Abstract
Albinism in shoots of tea plants is a common phenotypic expression which gives the tea infusion a pleasant umami taste. A novel natural albino mutant tea germplasm containing high amino acids content was found and named as ‘Huabai 1’. ‘Huabai 1’ has white jade tender shoots under low temperature and turns green with increased temperature. In order to understand the molecular mechanism of color change in leaf of ‘Huabai 1’, transcriptome analysis was performed to identify albino-associated differentially expressed genes (DEGs). A total of 483 DEGs were identified from white shoots of ‘Huabai 1’ compared to its green shoots. There were 15 DEGs identified to be involved in phenylpropanoid biosynthesis, which account for the majority of characterized DEGs. The metabolites related to phenylpropanoid biosynthesis revealed similar expression pattern of DEGs. Furthermore, metabolic pathways such as ubiquonone, tyrosine, and flavonoid biosynthesis associated with phenylpropanoid biosynthesis could also contribute to the color change in ‘Huabai 1’ tender shoots. Protein–protein interaction analysis revealed a hub protein NEDD8 (CSA009575) which interacted with many regulated genes in spliceosome, nitrogen metabolism, phenylpropanoid biosynthesis, and other pathways. In conclusion, the findings in this study indicate that the color change of ‘Huabai 1’ tender shoots is a combined effect of phenylpropanoid biosynthesis pathway and other metabolic pathways including flavonoid biosynthesis in tea plants. Chlorophyll biosynthesis-related genes LHCII and SGR may also play some roles in color change of ‘Huabai 1’.
Tea plants: Controlling color and flavor
Researchers have identified genes and related metabolic processes in tea plants that control the lack of green color known as albinism, which gives the tea a savory “umami” taste. The scientists, led by Xinghui Li at Nanjing Agricultural University in China, investigated genes associated with color changes in tender shoots of a novel albino variety of tea plant known as Huabai 1. The transition from white to green shoots is a temperature-dependent process associated with differences in the activity of 483 identified genes. Control of the color change is also linked to alterations in chemical reaction pathways involving a variety of plant compounds, including phenylpropanoids, flavonoids and the green chlorophylls most commonly associated with plant color. The insights may help plant breeders to create new varieties of teas with highly prized desirable flavors
Introduction
Leaf albinism is a common phenomenon which usually occurs in green plants as a result of chlorophyll deficiency1–3. In general, leaf albinism is an abnormal phenotypic expression, and is usually considered to be an undesirable trait in most crop breeding programs because it can cause several unfavorable outcomes such as yield loss and susceptibility to insects and pathogen attack4,5. However, in tea plants (Camellia sinensis), albinism is of great importance since it produces beneficial metabolites such as amino acids and catechins6. Studies have, therefore, shown that lower catechin and higher amino acid contents in albino tea cultivars account for its good flavor7,8.
Plant leaf albinism is influenced by various biochemical and environmental factors; however, its molecular mechanisms remain unclear. For instance, carotenoid compounds have been found to be involved in leaf color formation9, and suppression of phytoene desaturase gene resulted in leaf albinism in tobacco10. A number of studies have been conducted to uncover the different molecular mechanisms of leaf albinism in different albino tea cultivars. In ‘Baiye 1’, the leaves remain white under low temperature and turn green with increased temperature. The significant changes in metabolites involved in carbon fixation, phenylpropanoid and flavonoid biosynthesis result in leaf albinism in ‘Baiye 1’11. In addition, carbohydrate and energy metabolism, chloroplast biogenesis, and metabolic pathways based on transcriptional and proteomic levels were identified to elucidate the mechanism of color change in the albino tea cultivar ‘Baiye 1’12. In addition to ‘Baiye 1’, a light sensitive albino tea cultivar ‘Huangjinya’, whose leaf color shows yellow when exposed to light and turns green after shading, is mainly determined by the combined effects of flavonoid and carotenoid biosynthesis13.
The albino tea cultivar ‘Baiye 1’ is the mostly studied and the broadest cultivated because of its large economic value of albino tender shoots and high amino acids. Now, a novel albino tea germplasm, which possesses stable albino phenotype in the offspring by either seeds or asexual reproduction, has been identified and named ‘Huabai 1’. ‘Huabai 1’ shows pure white tender shoots, including white mesophyll and white vein. Dried tea made from albino tender shoots of ‘Huabai 1’ is beautiful, and the tea infusion tastes delicious. Dried tea of ‘Huabai 1’ contains high amino acids (84 mg/g) and tea polyphenol (177 mg/g) which contribute to the umami taste of tea infusion and high antioxidative effect, respectively (unpublished data). In addition, the albino period of ‘Huabai 1’ with economic value is longer by 50% than ‘Baiye 1’. Therefore, ‘Huabai 1’ is an alternative and ideal albino tea germplasm to consider in future breeding programs and plantations.
In order to explore the mechanism of shoot albinism in ‘Huabai 1’, transcriptome analysis of the white and green color shoots of ‘Huabai 1’ was conducted. The main differentially expressed genes (DEGs) and proteins identified in the phenylpropanoid biosynthesis and other metabolic pathways including flavonoid biosynthesis will facilitate the understanding of the mechanism involved in plant leaf albinism and albino tea plant breeding.
Materials and methods
Plant materials
Two-year-old ‘Huabai 1’ cuttings were cultivated in the experimental field of Nanjing Agricultural University (Nanjing, China). White tender shoots under low temperature (15/7 °C (day/night)) and green tender shoots under high temperature(30/19 °C (day/night)) were harvested and quickly frozen in liquid nitrogen and stored at −80 °C for RNA extraction and subsequent analysis of metabolites.
RNA extraction, cDNA library preparation, and sequencing
Total RNA was extracted using EASYspin Plus Plant RNA Extraction Kit (Aidlab Biotech, Beijing, China) according to the manufacture’s instruction. The quality and concentration of extracted RNA was assessed using Agilent 2100 Bio-analyzer (Agilent, USA) and NanoDrop ND-1000 spectrophotometer (NanoDrop, USA), respectively.
A total of 3 μg mixed RNA of each sample was used for cDNA library construction. Sequencing libraries were prepared using NEBNext®Ultra™ RNA Library Prep Kit for Illumina® (NEB, MA, USA) following manufacturer’s instruction. Briefly, the mRNA was purified using Oligo (dT). The purified mRNA was fragmented using NEBNext First Strand Synthesis Reaction Buffer. First strand cDNA was then synthesized using random hexamer primer, and then the second strand was synthesized using RNaseH, DNA Polymerase I, and dNTPs. After adenylation of 3′ end and purification of the cDNA library, polymerase chain reaction (PCR) was performed using Phusion High-Fidelity DNA polymerase. Finally, the PCR products were purified using AMPure XP system (Beckman Coulter, Indianapolis, IN, USA) and the library quality was assessed using the Agilent 2100 Bioanalyzer. After clustering with TruSeq PE Cluster Kit v3-cBot-HS (Illumia), the generated cDNA library was sequenced on Illumina HiSeqTM 2500 platform (Biomarker Biotech, Beijing, China) and paired-end reads were generated. Three biological replicates were conducted for each sample.
Quality control and transcriptome analysis
The clean data were produced by removing low quality reads, adapter, and reads with ploy-A. The clean reads were aligned to the tea plant genome14 (http://www.plantkingdomgdb.com/tea_tree/) by TopHat215. According to the reference genome, the unannotated genes were identified using Cufflinks software16. These genes were annotated by blast to NCBI non-redundant protein sequences (Nr) searching17, Pfam identification18, and to blast with Clusters of Orthologous Groups of proteins (KOG/COG)19,20, Swiss-Prot21, Kyoto Encyclopedia of Genes and Genomes (KEGG)22 and Gene Ontology (GO)23.
Differential expression analysis
Fragments Per Kilobase of transcript per Million fragments (FPKM) was used for the evaluation of expression of the transcripts24. Biological variability of the samples were assessed by Pearson’s Correlation Coefficient of which r2 close to 1 indicated a high correlation of the replicates25. DESeq was applied for differential expression analyses of genes26. Expressions, generated through Benjamini–Hochberg adjustment of p-value, with fold change ≥2 and false discovery rate (FDR) <0.01 were considered as DEGs.
Quantitative real-time PCR (qRT-PCR) verification analysis
To validate the reliability of RNA-Seq data, qRT-PCR was performed. The first strand cDNA was synthesized using the RevertAid First Strand cDNA Synthesis Kit (Thermo Scientific, USA). The qRT-PCR was performed in 20 μL reaction volume containing 1 μL of 100 ng cDNA, 0.5 μL each of 10 μM forward and reverse primers, 10 μL SYBR Premix Ex Taq II (Takara, Japan) and filled with 8 μL ddH2O. The primers of candidate DEGs used for qRT-PCR are listed in Table 1. The qPCR was conducted on Roche 480 II system (Switzerland) with the following thermos cycling: 95 °C for 30 s, 95 °C for 5 s, and 60 °C for 30 s for 40 cycles. All the samples were performed in three biological replicates. The glyceraldehyde-3-phosphate dehydrogenase gene (GAPDH), actin (ACTIN), and 18S ribosomal RNA (18S rRNA) were used as the reference genes27. The relative expressions of investigated genes were calculated using 2−ΔΔCT method28.
Table 1.
The primers used for quantitative RT-PCR verification
| Gene ID | Gene name | Forward (5′-3′) | Reverse (5′-3′) | Product size (bp) |
|---|---|---|---|---|
| CSA016595 | Galactinol synthase 2 | GGTCACGCTTCCTACTTCAT | GCATGTTCTCTTCCTGTCCA | 209 |
| CSA031871 | Thioredoxin-like protein CXXS1 | GTCCATTTTACTGCTTCTTG | CCATCCTTGTTGCTACCTCC | 136 |
| CSA021228 | Transparent testa 12 | GCATCCAAATCTACTACTAC | GGTGTTGTGTTTTGCTGATG | 141 |
| CSA030945 | Plant cadmium resistance 2 | GCAGAGATTGTTGACGAAGG | CTAAACAATCACCACAAGGG | 163 |
| CSA027637 | Reticuline oxidase-like protein | TCACACATCCAAGCAGCCAT | GGTCTCGTCTTCTATGCTGA | 168 |
| CSA011590 | KH domain-containing protein | GAAGAACCAATAGAGGACCC | ACATTACTGGAGAAACACAC | 178 |
| CSA013206 | Putative disease resistance protein RGA1 | GTCATTGTGTGGGAGGAGAT | CTTGAGATGGTATGTGGAAT | 184 |
| CSA002486 | Thaumatin-like protein | CTGACATAGTTGGCGAGTGC | CATCTGGGCACCTATCCTTG | 185 |
| CSA011958 | Coffea canephora DH200 | ATGTCGTCTCCAACTCCCTC | ATCAAAACCATTACAGGGCT | 155 |
| GAPDH | Glyceraldehyde-3-phosphate dehydrogenase | GTTTGGCGTCGTTGAGGGTC | GGCAGCACCTTACCAACAG | 164 |
| ACTIN | ACTIN | GAACCCGAAGGCGAATAGG | ACCATCACCAGAATCCAAGAC | 145 |
| 18S rRNA | 18S rRNA | TCTGCCCGTTGCTCTGATG | TCACCCGTCACCACCATAG | 134 |
Detection of the metabolites on phenylpropanoid biosynthesis
Metabolites were extracted from 100 mg tea shoots using 1.0 mL 70% methanol containing 0.1 mg/L Lidocaine and incubated at 4 °C overnight. After centrifugation at 10,000×g for 10 min, the supernatant was filtered with 0.22 μm pore size membrane for further liquid chromatography-tandem mass spectrometry analysis (LC-MS). Five microliters of each sample was detected using the Ultra Performance Liquid Chromatography (UPLC, Shim-pack UFLC SHIMADZU CBM20A, http://www.shimadzu.com.cn/) with Waters ACQUITY UPLC HSS T3 C18 1.8 µm column (2.1 mm×100 mm). Different ratios of water/acetonitrile were used as eluting agent in the following gradients: 95:5 V/V at 0 min, followed by 5:95 V/V at 11.0 min, 5:95 V/V at 12.0 min, 95:5 V/V at 12.1 min, and 95:5 V/V at 15.0 min. The flow rate was 0.4 mL/min, and the column temperature was 40 °C. Isolated samples were analyzed using the Applied Biosystems 4500 QTRAP MS system (http://www.appliedbiosystems.com.cn/). The peak area was recorded and normalized to characterize the metabolite differences between green and white ‘Huabai 1’ tender shoots.
Statistical analysis
Statistical analysis was performed using Microsoft Excel 2016 and GraphPad Prism 5.0 (https://www.graphpad.com/scientific-software/prism/). One-way ANOVA was used for significant difference analysis, and P < 0.05 was considered to be significantly different.
Results
Phenotypic characteristics of ‘Huabai 1’
Under low temperatures of 15/7 °C (day/night), tender shoots of ‘Huabai 1’ were pure white. However, at high temperatures of 30/19 °C (day/night), shoots turned green, while leaf veins remained white (Fig. 1a). The chlorophyll and carotenoid contents of the green shoots were significantly higher than in albino shoots (Figure 1B and Table S1).
Fig. 1. The phenotype and chlorophyll content of white and green ‘Huabai 1’ leaves.
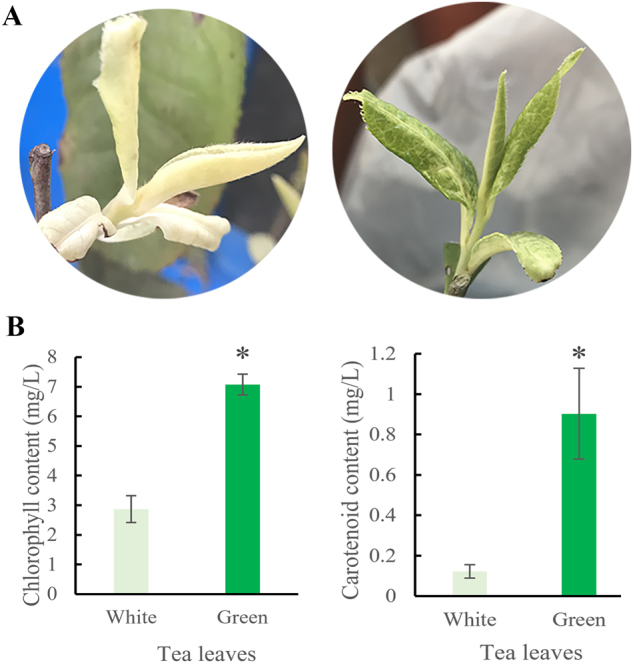
a leaf color of ‘Huabai 1’ new shoots. b Chlorophyll and carotenoid content of ‘Huabai 1’ new shoots; * represents significant difference
DEGs identification
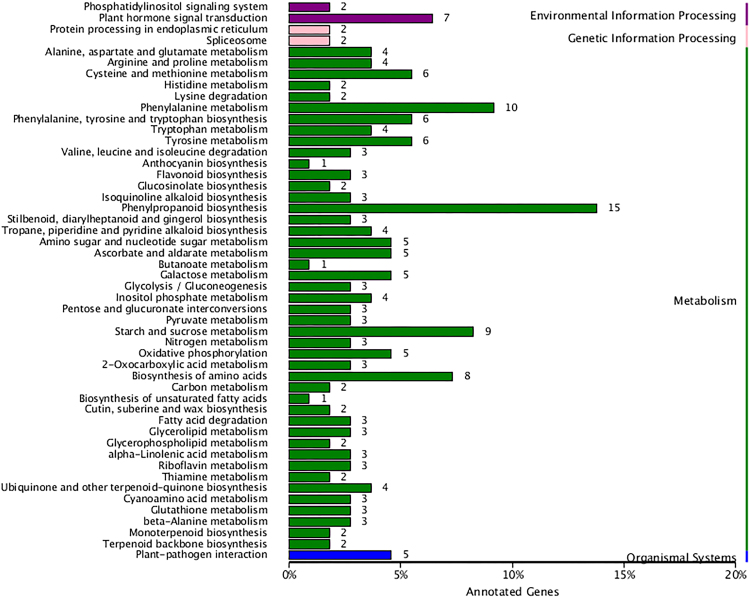
Q30 of the raw data ranged from 89.60 to 91.02% indicating a high-quality reads worthy of further analysis. More than 60% (63.93–65.24) reads of each sample mapped with tea genome (Table 2). The clean reads of the samples in the present study were deposited in the Sequence Read Archive (SRA) of NCBI database, and accession number SRP126084 was obtained. After comparing the green shoots to white shoots of ‘Huabai 1’, a total of 483 DEGs were identified. Among them, 471 genes were exclusively annotated with 182, 331, 170, 203, 471, 395, and 389 annotated genes from COG, GO, KEGG, KOG, NR, Swiss-Prot, and TrEMBL databases, respectively. KEGG pathway analysis revealed that most of the DEGs were enriched in metabolism pathway. There were 15 DEGs identified in the phenylpropanoid biosynthesis pathway, followed by phenylalanine metabolism (10 DEGs), starch and sucrose metabolism (9 DEGs), and biosynthesis of amino acids (8 DEGs) (Fig. 2).
Table 2.
The quality of the transcriptome of white and green ‘Huabai 1’ leaves
| Samples | GC content | %≥Q30 | Total reads | Mapped reads |
|---|---|---|---|---|
| White-1 | 44.56% | 89.79% | 49,281,276 | 31,503,245 (63.93%) |
| White-2 | 45.31% | 89.60% | 47,142,112 | 30,423,001 (64.53%) |
| White-3 | 45.40% | 89.77% | 44,300,068 | 28,726,569 (64.85%) |
| Green-1 | 45.23% | 91.02% | 43,114,402 | 28,126,192 (65.24%) |
| Green-2 | 45.02% | 90.14% | 48,858,098 | 31,515,833 (64.50%) |
| Green-3 | 45.10% | 90.42% | 45,656,596 | 29,720,851 (65.10%) |
Fig. 2.
Differentially expressed genes enriched on different KEGG pathways.
In order to verify the reliability of the DEG expression, 9 DEGs were randomly selected for qRT-PCR analysis. The results indicated similar expression patterns in transcript abundance analysis by RNA-Seq and qRT-PCR (Fig. 3).
Fig. 3. Quantitative RT-PCR verification for randomly selected differentially expressed genes.
RNA-Seq and qRT-PCR data were displayed as fold changes
DEGs and metabolites on phenylpropanoid biosynthesis
The expression of the genes involved in phenylpropanoid biosynthesis of white tender shoots showed significant higher levels than that of green shoots, especially for the genes related to deoxygenation and hydrogenation reduction reactions. As shown in Fig. 4, ferulate-5-hydroxylase (F5H), which encoded the cytochrome P450 isoform in association with syringyl lignin precursor hydroxylation, expressed significantly higher in white tender shoots than in green shoots. The expressions of cinnamyl-alcohol dehydrogenase (CAD) catalyzing the alcoholization, and Peroxidase (POD) in lignin synthesis, were also higher in white tender shoots. The relative changes of the metabolites downstream of phenylprponoid biosynthesis including sinapoyl-malate, sinapaldehyde, and sinapyl alcohol were consistent with the expressions of the corresponding genes. In addition, beta-glucosidase (β-GS) directed deglycosylation of β-D-glucosyl-Coumarate, and led to accumulation of coumarine (Fig. 4).
Fig. 4. The differentially expressed genes and metabolites on phenylpropanoid biosynthesis.
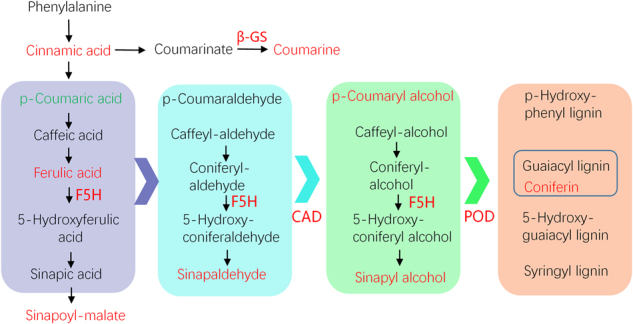
Red and green, respectively, mean up- and downregulation in white leaves compared to green leaves of ‘Huabai 1’
Other pathways related to phenylpropanoid biosynthesis
In addition to phenylpropanoid biosynthesis, several other metabolic pathways were also enriched with DEGs, including six in tyrosine, four in tryptophan, four in ubiquonone, three in flavonoid biosynthesis, and three in ‘stilbenoid, diaryheptanoid, and gingerol biosynthesis’. This result, therefore, suggests that leaf color change in ‘Huabai 1’ is a combined effect of the above-mentioned phenylpropanoid biosynthesis associated pathways (Fig. 5).
Fig. 5.
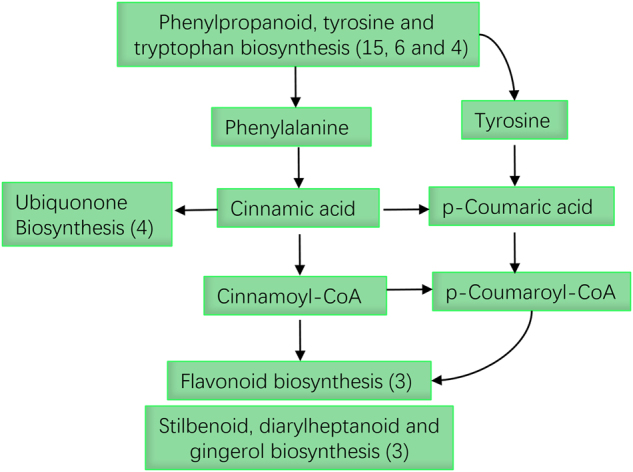
Phenylpropanoid biosynthesis-associated metabolic pathways in responding to color change of ‘Huabai 1’.
Chlorophyll biosynthesis-related genes
Chlorophyll deficiency is the direct expression of albino tea plants. Among 471 DEGs, two genes contributing to chlorophyll biosynthesis were identified: light-harvesting complex II chlorophyll a/b binding protein (LHCII, CSA035910) and STAY-GREEN (SGR, CSA024979). The expression of LHCII was higher in green tea shoots (2 folds) but SGR was higher in albino tea shoots (5.76 folds).
Protein–protein interaction analysis
Functional partnerships and interactions occur between proteins, and such interactions are usually at the core of cellular processing. In the present study, protein interaction networks revealed the presence of a hub protein, ‘neural precursor cell expressed developmentally downregulated 8’ (NEDD8), in albino leaves. This protein is also known as Related To Ubiquitin (RUB). It was found to have correlations with proteins of many metabolic pathways, of which the major ones included heat shock 70 kDa protein in spliceosome, ferredoxin-nitrite reductase and glutamate dehydrogenase in nitrogen metabolism, CAD in phenylpropanoid biosynthesis, and galactinol synthase in galactose metabolism (Fig. 6 and Table S2).
Fig. 6. Protein–protein interaction network analysis based on the differentially expressed genes.
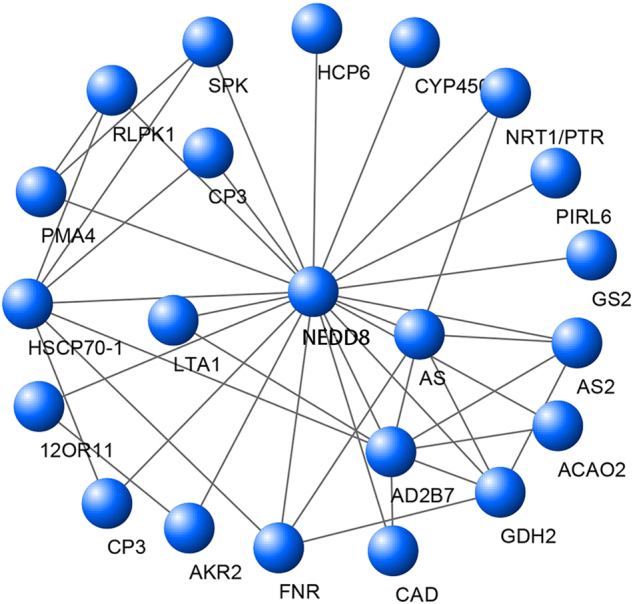
The definition and description of the proteins in this network are shown in Table S1
Discussion
Albinism in tender shoots is a desirable trait in most tea plant breeding programs due to its high amino acid and low catechins contents. ‘Baiye 1’ is not only the most studied albino tea cultivar in tea breeding program, but also the widely cultivated cultivar in most tea plantations29. However, the defects of shortened and unstable albinism are more pronounced in this cultivar. Thus, the ‘Huabai 1’ with pure white tender shoots and long albinism period is an alternative and ideal germplasm. In this study, transcriptome and metabolite analysis were employed to investigate the mechanism of albinism in ‘Huabai 1’ tea plants. A total of 15 DEGs were identified to be involved in phenylpropanoid biosynthesis. These DEGs were found to be the most influential factors that contributed to color changes of leaves in ‘Huabai 1’. Meanwhile, phenylpropanoid biosynthesis pathways were also observed to play a considerable role in color change in ‘Huabai 1’. In addition, a hub protein, NEDD8, was characterized through protein–protein interaction analysis. This protein has correlations to several metabolic pathways including spliceosome, nitrogen metabolism, and phenylpropanoid biosynthesis.
The phenylpropanoid metabolism generates a large number of secondary metabolites through many enzymes, like ligases, oxygenases, oxidoreductases, and transferases30. Lignin biosynthesis is a major branch of phenylpropanoid biosynthesis and produces lignin polymers31. Lignin is exclusively based on phenylpropanoid units derived from the oxidative polymerization of cinnamoyl alcohol derivatives. Cinnamyl alcohol dehydrogenase catalyzes the final step in monolignol biosynthesis, transferring the –aldehydes to –alcohols. Disruption of the CAD leads to altered lignification and promotes saccharification in Brachypodium distachyon32. As well, CAD can change the lignin structure and loss of function of CAD would lead to temperature sensitive defects33. In the present study, CAD of ‘Huabai 1’ was highly expressed in albino tea tender shoots. This observation can be explained by the mechanism involved in change in lignin structure as a result of loss of function of CAD, and its resultant effect on leaf coloration. The results in the present study, therefore, suggest that albinism in ‘Huabai 1’ is a cold sensitive phenotypic expression. This is due to higher expression of CAD in albino tea tender shoots than in green tender shoots. However, leaf albinism in ‘Huabai 1’ restored to normalcy with increased temperature.
With the exception of CAD, F5H is another dominant gene involved in lignin biosynthesis, which could control the ratio of syringyl (S)/guaiacyl (G) lignin. Downregulation of F5H produced more lignin with G units, whereas upregulation of F5H resulted in lignin with S units34. In white ‘Huabai 1’ tender shoots, the expression of F5H gene was higher than in green tender shoots. Meanwhile, the S-lignin precursors, including sinapoyl-malate, sinapaldehyde, and sinapyl alcohol in white tender shoots were also higher than in green tender shoots (Fig. 4). These results indicate a positive correlation between F5H and S-lignins precursors in tea plants. Therefore, the higher the F5H expressed, the more the S-lignins precursor content were produced. S-lignins were derived from the oxidative polymerization of sinapyl alcohol under POD catalyzation. Although higher sinapyl alcohol content and POD expression were observed in white ‘Huabai 1’ tender shoots, there was no significant changes in the S-lignin found. Therefore, it suggests that S-lignin may not be the unique result of sinapyl alcohol polymerization. This suggests that sinapyl alcohol can produce other derivatives apart from S-lignin. Tian et al.35 have identified four new sinapyl alcohol derivatives dichrocephols A–D from Lipo-soluble part of Dichrocephala benthamii. These derivatives may also exist in tea tender shoots.
According to the annotation of the DEGs, two chlorophyll catalytic process related genes LHCII and SGR were identified. LHCII protein embedded in chloroplast membranes and the aggregation of LHCII chlorophyll protein complex could control the light harvesting function of chloroplast membranes36. However, SGR expressed in senescing plastids and was required for the degradation of chlorophyll37. In addition, SGR could interact with LHCII and led to breakdown of LHCII-located chlorophyll (as the model shown in Fig. 7)37. Therefore, in ‘Huabai 1’ albino shoots, the higher expression of SGR and lower expression of LHCII suggest that the LHCII chlorophyll biosynthesis was inhibited and the breakdown of chlorophyll was accelerated. This may be part of cause of the color change in ‘Huabai 1’ shoots.
Fig. 7.
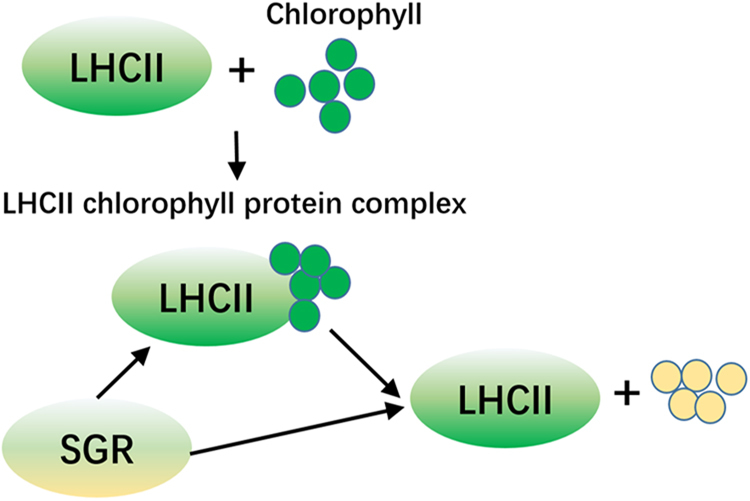
The model of role of LHCII and SGR proteins on chlorophyll catalytic process.
In this study, the color change in ‘Huabai 1’ was based on a network with the core gene of NEDD8. Like ubiquitin, NEDD8 is conjugated to the substrate protein through the isopeptide bond between C-terminal glycine and a lysine residue of the target protein (neddylation)38. NEDD8 modification is a post-translational modification of the cullin subunits of cullin-RING E3 ubiquitin ligases. Boh et al. reported that neddylation induced a conformation change of cullin RING ligases domains via structural analysis in vitro, and then promotes ubiquitin transfer onto the substrate39. In plants, the NEDD8‐conjugating enzyme mutation resulted in lethal phenotype, indicating that the NEDD8 conjugation pathway plays an important role in plant growth and development39. In Mirabilis jalapa, petroleum stress induced accumulation of a number of proteins including NEDD840. Therefore, the expression changes of NEDD8 and related proteins in ‘Huabai 1’ tender shoots would contribute to the leaf color change.
In conclusion, phenylpropanoid biosynthesis contributed to the color change in ‘Huabai 1’ tender shoots. Chlorophyll biosynthesis-related genes (LHCII and SGR) and a protein network with the core of NEDD8 may also be an influencing factor in leaf color change in ‘Huabai 1’.
Electronic supplementary material
Acknowledgements
This study was supported by the key R&D Plan of Liyang city for development of a novel albino tea germplasm ‘Huabai 1’, National Natural Science Foundation of China (31470690, 31570689), and the China Earmarked Fund for Modern Agro-industry Technology Research System (CARS-19).
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Supplementary Information accompanies this paper at 10.1038/s41438-018-0053-y.
References
- 1.Wu Z, et al. A chlorophyll-deficient rice mutant with impaired chlorophyllide esterification in chlorophyll biosynthesis. Plant Physiol. 2007;145:29–40. doi: 10.1104/pp.107.100321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Campbell BW, et al. Identical substitutions in magnesium chelatase paralogs result in chlorophyll-deficient soybean mutants. G3. 2015;5:123–131. doi: 10.1534/g3.114.015255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhu L, et al. Genetic characterisation and fine mapping of a chlorophyll-deficient mutant (BnaC.ygl) in Brassica napus. Mol. Breed. 2014;34:603–614. doi: 10.1007/s11032-014-0060-0. [DOI] [Google Scholar]
- 4.Choi HG, et al. Yield loss and quality degradation of strawberry fruits cultivated under the deficient insolation conditions by shading. Hortic. Environ. Biotech. 2014;55:263–270. doi: 10.1007/s13580-014-0039-0. [DOI] [Google Scholar]
- 5.Slattery RA, et al. Photosynthesis, light use efficiency, and yield of reduced-chlorophyll soybean mutants in field conditions. Front. Plant Sci. 2017;8:549. doi: 10.3389/fpls.2017.00549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Feng L, et al. Determination of quality constituents in the young leaves of albino tea cultivars. Food Chem. 2014;155:98–104. doi: 10.1016/j.foodchem.2014.01.044. [DOI] [PubMed] [Google Scholar]
- 7.Du YY, et al. A study on the chemical composition of albino tea cultivars. J. Hortic. Sci. Biotech. 2006;81:809–812. doi: 10.1080/14620316.2006.11512142. [DOI] [Google Scholar]
- 8.Wei K, et al. Comparison of catechins and purine alkaloids in albino and normal green tea cultivars (Camellia sinensis L.) by HPLC. Food Chem. 2012;130:720–724. doi: 10.1016/j.foodchem.2011.07.092. [DOI] [Google Scholar]
- 9.Yuan H, Zhang J, Nageswaran D, Li L. Carotenoid metabolism and regulation in horticultural crops. Hortic. Res. 2015;2:15036. doi: 10.1038/hortres.2015.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang M, Wang G, Ji J. Suppression of the phytoene desaturase gene influence on the organization and function of photosystem II (PSII) and antioxidant enzyme activities in tobacco. Environ. Exp. Bot. 2010;67:460–466. doi: 10.1016/j.envexpbot.2009.09.007. [DOI] [Google Scholar]
- 11.Li CF, et al. Differential Metabolic Profiles during the Albescent Stages of ‘Anji Baicha’ (Camellia sinensis) PLoS ONE. 2015;10:e0139996. doi: 10.1371/journal.pone.0139996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li Q, et al. Proteomic analysis of young leaves at three developmental stages in an albino tea cultivar. Proteome Sci. 2011;9:44. doi: 10.1186/1477-5956-9-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Song L, et al. Molecular link between leaf coloration and gene expression of flavonoid and carotenoid biosynthesis in Camellia sinensis cultivar ‘Huangjinya’. Front. Plant Sci. 2017;8:803. doi: 10.3389/fpls.2017.00803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xia EH, et al. The tea tree genome provides insights into tea flavor and independent evolution of caffeine biosynthesis. Mol. Plant. 2017;10:866–877. doi: 10.1016/j.molp.2017.04.002. [DOI] [PubMed] [Google Scholar]
- 15.Kim D, et al. TopHat2: accurate alignment of transcriptomes in the presence of insertions, deletions and gene fusions. Genome Biol. 2013;14:R36. doi: 10.1186/gb-2013-14-4-r36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Trapnell C, et al. Transcript assembly and quantification by RNA-Seq reveals unannotated transcripts and isoform switching during cell differentiation. Nat. Biotechnol. 2010;28:511–515. doi: 10.1038/nbt.1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Deng, Y. Y. et al. Integrated nr database in protein annotation system and Its localization. Computer Engineering32, 71–73 (2006).
- 18.Finn RD, et al. Pfam: the protein families database. Nucleic Acids Res. 2014;42:D222–D230. doi: 10.1093/nar/gkt1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tatusov RL, Galperin MY, Natale DA, Koonin EV. The COG database: a tool for genome-scale analysis of protein functions and evolution. Nucleic Acids Res. 2000;28:33–36. doi: 10.1093/nar/28.1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Koonin EV, et al. A comprehensive evolutionary classification of proteins encoded in complete eukaryotic genomes. Genome Biol. 2004;5:R7–R7. doi: 10.1186/gb-2004-5-2-r7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Apweiler R, et al. UniProt: the Universal Protein knowledgebase. Nucleic Acids Res. 2004;32:D115–D119. doi: 10.1093/nar/gkh131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kanehisa M, et al. The KEGG resource for deciphering the genome. Nucleic Acids Res. 2004;32:D277–D280. doi: 10.1093/nar/gkh063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ashburner M, et al. Gene ontology: tool for the unification of biology. The Gene Ontology Consortium. Nat. Genet. 2000;25:25–29. doi: 10.1038/75556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Florea L, Song L, Salzberg SL. Thousands of exon skipping events differentiate among splicing patterns in sixteen human tissues. F1000Res. 2013;2:188. doi: 10.12688/f1000research.2-188.v1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schulze SK, et al. SERE: single-parameter quality control and sample comparison for RNA-Seq. BMC Genomics. 2012;13:524. doi: 10.1186/1471-2164-13-524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Anders S, Huber W. Differential expression analysis for sequence count data. Genome Biol. 2010;11:R106. doi: 10.1186/gb-2010-11-10-r106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ma QP, Hao S, Chen X, Li XH. Validation of reliability for reference genes under various abiotic stresses in tea plant. Russ. J. Plant Physiol. 2016;63:423–432. doi: 10.1134/S1021443716030080. [DOI] [Google Scholar]
- 28.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 29.Li CF, et al. Biochemical and transcriptomic analyses reveal different metabolite biosynthesis profiles among three color and developmental stages in ‘Anji Baicha’ (Camellia sinensis) BMC Plant Biol. 2016;16:195. doi: 10.1186/s12870-016-0885-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vogt T. Phenylpropanoid biosynthesis. Mol. Plant. 2010;3:2–20. doi: 10.1093/mp/ssp106. [DOI] [PubMed] [Google Scholar]
- 31.Boerjan W, Ralph J, Baucher M. Lignin Biosynthesis. Annu. Rev. Plant. Biol. 2003;54:519–546. doi: 10.1146/annurev.arplant.54.031902.134938. [DOI] [PubMed] [Google Scholar]
- 32.Bouvier d’Yvoire M, et al. Disrupting the cinnamyl alcohol dehydrogenase 1 gene (BdCAD1) leads to altered lignification and improved saccharification in Brachypodium distachyon. Plant J. 2013;73:496–508. doi: 10.1111/tpj.12053. [DOI] [PubMed] [Google Scholar]
- 33.Zhao Q, et al. Loss of function of cinnamyl alcohol dehydrogenase 1 leads to unconventional lignin and a temperature-sensitive growth defect in Medicago truncatula. PNAS. 2013;110:13660–13665. doi: 10.1073/pnas.1312234110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Takeda Y, et al. Regulation of coniferaldehyde 5-hydroxylase expression to modulate cell wall lignin structure in rice. Planta. 2017;246:337–349. doi: 10.1007/s00425-017-2692-x. [DOI] [PubMed] [Google Scholar]
- 35.Tian X, et al. Sinapyl alcohol derivatives from the lipo-soluble part of Dichrocephala benthamii C. B. Clarke. Molecules. 2013;18:1720. doi: 10.3390/molecules18021720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pascal AA, et al. Molecular basis of photoprotection and control of photosynthetic light-harvesting. Nature. 2005;436:134. doi: 10.1038/nature03795. [DOI] [PubMed] [Google Scholar]
- 37.Sakuraba Y, et al. STAY-GREEN and chlorophyll catabolic enzymes interact at light-harvesting complex II for chlorophyll detoxification during leaf senescence in arabidopsis. Plant Cell. 2012;24:507–518. doi: 10.1105/tpc.111.089474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schwechheimer, C. & Mergner, J. The NEDD8 modification pathway in plants. Front. Plant Sci. 5, 103 (2014). [DOI] [PMC free article] [PubMed]
- 39.Boh BK, Smith PG, Hagen T. Neddylation-induced conformational control regulates cullin RING ligase activity in vivo. J. Mol. Biol. 2011;409:136–145. doi: 10.1016/j.jmb.2011.03.023. [DOI] [PubMed] [Google Scholar]
- 40.Chen S, et al. Quantitative proteomics analysis reveals the tolerance of Mirabilis jalapa L. to petroleum contamination. Environ. Sci. Pollut. Res. 2017;24:7375–7382. doi: 10.1007/s11356-017-8403-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.