Abstract
We evaluated the effects of Ivabradine on left ventricle (LV) ejection fraction (EF) and LV infarcted tissue in the rat myocardial ischemia-reperfusion model. Twenty rats were randomly assigned to group 1 (ischemia-reperfusion, no treatment, n=10) and group 2 (ischemia-reperfusion + Ivabradine 10 mg/kg, n=10). Ivabradine was administered for 28 days. Echocardiography was performed at 7 days and at 28 days after the induction of ischemia-reperfusion injury. Cardiac fibrosis induced by ischemia-reperfusion injury was evaluated by Masson's trichrome staining. The infarct size was quantified using the Image J program. At the 28-day follow-up, LVEF was significantly higher (36.02±6.16% vs. 45.72±2.62%, p<0.001) and fractional shortening was significantly higher (15.23±2.84% vs. 20.13±1.38%, p<0.001) in group 2 than group 1. Delta (28 day minus 7 day) EF was significantly higher in group 2 than group 1 (−4.36±3.49% vs. 4.31±5.63%, p<0.001). Also, heart rate (beats/min) was significantly lower in group 2 than group 1 (251.67±25.19 vs. 199.29±31.33, p=0.025). Group 2 had a smaller infarct size (40.70±8.94% vs. 30.19±5.89%, p<0.01) than group 1 at 28-day follow-up. Oral administration of Ivabradine could improve LV systolic function and reduce infarcted tissue area in rat myocardial ischemia-reperfusion model.
Keywords: Myocardial Ischemia, Myocardial Reperfusion Injury, Echocardiography, Ivabradine
INTRODUCTION
Myocardial infarction (MI) is significantly increasing compared with the past, and the definition of disease has evolved from the past as a cause of death and prognosis. In addition, treatment plans for MI are constantly evolving and changing.1
MI occurs when the coronary artery is occluded and the oxygen supply to the downstream myocardium becomes insufficient. Lack of oxygen supply induces necrosis in cardiac muscle cells that stimulate the complement cascade and initiate an inflammatory response. After MI, the heart muscle cells of the left ventricle (LV) are oxygenated and die and eventually the ventricular wall becomes thinner.2 The mortality rate of MI has been reduced by performing percutaneous coronary intervention to rehabilitate occluded blood vessels and recently being used medications.3,4
There is much interest and research for secondary prevention after MI after primary treatment by using various drugs. After reperfusion therapy for MI, angiotensin converting enzyme inhibitors, β blockers and angiotensin II receptors blocker are used to maintain cardiac function in order to prevent cardiovascular ischemia.5,6,7
Ivabradine acts by reducing the heart rate via specific inhibition of the funny channel. Ivabradine is a heart rate reducing drug that is used for the symptomatic management of stable heart related chest pain and heart failure that is not fully managed by beta blockers. Actually, Ivabradine has been shown to have prognostic benefits, and improve the functional and safety parameters in systolic heart failure.8,9 In patients with MI associated with reperfusion arrhythmia, long-term reperfusion of ischemia lasts for several hours during percutaneous coronary intervention.10 Ivabradine is available as a treatment strategy to reduce reperfusion arrhythmias.11
So far, no study has evaluated the effect of Ivabradine on ischemia-reperfusion injury. Therefore, the current study was designed to investigate the possible protective effects of Ivabradine such as reduction of infarct size and fibrosis as well as improvement of LV systolic function on ischemia-reperfusion injury in rats.
MATERIALS AND METHODS
1. Study animal
Sprague-Dawley rats (Samtako, Seoul, Republic of Korea), Male, weighing 250–260 g, aged 8 weeks, were used in all experiments. Ivabradine (Procoralan®, Servier, Neuilly-sur-Seine, France) was pulverized in a tablet mill and dissolved in 10 ml of water. A vortex (Vortex-Genie 2, Scientific Industries) was used to mix the drug well before drug administration. The body weight was measured daily before the administration of the drug and was administered proportionally to the body weight.
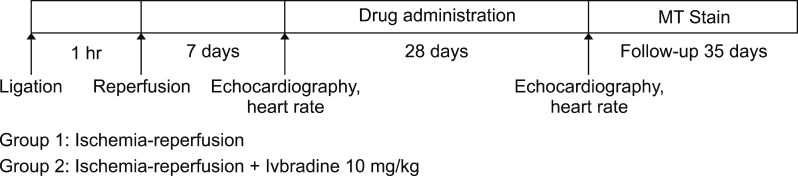
We administered Ivabradine at a high dose (10 mg/kg) orally for 28 days once per day after the induction of ischemia-reperfusion injury (Fig. 1). Twenty rats were randomly assigned to ischemia-reperfusion group (no treatment, n=10) and ischemia-reperfusion + Ivabradine 10 mg/kg group (n=10). The animal experiment protocol was approved by the Ethics Committee of the Chonnam National University Medaical School and the Ethics Committee of the Chonnam National University Hospital (CHU IACUCH-2017-23).
FIG. 1. Ischemia-reperfusion injury protocol.
2. Myocardial ischemia-reperfusion model
Myocardial ischemia-reperfusion was induced with a minor modification of the myocardial infarction Fliss method.12 Sprague-Dawley rats were anesthetized with an intramuscular femur injection of ketamine (50 mg/kg) and xylazine (5 mg/kg). After intubation, respiration maintenance was kept constant by ventilator (Model 683, Harvard apparatus, USA). Regional myocardial ischemia-reperfusion was induced by transient ligation of the proximal left anterior descending coronary artery (LAD) with suture using 5–0 silk. Finally, the heart was set in the original position in the thoracic cavity, and the skin was closed with silk. For the purpose of increasing survival rate and controlling body temperature at 37±0.5℃ a heating pad was used. After 1 hr of ischemia, we observed survival rates, and removed the linked silk in heart for myocardial reperfusion.
3. Drug administration
Ivabradine was dissolved in water, directly administrated into rats through an oral zonde needle (1.2×80 mm, 18G). Ivabradine was administered after the induction of myocardial ischemia-reperfusion injury with daily dose of 10 mg/kg for 28 days.
4. Left ventricular function measurement
The study measured the LV diastole and systole according to the main laws of the American Society of Echocardiography.13 Echocardiography was performed with a 15-MHz linear array transducer (iE33 system, Philips medical systems). Echocardiography was used for heart wall dimension measurements and to measure LV ejection fraction (EF) and myocardial remodeling.
5. Measurement of heart rate
We measured heart rate for 1 minute using echocardiography. Rats were anesthetized with intramuscular femur injection of ketamine and xylazine. Next, the left ventricular motion was measured for 1 minute using a linear array transducer. Heart rate was measured at 1 week and 4 weeks after the induction of ischemia-reperfusion injury.
6. Measurement of infarct size
After the abdomen was opened, cardiac arrest was induced through an abdominal aorta perfusion of 1ml of potassium perchlorate (KClO4), and heart tissue fixation was followed by a saline flush. The heart was quickly removed and heart tissues were immobilized in a 4% paraformaldehyde solution for 3 days, embedded in paraffin. The paraffin blocks were cut 10-um thick. Heart tissue sections were mounted on glass slides and processed with Masson's trichrome staining for measurement of infarct size. The infarct size was measured using an image analysis program called Image J.
7. Statistical analysis
We used Statistical Package for the Social Sciences (SPSS) 22.0 for Microsoft Windows (SPSS, Inc., Chicago, IL, USA) for all statistical analyses. All numerical variables were presented as mean value±standard deviation (SD) and were compared by independent samples t-test. A p value <0.05 was considered statistically significant.
RESULTS
1. Mortality rate
After the induction of MI, the mortality rate was very high (40% overall mortality, less than 35% EF) and the mortality rate during follow-up period was about 20%.
2. Evaluation of LV function by echocardiography
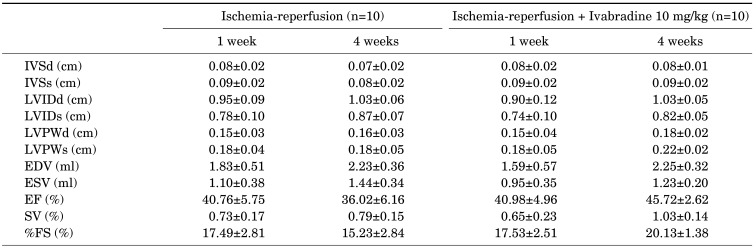
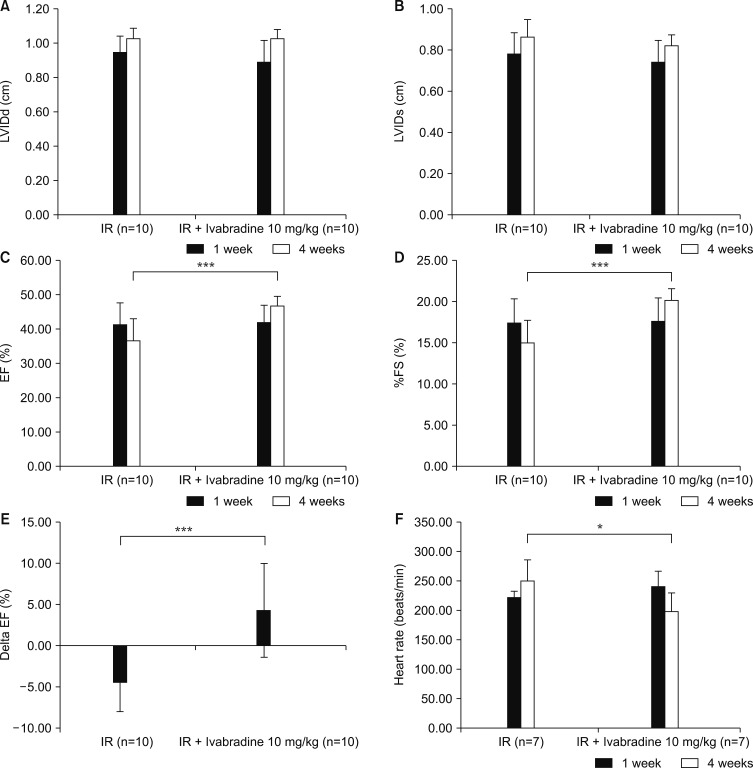
LVEF with M-mode of the short axis was measured in a total of twenty rats. LV functional evaluation was performed at 7-day and 4-week follow-ups after the induction of ischemia-reperfusion injury (Table 1). LV internal diameter at diastole (LVIDd), and LV internal diameter at systole (LVIDs) were similar between the ischemia-reperfusion group and the ischemia-reperfusion +Ivabradine group (Fig. 2A, B). LVEF (45.72±2.62% vs. 36.02±6.16%, p<0.001) and fractional shortening (20.13±1.38% vs. 15.23±2.84%, p<0.001) was significantly higher in the ischemia-reperfusion + Ivabradine group than the ischemia-reperfusion group (Fig. 2C, D). The delta EF was obtained by subtracting the 7 days measurement from the 28 days measurement. Delta EF was significantly higher in the ischemia-reperfusion +Ivabradine group than the ischemia-reperfusion group (4.31±5.63% vs. −4.36±3.49%, p<0.001) (Fig. 2E).
TABLE 1. Echocardiographic findings.
Values are mean±SD. IVSd: interventricular septal thickness at diastole, IVSs: interventricular septal thickness at systole, LVIDd: left ventricle internal diameter at diastole, LVIDs: left ventricle internal diameter at systole, LVPWd: left ventricle posterior wall thickness at diastole, LVPWs: left ventricle posterior wall thickness at systole, EDV: end-diastolic volume, ESV: end-systolic volume, EF: ejection fraction, SV: stroke volume, FS: fractional shortening.
FIG. 2. Changes in cardiac parameters by echocardiography. (A) Left ventricle internal diameter at diastole (LVIDd), (B) left ventricle internal diameter at systole (LVIDs), (C) ejection fraction (EF), (D) % fractional shortening (FS), (E) delta ejection fraction, and (F) heart rate. IR: ischemia-reperfusion. ***p<0.001, *p=0.025.
3. Measurement of heart rate by echocardiography
The heart rate of fourteen rats was measured using the M mode of echocardiography. Heart rate evaluation was performed at the 7-day and 4-week follow-ups after the induction of ischemia-reperfusion injury. The heart rate at the7-day mark was similar between the ischemia-reperfusion group and ischemia-reperfusion +Ivabradine groups (223.75±9.60 vs. 242.14±25.19, p=0.098). However, heart rates at the 4-week follow-up were significantly lower in the ischemia-reperfusion + Ivabradine group than the ischemia-reperfusion group (199.29±31.33 vs. 251.67±25.19, p=0.025) (Fig. 2F).
4. Measurement of cardiac fibrosis and infarct size
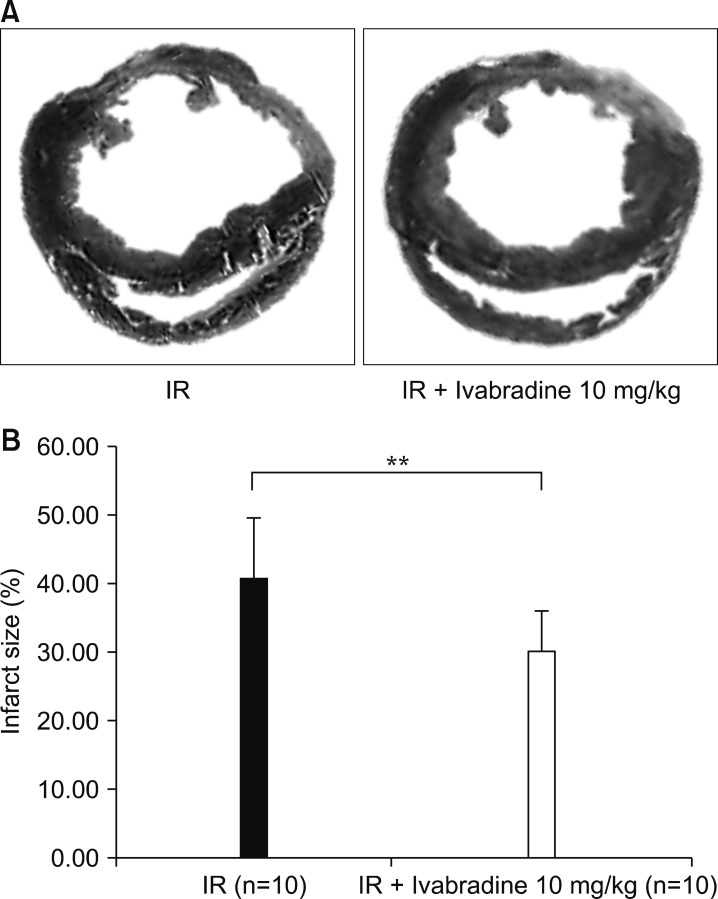
Cardiac fibrosis induced by ischemia-reperfusion was evaluated by Masson's trichrome staining (Fig. 3A). Fibrotic changes were detected as blue color. The infarct size was quantified using the Image J program. The ischemia-reperfusion + Ivabradine group had a significantly smaller infarct size than the ischemia-reperfusion group (30.19±5.89% vs. 40.70±8.94%, p<0.01) (Fig. 3B).
FIG. 3. (A) Masson's trichrome stain, and (B) measurement of infract size. IR: ischemia-reperfusion. **p<0.01.
DISCUSSION
This study was conducted to evaluate the effect of Ivabradine on the rat myocardial ischemia-reperfusion model. Improvement of LV systolic function and reduction of infarct size were observed after the use of Ivabradine in the present study.
Ischemia-reperfusion injuries can cause tissue damage that occurs when blood supply is restored after an oxygen deficiency event (oxygen and hypoxia) in the tissue due to insufficient blood supply.14,15 During the period of ischemia, deficiency of oxygen and nutrients from the blood causes inflammation and oxidative damage through the induction of oxidative stress rather than restoration of circulatory function to normal.16 Treatment methods for MI include medication, stem cell therapy, percutaneous coronary intervention, and bypass graft. There are many studies on cardiac rehabilitation through prevention of complications of progressive heart disease.17,18,19 In the present study, drug therapy was used as a treatment modality and considered as safe because it was easy, fast, and non-invasive in rat ishcemia-reperfusion model.
Ivabradine is a medication used for the symptomatic management of stable heart related chest pain and heart failure not fully managed by beta blockers.20 Ivabradine acts by reducing the heart rate via specific inhibition of the funny channel, a mechanism different from that of beta blockers and calcium channel blockers, two commonly prescribed antianginal drugs. Ivabradine is a cardiotonic agent. It is used for the symptomatic treatment of chronic stable angina pectoris in patients with normal sinus rhythm who cannot take beta blockers. It is also being used off-label in the treatment of inappropriate sinus tachycardia.21 Ivabradine acts on the If(f is for “funny”, so called because it had unusual properties compared with other current systems known at the time of its discovery) ion current, which is highly expressed in the sinoatrial node. If is a mixed Na+–K+ inward current activated by hyperpolarization and modulated by the autonomic nervous system. It is one of the most important ionic currents for regulating pacemaker activity in the sinoatrial (SA) node. Ivabradine selectively inhibits the pacemaker If current in a dose-dependent manner. Blocking this channel reduces cardiac pacemaker activity, selectively slowing the heart rate and allowing more time for blood to flow to the myocardium.22,23
In hypertensive rats, it has been reported that Ivabradine effectively reduces heart rate and thus affects carotid and aortic structure and function.24 Also, Ivabradine was found to improve left ventricular function and endogenous myocardial structure in congestive heart failure due to long-term heart rate reduction.25,26
The effects of Ivabradine were shown in many studies such as the Ivabradine for patients with stable coronary artery disease and left-ventricular systolic dysfunction (BEAUTIFUL) study,27 Ivabradine and outcomes in chronic heart failure (SHIFT) study,28 Efficacy of the I(f) current inhibitor Ivabradine in patients with chronic stable angina receiving beta-blocker therapy (ASSOCIATE) study,29 etc.
In the present study, we found that the effects of Ivabradine on LV systolic function and cardiac fibrosis were observed during the early period after inducing ischemia-reperfusion injury. There are postulated mechanisms for these benefit of Ivabradine. First, Ivabradine shares with β-blockers the property of decreasing heart rate and oxygen demand from the ischemic heart, which is presumably fundamentally important in mediating anti-ischemic effects.23 Second, Ivabradine increases the diastolic time and coronary blood flow.30 Third, in contrast to β-blockers, Ivabradine does not limit the decrease in coronary resistance induced by exercise.31 Fourth, Ivabradine can reduce the stunning of the myocardium via enhanced left ventricular wall thickening, 32,33 and reduction of post-systolic wall-thickening and conversion of post-systolic wall-thickening to ejectional thickening, more so than atenolol, resulting in an improvement in myocardial stunning.34
There are several limitations to be mentioned. First, we only focused on the morphological changes of the heart. Furthermore, the molecular part of the morphological change was not evaluated. Second, we observed the effects of Ivabradine on LV systolic function and cardiac fibrosis for only 1-month after MI. Therefore, further long-term study is needed. Third, we did not measure blood pressure. Fourth, we did not perform the hemodynamic study using a Milar catheter. And fifth, we did not evalute the effects of Ivabradine on the generation of free radicals and hydrogen peroxide, and mitochondrial function.
In conclusion, oral administration of Ivabradine could improve LV systolic function and reduce infarcted tissue area in rat myocardial ischemia-reperfusion model.
ACKNOWLEDGEMENTS
This study was supported by a grant of the Korean Health Technology R&D Project, Ministry of Health and Welfare, Republic of Korea (HI13C0163), by a grant of the Korean Health Technology R&D Project, Ministry of Health and Welfare, Republic of Korea (HI17C2150), by a grant of the Korean Health Technology R&D Project, Ministry of Health and Welfare, Republic of Korea (HI18C0173), by a grant of Bio & Medical Technology Development Program of the National Research Foundation (NRF) & funded by the Korean government (MSIT) (2018M3A9E2024584), by a grant of the Korean Health Technology R&D Project, Ministry of Health and Welfare, Republic of Korea (HI14C2069), and by a grant of the Korean Health Technology R&D Project, Ministry of Health and Welfare, Republic of Korea (HI13C1527).
Footnotes
CONFLICT OF INTEREST STATEMENT: None declared.
References
- 1.Anderson JL, Morrow DA. Actue myocardial infarction. N Engl J Med. 2017;376:2053–2064. doi: 10.1056/NEJMra1606915. [DOI] [PubMed] [Google Scholar]
- 2.Davies MJ, Thomas AC. Plaque fissuring–the cause of acute myocardial infarction, sudden ischaemic death, and crescendo angina. Br Heart J. 1985;53:363–373. doi: 10.1136/hrt.53.4.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Roe MT, Messenger JC, Weintraub WS, Cannon CP, Fonarow GC, Dai D, et al. Treatments, trends, and outcomes of acute myocardial infarction and percutaneous coronary intervention. J Am Coll Cardiol. 2010;56:254–263. doi: 10.1016/j.jacc.2010.05.008. [DOI] [PubMed] [Google Scholar]
- 4.Kim JH, Chae SC, Oh DJ, Kim HS, Kim YJ, Ahn Y, et al. Korea Acute Myocardial Infarction-National Institutes of Health Registry Investigators. Multicenter cohort study of acute myocardial infarction in Korea: interim analysis of the Korea acute myocardial infarction registry-National Institutes of Health Registry. Circ J. 2016;80:1427–1436. doi: 10.1253/circj.CJ-16-0061. [DOI] [PubMed] [Google Scholar]
- 5.Teo KK, Yusuf S, Furberg CD. Effects of prophylactic antiarrhythmic drug therapy in acute myocardial infarction. An overview of results from randomized controlled trials. JAMA. 1993;270:1589–1595. [PubMed] [Google Scholar]
- 6.Dargie HJ. Effect of carvedilol on outcome after myocardial infarction in patients with left-ventricular dysfunction: the CAPRICORN randomised trial. Lancet. 2001;357:1385–1390. doi: 10.1016/s0140-6736(00)04560-8. [DOI] [PubMed] [Google Scholar]
- 7.Verma S, Strauss M. Angiotensin receptor blockers and myocardial infarction. BMJ. 2004;329:1248–1249. doi: 10.1136/bmj.329.7477.1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tardif JC, O'Meara E, Komajda M, Böhm M, Borer JS, Ford I, et al. SHIFT Investigators. Effects of selective heart rate reduction with ivabradine on left ventricular remodelling and function: results from the SHIFT echocardiography substudy. Eur Heart J. 2011;32:2507–2515. doi: 10.1093/eurheartj/ehr311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Borer JS, Böhm M, Ford I, Robertson M, Komajda M, Tavazzi L, et al. SHIFT Investigators. Efficacy and safety of ivabradine in patients with severe chronic systolic heart failure (from the SHIFT study) Am J Cardiol. 2014;113:497–503. doi: 10.1016/j.amjcard.2013.10.033. [DOI] [PubMed] [Google Scholar]
- 10.Mehta RH, Harjai KJ, Grines L, Stone GW, Boura J, Cox D, et al. Primary Angioplasty in Myocardial Infarction (PAMI) Investigators. Sustained ventricular tachycardia or fibrillation in the cardiac catheterization laboratory among patients receiving primary percutaneous coronary intervention: incidence, predictors, and outcomes. J Am Coll Cardiol. 2004;43:1765–1772. doi: 10.1016/j.jacc.2003.09.072. [DOI] [PubMed] [Google Scholar]
- 11.Ng FS, Shadi IT, Peters NS, Lyon AR. Selective heart rate reduction with ivabradine slows ischaemia-induced electrophysiological changes and reduces ischaemia-reperfusion-induced ventricular arrhythmias. J Mol Cell Cardiol. 2013;59:67–75. doi: 10.1016/j.yjmcc.2013.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fliss H, Gattinger D. Apoptosis in ischemic and reperfused rat myocardium. Circ Res. 1996;79:949–956. doi: 10.1161/01.res.79.5.949. [DOI] [PubMed] [Google Scholar]
- 13.Sahn DJ, DeMaria A, Kisslo J, Weyman A. Recommendations regarding quantitation in M-mode echocardiography: results of a survey of echocardiographic measurements. Circulation. 1978;58:1072–1083. doi: 10.1161/01.cir.58.6.1072. [DOI] [PubMed] [Google Scholar]
- 14.Yue TL, Wang C, Gu JL, Ma XL, Kumar S, Lee JC, et al. Inhibition of extracellular signal-regulated kinase enhances Ischemia/Reoxygenation-induced apoptosis in cultured cardiac myocytes and exaggerates reperfusion injury in isolated perfused heart. Circ Res. 2000;86:692–699. doi: 10.1161/01.res.86.6.692. [DOI] [PubMed] [Google Scholar]
- 15.Ferrari R, Balla C, Malagù M, Guardigli G, Morciano G, Bertini M, et al. Reperfusion damage: a story of success, failure, and hope. Circ J. 2017;81:131–141. doi: 10.1253/circj.CJ-16-1124. [DOI] [PubMed] [Google Scholar]
- 16.Piper HM, Meuter K, Schäfer C. Cellular mechanisms of ischemia-reperfusion injury. Ann Thorac Surg. 2003;75:S644–S648. doi: 10.1016/s0003-4975(02)04686-6. [DOI] [PubMed] [Google Scholar]
- 17.Witt BJ, Jacobsen SJ, Weston SA, Killian JM, Meverden RA, Allison TG, et al. Cardiac rehabilitation after myocardial infarction in the community. J Am Coll Cardiol. 2004;44:988–996. doi: 10.1016/j.jacc.2004.05.062. [DOI] [PubMed] [Google Scholar]
- 18.Beltrami AP, Barlucchi L, Torella D, Baker M, Limana F, Chimenti S, et al. Adult cardiac stem cells are multipotent and support myocardial regeneration. Cell. 2003;114:763–776. doi: 10.1016/s0092-8674(03)00687-1. [DOI] [PubMed] [Google Scholar]
- 19.Segers VF, Lee RT. Stem-cell therapy for cardiac disease. Nature. 2008;451:937–942. doi: 10.1038/nature06800. [DOI] [PubMed] [Google Scholar]
- 20.Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE, Jr, Colvin MM, et al. 2016 ACC/AHA/HFSA focused update on new pharmacological therapy for heart failure: an update of the 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Task Force on clinical practice guidelines and the Heart Failure Society of America. J Am Coll Cardiol. 2016;68:1476–1488. doi: 10.1016/j.jacc.2016.05.011. [DOI] [PubMed] [Google Scholar]
- 21.Yusuf S, Camm AJ. Sinus tachyarrhythmias and the specific bradycardic agents: a marriage made in heaven? J Cardiovasc Pharmacol Ther. 2003;8:89–105. doi: 10.1177/107424840300800202. [DOI] [PubMed] [Google Scholar]
- 22.Thollon C, Cambarrat C, Vian J, Prost JF, Peglion JL, Vilaine JP. Electrophysiological effects of S 16257, a novel sino-atrial node modulator, on rabbit and guinea-pig cardiac preparations: comparison with UL-FS 49. Br J Pharmacol. 1994;112:37–42. doi: 10.1111/j.1476-5381.1994.tb13025.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sulfi S, Timmis AD. Ivabradine–the first selective sinus node I(f) channel inhibitor in the treatment of stable angina. Int J Clin Pract. 2006;60:222–228. doi: 10.1111/j.1742-1241.2006.00817.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Albaladejo P, Carusi A, Apartian A, Lacolley P, Safar ME, Bénétos A. Effect of chronic heart rate reduction with ivabradine on carotid and aortic structure and function in normotensive and hypertensive rats. J Vasc Res. 2003;40:320–328. doi: 10.1159/000072696. [DOI] [PubMed] [Google Scholar]
- 25.Mulder P, Barbier S, Chagraoui A, Richard V, Henry JP, Lallemand F, et al. Long-term heart rate reduction induced by the selective I(f) current inhibitor ivabradine improves left ventricular function and intrinsic myocardial structure in congestive heart failure. Circulation. 2004;109:1674–1679. doi: 10.1161/01.CIR.0000118464.48959.1C. [DOI] [PubMed] [Google Scholar]
- 26.Tsutsui H, Momomura S, Yamashina A, Ogawa H, Shimokawa H, Kihara Y, et al. study investigators. Heart rate control with if inhibitor, ivabradine, in Japanese patients with chronic heart failure: a randomized, double-blind, placebo-controlled phase II study. Circ J. 2016;80:668–676. doi: 10.1253/circj.CJ-15-1112. [DOI] [PubMed] [Google Scholar]
- 27.Fox K, Ford I, Steg PG, Tendera M, Ferrari R BEAUTIFUL Investigators. Ivabradine for patients with stable coronary artery disease and left-ventricular systolic dysfunction (BEAUTIFUL): a randomised, double-blind, placebo-controlled trial. Lancet. 2008;372:807–816. doi: 10.1016/S0140-6736(08)61170-8. [DOI] [PubMed] [Google Scholar]
- 28.Swedberg K, Komajda M, Böhm M, Borer JS, Ford I, Dubost-Brama A, et al. Ivabradine and outcomes in chronic heart failure (SHIFT): a randomised placebo-controlled study. Lancet. 2010;376:875–885. doi: 10.1016/S0140-6736(10)61198-1. [DOI] [PubMed] [Google Scholar]
- 29.Tardif JC, Ponikowski P, Kahan T ASSOCIATE Study Investigators. Efficacy of the I(f) current inhibitor ivabradine in patients with chronic stable angina receiving beta-blocker therapy: a 4-month, randomized, placebo-controlled trial. Eur Heart J. 2009;30:540–548. doi: 10.1093/eurheartj/ehn571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Colin P, Ghaleh B, Monnet X, Su J, Hittinger L, Giudicelli JF, et al. Contributions of heart rate and contractility to myocardial oxygen balance during exercise. Am J Physiol Heart Circ Physiol. 2003;284:H676–H682. doi: 10.1152/ajpheart.00564.2002. [DOI] [PubMed] [Google Scholar]
- 31.Simon L, Ghaleh B, Puybasset L, Giudicelli JF, Berdeaux A. Coronary and hemodynamic effects of S 16257, a new bradycardic agent, in resting and exercising conscious dogs. J Pharmacol Exp Ther. 1995;275:659–666. [PubMed] [Google Scholar]
- 32.Monnet X, Ghaleh B, Colin P, de Curzon OP, Giudicelli JF, Berdeaux A. Effects of heart rate reduction with ivabradine on exercise-induced myocardial ischemia and stunning. J Pharmacol Exp Ther. 2001;299:1133–1139. [PubMed] [Google Scholar]
- 33.Monnet X, Colin P, Ghaleh B, Hittinger L, Giudicelli JF, Berdeaux A. Heart rate reduction during exercise-induced myocardial ischaemia and stunning. Eur Heart J. 2004;25:579–586. doi: 10.1016/j.ehj.2003.10.010. [DOI] [PubMed] [Google Scholar]
- 34.Lucats L, Ghaleh B, Monnet X, Colin P, Bizé A, Berdeaux A. Conversion of post-systolic wall thickening into ejectional thickening by selective heart rate reduction during myocardial stunning. Eur Heart J. 2007;28:872–879. doi: 10.1093/eurheartj/ehm030. [DOI] [PubMed] [Google Scholar]