The role that the bone marrow microenvironment plays in differentiation, migration, proliferation, survival and drug resistance of malignant plasma cells has attracted significant attention in the attempt to identify new druggable targets in multiple myeloma (MM).1
Heparanase is an endo-β-d-glucuronidase that trims the heparan sulfate chains of proteoglycans, thereby affecting cell signaling and gene expression and promoting extracellular matrix remodeling within the tumor microenvironment.2–4 Heparanase is strongly upregulated in the great majority of MM patients and is associated with elevated microvessel density and enhanced shedding of the heparan sulfate proteoglycan syndecan-1,5 events that are highly relevant to disease progression.6,7 In preclinical models of MM, heparanase was shown to be a master regulator of aggressive tumor behavior and bortezomib and melphalan were each found to enhance heparanase expression and secretion. MM cells expressing high levels of heparanase are less susceptible to cytotoxic effects of bortezomib or melphalan.8–10
Roneparstat (laboratory codes: G4000, SST0001; Leadiant Biosciences, formerly sigma tau Research Switzerland SA) is a chemically modified 100% N-desulphated, N-reacetylated and 25% glycol-split heparin with very low anticoagulant activity and a molecular weight between 15,000 and 25,000 Da. It is a very potent and pure competitive heparanase inhibitor.11,12 Roneparstat showed a significant anti-myeloma effect in murine models, either alone or in combination with dexamethasone, bortezomib or melphalan.10,13 Based on this preclinical evidence an open-label, multicenter, phase I, first-in-human study was designed to assess the safety and tolerability profile of roneparstat in patients with relapsed/refractory MM (EudraCT number 2012-001127-12 and clinicaltrials.gov identifier: NCT01764880).
Patients with advanced relapsed/refractory MM were eligible to be enrolled. Two treatment schedules were used: every day for 5 days (schedule A) and every day for 5 days in week 1 and week 2 (schedule B), in a cycle of 28 days, according to a 3+3 design (see Table 1). The drug was administered subcutaneously.
Table 1.
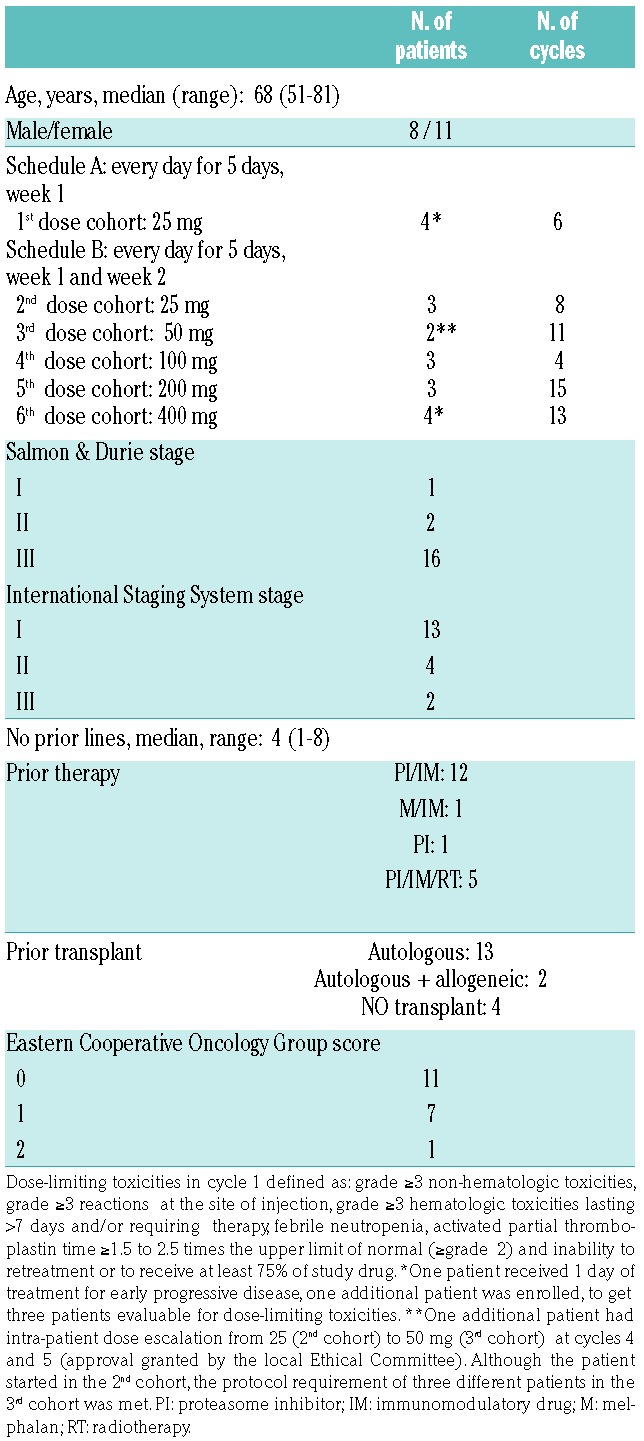
Patients’ baseline characteristics, enrollment by cohort of treatment and cycles administered.
Dose-limiting toxicities were characterized according to National Cancer Institute Common Terminology Criteria for Adverse Events (NCI CTCAE) version 4.0. The safety of the drug was assessed based on records of adverse events, evaluation of local tolerability, physical examination, vital signs and laboratory tests. Patients could receive standard supportive therapy with dexamethasone. The use and doses of dexamethasone were determined by the treating physicians’ judgment.
Blood samples were collected during cycle 1, on day 1 over 24 h, and on the last day of treatment (day 5 or day 12) over 72 h. Pharmacokinetic sampling was performed during the first cycle. Plasma samples were analyzed by a fluorescent probe assay (Heparin Red).14
The assessment of anti-MM activity was based on a surrogate parameter, the monoclonal protein modifications in serum and urine, evaluated according to the International Myeloma Working Group guidelines.
Nineteen patients with advanced relapsed/refractory MM were enrolled into the study and completed a total of 57 cycles (514 doses), for a median of two cycles (range, 1–11). Four patients received more than five cycles. The patients’ baseline characteristics and enroll ment by cohort of treatment are reported in Table 1.
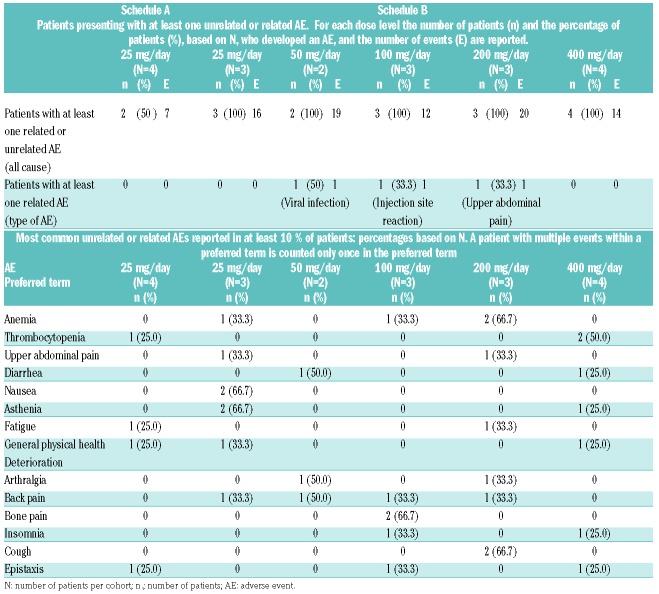
Roneparstat was well tolerated and safe at all doses tested. Seventeen patients reported a total of 88 adverse events. The most common adverse events, occurring in at least 10% of patients, are reported in Table 2. Most of the adverse events were grade 1 or 2 and unrelated to the treatment. There were three treatment-related adverse events in three patients (viral infection, injection site reaction, abdominal pain): these were judged to be grade 1/2, transient and resolved with conservative therapy.
Table 2.
Therapy-unrelated and related adverse events in the safety population (19 patients); the number of patients per cohort is reported as (N).
Grade 3/4 adverse events included general physical health deterioration (3 patients, 15.8%), anemia, thrombocytopenia and bone pain (2 patients each, 10.5%); neutropenia, gastric hemorrhage, and hyperglycemia (1 patient each, 5.3%). None of these events was assessed by the investigators as related to the study treatment.
Five patients experienced six serious adverse events, with only one (viral infection) considered to be related to the study drug, although a correlation with the concomitant treatment with dexamethasone could not be excluded. The remaining serious adverse events were treatment-unrelated: general physical health deterioration in two patients, pneumonia in one patient, a suspected gastrointestinal hemorrhage in one patient (which endoscopic evaluation did not confirm) and gastric hemorrhage in one patient (the patient was enrolled with a gastric myeloma lesion and developed progressive disease while on roneparstat therapy). The serious adverse events were transient and the patients recovered with conventional therapy except one whose deterioration in general physical health was ongoing at the end of the study.
No patients succumbed due to toxicity of the study drug. Nine patients died during the study, all from tumor progression.
Fifteen patients reported 31 local reactions at the injection site. Local side effects were less than grade 2, transient and resolved with conventional therapy, when needed. Only one (redness) was grade 2.
Due to the similarity of roneparstat with heparin, the risk of bleeding was monitored carefully. There were no evident relationships between either thrombin time or international normalized ratio (INR) and roneparstat administration following single or repeated dosing. For activated partial thromboplastin time (aPTT) there was no association with dose upon single dosing; after repeated dosing aPTT pharmacodynamic parameters (Emax and AUEC0−t) were higher at 200 and 400 mg/day than at lower doses, without reaching clinically meaningful levels.
The coagulation results by worst CTCAE grade showed one grade 1 aPTT level in one patient (5.3%), one grade 1 INR value in one patient (5.3%) and one grade 2 INR value in four patients (21.1%).
In the 17 patients evaluable for dose-limiting toxicities, no clinically relevant toxicities occurred and, indeed, no dose-limiting toxicities were observed: a true maximum tolerated dose was not reached. Dose escalation was stopped based on safety and pharmacokinetic data, indicating that patients could be exposed to the study drug at dose levels of 200 and 400 mg/day without any clinically relevant toxicities.
Reproducible plasma levels of roneparstat were measurable in cycle 1 at the two highest dose levels, as shown in Online Supplementary Figure S1, which depicts plasma concentrations in individual patients on day 1 and day 12 (single and repeat dosing, respectively). There was a dose related increase in mean Cmax between 200 mg/day and 400 mg/day, both on day 1 (1.67 μg/mL versus 2.45 μg/mL) and day 12 (2.07 μg/mL versus 5.95 μg/mL). The mean exposure (AUC0−t) at day 1 after repeated dosing was 16.2 μg.h/mL and 37.25 μg.h/mL, while at day 12 it was 15.4 μg.h/mL and 133 μg.h/mL for 200 mg and 400 mg/day, respectively. Upon repeated dosing, tmax was achieved at approximately 3 h after administration of the dose. On day 12 and at 400 mg/day, the estimated half-life was approximately 14−20 h (2 patients).
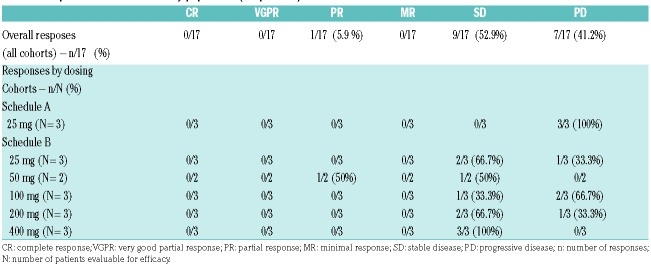
Seventeen patients who received at least 1 cycle of roneparstat were evaluable for overall response (Table 3). One partial response (5.9%) and nine cases of stable disease (52.9%) were observed. The remaining patients (41.2%) had progressive disease. The partial response occurred in a patient who received roneparstat 50 mg, who had relapsed after three prior lines of therapy with a continuous increase of the monoclonal component. Response was characterized by a rapid decrease of the monoclonal component, from 1.75 g/dL at baseline to 0.99 g/dL at cycle 1 and 0.71 g/dL at cycle 6. The patient remained on therapy until cycle 9 with sustained clinical benefit. Two of the nine cases of stable disease were sustained with significant clinical benefit (10 and 7 months) following 200 mg and 400 mg of roneparstat. The patient with a partial response and one with prolonged stable disease received concomitant, low doses of dexamethasone (up to 40 mg/week), while the other patient with prolonged stable disease did not receive any dexamethasone.
Table 3.
Response rates in the efficacy population (17 patients).
This is the first trial evaluating a heparanase inhibitor in hematologic malignancies. Heparanase represents an increasingly studied but still largely unexploited target for anticancer therapy. Our data show that roneparstat has an excellent safety profile, without causing clinically relevant systemic reactions, and an excellent tolerability profile. Systemic exposure appears measurable in a repro ducible and linear fashion at the doses of 200 and 400 mg/day. This study allowed identification of doses within the range of 300 to 400 mg/day as suitable for further development of the drug.
Roneparstat showed little efficacy in this specific experimental setting. Heparanase inhibition is not expected to cause direct tumor cell killing and evidence of efficacy was beyond the main scope of this trial, in part because of the heavily pretreated population of patients with advanced disease, the trial size, and its design allowing concomitant administration of dexamethasone. However, the safe and well-tolerated profile of roneparstat that emerged from this clinical experience, combined with the extensive preclinical evidence on the ability of heparanase inhibition to influence the bone marrow microenvironment in myeloma patients, and the synergistic effect of roneparstat when associated with bortezomib or melphalan do raise the possibility of capitalizing on and improving the role of heparanase inhibition in myeloma treatment. In fact, the involvement of heparanase in regulating the cross-talk between the tumor and the host myeloma microenvironment and the preclinical activity of roneparstat in combination regimens8–10 have been widely described (see Online Supplementary Material). Of particular interest, Ramani et al.10 reported a very significant effect on tumor burden when roneparstat was combined with bortezomib or melphalan in the treatment of mice bearing an aggressive myeloma in an in vivo model formed by CAG human myeloma cells expressing high levels of heparanase (CAG-HPSE cells). Therefore, even though this phase I study does not provide evidence of a potential direct anti-myeloma effect of ronepartstat in humans, exploration of roneparstat in combination regimens for the treatment of MM is justified and should be the next step in this field.
Supplementary Material
Acknowledgments
The authors are grateful to prof. Israel Vlodavsky for his pioneering work in the heparanase field. His continuous and valuable scientific contribution has been the pillar that made possible the transition from basic science to the development of heparanase inhibitors as novel therapeutic agents.
Footnotes
Information on authorship, contributions, and financial & other disclosures was provided by the authors and is available with the online version of this article at www.haematologica.org.
References
- 1.Kawano Y, Moschetta M, Manier S, Glavey S, et al. Targeting the bone marrow microenvironment in multiple myeloma. Immunol Rev. 2015; 263(1):160−172. [DOI] [PubMed] [Google Scholar]
- 2.Vlodavsky I, Singh P, Boyango I, et al. Heparanase: from basic research to therapeutic applications in cancer and inflammation. Drug Resist Updat. 2016; 29:54−75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sanderson RD, Elkin M, Rapraeger AC, Ilan N, Vlodavsky I. Heparanase regulation of cancer, autophagy and inflammation: new mechanisms and targets for therapy. FEBS J. 2017; 284(1):42−55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Masola V, Secchi MF, Gambaro G, Onisto M. Heparanase as a target in cancer therapy. Curr Cancer Drug Targets. 2014; 14(3):286−293. [DOI] [PubMed] [Google Scholar]
- 5.Kelly T, Miao HQ, Yang Y, et al. High heparanase activity in multiple myeloma is associated with elevated microvessel density. Cancer Res. 2003; 63(24):8749−8756. [PubMed] [Google Scholar]
- 6.Yang Y, Yaccoby S, Liu W, et al. Soluble syndecan-1 promotes growth of myeloma tumors in vivo. Blood. 2002; 100(2):610−617. [DOI] [PubMed] [Google Scholar]
- 7.Yang Y, Macleod V, Miao HQ, et al. Heparanase enhances syndecan-1 shedding: a novel mechanism for stimulation of tumor growth and metastasis. J Biol Chem. 2007; 282(18):13326−13333. [DOI] [PubMed] [Google Scholar]
- 8.Purushothaman A, Chen L, Yang Y, Sanderson RD. Heparanase stimulation of protease expression implicates it as a master regulator of the aggressive tumor phenotype in myeloma. J Biol Chem. 2008; 283(47):32628−32636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ramani VC, Vlodavsky I, Ng M, et al. Chemotherapy induces expression and release of heparanase leading to changes associated with an aggressive tumor phenotype. Matrix Biol. 2016; 55:22−34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ramani VC, Zhan F, He J, et al. Targeting heparanase overcomes chemoresistance and diminishes relapse in Myeloma. Oncotarget. 2016; 7(2):1598−1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Naggi A, Casu B, Perez M, et al. Modulation of the heparanaseinhibiting activity of heparin through selective desulfation, graded N-acetylation, and glycol splitting. J Biol Chem. 2005; 280(13):12103−12113. [DOI] [PubMed] [Google Scholar]
- 12.Pala D, Rivara S, Mor M, et al. Kinetic analysis and molecular modeling of the inhibition mechanism of roneparstat (SST0001) on human heparanase. Glycobiology. 2016; 26(6):640−654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ritchie JP, Ramani VC, Ren Y, et al. SST0001, a chemically modified heparin, inhibits myeloma growth and angiogenesis via disruption of the heparanase/syndecan-1 axis. Clin Cancer Res. 2011; 17(6):1382−1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nobili L, Goegan M, Pezzetta D, Pace S, Barbieri P, Molinari A. Validation of a homogeneous fluorescence quenching assay for the direct quantification of a heparin-like compound in human plasma. EBF. November 19−21, 2014; abstract. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.