Abstract
Breast cancer (BC) is the most common cancer and principal cause of death among females worldwide. Invasion and metastasis are major causes which influence the survival and prognosis of BC. Therefore, to understand the molecule mechanism underlying invasion and metastasis is paramount for developing strategies to improve survival and prognosis in BC patients. Recent studies have reported that long non-coding RNAs (lncRNAs) play critical roles in the regulation of BC invasion and metastasis through a variety of molecule mechanisms that endow cells with an aggressive phenotype. In this article, we focused on the function of lncRNAs on BC invasion and metastasis through participating in epithelial-to-mesenchymal transition, strengthening cancer stem cells generation, serving as competing endogenous lncRNAs, influencing multiple signaling pathways as well as regulating expressions of invasion–metastasis related factors, including cells adhesion molecules, extracellular matrix, and matrix metallo-proteinases. The published work described has provided a better understanding of the mechanisms underpinning the contribution of lncRNAs to BC invasion and metastasis, which may lay the foundation for the development of new strategies to prevent BC invasion and metastasis.
Keywords: Breast cancer (BC), Invasion, Long non-coding RNAs (lncRNAs), Metastasis
Introduction
Breast cancer (BC), as the most common malignant tumor among women, is one of the leading causes of cancer deaths worldwide. In 2017, approximately 252710 new cases of invasive BC and 40610 BC deaths are expected to occur among US women [1]. BC invasion and metastasis are the main causes of BC-related deaths. Bone, lung, brain, and liver are the primary target sites of BC metastasis [2]. BC metastasis is the spread of cancer cells to tissues and organs beyond where the tumor originated and the formation of new tumors which may eventually result in the death of most BC patients [3]. At least half of the cancer patients already present clinically detectable metastatic disease when the time of cancer diagnosis [4]. A higher number of cancer patients might also have micrometastases that is beyond conventional detection techniques. Thus, cancer metastasis is the most threatening event in cancer patients [5]. BC invasion and metastasis as intricate process means that cancer cells escape from the primary cancer and penetrate the blood circulation [6]. The process involves both the selection of traits that are advantageous to cancer cells and the concomitant recruitment of traits in the tumor stroma that accommodate invasion by metastatic cells [7,8]. The course of BC invasion and metastasis entails a series of molecules such as cells adhesion molecules (CAMs), extracellular matrix (ECMs), and matrix metallo-proteinases (MMPs). It also involves the biological progresses including epithelial-to-mesenchymal transition (EMT) and cancer stem cells (CSCs) formation that cooperate on the formation of secondary tumors in distant organs [2,9]. Long non-coding RNAs (lncRNAs) are a novel class of RNA transcripts that are longer than 200 nucleotides (nt) in length without protein-coding capacity. The major functions of lncRNAs include: (1) participating in chromosome rearrangement and histone modification, (2) transcribing and interfering, (3) stabilizing mRNA, and (4) modifying alternative splicing sequence [10]. Recent studies have shown that lncRNAs exerted critically roles in multiple cancer biological processes, including carcinogenesis, apoptosis, differentiation, proliferation, invasion as well as metastasis [11]. Accumulating evidence has shown that ectopic expression of lncRNAs served as carcinogenic factors or tumor suppressors in BC invasion and metastasis [12,13]. In this review, we will sum up the precise mechanism of lncRNAs function on BC invasion and metastasis and reveal the clinical significance of dysregulated lncRNAs in BC metastasis. Knowledge obtained from this review could assist in the development of new strategies to treat or prevent the metastatic BC.
LncRNAs participate in process of BC invasion and metastasis
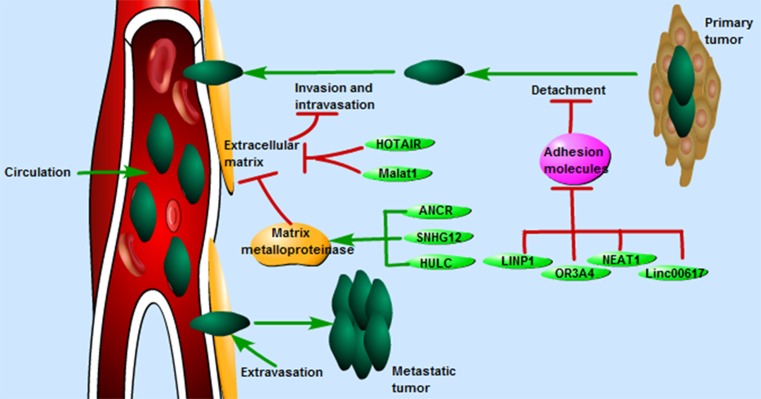
The process of BC invasion and metastasis is complex, which incorporates molecular factors, multiple cells, and stages. Some cancer cells detach from primary tumor through the repression of CAMs and the disruption of intercellular adhesion (detachment), followed by these cells invading through the ECMs and breaking down of ECMs (invasion), thus entering the circulation (intravasation). From this point, these cancer cells move away from the primary tumor and circulate in the blood circulation. Some cancer cells will adopt a process to leave the blood circulation (extravasation), in which cells adhere and penetrate the blood vessel again, eventually develop a secondary tumor at the other site [14,15] (Figure 1).
Figure 1. The process of BC invasion and metastasis includes detachment, invasion, intravasation, circulation, and extravasation.
LncRNAs such as NEAT1, linc00617, OR3A4, LINP1, HOTAIR, Malat1, SNHG12, HULC, ANCR, and BANCR were reported to participate the process of BC invasion and metastasis by regulating different molecules including CAMs, ECMs, and MMPs.
CAMs
CAMs include immunoglobulin superfamily, cadherins, integrins, and selectins which provide essential links between the extracellular environment and the intracellular signaling pathways. Thus, CAMs play key roles in cells behaviors such as differentiation, apoptosis, invasion, and even metastasis [16,17]. Among them, E-cadherin (E-cad) as the most well-studied member of the CAMs displays a crucial type of cell–cell adhesion to hold cancer cells tight together. An increasing number of studies have shown that down-regulation of E-cad by lncRNAs could decrease the strength of cancer cellular adhesion, resulting in the increase in cellular motility. This in turn may allow cells to cross the CAMs and invade surrounding blood vessels in various cancers. For example, overexpression of lncRNA SPRY4-IT1 could increase in vitro motility of esophageal squamous cells carcinoma cells via decrease in E-cad [18]. In colon cancer, lncRNA activated by TGF-β (lncRNA-ATB) mediated E-cad repression may promote the progression of cancer and predicts poor prognosis [19]. In another study, lncRNA H19 levels were remarkably increased in bladder cancer tissues, and up-regulated H19 promoted bladder cancer cells migration by down-regulation of E-cad [20]. In BC, lncRNAs such as NEAT1, linc00617, OR3A4, and LINP1 were up-regulated in BC samples and significantly promoted the invasion and metastasis capacity in BC cells through decreasing the expression of E-cad [21–24].
ECMs
ECMs such as collagen, fibrinogen, laminin, fibronectin, and vitronectin provide structural and biochemical support to the surrounding cells. ECMs are essential for many cellular processes, including development, migration, and proliferation [25–28]. LncRNAs play key roles in the complex dynamics of cancer cells invasion and metastasis through the regulation of ECMs. The elevation of lncRNA HOTAIR mediated invasion and metastasis of BC. HOTAIR expression exhibited robust induction in laminin-rich ECMs through the canonical ECM signaling pathway, namely integrins and Src kinase [29]. In addition, lncRNA Malat1 knockdown could also increase adhesion and inhibit migration of BC cells through the up-regulation of Tenascin Xb (Tnxb), Tnxb as an ECM protein that has been shown to have anti-metastatic property [12].
MMPs
MMPs act important roles in tissue remodeling associated with various biology processes such as morphogenesis, angiogenesis, tissue repair, and metastasis. MMPs are also important components of cells invasion capable of degrading a range of ECMs proteins allowing cancer cells to migrate and invade [30–32]. Recent researches confirmed that lncRNAs regulate tumor invasion and metastasis partly because of their abilities to influence the expressions of MMPs. Such as, lncRNA SNHG12 was strongly up-regulated in Triple-negative BC (TNBC). Moreover, SNHG12 could promote BC cells migration by increasing expression of MMP13 [33]. In addition, HULC was another up-regulated lncRNA in TNBC tissues and cells lines. The increased HULC effectively promoted TNBC cells metastasis through regulating the expressions of MMP-2 and MMP-9 [34]. MMP-2 and MMP-9 could degrade basement-membrane collagen at the site of local invasion, thereby promoting cancer cells invasion and metastasis [35]. The expression of MMP-2 and MMP-9 could also be regulated by lncRNA ANCR. ANCR could act as a negative regulator of metastasis in BC cells through repressing MMP-9 and MMP-2 expression [36]. The expression level of lncRNA BANCR in BC tissues was significantly increased. After BANCR knockdown in BC MCF-7 cells, the cells invasion and metastasis capacity was significantly inhibited by decreasing expression of MMP-2 and MMP-9 [37].
Collectively, these findings revealed that lncRNAs such as NEAT1, linc00617, OR3A4, LINP1, HOTAIR, Malat1, SNHG12, HULC, ANCR, and BANCR affected BC cells invasion and metastasis through regulating different molecules including CAMs, ECMs, and MMPs (Figure 1). Therefore, lncRNAs may serve as therapeutic targets for BC, particularly in patients with BC metastasis.
LncRNAs participate in BC metastasis via affecting EMT
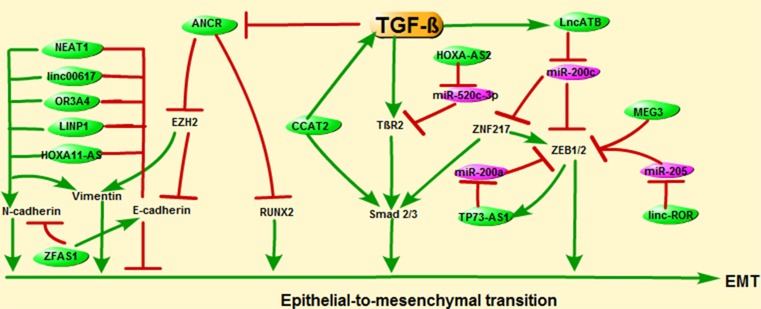
EMT is a progression of cellular plasticity critical for BC cells migration and metastasis. It is characterized by the combined an increased expression of mesenchymal markers, such as vimentin, N-cadherin, fibronectin, and loss of epithelial cells junction proteins, including E-cad, claudins, α-catenin, ZO-1, and occludin [38–40]. Transforming growth factor-β (TGF-β) signaling pathway is a key regulator of various cancer biology, including cancer cells migration and invasion. TGF-β signaling pathway acts as an important regulator for EMT process through influencing the expressions of EMT-associated genes such as Zinc finger E-box-binding homeobox (ZEB), C/EBPb, RhoA, E-cad, vimentin, SNAIL, etc. [6].
Accumulating evidence shows that lncRNAs act crucial roles on EMT to influence BC metastasis. For instance, lncRNA ANCR could restrain the TGF-β1-induced EMT by inhibiting the phosphorylation of runt-related transcription factor 2 (RUNX2). On the contrary, TGF-β could decrease ANCR expression through decreasing the histone acetylation at ANCR promoter [41]. Moreover, ANCR could facilitate the ubiquitination and degradation of EZH2 (the Enhancer of Zeste Homolog 2), hence inhibit the invasion and metastasis of BC cells. The forced expression of EZH2 (an EMT inducer) increased the vimentin expression and decreased the E-cad expression [36]. LncRNA CCAT2 also plays an essential role in BC tumorigenesis, growth, and metastasis. The level of CCAT2 in BC tissues was significantly increased compared with adjacent normal tissues. The increased amount of CCAT2 promoted the proliferation, invasion, and migration by significantly reinforcing the expressions of TGF-β and Smad2 in BC cells [42]. Moreover, lncRNA-ATB was the most remarkably up-regulated lncRNA in trastuzumab-resistant (TR) SKBR-3 cells. The up-regulated lncRNA-ATB could promote invasion–metastasis cascade in BC by competitively binding miR-200c, increasing ZNF-217 and ZEB1, and then inducing EMT [43]. What is more, the increased ZNF217 could further increase the activity of TGF-β signaling by transcriptionally activating Smad 2/3 and ZEB [44]. Furthermore, lncRNA HOXA cluster antisense RNA2 (HOXA-AS2) was also up-regulated in BC tissues and cells lines. High level of HOXA-AS2 could inhibit miR-520c-3p, then release its target, TGF-β receptor 2 (TβR2), thus promote the migration and invasion of BC cells [45]. Zou et al. found that lncRNA TP73-AS1 promoted BC cells invasion and migration through competing with ZEB1 3’UTR for miR-200a binding. Moreover, ZEB1 could activate the expression of TP73-AS1 via binding to the promoter region of TP73-AS1. TP73-AS1/miR-200a/ ZEB1 as a regulating loop in BC cells could promote BC cells invasion and migration [46]. The recent study also demonstrated that linc-ROR as a competing endogenous RNA to mir-205 could prevent the degradation of ZEB1/2 [47]. LncRNA MEG3 was significantly down-regulated in BC tissues compared with adjacent normal tissues. MEG3 could inhibit BC cells proliferation and invasion capacities by down-regulating the levels of ZEB1/2 [20].
The regulatory roles of lncRNAs on EMT are based on loss of expression of the E-cad and increase in expression of mesenchymal markers, such as N-cadherin, vimentin, which collectively result in the acquisition of a characteristic mesenchymal and migratory phenotype of BC cells. LncRNAs such as HOXA11-AS, NEAT1, linc00617, OR3A4, and LINP1 were reported to be related to EMT via decreasing the E-cad and increasing the mesenchymal markers vimentin and N-cadherin [21–24,48]. On the contrary, the up-regulation of lncRNA ZFAS1 could inhibit BC cells migration and invasion by regulating EMT. As expected, overexpression of ZFAS1 increased the expression of the epithelial marker E-cad while decreasing the expression of the mesenchymal markers N-cadherin and vimentin in MDA-MB-231 cells (Figure 2) [49].
Figure 2. EMT is a progression of cellular plasticity critical for BC cells migration and metastasis.
LncRNAs such as ANCR, CCAT2, lncRNA-ATB, and HOXA-AS2 participate in BC invasion and metastasis through TGF-β-induced EMT. In addition, EMT-associated genes such as ZEB1/2, E-cad, N-cad, and vimentin were reported to be regulated by lncRNAs TP73-AS1, linc-ROR, MEG3, HOXA11-AS, NEAT1, linc00617, OR3A4, and LINP1.
Together, these results suggest that lncRNAs such as ANCR, CCAT2, lncRNA-ATB, and HOXA-AS2 participate in BC invasion and metastasis through TGF-β-induced EMT. In addition, EMT-associated genes such as ZEB1/2, E-cad, N-cad, and vimentin were reported to be regulated by lncRNAs TP73-AS1, linc-ROR, MEG3, HOXA11-AS, NEAT1, linc00617, OR3A4, and LINP1 (Figure 2). Thus, lncRNAs are involved in invasion and metastasis of BC cells by promoting EMT, providing potential therapeutic targets for BC.
LncRNAs influence BC metastasis by regulating CSCs generation
CSCs are a subpopulation of cancer cells with self-renewal capacity and high invasion and migration capacity as well as limitless proliferative potential [50,51]. Recent studies illustrate a direct link between lncRNAs and stemness of cancer cells. LncRNAs have emerged as important new players in the regulation of CSCs stemness acquisition and maintenance [52,53]. Recently, lncRNA linc00617 was reported to promote BC invasion and metastasis through increasing the percentage of the stem cells phenotype CD44(+)/CD24(−) subpopulation cells. Furthermore, the up-regulated linc00617 promoted generation of stem-like cells and elevation of self-renewal ability in BC cells [22]. Also, the increased lncRNA linc-ROR was confirmed to affect the acquisition of CSC-like phenotype and the self-renewal capacity in BC cells. Linc-ROR promoted metastasis of BC cells by enhancing the ability to form multiple large-sized mammospheres [47]. What is more, lncRNA-Hh which was transcriptionally regulated by Twist could directly target GAS1 to stimulate the activation of hedgehog pathway. The activated hedgehog pathway increased the expression of GLI1, SOX2, and OCT4 to play a regulatory role in CSCs maintenance of BC cells [54]. In addition, lncRNA MALAT1 played a critical role in the acquisition of CSC-like phenotype of BC cells. Lysine-specific demethylase 5B protein (KDM5B) could enhance the maintenance of aggressive BC cells malignant phenotype via modulation of lncRNA MALAT1 activity [55].
Thus, these results suggest that lncRNAs including linc00617, linc-ROR, lncRNA-Hh, and MALAT1 play important roles in CSCs generation to increase invasion and migration capacity of BC cells. They may be explored as a prognostic and diagnostic molecule in BC patients with metastasis.
LncRNAs serve as ceRNAs to influence BC metastasis
Dysregulated miRNAs participate in multiple biological processes such as apoptosis, proliferation, invasion, and metastasis by repressing translation or induce degradation of their target mRNAs [56,57]. LncRNAs act crucial roles on BC metastasis partly via sponging to miRNAs or regulating the expression of miRNAs. For instance, NEAT1 could promote BC cells growth, migration, and invasion by inhibiting miR-448 and up-regulating ZEB1. NEAT1 could serve as a competing endogenous lncRNA (ceRNA) to modulate ZEB1 by sponging miR-448 in BC [58]. In addition, miR-218 was reported to be another direct target of NEAT1. NEAT1 promoted BC cells invasion by negatively regulating expression of miR-218 [59]. It was also found that down-regulation of NEAT1 could also inhibit EMT program of BC cells through miR-211/high mobility group AT-hook 2 (HMGA2) axis and there was a reciprocal repression between NEAT1 and miR-211 [60]. HMGA2 has been reported to promote the action of transcriptional enhancers through binding to AT-rich regions in DNA and altering chromatin architecture. It is highly expressed in most cancers, including ovary, pancreas, lung, and BC, suggesting that HMGA2 could promote tumor progression in BC [61,62]. HMGA2 was identified as a target of miR-20a-5p, which significantly induced carcinogenesis of BC. MiR-20a-5p as a target of lncRNA HOTAIR had a negative correlation with HMGA2. HOTAIR affected BC cells growth, metastasis, and apoptosis via the miR-20a-5p/HMGA2 axis in BC cells [63]. Moreover, the pro-metastatic role of lncRNA MALAT1 on BC migration and invasion was mediated by miR-129-5p. MiR-129-5p is a direct inhibitory target of MALAT1 and its expression has an inverse correlation with MALAT1 [64]. Another study showed that the ectopic lncRNA SNHG15 was correlated with TNM stage, lymph node metastasis, and survival in BC patients. Increased SNHG15 significantly reinforced the abilities of BC cells migration and invasion. SNHG15 could act as a competing endogenous RNA to sponge miR-211-3p [65]. In MCF-7 cells, repression of HOST2 was remarkably elevated in the expression of miRNA let-7b. Down-regulation of HOST2 could decrease BC cells motility, migration, and invasion by inhibiting let-7b in BC patients [66]. On the contrary, lncRNA XIST was significantly down-regulated in BC tissues and cells lines. Overexpression of XIST remarkably inhibited BC cells growth, migration, and invasion via sponging to miR-155 in BC. Moreover, caudal-type homeobox 1 (CDX1) was identified as a direct target of miR-155 and miR-155/CDX1 rescued the effects of XIST in BC cells [67]. BC migration and invasion could also be regulated by lncRNA SUMO1P3. Further study confirmed that SUMO1P3 functioned as an oncogenic lncRNA via binding to miR-320a, which had been identified as a tumor suppressor in many cancers [68]. LncRNA cancer susceptibility candidate 2 (CASC2) has been demonstrated to be a tumor suppressor in several types of cancer [69]. In the present study, the expression level of CASC2 was significantly decreased in BC tissues compared with adjacent normal tissues. The up-regulated CASC2 decreased the viability, migration, and invasion, and promoted apoptosis of BC cells through acted as a ceRNA for miR-96-5p, while miR-96-5p overexpression could increase BC cells viability, migration, and invasion by repressing the expression of its target gene, synoviolin (SYVN1) [70] (Table 1).
Table 1. LncRNAs participate in BC metastasis by sponging to miRNA.
| LncRNA | Expression | Sponging miRNA | Function | Reference |
|---|---|---|---|---|
| NEAT1 | Up | miR-448, miR-218, miR-211 | NEAT1 facilitated cell growth and invasion via negatively regulating miR-218, as well as by regulating miR-211/HMGA2 axis and miR-448/ZEB1 axis in BC. | [58–60] |
| HOTAIR | Up | miR-20a-5p | HOTAIR affected BC cell growth, metastasis, and apoptosis via the miR-20a-5p/HMGA2 axis. | [63] |
| MALAT1 | Up | miR-129-5p | MALAT1 promoted triple-negative BC invasion via targeting miR-129-5p. | [64] |
| SNHG15 | Up | miR-211-3p | SNHG15 promoted BC cell migration and invasion by sponging miR-211-3p. | [65] |
| HOST2 | Up | let-7b | HOST2 decreased BC cell motility, migration, and invasion by inhibiting let-7b. | [66] |
| XIST | Down | miR-155 | XIST inhibited BC cell growth, migration, and invasion via miR-155/CDX1 axis. | [67] |
| SUMO1P3 | Up | miR-320a | SUMO1P3 facilitated BC progression by negatively regulating miR-320a. | [68] |
| CASC2 | Down | miR-96-5p | CASC2 inhibited the growth and metastasis of BC through the miR-96-5p/SYVN1 axis. | [70] |
LncRNAs regulate multiple signaling pathways in BC metastasis
Changes in the multiple signaling pathways directing their regulation can lead to the pathological process of BC cells invasion and metastasis. For example, lncRNA LINP1 could promote BC cells metastasis and influence the expression of EMT-related markers. P53 overexpression inhibited BC cells migration partly by decreasing LINP1 expression. P53 is a regulator of LINP1 while increased LINP1 could in turn attenuate the anti-metastatic effects of p53 [24]. P53 signaling pathway has a key role in the negative regulation of tumor angiogenesis, migration, and cells motility [71,72]. P21 as a classical downstream gene of the p53 signaling pathway was also associated various cancers progression, including the migration and invasion of BC [73–76]. LncRNA lincIN acted as a new regulator of BC metastasis at both transcriptional and translational levels. The up-regulated lincIN could repress p21 protein expression by inhibiting translation of p21 [77]. The lncRNA associated with BC brain metastases (Lnc-BM) is prognostic of the progression of brain metastasis in BC patients. In preclinical murine models, elevated Lnc-BM expression drove BC brain metastases, while depletion of Lnc-BM effectively treated BC brain metastases. Lnc-BM could increase JAK2 kinase activity to mediate IL-6- and oncostatin M-triggered STAT3 phosphorylation. In BC cells, Lnc-BM could trigger the activation of downstream signaling pathway of STAT3 that included the proteins CCL2 and ICAM1. The increased CCL2 and ICAM1 could attract macrophages and mediate vascular co-option in the brain, respectively. The attracted macrophages in turn released oncostatin M and IL-6, thereby further activating the Lnc-BM/JAK2/STAT3 pathway and enhancing BC brain metastases [78]. LncRNA CCAT2 was dysregulated in several cancers such as gastric cancer, colon cancer, and BC. Increased CCAT2 was associated with tumor metastasis, growth, and chromosomal instability [79]. The high expression level of CCAT2 could increase proliferation and invasion BC cells by activating the Wnt signaling pathway. CCAT2 could enhance the expression of downstream genes of Wnt/β-catenin signaling pathway, such as CCND1 and c-myc. In addition, the increased CCAT2 expression could activate the Wnt/β-catenin signaling pathway, and combination of increased CCAT2 and c-myc could synergistically improve the activation of Wnt signaling pathway [80]. In addition, DKK1, an inhibitor of Wnt signaling pathway, could inhibit the migration and invasion of BC [81,82]. Up-regulation of lncRNA NBAT1 could significantly increase the expression of DKK1 to inhibit migration and invasion of BC [83].
Clinical significance of lncRNAs in BC metastasis
Accumulating evidence suggested that lncRNAs were abnormal expressed in various cancers and associated with cancer metastasis [84,85]. Dysregulated lncRNAs are also significantly related with multiple clinicopathological characteristics of BC, such as histological grade, ERBB2 expression, steroid-receptor expression, tumor size, TNM stage, and even lymph-node-metastasis (LNM) [12,86]. The expression of lncRNA H19 decreased significantly in adjacent normal tissues compared with BC tissues and plasma (P<0.05), and plasma levels of lncRNA H19 were significantly correlated with LNM (P=0.006) [87]. LncRNA TUG1 expression was significantly decreased in BC tissues compared with controls, and low TUG1 expression was significantly correlated with LNM (P=0.044) [88]. LncRNA FGF14 antisense RNA 2 (FGF14-AS2) was significantly restrained in BC tissues compared with adjacent normal tissues. Down-regulation of lncRNA FGF14-AS2 was correlated with more lymph node metastasis (P=0.000) [89]. BC patients with increased expressions of AC010729.1 and RP11-482H16.1 had a shorter metastasis-free survival (MFS) compared with patients with low expressions of AC010729.1 and RP11-482H16.1. Further analysis showed that AC010729.1 and RP11-482H16.1 could be useful prognostic markers to predict metastatic risk in BC patients [90]. LncRNA MALAT1 expressions were remarkably increased in 85.9% (67/78) of cancer tissues compared with adjacent normal tissues (P<0.01). Furthermore, the increased lncRNA MALAT1 in BC tissues was significantly associated with LNM (P=0.037) [91]. In matched metastatic and primary cancers, the expression of lncRNA HOTAIR increased in the metastatic carcinomas and its expression was a predictor of poor survival of BC patients [92]. Compared with BC tissues, lncRNA Z38 was down-regulated in normal breast tissues. High level of Z38 was significantly related to TNM stage and LNM [93]. The level of MEG3 was significantly decreased in BC tissues compared with adjacent normal tissues. Down-regulated MEG3 was significantly associated with TNM stage and LNM in BC patients [20]. The level of lncRNA OR3A4 was up-regulated in BC tissues compared with the adjacent normal tissues. The expression level of OR3A4 was found to be associated with LNM, differentiation grade, HER-2/neu status, and TNM stage [23]. LncRNA LINC01296 was aberrantly expressed in both BC tissue samples and cells. Increased LINC01296 was positive correlated with larger tumor size, positive LNM, and advanced TNM stage of patients with BC. Additionally, Cox regression analysis confirmed that LINC01296 could be an independent prognostic indicator for patients with BC [94]. LncRNA p10247 expression level in BC tissues was significantly up-regulated. More importantly, the overexpressed p10247 was associated with the development and progression of BC. The up-regulated expression of p10247 was significantly associated with progression of clinical stage (P=0.045) and LNM (P=0.039) [95] (Table 2).
Table 2. Clinical significance of LncRNAs in BC metastasis.
| LncRNA | Expression | Clinical significance | Reference |
|---|---|---|---|
| MEG3 | Down | Down-regulated MEG3 was significantly associated with TNM stage and LNM in BC patients. | [20] |
| OR3A4 | Up | The expression level of OR3A4 was found to be associated with LNM, differentiation grade, HER-2/neu status, and TNM stage. | [23] |
| H19 | Up | Plasma levels of lncRNA H19 were significantly correlated with LNM. | [87] |
| TUG1 | Down | Low TUG1 expression was significantly correlated with LNM. | [88] |
| FGF14-AS2 | Down | Down-regulation of lncRNA FGF14-AS2 was correlated with more lymph node metastasis. | [89] |
| AC010729.1 | – | Increased expressions of AC010729.1 and RP11-482H16.1 had a shorter MFS compared with patients with low expressions of AC010729.1 and RP11-482H16.1. | [90] |
| RP11-482H16.1 | |||
| AC010729.1 | |||
| RP11-482H16.1 | |||
| MALAT1 | Up | Up-regulated MALAT1 was related to LNM. | [91] |
| HOTAIR | Up | HOTAIR increased in the metastatic carcinomas and its expression in BC was a predictor of poor survival. | [92] |
| Z38 | Up | High level of Z38 was significantly related to advanced TNM stage and LNM. | [93] |
| LINC01296 | Up | Increased LINC01296 was positive correlated with larger tumor size, positive LNM, and advanced TNM stage of patients with BC. | [94] |
| p10247 | Up | The up-regulated expression of p10247 was significantly associated with progression of clinical stage and LNM. | [95] |
Prospects
The emerging evidence demonstrated that lncRNAs played key roles in various biological processes of BC. However, only a few lncRNAs have been well characterized in BC invasion and metastasis. While surgery can successfully treat primary BC, invasion and metastasis have proven to be a largely insurmountable challenge [96–98]. One primary challenge in BC patients is that metastasis has already occurred prior to diagnosis. Thus, it is essential to find useful prognostic markers to predict metastatic risk in BC patients, which may allow BC patients to choose an individualized treatment. LncRNAs are stable expression in tissue and plasma which may provide markers for a noninvasive and rapid diagnosis for metastatic BC. However, it is well known that the molecular mechanisms of lncRNAs are complicated, including chromatin remodeling, cells cycle control, genetic imprinting, splicing regulation, translational regulation as well as mRNA decay. The detailed molecular mechanisms underlying the function of lncRNAs on BC invasion and metastasis are still unresolved mysteries. Thus, future researches are needed to reveal the underlying mechanisms of dysregulated lncRNAs influence BC metastasis. In the wake of developments in molecular biologic techniques, such as next-generation sequencing and microarray, perhaps the mystery of lncRNAs in BC invasion and metastasis will be unveiled soon. An improved understanding of the BC invasion and metastasis regulated by lncRNAs could assist the development of new strategies to treat or prevent metastatic BC.
Conclusion
In this review, we focused on the roles of lncRNAs as regulators during BC invasion and metastasis. Dysregulated lncRNAs participate in BC invasion and metastasis through participating in EMT transition, strengthening CSCs generation, serving as ceRNAs, influencing multiple signaling pathways as well as regulating expressions of CAMs, ECMs, and MMPs. We summarized the function and molecular mechanism of lncRNAs in BC invasion and metastasis as well as discussing the clinical significance of lncRNAs in BC staging and LNM. LncRNAs may act as pro-metastatic or anti-metastatic roles in BC. Through a clear understanding of the mechanism of lncRNAs in BC invasion and metastasis, lncRNAs could be promising therapeutic targets and novel molecular biomarkers for metastatic BC.
Abbreviations
- BC
breast cancer
- CAM
cells adhesion molecule
- CASC2
cancer susceptibility candidate 2
- CDX1
caudal-type homeobox 1
- ceRNA
competing endogenous lncRNA
- CSC
cancer stem cell
- E-cad
E-cadherin
- ECM
extracellular matrix
- EMT
epithelial-to-mesenchymal transition
- EZH2
enhancer of Zeste Homolog 2
- HMGA2
high mobility group AT-hook 2
- lncRNA
long non-coding RNA
- LNM
lymph-node-metastasis
- MFS
metastasis-free survival
- MMP
matrix metallo-proteinase
- RUNX2
runt-related transcription factor 2
- SYVN1
synoviolin
- TβR2
TGF-β receptor 2
- TGF-β
transforming growth factor-β
- Tnxb
Tenascin Xb
- TR
trastuzumab-resistant
- ZEB
Zinc finger E-box-binding homeobox
Funding
This research was supported by the National Key Research and Development Program of China [grant number 2016YFC0905900); the “333” Talent Project of Jiangsu Province [grant number 4 (2016)]; the National Key Clinical Specialist Construction Programs of China [grant number 544 (2013)]; and Natural Science Foundation of Jiangsu Province [grant number BK20151579].
Competing interests
The authors declare that there are no competing interests associated with the manuscript.
References
- 1.DeSantis C.E., Ma J., Goding Sauer A., Newman L.A. and Jemal A. (2017) Breast cancer statistics, 2017, racial disparity in mortality by state. CA Cancer J. Clin. 67, 439–448, 10.3322/caac.21412 [DOI] [PubMed] [Google Scholar]
- 2.Yousefi M., Nosrati R., Salmaninejad A., Dehghani S., Shahryari A. and Saberi A. (2018) Organ-specific metastasis of breast cancer: molecular and cellular mechanisms underlying lung metastasis. Cell. Oncol. (Dordrecht) 41, 123–140, 10.1007/s13402-018-0376-6 [DOI] [PubMed] [Google Scholar]
- 3.Miller K.D., Siegel R.L., Lin C.C., Mariotto A.B., Kramer J.L., Rowland J.H. et al. (2016) Cancer treatment and survivorship statistics, 2016. CA Cancer J. Clin. 66, 271–289, 10.3322/caac.21349 [DOI] [PubMed] [Google Scholar]
- 4.Graham J., Pitz M., Gordon V., Grenier D., Amir E. and Niraula S. (2016) Clinical predictors of benefit from fulvestrant in advanced breast cancer: a meta-analysis of randomized controlled trials. Cancer Treat. Rev. 45, 1–6, 10.1016/j.ctrv.2016.02.004 [DOI] [PubMed] [Google Scholar]
- 5.Neal R.D., Tharmanathan P., France B., Din N.U., Cotton S., Fallon-Ferguson J. et al. (2015) Is increased time to diagnosis and treatment in symptomatic cancer associated with poorer outcomes? Systematic review Br. J. Cancer 112, S92–S107, 10.1038/bjc.2015.48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen W., Zhou S., Mao L., Zhang H., Sun D., Zhang J. et al. (2016) Crosstalk between TGF-beta signaling and miRNAs in breast cancer metastasis. Tumour Biol. 37, 10011–10019, 10.1007/s13277-016-5060-8 [DOI] [PubMed] [Google Scholar]
- 7.Liu Q., Zhang H., Jiang X., Qian C., Liu Z. and Luo D. (2017) Factors involved in cancer metastasis: a better understanding to “seed and soil” hypothesis. Mol. Cancer 16, 176, 10.1186/s12943-017-0742-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Micalizzi D.S., Maheswaran S. and Haber D.A. (2017) A conduit to metastasis: circulating tumor cell biology. Genes Dev. 31, 1827–1840, 10.1101/gad.305805.117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pindiprolu S., Krishnamurthy P.T. and Chintamaneni P.K. (2018) Pharmacological targets of breast cancer stem cells: a review. Naunyn-Schmiedeberg’s Archives Pharmacol. 391, 463–479, 10.1007/s00210-018-1479-3 [DOI] [PubMed] [Google Scholar]
- 10.Idogawa M., Ohashi T., Sasaki Y., Nakase H. and Tokino T. (2017) Long non-coding RNA NEAT1 is a transcriptional target of p53 and modulates p53-induced transactivation and tumor-suppressor function, Int. J. Cancer. 140, 2785–2791 [DOI] [PubMed] [Google Scholar]
- 11.Schmitt A.M. and Chang H.Y. (2016) Long noncoding RNAs in cancer pathways. Cancer Cell 29, 452–463, 10.1016/j.ccell.2016.03.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Arun G., Diermeier S., Akerman M., Chang K.C., Wilkinson J.E., Hearn S. et al. (2016) Differentiation of mammary tumors and reduction in metastasis upon Malat1 lncRNA loss. Genes Dev. 30, 34–51, 10.1101/gad.270959.115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bhan A., Soleimani M. and Mandal S.S. (2017) Long noncoding RNA and cancer: a new paradigm. Cancer Res. 77, 3965–3981, 10.1158/0008-5472.CAN-16-2634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stuelten C.H., Parent C.A. and Montell D.J. (2018) Cell motility in cancer invasion and metastasis: insights from simple model organisms. Nat. Rev. Cancer 18, 296–312, 10.1038/nrc.2018.15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sakamoto S. and Ichikawa T. (2014) Mechanism of prostate cancer invasion and metastasis. Nihon Rinsho Jpn. J. Clin. Med. 72, 2086–2089 [PubMed] [Google Scholar]
- 16.Catalano V., Turdo A., Di Franco S., Dieli F., Todaro M. and Stassi G. (2013) Tumor and its microenvironment: a synergistic interplay. Semin. Cancer Biol. 23, 522–532, 10.1016/j.semcancer.2013.08.007 [DOI] [PubMed] [Google Scholar]
- 17.Guan X. (2015) Cancer metastases: challenges and opportunities. Acta. Pharm. Sin. B 5, 402–418 10.1016/j.apsb.2015.07.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang C.Y., Li R.K., Qi Y., Li X.N., Yang Y., Liu D.L. et al. (2016) Upregulation of long noncoding RNA SPRY4-IT1 promotes metastasis of esophageal squamous cell carcinoma via induction of epithelial-mesenchymal transition. Cell Biol. Toxicol. 32, 391–401 10.1007/s10565-016-9341-1 [DOI] [PubMed] [Google Scholar]
- 19.Yue B., Qiu S., Zhao S., Liu C., Zhang D., Yu F. et al. (2016) LncRNA-ATB mediated E-cadherin repression promotes the progression of colon cancer and predicts poor prognosis. J. Gastroenterol. Hepatol. 31, 595–603 10.1111/jgh.13206 [DOI] [PubMed] [Google Scholar]
- 20.Zhang W., Shi S., Jiang J., Li X., Lu H. and Ren F. (2017) LncRNA MEG3 inhibits cell epithelial-mesenchymal transition by sponging miR-421 targeting E-cadherin in breast cancer. Biomed. Pharmacother. 91, 312–319 10.1016/j.biopha.2017.04.085 [DOI] [PubMed] [Google Scholar]
- 21.Zhang M., Wu W.B., Wang Z.W. and Wang X.H. (2017) lncRNA NEAT1 is closely related with progression of breast cancer via promoting proliferation and EMT. Eur. Rev. Med. Pharmacol. Sci. 21, 1020–1026 [PubMed] [Google Scholar]
- 22.Li H., Zhu L., Xu L., Qin K., Liu C., Yu Y. et al. (2017) Long noncoding RNA linc00617 exhibits oncogenic activity in breast cancer. Mol. Carcinog. 56, 3–17 10.1002/mc.22338 [DOI] [PubMed] [Google Scholar]
- 23.Liu G., Hu X. and Zhou G. (2017) Long non-coding RNA OR3A4 promotes proliferation and migration in breast cancer. Biomed. Pharmacother. 96, 426–433 10.1016/j.biopha.2017.10.011 [DOI] [PubMed] [Google Scholar]
- 24.Liang Y., Li Y., Song X., Zhang N., Sang Y., Zhang H. et al. (2018) Long noncoding RNA LINP1 acts as an oncogene and promotes chemoresistance in breast cancer. Cancer Biol. Therapy 19, 120–131 10.1080/15384047.2017.1394543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cazzaniga W., Nebuloni M., Longhi E., Locatelli I., Allevi R., Luciano R. et al. (2016) Human prostate tissue-derived extracellular matrix as a model of prostate microenvironment. Eur. Urology Focus 2, 400–408 10.1016/j.euf.2016.02.016 [DOI] [PubMed] [Google Scholar]
- 26.Alfano M., Nebuloni M., Allevi R., Zerbi P., Longhi E., Luciano R. et al. (2016) Linearized texture of three-dimensional extracellular matrix is mandatory for bladder cancer cell invasion. Sci. Rep. 6, 36128 10.1038/srep36128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kumar S., Kapoor A., Desai S., Inamdar M.M. and Sen S. (2016) Proteolytic and non-proteolytic regulation of collective cell invasion: tuning by ECM density and organization. Sci. Rep. 6, 19905 10.1038/srep19905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Genovese L., Zawada L., Tosoni A., Ferri A., Zerbi P., Allevi R. et al. (2014) Cellular localization, invasion, and turnover are differently influenced by healthy and tumor-derived extracellular matrix. Tissue Eng. Part A 20, 2005–2018 10.1089/ten.tea.2013.0588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li M., Li X., Zhuang Y., Flemington E.K., Lin Z. and Shan B. (2017) Induction of a novel isoform of the lncRNA HOTAIR in Claudin-low breast cancer cells attached to extracellular matrix. Mol. Oncol. 11, 1698–1710 10.1002/1878-0261.12133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Singh R., Mandhani A., Agrawal V. and Garg M. (2018) Positive correlation between matrix metalloproteinases and epithelial-to-mesenchymal transition and its association with clinical outcome in bladder cancer patients, Cancer Microenviron., 11, 23–39, 10.1007/s12307-017-0199-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Piperigkou Z., Manou D., Karamanou K. and Theocharis A.D. (2018) Strategies to target matrix metalloproteinases as therapeutic approach in cancer. Methods Mol. Biol. 1731, 325–348 10.1007/978-1-4939-7595-2_27 [DOI] [PubMed] [Google Scholar]
- 32.Das A., Monteiro M., Barai A. and Kumar S. (2017) MMP proteolytic activity regulates cancer invasiveness by modulating integrins. 7, 14219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang O., Yang F., Liu Y., Lv L., Ma R., Chen C. et al. (2017) C-MYC-induced upregulation of lncRNA SNHG12 regulates cell proliferation, apoptosis and migration in triple-negative breast cancer. Am. J. Transl. Res. 9, 533–545 [PMC free article] [PubMed] [Google Scholar]
- 34.Shi F., Xiao F., Ding P., Qin H. and Huang R. (2016) Long noncoding RNA highly up-regulated in liver cancer predicts unfavorable outcome and regulates metastasis by MMPs in triple-negative breast cancer. Arch. Med. Res. 47, 446–453 10.1016/j.arcmed.2016.11.001 [DOI] [PubMed] [Google Scholar]
- 35.Eoh K.J., Paek J., Kim S.W., Kim H.J., Lee H.Y., Lee S.K. et al. (2017) Long non-coding RNA, steroid receptor RNA activator (SRA), induces tumor proliferation and invasion through the NOTCH pathway in cervical cancer cell lines. Oncol. Rep. 38, 3481–3488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li Z., Hou P., Fan D., Dong M., Ma M., Li H. et al. (2017) The degradation of EZH2 mediated by lncRNA ANCR attenuated the invasion and metastasis of breast cancer. Cell Death Differ. 24, 59–71 10.1038/cdd.2016.95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lou K.X., Li Z.H., Wang P., Liu Z., Chen Y., Wang X.L. et al. (2018) Long non-coding RNA BANCR indicates poor prognosis for breast cancer and promotes cell proliferation and invasion. Eur. Rev. Med. Pharmacol. Sci. 22, 1358–1365 [DOI] [PubMed] [Google Scholar]
- 38.O’Brien S.J., Carter J.V., Burton J.F., Oxford B.G., Schmidt M.N., Hallion J.C. et al. (2018) The role of the miR-200 family in epithelial-mesenchymal transition in colorectal cancer: a systematic review [DOI] [PubMed] [Google Scholar]
- 39.Karlsson T., Sundar R., Widmark A., Landstrom M. and Persson E. (2018) Osteoblast-derived factors promote metastatic potential in human prostate cancer cells, in part via non-canonical transforming growth factor beta (TGFbeta) signaling [DOI] [PubMed] [Google Scholar]
- 40.Ceausu A.R., Ciolofan A., Cimpean A.M., Magheti A., Mederle O. and Raica M. (2018) The mesenchymal-epithelial and epithelial-mesenchymal cellular plasticity of liver metastases with digestive origin. Anticancer Res. 38, 811–816 [DOI] [PubMed] [Google Scholar]
- 41.Li Z., Dong M., Fan D., Hou P., Li H., Liu L. et al. (2017) LncRNA ANCR down-regulation promotes TGF-beta-induced EMT and metastasis in breast cancer. Oncotarget 8, 67329–67343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wu Z.J., Li Y., Wu Y.Z., Wang Y., Nian W.Q., Wang L.L. et al. (2017) Long non-coding RNA CCAT2 promotes the breast cancer growth and metastasis by regulating TGF-beta signaling pathway. Eur. Rev. Med. Pharmacol. Sci. 21, 706–714, [PubMed] [Google Scholar]
- 43.Shi S.J., Wang L.J., Yu B., Li Y.H., Jin Y. and Bai X.Z. (2015) LncRNA-ATB promotes trastuzumab resistance and invasion-metastasis cascade in breast cancer. Oncotarget 6, 11652–11663, 10.18632/oncotarget.3457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hilmarsdottir B., Briem E., Bergthorsson J.T., Magnusson M.K. and Gudjonsson T. (2014) Functional role of the microRNA-200 family in breast morphogenesis and neoplasia. Genes 5, 804–820, 10.3390/genes5030804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fang Y., Wang J., Wu F., Song Y., Zhao S. and Zhang Q. (2017) Long non-coding RNA HOXA-AS2 promotes proliferation and invasion of breast cancer by acting as a miR-520c-3p sponge. Oncotarget 8, 46090–46103, 10.18632/oncotarget.17552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zou Q., Zhou E., Xu F., Zhang D., Yi W. and Yao J. (2018) A TP73-AS1/miR-200a/ZEB1 regulating loop promotes breast cancer cell invasion and migration, J. Cell. Biochem.. 119, 2189–2199, 10.1002/jcb.26380 [DOI] [PubMed] [Google Scholar]
- 47.Hou P., Zhao Y., Li Z., Yao R., Ma M., Gao Y. et al. (2014) LincRNA-ROR induces epithelial-to-mesenchymal transition and contributes to breast cancer tumorigenesis and metastasis. Cell Death Dis. 5, e1287, 10.1038/cddis.2014.249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Li W., Jia G., Qu Y., Du Q., Liu B. and Liu B. (2017) Long non-coding RNA (LncRNA) HOXA11-AS promotes breast cancer invasion and metastasis by regulating epithelial-mesenchymal transition. Med. Sci. Monitor: Int. Med. J. Exp. Clin. Res. 23, 3393–3403, 10.12659/MSM.904892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fan S., Fan C., Liu N., Huang K., Fang X. and Wang K. (2018) Downregulation of the long non-coding RNA ZFAS1 is associated with cell proliferation, migration and invasion in breast cancer. Mol. Med. Rep. 17, 6405–6412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Deshmukh A. and Arfuso F. (2018, Cancers) Regulation of cancer stem cell metabolism by secreted frizzled-related protein 4 (sFRP4) 10, 10.3390/cancers10020040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Valle S. and Martin-Hijano L. (2018) The ever-evolving concept of the cancer stem cell in pancreatic cancer 10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang L., Dong P., Wang W., Huang M. and Tian B. (2017) Gemcitabine treatment causes resistance and malignancy of pancreatic cancer stem-like cells via induction of lncRNA HOTAIR. Exp. Therapeutic Med. 14, 4773–4780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Luczak A., Supernat A., Lapinska-Szumczyk S., Jachimowicz D., Majewska H., Gulczynski J. et al. (2016) HOTAIR in relation to epithelial-mesenchymal transition and cancer stem cells in molecular subtypes of endometrial cancer. Int. J. Biol. Markers 31, e245–51 10.5301/jbm.5000187 [DOI] [PubMed] [Google Scholar]
- 54.Zhou M., Hou Y., Yang G., Zhang H., Tu G., Du Y.E. et al. (2016) LncRNA-Hh strengthen cancer stem cells generation in twist-positive breast cancer via activation of hedgehog signaling pathway. Stem Cells 34, 55–66 10.1002/stem.2219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bamodu O.A., Huang W.C., Lee W.H., Wu A., Wang L.S., Hsiao M. et al. (2016) Aberrant KDM5B expression promotes aggressive breast cancer through MALAT1 overexpression and downregulation of hsa-miR-448. BMC Cancer 16, 160 10.1186/s12885-016-2108-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ni J., Bucci J., Chang L., Malouf D., Graham P. and Li Y. (2017) Targeting MicroRNAs in prostate cancer radiotherapy. Theranostics 7, 3243–3259 10.7150/thno.19934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gandellini P., Doldi V. and Zaffaroni N. (2017) microRNAs as players and signals in the metastatic cascade: implications for the development of novel anti-metastatic therapies. Semin. Cancer Biol. 44, 132–140 10.1016/j.semcancer.2017.03.005 [DOI] [PubMed] [Google Scholar]
- 58.Jiang X., Zhou Y., Sun A.J. and Xue J.L. (2018) NEAT1 contributes to breast cancer progression through modulating miR-448 and ZEB1. J. Cell Physiol. 10.1002/jcp.26470 [DOI] [PubMed] [Google Scholar]
- 59.Zhao D., Zhang Y., Wang N. and Yu N. (2017) NEAT1 negatively regulates miR-218 expression and promotes breast cancer progression. Cancer Biomark. 20, 247–254 10.3233/CBM-170027 [DOI] [PubMed] [Google Scholar]
- 60.Li X., Wang S., Li Z., Long X., Guo Z., Zhang G. et al. (2017) The lncRNA NEAT1 facilitates cell growth and invasion via the miR-211/HMGA2 axis in breast cancer. Int. J. Biol. Macromol. 105, 346–353 10.1016/j.ijbiomac.2017.07.053 [DOI] [PubMed] [Google Scholar]
- 61.Sgarra R., Pegoraro S., Ros G., Penzo C., Chiefari E., Foti D. et al. (2018) High Mobility Group A (HMGA) proteins: molecular instigators of breast cancer onset and progression. Biochim. Biophys. Acta. 1869, 216–229 [DOI] [PubMed] [Google Scholar]
- 62.Pallante P., Sepe R., Puca F. and Fusco A. (2015) High mobility group a proteins as tumor markers. Front. Med. 2, 15 10.3389/fmed.2015.00015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhao W., Geng D., Li S., Chen Z. and Sun M. (2018) LncRNA HOTAIR influences cell growth, migration, invasion, and apoptosis via the miR-20a-5p/HMGA2 axis in breast cancer, Cancer Med.. 7, 842–855, 10.1002/cam4.1353 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 64.Zuo Y., Li Y., Zhou Z., Ma M. and Fu K. (2017) Long non-coding RNA MALAT1 promotes proliferation and invasion via targeting miR-129-5p in triple-negative breast cancer. Biomed. Pharmacother. 95, 922–928 10.1016/j.biopha.2017.09.005 [DOI] [PubMed] [Google Scholar]
- 65.Kong Q. and Qiu M. (2018) Long noncoding RNA SNHG15 promotes human breast cancer proliferation, migration and invasion by sponging miR-211-3p. Biochem. Biophys. Res. Commun. 495, 1594–1600 10.1016/j.bbrc.2017.12.013 [DOI] [PubMed] [Google Scholar]
- 66.Lu P.W., Li L., Wang F. and Gu Y.T. (2018) Effects of long non-coding RNA HOST2 on cell migration and invasion by regulating MicroRNA let-7b in breast cancer, J. Cell. Biochem.. 119, 4570–4580, 10.1002/jcb.26606 [DOI] [PubMed] [Google Scholar]
- 67.Zheng R., Lin S., Guan L., Yuan H., Liu K., Liu C. et al. (2018) Long non-coding RNA XIST inhibited breast cancer cell growth, migration, and invasion via miR-155/CDX1 axis. Biochem. Biophys. Res. Commun. 498, 1002–1008 10.1016/j.bbrc.2018.03.104 [DOI] [PubMed] [Google Scholar]
- 68.Liu J., Song Z., Feng C., Lu Y., Zhou Y., Lin Y. et al. (2017) The long non-coding RNA SUMO1P3 facilitates breast cancer progression by negatively regulating miR-320a. Am. J. Transl. Res. 9, 5594–5602, [PMC free article] [PubMed] [Google Scholar]
- 69.Simonian M., Sharifi M., Nedaeinia R., Mosallaie M., Khosravi S., Avan A. et al. (2018) Evaluation of miR-21 inhibition and its impact on cancer susceptibility candidate 2 long noncoding RNA in colorectal cancer cell line. Adv. Biomed. Res. 7, 14 10.4103/abr.abr_214_16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gao Z., Wang H., Li H., Li M., Wang J., Zhang W. et al. (2018) Long non-coding RNA CASC2 inhibits breast cancer cell growth and metastasis through the regulation of the miR-96-5p/SYVN1 pathway. Int. J. Oncol. 10.3892/ijo.2018.4522 [DOI] [PubMed] [Google Scholar]
- 71.Merkel O., Taylor N., Prutsch N., Staber P.B., Moriggl R., Turner S.D. et al. (2017) When the guardian sleeps: reactivation of the p53 pathway in cancer. Mutat. Res. 773, 1–13, 10.1016/j.mrrev.2017.02.003 [DOI] [PubMed] [Google Scholar]
- 72.Teodoro J.G., Parker A.E., Zhu X. and Green M.R. (2006) p53-mediated inhibition of angiogenesis through up-regulation of a collagen prolyl hydroxylase. Science 313, 968–971, 10.1126/science.1126391 [DOI] [PubMed] [Google Scholar]
- 73.Kim E.M., Jung C.H., Kim J., Hwang S.G., Park J.K. and Um H.D. (2017) The p53/p21 complex regulates cancer cell invasion and apoptosis by targeting Bcl-2 family proteins. Cancer Res. 77, 3092–3100, 10.1158/0008-5472.CAN-16-2098 [DOI] [PubMed] [Google Scholar]
- 74.El-Deiry W.S. (2016) p21(WAF1) Mediates cell-cycle inhibition, relevant to cancer suppression and therapy. Cancer Res. 76, 5189–5191, 10.1158/0008-5472.CAN-16-2055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zaremba-Czogalla M., Hryniewicz-Jankowska A., Tabola R., Nienartowicz M., Stach K., Wierzbicki J. et al. (2018) A novel regulatory function of CDKN1A/p21 in TNFalpha-induced matrix metalloproteinase 9-dependent migration and invasion of triple-negative breast cancer cells. Cell. Signal. 47, 27–36, 10.1016/j.cellsig.2018.03.010 [DOI] [PubMed] [Google Scholar]
- 76.Lui A.J., Geanes E.S., Ogony J., Behbod F., Marquess J., Valdez K. et al. (2017) IFITM1 suppression blocks proliferation and invasion of aromatase inhibitor-resistant breast cancer in vivo by JAK/STAT-mediated induction of p21. Cancer Lett. 399, 29–43, 10.1016/j.canlet.2017.04.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Jiang Z., Slater C.M., Zhou Y., Devarajan K., Ruth K.J., Li Y. et al. (2017) LincIN, a novel NF90-binding long non-coding RNA, is overexpressed in advanced breast tumors and involved in metastasis, Breast Cancer Res.. 19, 62, 10.1186/s13058-017-0853-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wang S., Liang K., Hu Q., Li P., Song J., Yang Y. et al. (2017) JAK2-binding long noncoding RNA promotes breast cancer brain metastasis. J. Clin. Invest. 10.1172/JCI91553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ling H., Spizzo R., Atlasi Y., Nicoloso M., Shimizu M., Redis R.S. et al. (2013) CCAT2, a novel noncoding RNA mapping to 8q24, underlies metastatic progression and chromosomal instability in colon cancer. Genome Res. 23, 1446–1461, 10.1101/gr.152942.112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Cai Y., He J. and Zhang D. (2015) Long noncoding RNA CCAT2 promotes breast tumor growth by regulating the Wnt signaling pathway. Onco. Targets Therapy 8, 2657–2664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Baillo A., Giroux C. and Ethier S.P. (2011) Knock-down of amphiregulin inhibits cellular invasion in inflammatory breast cancer. J. Cell Physiol. 226, 2691–2701, 10.1002/jcp.22620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Menezes M.E., Mitra A., Shevde L.A. and Samant R.S. (2012) DNAJB6 governs a novel regulatory loop determining Wnt/beta-catenin signalling activity. Biochem. J. 444, 573–580, 10.1042/BJ20120205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hu P., Chu J., Wu Y., Sun L., Lv X., Zhu Y. et al. (2015) NBAT1 suppresses breast cancer metastasis by regulating DKK1 via PRC2. Oncotarget 6, 32410–32425, 10.18632/oncotarget.5609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.White N.M. and Maher C.A. (2017) The potential use of lncRNAs found in the 8q24 region as biomarkers for colon cancer. Ann. Oncol.: Off. J. Eur. Soc. Med Oncol./ESMO 28, 1688–1689, 10.1093/annonc/mdx337 [DOI] [PubMed] [Google Scholar]
- 85.Diermeier S.D., Chang K.C., Freier S.M., Song J., El Demerdash O., Krasnitz A. et al. (2016) Mammary tumor-associated RNAs impact tumor cell proliferation, invasion, and migration. Cell Rep. 17, 261–274, 10.1016/j.celrep.2016.08.081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Huang J., Zhou N., Watabe K., Lu Z., Wu F., Xu M. et al. (2014) Long non-coding RNA UCA1 promotes breast tumor growth by suppression of p27 (Kip1). Cell Death Dis. 5, e1008, 10.1038/cddis.2013.541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zhang K., Luo Z., Zhang Y., Zhang L., Wu L., Liu L. et al. (2016) Circulating lncRNA H19 in plasma as a novel biomarker for breast cancer. Cancer Biomark. 17, 187–194, 10.3233/CBM-160630 [DOI] [PubMed] [Google Scholar]
- 88.Fan S., Yang Z., Ke Z., Huang K., Liu N., Fang X. et al. (2017) Downregulation of the long non-coding RNA TUG1 is associated with cell proliferation, migration, and invasion in breast cancer. Biomed. Pharmacother. 95, 1636–1643, 10.1016/j.biopha.2017.09.076 [DOI] [PubMed] [Google Scholar]
- 89.Yang F., Liu Y.H., Dong S.Y., Ma R.M., Bhandari A., Zhang X.H. et al. (2016) A novel long non-coding RNA FGF14-AS2 is correlated with progression and prognosis in breast cancer. Biochem. Biophys. Res. Commun. 470, 479–483, 10.1016/j.bbrc.2016.01.147 [DOI] [PubMed] [Google Scholar]
- 90.Sun J., Chen X., Wang Z., Guo M., Shi H., Wang X. et al. (2015) A potential prognostic long non-coding RNA signature to predict metastasis-free survival of breast cancer patients. Sci. Rep. 5, 16553, 10.1038/srep16553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Miao Y., Fan R., Chen L. and Qian H. (2016) Clinical significance of long non-coding RNA MALAT1 expression in tissue and serum of breast cancer. Ann. Clin. Lab. Sci. 46, 418–424, [PubMed] [Google Scholar]
- 92.Chisholm K.M., Wan Y., Li R., Montgomery K.D., Chang H.Y. and West R.B. (2012) Detection of long non-coding RNA in archival tissue: correlation with polycomb protein expression in primary and metastatic breast carcinoma. PLoS One 7, e47998, 10.1371/journal.pone.0047998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Nie Z.L., Wang Y.S., Mei Y.P., Lin X., Zhang G.X., Sun H.L. et al. (2018) Prognostic significance of long noncoding RNA Z38 as a candidate biomarker in breast cancer 32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Jiang M., Xiao Y., Liu D., Luo N., Gao Q. and Guan Y. (2018) Overexpression of long noncoding RNA LINC01296 indicates an unfavorable prognosis and promotes tumorigenesis in breast cancer. Gene 10.1016/j.gene.2018.07.004 [DOI] [PubMed] [Google Scholar]
- 95.Yang Y.X., Wei L., Zhang Y.J., Hayano T., Pineiro Pereda M.D.P., Nakaoka H. et al. (2018) Long non-coding RNA p10247 , high expressed in breast cancer (lncRNA-BCHE), is correlated with metastasis. Clin. Exp. Metastasis 35, 10247–121. 10.1007/s10585-018-9901-2 [DOI] [PubMed] [Google Scholar]
- 96.Bing A.U., Kerr G.R., Jack W., Chetty U., Williams L.J., Rodger A. et al. (2016) Pooled long-term outcomes from two randomized trials of axillary node sampling with axillary radiotherapy versus axillary node clearance in patients with operable node-positive breast cancer. Br. J. Surg. 103, 81–87 10.1002/bjs.9952 [DOI] [PubMed] [Google Scholar]
- 97.Twelves C., Cortes J., Kaufman P.A., Yelle L., Awada A., Binder T.A. et al. (2015) “New” metastases are associated with a poorer prognosis than growth of pre-existing metastases in patients with metastatic breast cancer treated with chemotherapy. Breast Cancer Res. 17, 150 10.1186/s13058-015-0657-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Giuliano A.E., Ballman K.V., McCall L., Beitsch P.D., Brennan M.B., Kelemen P.R. et al. (2017) Effect of axillary dissection vs no axillary dissection on 10-year overall survival among women with invasive breast cancer and sentinel node metastasis: the ACOSOG Z0011 (Alliance) randomized clinical trial. JAMA 318, 918–926 10.1001/jama.2017.11470 [DOI] [PMC free article] [PubMed] [Google Scholar]