Abstract
As part of the UK NCRI AML17 trial, adult patients with acute myeloid leukemia in remission could be randomized to receive the mammalian target of rapamycin inhibitor everolimus, sequentially with post-induction chemotherapy. Three hundred and thirty-nine patients were randomised (2:1) to receive everolimus or not for a maximum of 84 days between chemotherapy courses. The primary endpoint was relapse-free survival. At 5 years there was no difference in relapse-free survival [29% versus 40%; odds ratio 1.19 (0.9-1.59) P=0.2], cumulative incidence of relapse [60% versus 54%: odds ratio 1.12 (0.82-1.52): P=0.5] or overall survival [45% versus 58%: odds ratio 1.3 (0.94-1.81): P=0.11]. The independent Data Monitoring Committee advised study termination after randomization of 339 of the intended 600 patients because of excess mortality in the everolimus arm without any evidence of beneficial disease control. The delivery of the everolimus dose was variable, but there was no evidence of clinical benefit in patients with adequate dose delivery compared with no treatment. This study suggests that the addition of mammalian target of rapamycin inhibition to chemotherapy provides no benefit.
Introduction
The majority (70-85%) of younger patients with acute myeloid leukemia (AML) will enter complete morphological remission with any one of a variety of induction treatments. However, nearly half will relapse. It is being increasingly recognized that a substantial proportion of those subjects in morphological remission do actually have residual disease, as determined by techniques of minimal/measurable residual disease assessment (flow cytometry or quantitative polymerase chain reaction1,2). In our previous studies we endeavored to define the optimum post-remission chemotherapy. To date we have concluded that, apart from transplantation, following two induction courses of anthracycline-containing therapy, two consolidation courses of cytarabine (Ara-C) is adequate.3 One of the aims of the UK NCRI AML17 trial was to explore the effects of a further reduction in the total number of chemotherapy courses from four to three, as well as the addition of molecularly targeted treatments to consolidation therapy. Among these was the incorporation of the inhibitor of the mammalian target of rapamycin (mTOR), everolimus.
There is plausible pre-clinical evidence both in vitro and in vivo that mTOR inhibition could be beneficial in AML. mTOR is a serine/threonine protein kinase that is predominantly modulated by PI3K-AKT-dependent mechanisms and acts as a central regulator of cellular metabolism, growth and survival.4 Dysregulation of the mTOR pathway is closely associated with cancers including AML,5,6 and other human diseases. Part of the rationale is the evidence of constitutive activation of the PI3K-AKT pathway in 90% of AML samples and the demonstration that this activation is central to the survival of AML blasts but not to that of normal CD34+ cells.7 The concept that everolimus may have the potential to eliminate leukemia-initiating stem cells while sparing normal hematopoietic stem cells is also appealing. In vivo evidence in NOD/SCID mice has suggested that mTOR regulates a critical cell survival pathway in AML stem cells.8,9 In preliminary unrandomized clinical trial, the mTOR inhibitor sirolimus was administered as a single agent to nine relapsed, refractory or poor-risk AML patients for 28 days resulting in partial responses in four, and stable disease in a fifth patient.10 Dephosphorylation of downstream effectors of mTOR was demonstrated. In an ongoing UK trial, 11 elderly patients with primary, relapsed AML have been treated with the combination of low-dose Ara-C and sirolimus. Following a single 28-day course of treatment, of the seven patients eligible for analysis, one had achieved a complete remission, four had obtained a partial remission, one had profoundly hypocellular bone marrow and one patient was a non-responder (Das Gupta, unpublished data). Patients in this trial reliably maintained trough sirolimus levels of 8-16 ng/mL, which are consistent with the published concentrations required to inhibit AML cell growth in vitro. The feasibility of combining mTOR inhibition (sirolimus) with intensive chemotherapy had also been assessed in AML patients in conjunction with the more intensive MEC (mitoxantrone, etoposide and cytarabine) chemotherapy regimen in a phase I dose escalation study and reported in abstract form. In this study standard renal transplant doses of sirolimus were well tolerated and did not increase the non-hematologic toxicity of MEC chemotherapy with a median time to neutrophil recovery of 27 days.11 Based on this background information, the NCRI AML17 trial included the option for eligible patients to be randomized to receive, or not, the mTOR inhibitor everolimus daily between consolidation chemotherapy courses.
Methods
The UK NCRI AML 17 trial (ISRNCTN 55675535) was a large, prospective, phase 3, multicenter trial for patients with newly-diagnosed AML or high-risk myelodysplastic syndrome (>10% marrow blasts), generally under the age of 60 years, open from April 2009 to December 2014 in more than 130 centers in the United Kingdom, Denmark and New Zealand. Through randomization of the participants, it addressed several issues (Online Supplementary Figure S1). Between October 2009 and October 2012, 499 adult patients who did not have acute promyelocytic leukemia had received a first induction course of treatment: those who did not have core-binding factor leukemia, high-risk disease (defined using a multifactorial score12) and were not in the lestaurtinib randomization for patients with FLT3 mutations, could be randomized to receive everolimus, or not, in a 2:1 ratio, between subsequent consolidation chemotherapy courses. The treatment schedules have been set out elsewhere.13 Allogeneic stem cell transplantation was permitted for patients with intermediate- or poor-risk disease with a recommendation of myelo-ablative conditioning for patients aged <35 years and reduced intensity conditioning for intermediate-risk patients aged >45 years, with investigators able to choose an ablative or reduced intensity approach for patients between 35 and 45 years.
Of all adult patients entering the AML17 trial while the everolimus randomization was available, 34% were eligible for such randomization. These patients were randomized to receive, or not, oral everolimus (10 mg daily from 2 days after each chemotherapy course for up to 28 days or until 2 days before the start of the subsequent course, whichever was shorter) between each course of consolidation chemotherapy. In patients allocated three courses of treatment, a final 28-day course of everolimus was given after a 1-week break. In patients with side effects thought to be due to everolimus, subsequent daily doses could be reduced by 50%. If this did not improve tolerability, dosing could be further reduced to alternate days; if these reduced doses were not tolerated, subsequent doses were to be omitted. After 65% (n=146) of the patients randomized to everolimus had been assessed, the independent data monitoring committee recommended, because of increased side effects and reduced compliance, that the starting daily dose of everolimus be reduced to 5 mg with the option to increase to 10 mg if well-tolerated.
Extensive Sanger sequencing (111 genes) was undertaken in 123 patients; NPM1 status was available in 302 patients.
Patients were requested to provide a trough blood sample taken immediately prior to everolimus dosing on day 14 of each treatment course to measure the level of mTOR inhibitory activity in their plasma. The methods for measuring this activity are summarized in Online Supplementary Figure S2 and will be reported more fully elsewhere.
Statistical considerations
All analyses are on an intention-to-treat basis. Categorical endpoints were compared using Mantel-Haenszel tests, giving Peto odds ratios and confidence intervals. Continuous/scale variables were analyzed by Wilcoxon rank sum tests and time-to-event outcomes using the log-rank test, with Kaplan-Meier survival curves. Odds/hazard ratios (OR/HR) <1 indicate benefit for everolimus. All survival percentages refer to 5 years unless otherwise stated.
Stratified analyses were performed with suitable tests for interaction14 and interpreted cautiously.
It was planned to recruit 600 patients to the everolimus randomization, which would have given 85% power to detect a 12.5% difference in the primary endpoint of relapse-free survival, from 50% to 62.5% (HR 0.68). Follow-up is complete until March 1st, 2016 [median follow-up from diagnosis 53.5 months (range, 4.3 – 76.8 months)].
The trial was conducted in accordance with the Declaration of Helsinki, sponsored by Cardiff University and approved by Wales REC3 on behalf of all UK investigators, by the Danish Medicines Agency for sites in Denmark, and by MEDSAFE for sites in New Zealand.
Results
Patients’ characteristics
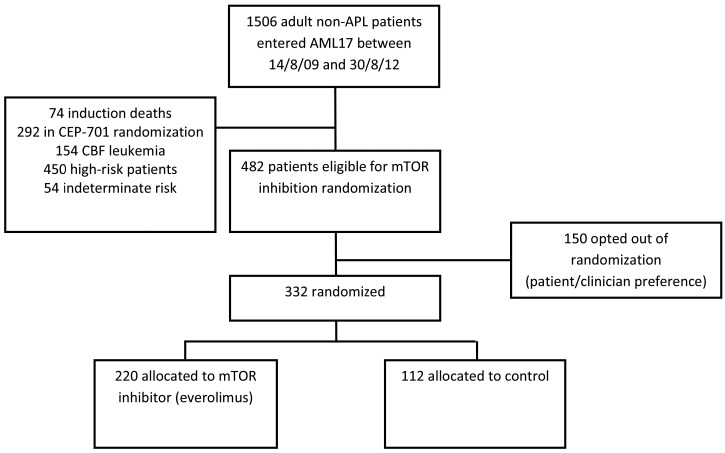
The randomization opened in October 2009. In 2012, the independent data monitoring committee recommended closure of the randomization because of an excess of early mortality in remission with everolimus and no associated evidence of relapse reduction. Between October 2009 and October 2012, 332 of 482 eligible patients were randomized (Figure 1). Their characteristics are shown in Table 1. There were no significant differences in survival outcomes between eligible patients who entered the randomization and those who did not (P=0.8), although patients with higher white blood cell counts, worse performance status and secondary disease were marginally less likely to enter the randomization. The median age was 47 years (range, 16-69). The majority presented with de novo AML and had a WHO performance score of <2. The other protocol treatments given to patients in the everolimus randomization are shown in Table 1. In addition to standard daunorubicin/Ara-C induction, etoposide and gemtuzumab ozogamicin was given to 43% and 45% of patients, respectively, in induction with no difference between the arms.
Figure 1.
CONSORT diagram. APL: acute promyelocytic leukemia; CEP-701: lestaurtinib; CBF: core-binding factor; MTOR: mammalian target of rapamycin;
Table 1.
Patients’ characteristics.
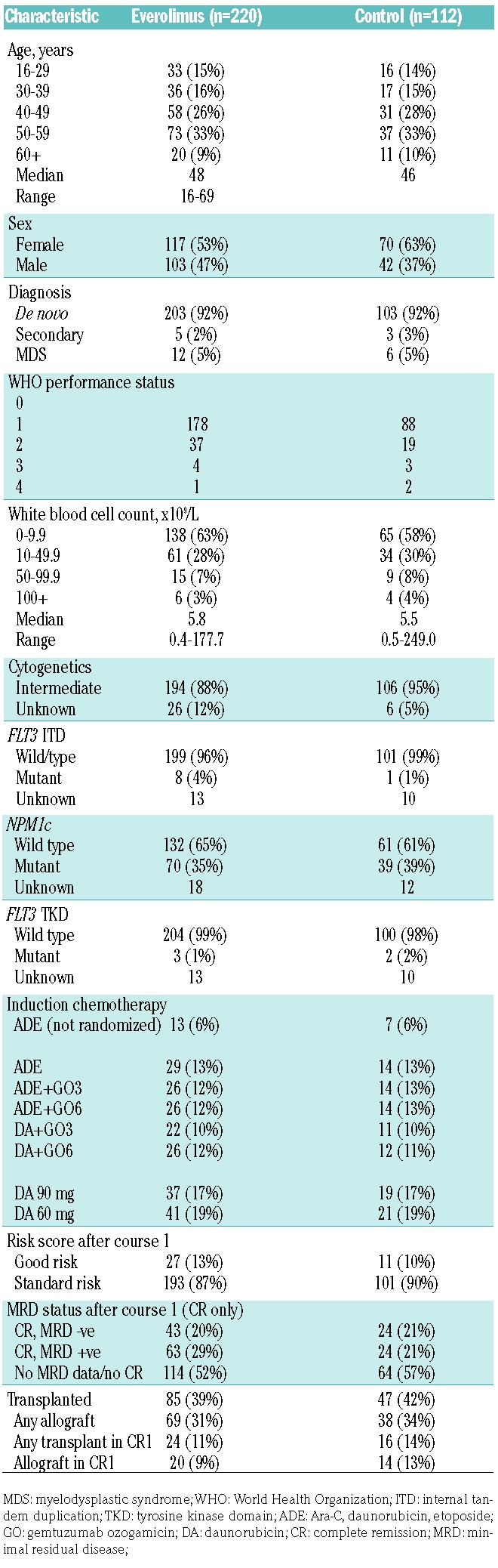
Overall, 132/332 (40%) of patients received a transplant (everolimus 39%, control 42%, P=0.6), with a minority of these (34/132) being allografts in first remission (20 versus 14, P=0.3). There was no evidence of differences in transplantation rates or types of transplants between the arms (any stem cell transplant 39% versus 42%, P=0.6; allograft 31% versus 34%, P=0.6; allograft in first complete remission 9% versus 13%, P=0.3) (Table 1).
Extensive Sanger sequencing (111 genes) was undertaken in 123 patients: the gene panel and distribution are shown in Online Supplementary Figure S3. In addition NPM1 status, determined using previously published methods, was known for 302 patients.
Treatment compliance
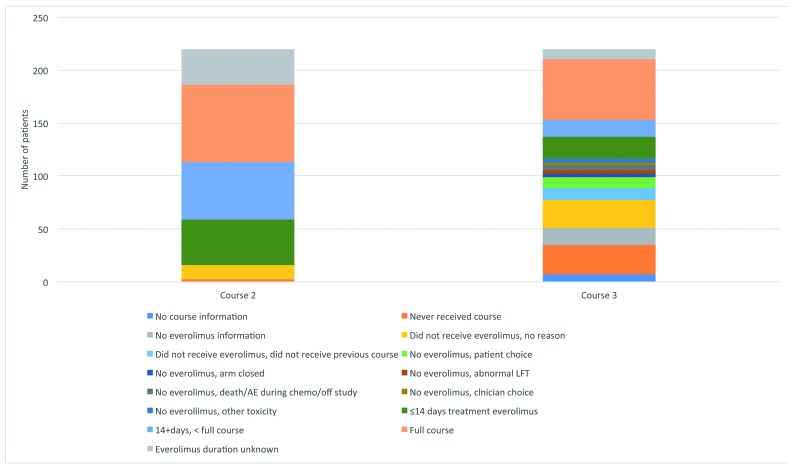
Of the 220 patients allocated to receive everolimus, 16 never started therapy. Approximately 25% of patients did not receive 14 days of everolimus; about half completed the first 28-day course. At the time of the second course of everolimus (course 3 of chemotherapy), 35% of patients for whom information on the second everolimus course was available did not receive the drug (Figure 2). Reasons were given for about two-thirds of patients (39/61): 11 patients had not completed the previous course; 11 patients chose to discontinue the therapy (often because of toxicity in the previous course); in three cases the data monitoring committee had recommended closure of the study with cessation of everolimus treatment; in five cases patients did not reach the starting point for everolimus therapy on protocol; in two cases the clinician decided, and in four other cases, everolimus was not given due to a variety of toxicities.
Figure 2.
Compliance with treatment. AE: adverse event; LFT: liver fuction tests.
Toxicity
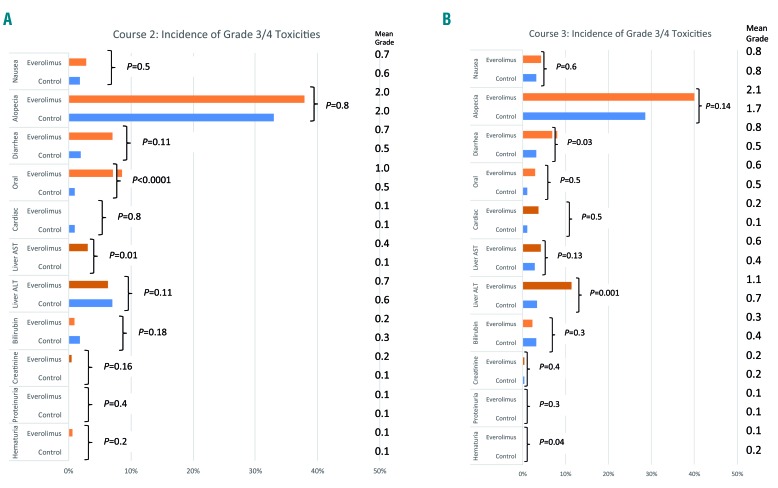
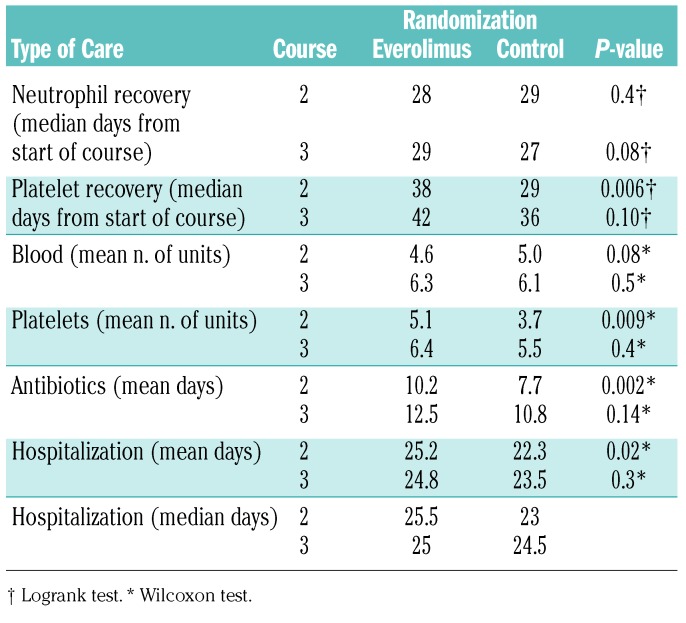
The recorded toxicities are shown in Figure 3. There were more hematologic toxicities in the everolimus arm and these were most obvious after the first everolimus course, with a median time to platelet count recovery to >100×109/L being 9 days longer (39 versus 29 days; P=0.006); this was reflected by a significantly greater requirement for platelet support (Table 3). The kinetics of neutrophil recovery was unaffected by everolimus, but there was significantly more use of antibiotics and a longer stay in hospital with the first course of everolimus, as well as increased oral toxicity (course 1) and higher alanine transaminase levels (course 2).
Figure 3.
Toxicities associated with treatment in courses 2 and 3. (A) Course 2 (B) Course 3.
Table 3.
Recovery and supportive care in everolimus randomization.
Cumulative risk of relapse and death in remission
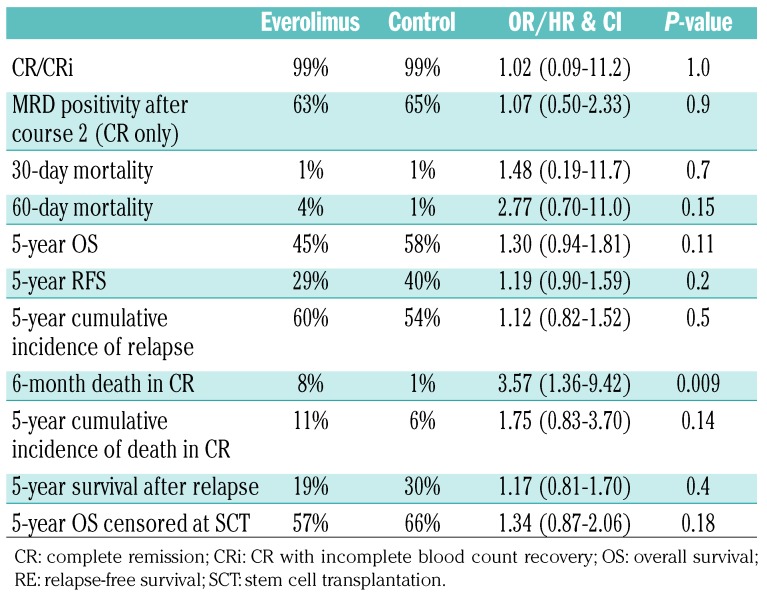
The overall outcomes are shown in Table 2. The cumulative incidence of relapse at 5 years (Figure 4A) did not differ significantly between arms [60% versus 54%, HR 1.12 (0.82-1.52), P=0.5]. There was a significant excess of deaths in remission in the everolimus arm in the first 6 months following randomization [8% versus 1%, HR 3.57 (1.36-9.42), P=0.009], with no significant differences thereafter, leading to a non-significant excess of overall mortality with everolimus [11% versus 6%, HR 1.75 (0.83-3.70), P=0.14] (Figure 4B). In the first 6 months there were 17 deaths in remission in the everolimus arm versus 1 death in the control arm: the causes of these deaths were infection (9 versus 1), infection + hemorrhage (3 versus 0), hemorrhage/cardiovascular accident (3 versus 0), cardiac (1 versus 0) and multiple (1 versus 0). Beyond 6 months, there were six deaths in each of the two arms, with the causes of these deaths in remission being infection (1 versus 1), cardiac (1 versus 0), hepatic (1 versus 0), second cancer (1 versus 0), graft-versus-host disease (0 versus 1), multiple (0 versus 2) and unknown/other (2 versus 2).
Table 2.
Clinical outcomes by treatment arm.
Figure 4.
Relapse, death in remission, relapse-free survival and overall survival within the everolimus randomisation: (A) Cumulative incidence of relapse; (B) cumulative incidence of death in remission; (C) relapse-free survival; (D) overall survival.
Relapse-free and overall survival
Both relapse-free and overall survival rates were non-significantly inferior in the everolimus arm (Figure 4C,D), reflecting the adverse hazard ratios for both relapse and death in remission, with no evidence of differences in salvage between arms after relapse [relapse-free survival: 29% versus 40%, HR 1.19 (0.90-1.59), P=0.2; overall survival: 45% versus 58%, HR 1.30 (0.94-1.81), P=0.11]. A sensitivity analysis censoring patients at the time of stem cell transplantation showed results which were consistent with the overall analysis (Table 2).
Exploratory analyses
Correlations with everolimus plasma inhibitory activity, determined by the assay used in this study, did not show convincing patterns. Even patients whose samples showed deep and sustained inhibition did not have an associated reduction in relapse (Online Supplementary Figure S2). There was no relationship between the level of inhibition and toxicity or excess mortality. Prior induction chemotherapy, age, gender, white blood cell count, and minimal residual disease status after course one all had no impact on outcomes (Online Supplementary Figure S4A). In addition no relationship was found between other treatment modalities given and response, and no gene mutation, including the 111 genes assayed by Sanger sequencing in 123 patients, was shown to be associated with a differential response (Online Supplementary Figure S5). Because of concerns about compliance with everolimus treatment, relapse-free survival was compared between patients with satisfactory drug delivery (defined as at least 14 days of treatment per course), those with inadequate drug delivery (less than 14 days treatment per course) and those allocated to no treatment. Although patients in whom drug delivery was inadequate (n=85) had a worse relapse-free survival (29%) there was no difference in relapse-free survival between patients with satisfactory drug delivery (n=63) at 41% and no everolimus treatment (n=99) at 40% (Online Supplementary Figure S4).
Discussion
In this trial there was no benefit of the addition of everolimus to post-induction chemotherapy, despite the pre-clinical in vitro and in vivo rationale for the use of this mTOR inhibitor. The main explanations appear to be the observed excess toxicity, which was primarily gastrointestinal (mucositis and diarrhea), and biochemical evidence of liver toxicity at the dose chosen. Infection was a major issue in the first 6 months of treatment with 12 versus 1 deaths attributed to infection in the everolimus and control arms, respectively. This did not appear to be the result of prolonged neutropenia but may be attributable to the immunosuppressive effects of everolimus when given with chemotherapy, which reflects what has been seen with the use of the mTOR inhibitor in solid tumors.15 This in turn contributed to sub-optimal drug delivery for many patients. The chosen schedule of 10 mg daily was not feasible in this setting, but drug delivery improved when a 5 mg daily dose was introduced. Other studies in leukemia have used equivalent schedules16,17 or a loading dose (12 mg) followed by 4 mg/day for 7 days per cycle11 or lower doses in combination with low-dose Ara-C.18 However even when the subgroup of good compliers was compared separately, there was no evidence of improved disease control.
We had hoped that the development of an assay to quantitate plasma inhibitory activity would provide insight into response or toxicity, but unlike the experience of plasma inhibitory activity in the setting of an FLT3 inhibitor,19,20 consistent correlations were not found. In a phase 2 study of patients with relapsed AML treated with clofarabine and temsirolomus, correlation of response to dephosphorylation of pS6RP (S6 ribosomal protein) was demonstrated.21 However the target cells were the patients’ own blasts, which were not available in the current study and it was unclear whether the clinical outcome was superior to that which clofarabine alone could achieve.
Finally the mTOR inhibitors tested to date have been inhibitors of the TORC1 pathway. This may be by-passed by the TORC2 pathway which is insensitive to this class of mTOR inhibitors, but may be sensitive to agents which produce dual inhibition.
Supplementary Material
Acknowledgments
The authors are grateful to Novartis for the provision of everolimus, to Cancer Research UK for research funding of the trial and to the investigators, research staff and patients in the participating sites:
Aalborg Hospital: Maria Kallenbach; Aarhus University Hospital: Hans Beier Ommen, Jan Maxwell Norgaard; Aberdeen Royal Infirmary: Dominic Culligan; Addenbrookes Hospital: George Follows, Jenny Craig; Auckland City Hospital: Lucy Pemberton, Richard Doocey, Sophie Lee, Timothy Hawkins; Barnet General Hospital: Andres Virchis; Barts and the London NHS Trust: Jamie Cavenagh, Matthew Smith; Basingstoke and North Hampshire Foundation NHS Trust: Sylwia Simpson; Beatson West of Scotland Cancer Centre: Mark Drummond; Belfast City Hospital: Claire Arnold, Mary Francis McMullin, Robert Cuthbert; Birmingham Heartlands Hospital: Donald Milligan, Guy Pratt, Matthew Lumley, Shankara Paneesha; Blackpool Victoria Hospital NHS Foundation Trust: Paul Cahalin; Borders General Hospital: John Tucker; Bradford Royal infirmary: Adrian Wiliams, Lisa Newton, Sam Ackroyd; Bristol Haematology and Oncology Centre: Priyanka Mehta; Chesterfield Royal Hospital: Mark Wodzinski, Robert Cutting; Christchurch Hospital: Ruth Spearing, Steve Gibbons; Christie Hospital NHS Trust: Mike Dennis; Countess of Chester Hospital: Salah Tuegar; Crosshouse Hospital: Julie Gillies; Derby Hospitals NHS Foundation Trust: Juanah Addada; Derriford Hospital: Hannah Hunter, Tim Nokes; Doncaster Royal Infirmary: Stuti Kaul; Dorset County Hospital NHS Foundation Trust: Akeel Moosa; East Kent Hospitals University NHS Foundation Trust: Jindriska Lindsay, Vijay Ratnayake; East Sussex Hospitals NHS Trust: Richard Grace; Falkirk and District Royal Infirmary: Christopher Brammer, Marie Hughes; Glan Clwyd Hospital: Christine Hoyle, Earnest Heartin, Margaret Goodrick; Gloucestershire Royal Hospital: Adam Rye, Sally Chown; Great Western Hospital: Alex Sternberg, Atherton Gray, Norrbert Blessing; Guys and St Thomas’ Foundation Trust: Kavita Raj, Robert Carr; Hairmyres Hospital: Iain Singer; Heatherwood and Wexham Park NHS Foundation Trust: Nicola Bienz, Simon Moule; Hereford County Hospital: Sara Willoughby; Herlev Hospital: Morten Krogh Jensen, Peter Moller; Hillingdon Hospital: Riaz Janmohamed, Richard Kaczmarski; Hull Royal Infirmary: Sahra Ali; James Cook University Hospital: Ray Dang; James Paget University Hospital: Cesar Gomez, Shala Sadullah; John Radcliffe Hospital: Paresh Vyas; Kettering General Hospital: Isaac Wilson-Morkeh, Matthew Lyttelten; Leicester Royal Infirmary: Ann Hunter, Murray Martin; Lincoln County Hospital: Kandeepan Saravanamuttu; Maidstone Hospital: Evangelia Dimitriadou; Manchester Royal Infirmary: Eleni Tholouli, Guy Lucas; Milton Keynes Hospital NHS Foundation Trust: Moez Dungarwalla; Monklands Hospital: John Murphy, Lindsey Mitchell, Pamela Paterson; New Cross Hospital: Sunil Hada, Supratik Basu; Ninewells Hospital and Medical Centre: Keith Gelly; Norfolk and Norwich University Hospital NHS Foundation Trust: Matthew Lawes; Northampton General Hospital: Angela Bowen, Sajan Mittal, Suchitra Krishnamurthy@Ngh.Nhs.Uk; Nottingham University Hospitals NHS Trust: Emma Dasgupta, Jenny Byrne, Kate Forman, Nigel Russell; Odense University Hospital: Claus Marcher, Lone Friis, Poul Gram Hansen; Peterborough District Hospital: Kanchan Rege; Pinderfields General Hospital: David Wright, Mary Chapple, Paul Moreton; Poole General Hospital: Fergus Jack; Queen Alexandra Hospital: Mary Ganczakowski, Tanya Cranfield; Queen Elizabeth Hospital, Birmingham: Charles Craddock, Jim Murray; Queen Elizabeth Hospital, Norfolk: Jane Keidan; Queens Hospital, Romford: Claire Hemmaway; Raigmore Hospital NHS Highland: Chris Lush, Peter Forsyth; Rigshospitalet: Carsten Niemann, Lars Kjeldsen, Ole Wei Bjerrum, Ove Juul Nielsen, Peter Kampmann; Rotherham General Hospital: Arun Alfred; Royal Berkshire Hospital: Henri Groch, Stuart Mucklow; Royal Cornwall Hospital: Bryson Pottinger, Richard Noble; Royal Devon and Exeter Hospital: Claudius Rudin, Malcolm Hamilton, Paul Kerr; Royal Free Hospital: Panos Kottaridis; Royal Hallamshire Hospital: Chris Dalley, John Snowden; Royal Liverpool University Hospital: Rahuman Salim, Richard Clark; Royal Marsden Hospital: Mark Ethell; Royal Oldham Hospital: Allameddine Allameddine, David Osborne, Hayley Greenfield, Sumaya Elhanash, Vivek Sen; Royal Surrey County Hospital: Johannes Devos, Louise Hendry; Royal Sussex County Hospital: Timothy Corbett; Russell’s Hall Hospital: Jeff Neilson; Salford Royal Hospital: John Houghton, Simon Jowitt, Sonya Zaman; Salisbury Hospital NHS Foundation Trust: Jonathan Cullis, Tamara Everington; Sandwell Hospital: Farooq Wandoo, Yasmin Hasan; Singleton Hospital: Saad Ismail; South Devon Healthcare NHS Foundation Trust: Deborah Turner, Nicholas Rymes; Southampton University Hospital NHS Trust: Deborah Richardson, Kim Orchard, Matthew Jenner; St Helens and Knowsley NHS Trust: Toby Nicholson; St James University Hospital: David Bowen; St Richard’s Hospital: Sarah Janes; Stafford Hospital: Andrew Amos; Stoke Mandeville Hospital: Helen Eagleton; Sunderland Royal Hospital: Annette Nicolle, Scott Marshall; Taunton and Somerset Foundation Trust: Sarah Allford; The Newcastle upon Tyne NHS Foundation Trust: Gail Jones, Graham Jackson; University College London Hospitals: Anthony Goldstone, Asim Khwaja, Kirit Ardeshna, Nishal Patel; University Hospital Aintree: Barbara Hammer, Walid Sadik; University Hospital Coventry and Warwickshire NHS Trust: Mekkali Narayanan, Nicholas Jackson, Peter Rose, Syed Bokhari; University Hospital of North Staffordshire NHS Trust: Andrew Stewart, Kamaraj Karunanithi, Neil Phillips, Srinivas Pillai; University Hospital of North Tees and Hartlepool: Zor Maung; University Hospital of Wales: Jonathan Kell, Steve Knapper; Victoria Hospital NHS Fife: Stephen Rogers; Waikato Hospital: Hugh Goodman, Humphrey Pullon; Wellington Hospital: John Carter; Western General Hospital: Peter Johnson, Ph Roddie, Annielle Hung; Worcestershire Royal Hospital: Juliet Mills; Worthing Hospital: Santosh Narat.
Footnotes
Check the online version for the most updated information on this article, online supplements, and information on authorship & disclosures: www.haematologica.org/content/103/10/1654
References
- 1.Buckley SA, Wood BL, Othus M, et al. Minimal residual disease prior to allogeneic hematopoietic cell transplantation in acute myeloid leukemia: a meta-analysis. Haematologica. 2017;102(5):865–873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ivey A, Hills RK, Simpson MA, et al. Assessment of minimal residual disease in standard-risk AML N Engl J Med. 2016; 374(5):422–433. [DOI] [PubMed] [Google Scholar]
- 3.Burnett AK, Hills RK, Milligan DW, et al. Attempts to optimize induction and consolidation treatment in acute myeloid leukemia: results of the MRC AML12 trial. J Clin Oncol. 2010;28(4):586–595. [DOI] [PubMed] [Google Scholar]
- 4.Dobashi Y, Watanabe Y, Miwa C, Suzuki S, Koyama S. Mammalian target of rapamycin: a central node of complex signaling cascades. Int J Clin Exp Pathol. 2011;4(5):476–495. [PMC free article] [PubMed] [Google Scholar]
- 5.Zoncu R, Efeyan A, Sabatini DM. mTOR: from growth signal integration to cancer, diabetes and ageing. Nat Rev Mol Cell Biol. 2011;12(1):21–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Park S, Chapuis N, Tamburini J, et al. Role of the PI3K/AKT and mTOR signaling pathways in acute myeloid leukemia. Haematologica. 2010;95(5):819–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Recher C, Beyne-Rauzy O, Demur C, et al. Antileukemic activity of rapamycin in acute myeloid leukemia. Blood. 2005;105(6):2527–2534. [DOI] [PubMed] [Google Scholar]
- 8.Xu Q, Simpson SE, Scialla TJ, Bagg A, Carroll M. Survival of acute myeloid leukemia cells requires PI3 kinase activation. Blood. 2003;102(3):972–980. [DOI] [PubMed] [Google Scholar]
- 9.Xu Q, Thompson JE, Carroll M. mTOR regulates cell survival after etoposide treatment in primary AML cells. Blood. 2005;106(13):4261–4268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Luger S, Perl A, Kemner A, et al. A phase I dose escalation study of the mTOR inhibitor sirolimus and MEC chemotherapy targetting signal transduction in leukemic stem cells for acute myeloid leukemia. Blood. 2006;108(11):161. [Google Scholar]
- 11.Perl AE, Kasner MT, Tsai DE, et al. A phase I study of the mammalian target of rapamycin inhibitor sirolimus and MEC chemotherapy in relapsed and refractory acute myelogenous leukemia. Clin Cancer Res. 2009;(15):6732–6739. [DOI] [PubMed] [Google Scholar]
- 12.Burnett AK, Hills RK, Wheatley K, et al. A sensitive risk score for directing treatment in younger patients with AML Blood. 2006; 108(11):18. [Google Scholar]
- 13.Knapper S, Russell N, Gilkes A, et al. A randomized assessment of adding the kinase inhibitor lestaurtinib to first-line chemotherapy for FLT3-mutated AML. Blood. 2017; 129(9):1143–1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Early Breat Cancer Trialists Collaborative Group (EBCTCG). Treatment of early breast cancer. 1. Worldwide evidence 1985–1990, Oxford University Press, USA, 1990 [Google Scholar]
- 15.Kaymakcalan MD, Je Y, Sonpavde G, et al. Risk of infections in renal cell carcinoma (RCC) and non-RCC patients treated with mammalian target of rapamycin inhibitors. Br J Cancer. 2013;108(12):2478–2484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Daver N, Boumber Y, Kantarjiian H, et al. A Phase l/ll study of the mTOR inhibitor everolimus in combination with hyperCVAD chemotherapy in patients with relapsed/refractory acute lymphoblastic leukemia. Clin Cancer Res. 2015;21(12): 2704–2714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yee AL, Zeng Z, Konople Phase I/II study of the mammalian target or rapamycin inhibitor everolimus (RAD001) inn patients with relapsed or refractory hematologic malignancies. Clin Cancer Res. 2006;12(17): 5165–5173. [DOI] [PubMed] [Google Scholar]
- 18.Wei AH, Sadawarte S, Catalono J. Phase Ib study combining mTOR inhibitor everolimus (RAD001) with low-dose cytarabine in untreated elderly AML. Blood. 2010;116(21):3299. [Google Scholar]
- 19.Levis M, Brown P, Smith BD, et al. Plasma inhibitory activity (PIA): a pharmacodynamic assay reveals insights into the basis for cytotoxic response to FLT3 inhibitors. Blood. 2006;108(10):3477–3483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Knapper S, Burnett AK, Littlewood T, et al. A Phase 2 trial of the FLT3 inhibitor lestaurtinib (CEP701) as first-line treatment for older patients with acute myeloid leukemia not considered fit for intensive chemotherapy. Blood. 2006;108(10):3262–3270. [DOI] [PubMed] [Google Scholar]
- 21.Amadori S, Stasi R, Martelli AM. Temsirolimus, an mTOR inhibitor, in combination with lower-dose clofarabine as salvage therapy for older patients with acute myeloid leukaemia: results of a phase II GIMEMA study (AML1107) Br J Haematol. 2012;156(2):205–212. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.