1, VLA-4, and VLA-5 on immature human CD34(+) cells: role in transendothelial/stromal migration and engraftment of NOD/SCID mice. Blood. 2000;95(11):3289–3296. [PubMed] [Google Scholar]
- 14.Majeti R, Park CY, Weissman IL. Identification of a hierarchy of multipotent hematopoietic progenitors in human cord blood. Cell Stem Cell. 2007;1(6):635–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Notta F, Doulatov S, Laurenti E, Poeppl A, Jurisica I, Dick JE. Isolation of single human hematopoietic stem cells capable of long-term multilineage engraftment. Science. 2011;333(6039):218–221. [DOI] [PubMed] [Google Scholar]
- 16.Bhatia M. AC133 expression in human stem cells. Leukemia. 2001;15(11):1685–1688. [DOI] [PubMed] [Google Scholar]
- 17.Bauer N, Fonseca AV, Florek M, et al. New insights into the cell biology of hematopoietic progenitors by studying prominin-1 (CD133). Cells Tissues Organs. 2008;188(1–2):127–138. [DOI] [PubMed] [Google Scholar]
- 18.Drake AC, Khoury M, Leskov I, et al. Human CD34+ CD133+ hematopoietic stem cells cultured with growth factors including Angptl5 efficiently engraft adult NOD-SCID Il2rgamma−/− (NSG) mice. PloS One. 2011;6(4):e18382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ivanovic Z, Duchez P, Morgan DA, et al. Whole-blood leuko-depletion filters as a source of CD34+ progenitors potentially usable in cell therapy. Transfusion. 2006;46(1):118–125. [DOI] [PubMed] [Google Scholar]
- 20.Peytour Y, Guitart A, Villacreces A, et al. Obtaining of CD34+ cells from healthy blood donors: development of a rapid and efficient procedure using leukoreduction filters. Transfusion. 2010;50(10):2152–2157. [DOI] [PubMed] [Google Scholar]
- 21.Duchez P, Chevaleyre J, Brunet de la Grange P, et al. Cryopreservation of hematopoietic stem and progenitor cells amplified ex vivo from cord blood CD34+ cells. Transfusion. 2013;53(9):2012–2019. [DOI] [PubMed] [Google Scholar]
- 22.Sutherland DR, Anderson L, Keeney M, Nayar R, Chin-Yee I. The ISHAGE guidelines for CD34+ cell determination by flow cytometry. International Society of Hematotherapy and Graft Engineering. J Hematother. 1996;5(3):213–226. [DOI] [PubMed] [Google Scholar]
- 23.Frisch BJ, Calvi LM. Hematopoietic stem cell cultures and assays. Methods Mol Biol. 2014;1130:315–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ivanovic Z, Duchez P, Chevaleyre J, et al. Clinical-scale cultures of cord blood CD34(+) cells to amplify committed progenitors and maintain stem cell activity. Cell Transplant. 2011;20(9):1453–1463. [DOI] [PubMed] [Google Scholar]
- 25.Robert-Richard E, Ged C, Ortet J, et al. Human cell engraftment after busulfan or irradiation conditioning of NOD/SCID mice. Haematologica. 2006;91(10):1384. [PubMed] [Google Scholar]
- 26.Chevaleyre J, Duchez P, Rodriguez L, et al. Busulfan administration flexibility increases the applicability of scid repopulating cell assay in NSG mouse model. PloS One. 2013;8(9):e74361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ivanovic Z, Hermitte F, Brunet de la Grange P, et al. Simultaneous maintenance of human cord blood SCID-repopulating cells and expansion of committed progenitors at low O2 concentration (3%). Stem Cells. 2004;22(5):716–724. [DOI] [PubMed] [Google Scholar]
- 28.Woodard LE, Nimmagadda S. CXCR4-based imaging agents. J Nucl Med. 2011;52(11):1665–1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang JC, Doedens M, Dick JE. Primitive human hematopoietic cells are enriched in cord blood compared with adult bone marrow or mobilized peripheral blood as measured by the quantitative in vivo SCID-repopulating cell assay. Blood. 1997;89(11):3919–3924. [PubMed] [Google Scholar]
- 30.Hu Y, Smyth GK. ELDA: extreme limiting dilution analysis for comparing depleted and enriched populations in stem cell and other assays. J Immunol Methods. 2009;347(1–2):70–78. [DOI] [PubMed] [Google Scholar]
- 31.Denning-Kendall PA, Evely R, Singha S, Chapman M, Bradley BA, Hows JM. In vitro expansion of cord blood does not pre vent engraftment of severe combined immunodeficient repopulating cells. Br J Haematol. 2002;116(1):218–228. [DOI] [PubMed] [Google Scholar]
- 32.Duchez P, Rodriguez L, Chevaleyre J, et al. Interleukin-6 enhances the activity of in vivo long-term reconstituting hematopoietic stem cells in “hypoxic-like” expansion cultures ex vivo. Transfusion. 2015;55(11):2684–2691. [DOI] [PubMed] [Google Scholar]
- 33.Jing D, Fonseca AV, Alakel N, et al. Hematopoietic stem cells in co-culture with mesenchymal stromal cells–modeling the niche compartments in vitro. Haematologica. 2010;95(4):542–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pantin J, Purev E, Tian X, et al. Effect of high-dose plerixafor on CD34+ cell mobilization in healthy stem cell donors: results of a randomized crossover trial. Haematologica. 2017;102(3):600–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lataillade JJ, Clay D, Dupuy C, et al. Chemokine SDF-1 enhances circulating CD34(+) cell proliferation in synergy with cytokines: possible role in progenitor survival. Blood. 2000;95(3):756–768. [PubMed] [Google Scholar]
- 36.Rosu-Myles M, Gallacher L, Murdoch B, et al. The human hematopoietic stem cell compartment is heterogeneous for CXCR4 expression. Proc Natl Acad Sci USA. 2000;97(26):14626–14631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kahn J, Byk T, Jansson-Sjostrand L, et al. Overexpression of CXCR4 on human CD34+ progenitors increases their proliferation, migration, and NOD/SCID repopulation. Blood. 2004;103(8):2942–2949. [DOI] [PubMed] [Google Scholar]
- 38.Herrera C, Sanchez J, Torres A, Pascual A, Rueda A, Alvarez MA. Pattern of expression of CXCR4 and adhesion molecules by human CD34+ cells from different sources: role in homing efficiency in NOD/SCID mice. Haematologica. 2004;89(9):1037–1045. [PubMed] [Google Scholar]
- 39.Kollet O, Petit I, Kahn J, et al. Human CD34(+)CXCR4(−) sorted cells harbor intracellular CXCR4, which can be functionally expressed and provide NOD/SCID repopulation. Blood. 2002;100(8):2778–2786. [DOI] [PubMed] [Google Scholar]
- 40.Christopherson KW, 2nd, Uralil SE, Porecha NK, Zabriskie RC, Kidd SM, Ramin SM. G-CSF- and GM-CSF-induced upregulation of CD26 peptidase downregulates the functional chemotactic response of CD34+CD38- human cord blood hematopoietic cells. Exp Hematol. 2006;34(8):1060–1068. [DOI] [PubMed] [Google Scholar]
- 41.Campbell TB, Hangoc G, Liu Y, Pollok K, Broxmeyer HE. Inhibition of CD26 in human cord blood CD34+ cells enhances their engraftment of nonobese diabetic/severe combined immunodeficiency mice. Stem Cells Dev. 2007;16(3):347–354. [DOI] [PubMed] [Google Scholar]
- 42.Christopherson KW, 2nd, Paganessi LA, Napier S, Porecha NK. CD26 inhibition on CD34+ or lineage- human umbilical cord blood donor hematopoietic stem cells/hematopoietic progenitor cells improves long-term engraftment into NOD/SCID/Beta2null immunodeficient mice. Stem Cells Dev. 2007;16(3): 355–360. [DOI] [PubMed] [Google Scholar]
- 43.Broxmeyer HE, Hangoc G, Cooper S, Campbell T, Ito S, Mantel C. AMD3100 and CD26 modulate mobilization, engraftment, and survival of hematopoietic stem and progenitor cells mediated by the SDF-1/CXCL12-CXCR4 axis. Ann N Y Acad Sci. 2007;1106:1–19. [DOI] [PubMed] [Google Scholar]
- 44.Ratajczak MZ, Suszynska M. Emerging strategies to enhance homing and engraftment of hematopoietic stem cells. Stem Cell Rev. 2016;12(1):121–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ratajczak MZ. A novel view of the adult bone marrow stem cell hierarchy and stem cell trafficking. Leukemia. 2015;29(4):776–782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ratajczak MZ, Adamiak M. Membrane lipid rafts, master regulators of hematopoietic stem cell retention in bone marrow and their trafficking. Leukemia. 2015;29(7): 1452–1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.De Bruyne E, Andersen TL, De Raeve H, et al. Endothelial cell-driven regulation of CD9 or motility-related protein-1 expression in multiple myeloma cells within the murine 5T33MM model and myeloma patients. Leukemia. 2006;20(10):1870–1879. [DOI] [PubMed] [Google Scholar]
- 48.Brendel C, Neubauer A. Characteristics and analysis of normal and leukemic stem cells: current concepts and future directions. Leukemia. 2000;14(10):1711–1717. [DOI] [PubMed] [Google Scholar]
- 49.Radtke S, Gorgens A, Kordelas L, et al. CD133 allows elaborated discrimination and quantification of haematopoietic progenitor subsets in human haematopoietic stem cell transplants. Br J Haematol. 2015;169(6):868–878. [DOI] [PubMed] [Google Scholar]
- 50.Gorgens A, Radtke S, Mollmann M, et al. Revision of the human hematopoietic tree: granulocyte subtypes derive from distinct hematopoietic lineages. Cell Rep. 2013;3(5):1539–1552. [DOI] [PubMed] [Google Scholar]
- 51.Takahashi M, Matsuoka Y, Sumide K, et al. CD133 is a positive marker for a distinct class of primitive human cord blood-derived CD34-negative hematopoietic stem cells. Leukemia. 2014;28(6):1308–1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Giebel B, Corbeil D, Beckmann J, et al. Segregation of lipid raft markers including CD133 in polarized human hematopoietic stem and progenitor cells. Blood. 2004;104(8):2332–2338. [DOI] [PubMed] [Google Scholar]
- 53.Bornhauser M, Eger L, Oelschlaegel U, et al. Rapid reconstitution of dendritic cells after allogeneic transplantation of CD133+ selected hematopoietic stem cells. Leukemia. 2005;19(1):161–165. [DOI] [PubMed] [Google Scholar]
- 54.Ehninger A, Trumpp A. The bone marrow stem cell niche grows up: mesenchymal stem cells and macrophages move in. J Exp Med. 2011;208(3):421–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lucas D, Battista M, Shi PA, Isola L, Frenette PS. Mobilized hematopoietic stem cell yield depends on species-specific circadian timing. Cell Stem Cell. 2008;3(4):364–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jobin C, Cloutier M, Simard C, Neron S. Heterogeneity of in vitro-cultured CD34+ cells isolated from peripheral blood. Cytotherapy. 2015;17(10):1472–1484. [DOI] [PubMed] [Google Scholar]
- 57.Pang WW, Price EA, Sahoo D, et al. Human bone marrow hematopoietic stem cells are increased in frequency and myeloid-biased with age. Proc Natl Acad Sci USA. 2011;108(50):20012–20017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cho RH, Sieburg HB, Muller-Sieburg CE. A new mechanism for the aging of hematopoietic stem cells: aging changes the clonal composition of the stem cell compartment but not individual stem cells. Blood. 2008;111(12):5553–5561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Antonchuk J, Sauvageau G, Humphries RK. HOXB4-induced expansion of adult hematopoietic stem cells ex vivo. Cell. 2002;109(1):39–45. [DOI] [PubMed] [Google Scholar]
- 60.Krosl J, Austin P, Beslu N, Kroon E, Humphries RK, Sauvageau G. In vitro expansion of hematopoietic stem cells by recombinant TAT-HOXB4 protein. Nat Med. 2003;9(11):1428–1432. [DOI] [PubMed] [Google Scholar]
- 61.Lee J, Shieh JH, Zhang J, et al. Improved ex vivo expansion of adult hematopoietic stem cells by overcoming CUL4-mediated degradation of HOXB4. Blood. 2013;121(20):4082–4089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Codispoti B, Rinaldo N, Chiarella E, et al. Recombinant TAT-BMI-1 fusion protein induces ex vivo expansion of human umbilical cord blood-derived hematopoietic stem cells. Oncotarget. 2017;8(27): 43782–43798. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.