Abstract
Aims
Pulmonary congestion remains a diagnostic challenge in patients with heart failure (HF). The recommended method, chest X‐ray (CXR), lacks in accuracy, whereas quantitative tomographic lung scintigraphy [ventilation/perfusion single‐photon emission computed tomography (V/P SPECT)] has shown promising results but needs independent validation. The aim of this study is to evaluate V/P SPECT as a non‐invasive method to assess and quantify pulmonary congestion in HF patients, using right heart catheterization as reference method. The secondary objective was to investigate the performance of V/P SPECT in the clinical setting compared with CXR.
Methods and results
Forty‐six consecutive patients with HF that were under consideration for heart transplantation were studied prospectively. All participants were examined with V/P SPECT, CXR, and right heart catheterization. Pulmonary artery wedge pressure served as reference method. Quantitative perfusion gradients were derived from V/P SPECT images. Ventilation/perfusion single‐photon emission computed tomography images were also assessed both by expert readers and clinical nuclear medicine physicians. Expert readers correctly identified 87% of all patients with an elevated pulmonary artery wedge pressure > 15 mmHg. The average sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) for V/P SPECT assessed by the expert readers were 87%, 72%, 85%, and 75%, respectively. In the clinical nuclear medicine setting, V/P SPECT had 87% sensitivity, 63% specificity, 81% PPV, and 71% NPV. Clinically, V/P SPECT outperformed CXR, which had 27% sensitivity, 75% specificity, 67% PPV, and 35% NPV.
Conclusions
Ventilation/perfusion single‐photon emission computed tomography can be used as a non‐invasive method to diagnose and quantify pulmonary congestion in patients with HF and is more accurate than CXR in diagnosing pulmonary congestion in the clinical setting.
Keywords: Heart failure, Pulmonary congestion, Pulmonary perfusion distribution, Right heart catheterization, V/P SPECT, Lung scintigraphy
Introduction
Left heart failure (HF) syndromes, while diverse and multifaceted, are all distinguished by pathological elevation of left heart filling pressure. The pathophysiological complexity of HF makes it difficult to diagnose, especially as symptoms and signs are unspecific and similar to those of other diseases.1
One major sign of severe left HF is pulmonary congestion. Pulmonary congestion is recognized as a challenge to diagnose and grade objectively. Available guidelines recommend the use of chest X‐ray (CXR) or the presence of rales on physical examination, although the sensitivity and specificity of these examinations for the diagnosis of pulmonary congestion are low.1, 2, 3, 4, 5 Even among HF patients with known pulmonary congestion, studies show that CXR can be normal in 50–60% of cases.3, 4
The reference method to assess pulmonary congestion is right heart catheterization (RHC) with measurement of the pulmonary artery wedge pressure (PAWP). Right heart catheterization is invasive and therefore only used in selected patients with severe disease.1 Hence, better diagnostic alternatives are needed.
Tomographic lung scintigraphy or ventilation/perfusion single‐photon emission computed tomography (V/P SPECT) is a non‐invasive imaging method, primarily used in the diagnosis of pulmonary embolism. The method shows the distribution of ventilation, using Technegas, and pulmonary perfusion, using 99mTc‐macroaggregated albumin.6, 7, 8
Ventilation/perfusion single‐photon emission computed tomography showed promise in the evaluation of pulmonary congestion in an earlier study8 but has not been validated against an independent method like RHC.
Here, we conducted a prospective study to evaluate the potential of V/P SPECT as a non‐invasive method to assess and quantify pulmonary congestion in patients with severe HF who were under consideration for heart transplantation and used RHC as reference method. Furthermore, we investigated the performance of V/P SPECT compared with CXR in the clinical setting.
Methods
A total of 46 patients (10 female, age 54.7 ± 9.1 years) with severe HF were prospectively enrolled between October 2013 and January 2017. The patients, all under consideration for heart transplantation, were examined with RHC, V/P SPECT, CXR, and laboratory tests at Skåne University Hospital in Lund, Sweden. The V/P SPECT examination was only performed as part of this study. The result from the study has, however, lead to a change in our routines, and V/P SPECT is now performed on all patients under evaluation for heart transplantation.
The study was performed with informed consent in accordance with the Declarations of Helsinki and was approved by the ethics board in Lund, Sweden. All examinations were performed within 72 h except in 11 patients [examination span 4–30 days for 10 patients and 40 days for one patient (the mean time between the different imaging modalities was 4 ± 7 days)].
Right heart catheterization
The RHCs were performed at the Haemodynamic Lab at Skåne University Hospital in Lund, which is one out of two centres in Sweden with national responsibility for heart and lung transplantation and regional responsibility for pulmonary hypertension (PH) care. At the Haemodynamic Lab, 450–500 RHCs are performed on a yearly basis, divided upon four doctors with 10–25 years' experience each of performing the catheterizations.
Right heart catheterization was performed via the right internal jugular vein (chiefly), using a Swan–Ganz catheter (Edwards Lifesciences, Irvine, CA, USA). Haemodynamic parameters including mean and diastolic pulmonary artery pressure (mPAP and dPAP), mean PAWP, mean right atrial pressure (mRAP), and mean arterial pressure were recorded. Heart rate was recorded from electrocardiogram. Cardiac output (CO) was measured by thermodilution. Cardiac index, stroke volume (SV), transpulmonary gradient (TPG), diastolic pulmonary vascular pressure gradient (DPG), and pulmonary vascular resistance (PVR) were calculated using the following formulas: cardiac index = CO∕body surface area; SV = CO∕heart rate; TPG = mPAP − PAWP; DPG = dPAP – PAWP; and PVR = TPG∕CO. Mean pulmonary artery pressure ≥ 25 mmHg was used as threshold value for PH.9 Pulmonary artery wedge pressure is normally ≤12 mmHg. For statistical analysis, PAWP was defined as elevated to a level indicating pulmonary congestion when >15 mmHg.9 This PAWP level was chosen because it is the level used in guidelines to discriminate between pre‐capillary and post‐capillary PH.9
Ventilation/perfusion single‐photon emission computed tomography
Examination protocol
Ventilation/perfusion single‐photon emission computed tomography was performed as a 1 day protocol using a dual‐head gamma camera (Discovery NM/CT 670, GE Healthcare Sverige AB, Danderyd, Sweden), and the examination was performed in accordance with European guidelines.6, 10 An extended low‐energy general purpose collimator was used. All patients were examined in supine position. The ventilation (V) study was performed after inhalation of 30 MBq of Technegas (Cyclomedica Ltd, Dublin, Ireland). Images were acquired in 120 projections. The acquisition time for the ventilation study was 10 s in each projection. Thereafter, lung perfusion was assessed by intravenous injection of 140 MBq of technetium‐99m‐labelled macroaggregated albumin (TechneScan LyoMAA; Mallinckrodt Medical BV, Petten, Netherlands) in maintained patient position. Perfusion images were acquired for 5 s in each projection. Ventilation/perfusion single‐photon emission computed tomography images were then iteratively reconstructed using ordered subset expectation maximization.10 Oasis Pulmogam software (Segami Corp., Columbia, MD, USA) was used for visual evaluation.
Assessment of ventilation/perfusion single‐photon emission computed tomography images
A qualitative (visual) and quantitative (perfusion gradients derived from V/P SPECT images) assessment of the V/P SPECT images were made. The qualitative assessment was made by two expert readers and by nuclear physicians as a part of their daily clinical work in the department. The expert readers did not participate in the clinical evaluation of the V/P SPECT examinations.
Visual assessment of pulmonary congestion
The visual interpretation criteria of pulmonary congestion in V/P SPECT have been described previously.8 In healthy individuals, because of gravity, pulmonary perfusion is predominantly distributed to dependent parts of the lungs, that is, to posterior parts of the lungs in the supine position. In patients with pulmonary congestion, however, the elevated pressure and surrounding interstitial oedema cause narrowing and an increased resistance to flow in vessels in the lower parts of the lungs. Hence, pulmonary perfusion is redistributed from posterior to anterior parts of the lungs in the supine position, and this can be shown and quantified with technetium‐99m‐labelled macroaggregated albumin. Ventilation is usually not affected to the same degree. The perfusion defects that occur in the posterior parts of the lungs are not of segmental character as seen in pulmonary embolism.
Expert readers
Two nuclear medicine physicians (M. B. and J. J.) with more than 15 years' experience of V/P SPECT independently assessed all V/P SPECT images visually for signs of pulmonary congestion. The expert readers were blinded to the perfusion gradients, the results of additional examinations, and clinical information. One of the expert readers reassessed all the images in random order 3 weeks later. The two expert readers were not assessing these patients in the clinical routine.
Nuclear physicians in the clinical routine
Specialists in nuclear medicine or residents with various degrees of training in V/P SPECT visually assessed all V/P SPECT images as part of their daily clinical work in the department. The physicians received no special training other than what is generally taught when learning V/P SPECT at the department. The information in the referrals did not include RHC or CXR data.
Perfusion gradients
All V/P SPECT examinations were also evaluated with a previously published user‐independent quantitative algorithm for calculating perfusion gradients.8 In short, the algorithm uses the tomographic perfusion imaging data from both lungs to calculate perfusion gradients in the posterior–anterior direction by three‐dimensional linear regression. The algorithm uses the V/P SPECT perfusion data from both lungs. The ventilation distribution has no impact on the perfusion gradients. In the first step, the algorithm automatically excludes areas in the hili with large vessels and airways. It also excludes the peripheral border of the lungs (approximately 1 cm) to avoid artefacts owing to breathing and partial volume effect. The algorithm calculates the perfusion gradient as the slope of the regression line in the posterior–anterior direction. This means that the perfusion gradient represents the percentual change in counts per centimetre lung compared with the normalized maximum activity. The algorithm has been validated in a phantom and tested in patients.8 A negative gradient is considered to represent a normal perfusion pattern, while patients with positive perfusion gradients are considered to have a redistributed pulmonary perfusion indicating pulmonary congestion.8 In the present study, we, therefore, chose a threshold value of 0% counts/cm to discriminate between normal perfusion pattern and pulmonary congestion.
Chest X‐ray
Posterior–anterior CXR images of the lungs were collected in upright patient position using a digital X‐ray unit (140 kV, 200 mA). A qualitative (visual) assessment of the CXR images was made by thoracic radiology specialists with more than 5 years' experience. They evaluated the CXR images for signs of pulmonary congestion1 as part of their clinical routine work. Signs of pulmonary congestion included upper lobe venous diversion, peribronchial cuffing, Kerley B lines, thickening of interlobar fissures, pleural effusion, and an increased cardiothoracic ratio. The radiology specialists had access to all clinical information except RHC data.
Statistical analysis
SPSS statistics v23.0 (IBM Corp, Armonk, NY, USA) and GraphPad Prism 6.0 (GraphPad Software, La Jolla, CA, USA) were used for statistical analyses. Differences between groups regarding PAWP were evaluated using independent t‐test (statistical significance was chosen as P < 0.05). Intra‐individual and inter‐individual agreements for expert readers and clinical reports of V/P SPECT and CXR are presented visually and by chance adjusted Cohen's kappa test. The strength of agreement of Cohen's kappa is described according to Altman as 0–0.20 = poor, 0.21–0.40 = fair, 0.41–0.60 = moderate, 0.61–0.80 = good, and 0.81–1.0 = very good. Values are presented as mean ± standard deviation.
Results
Right heart catheterization
Individual haemodynamic data of the 46 patients together with PH classification in accordance with both the 2009 and the 2015 ESC/ERS guidelines are shown in Supporting Information, Table S1 .9, 11 Pulmonary arterial systolic pressure ranged from 14 to 53 mmHg with a median pulmonary arterial systolic pressure of 29 mmHg. Thirty out of the 46 patients had PH at the time of RHC. Two patients were classified as having pre‐capillary PH, and the remaining 28 patients were classified as post‐capillary PH. Among the 16 patients without PH, two patients had a PAWP >15 mmHg, and four patients had an elevated PAWP in the range 14–15 mmHg. The two patients with a PAWP of 15 mmHg had an elevated mRAP.
Ventilation/perfusion single‐photon emission computed tomography
Figure 1 illustrates the pulmonary perfusion patterns in a normal lung and in patients with HF with and without pulmonary congestion. Single sagittal V/P SPECT slices from the left lung are shown. Figure 2 shows V/P SPECT and corresponding CXR images compared with PAWP and perfusion gradients in two patients with HF, with and without pulmonary congestion.
Figure 1.
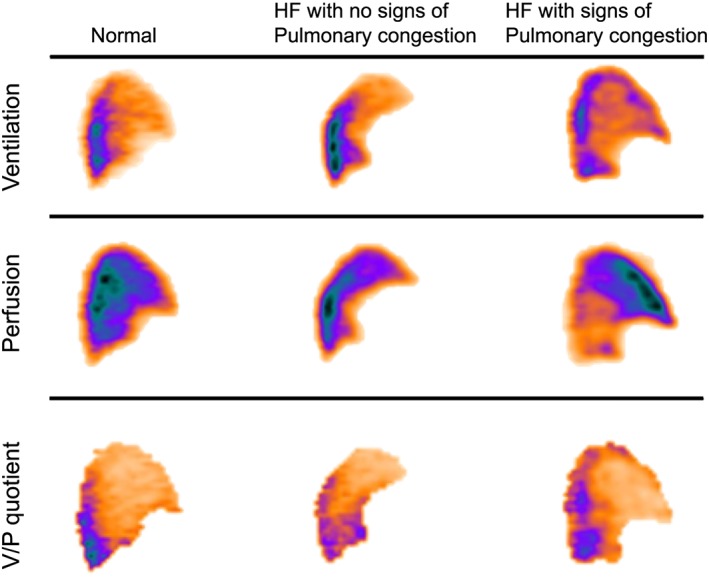
Sagittal ventilation/perfusion (V/P) single‐photon emission computed tomography slices from the left lung of patients with heart failure (HF), with and without signs of pulmonary congestion, compared with a representative normal lung. In the normal lung and in the patient with HF but no signs of pulmonary congestion, pulmonary perfusion is predominantly distributed to posterior, that is, dependent parts of the lungs. In the lungs of the patient with pulmonary congestion and elevated pulmonary wedge pressure, perfusion is redistributed to anterior, non‐dependent, parts of the lung. Ventilation and V/P quotient images are included for reference purposes.
Figure 2.
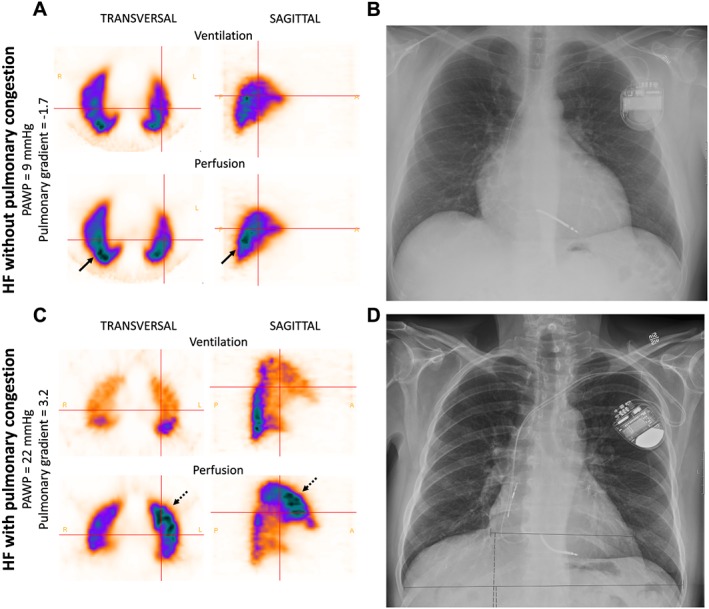
Ventilation/perfusion single‐photon emission computed tomography (V/P SPECT) and chest X‐ray (CXR) images of two patients with heart failure. (A) A patient with heart failure (HF) but no signs of pulmonary congestion. Pulmonary artery wedge pressure (PAWP) is normal. In the transversal and sagittal planes on V/P SPECT, perfusion is predominantly distributed to the dependent posterior parts of the lungs (arrows). The perfusion gradient is −1.7, which is normal. (B) The CXR shows enlargement of the heart but was otherwise regarded as negative regarding pulmonary congestion. (C) Transversal and sagittal V/P SPECT slices of the lungs of a patient with HF and pulmonary congestion. PAWP was elevated to 22 mmHg. The perfusion images show redistribution of pulmonary perfusion to the anterior parts of the lungs in the supine position (dotted arrows). The perfusion gradient is 3.2, which also supports the diagnosed pulmonary congestion. (D) The CXR shows an enlarged heart but was otherwise assessed as negative for pulmonary congestion.
Ventilation/perfusion single‐photon emission computed tomography assessment by expert readers
All patients that were classified as having pulmonary congestion by visual assessment by the expert readers had a PAWP >12 mmHg (Figure 3 A and B). The patients classified as having pulmonary congestion had significantly higher PAWP compared with those that were not (P < 0.001). The overlap in PAWP between the two groups was small. All patients with post‐capillary PH, except two, were identified by V/P SPECT whether classified as isolated or combined with a precapillary component (Supporting Information, Table S1 ). Among the patients without PH, V/P SPECT showed pulmonary congestion in four out of 16 patients (Expert Reader 2). These four patients had a PAWP in the range of 14–19 mmHg, and two of these patients had an elevated mRAP as well. The two expert readers' diagnoses were false negative in five and three patients, respectively. Expert Reader 2 had 90% sensitivity, 75% specificity, 87% positive predictive value (PPV), 80% negative predictive value (NPV), and 85% accuracy in diagnosing pulmonary congestion with PAWP > 15 mmHg as threshold value. The diagnostic performance for both expert readers is shown in Figure 4 A.
Figure 3.
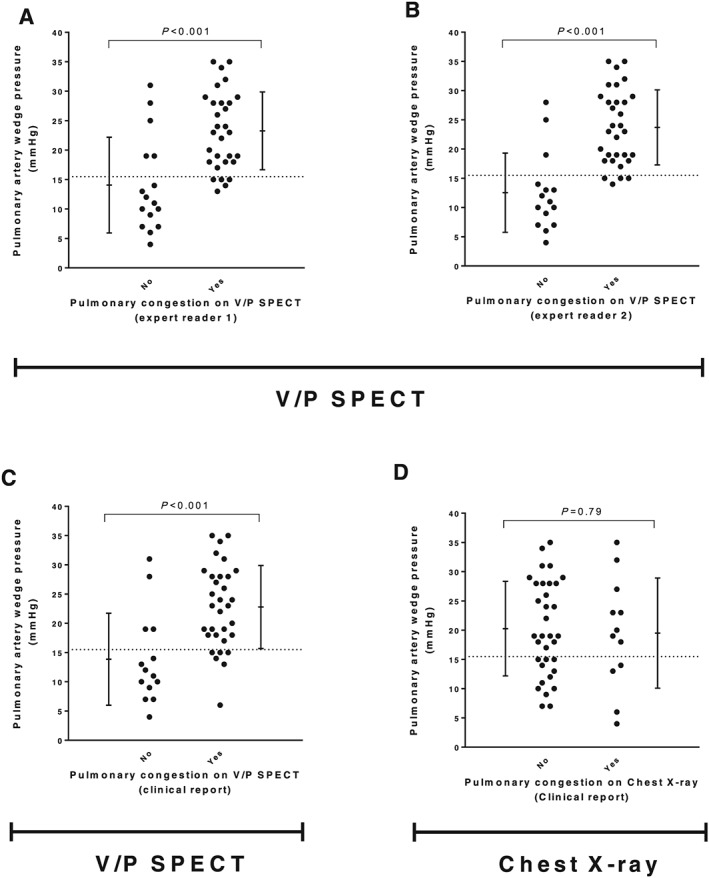
The upper panels (A and B) show how signs of pulmonary congestion on V/P SPECT images, assessed by expert readers (blinded to the perfusion gradients), compared with pulmonary artery wedge pressures. The two bottom panels (C and D) show the same comparison for V/P SPECT and chest X‐ray images assessed by physicians in the clinical routine. The dashed line represents the chosen threshold value for pulmonary congestion (pulmonary artery wedge pressure > 15 mmHg). The error bars show mean ± 1 SD.
Figure 4.
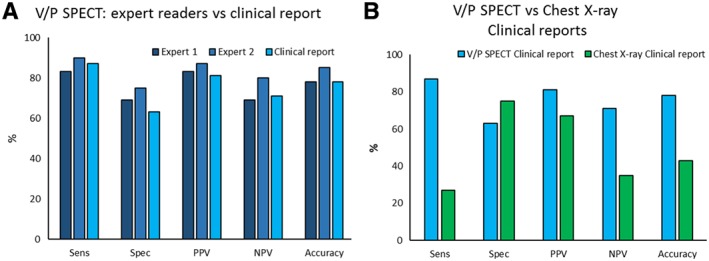
The diagnostic performance of visual assessment of ventilation/perfusion single‐photon emission computed tomography (V/P SPECT) and chest X‐ray for diagnosing pulmonary congestion, using pulmonary artery wedge pressure > 15 mmHg as threshold value. (A) The bars in the two darker blue colours show the performance of V/P SPECT assessed by the expert readers, and the light blue bars show V/P SPECT assessed by physicians in the clinical routine. (B) The light blue bars are identical to the ones in panel A and shows V/P SPECT when assessed in the clinical routine, now compared with chest X‐ray (green bars) assessed by radiology specialists in the clinical routine. Sensitivity (Sens), specificity (Spec), positive predictive value (PPV), negative predicted value (NPV), and accuracy.
Ventilation/perfusion single‐photon emission computed tomography assessment by nuclear medicine physicians in the clinic
The clinical performance of V/P SPECT showed good concordance with the expert readers, with 87% sensitivity, 63% specificity, 81% PPV, 71% NPV, and 78% accuracy, respectively, using a threshold value of PAWP > 15 mmHg as reference (Figure 4 A). The patients classified as having pulmonary congestion by the clinical nuclear medicine physicians had significantly higher PAWP compared with those who were not (P < 0.001) (Figure 3 C and Supporting Information, Table S1 ). All patients that were classified as having pulmonary congestion, except two, had a PAWP ≥ 14 mmHg. The overall performance of the clinical V/P SPECT reports was superior to that of CXR in diagnosing pulmonary congestion in this group of patients with known HF (Figure 4 B).
Agreement between readers and when reassessing ventilation/perfusion single‐photon emission computed tomography
A perfect intra‐individual agreement (κ = 1.0) was found when Expert Reader 2 reassessed the V/P SPECT images. There was a very good agreement (κ = 0.85) between the two expert readers. The agreement between Expert Reader 1 and the clinical reports was very good (κ = 0.90), and the agreement was good between Expert Reader 2 and the clinical reports (κ = 0.75).
Perfusion gradients
All patients with a PAWP > 15 mmHg, except two, had positive quantitative perfusion gradients and were identified correctly as having pulmonary congestion (Figure 5 ). The rest of the patients with normal perfusion gradients all had a PAWP ≤ 14 mmHg. The sensitivity, specificity, PPV, NPV, and accuracy for the automatic perfusion gradients in diagnosing pulmonary congestion were 93%, 50%, 78%, 80%, and 78%, respectively.
Figure 5.
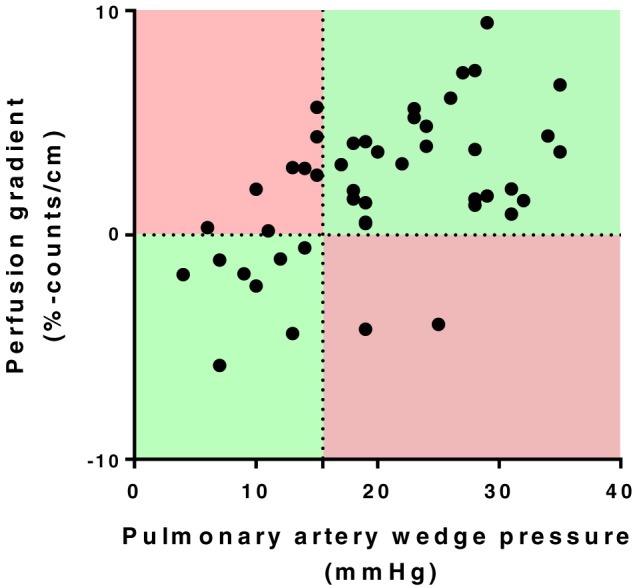
Pulmonary artery wedge pressures compared with perfusion gradients that are automatically calculated from ventilation/perfusion single‐photon emission computed tomography images. The horizontal dashed line represents the threshold value where perfusion gradients indicate pulmonary congestion (0% counts/cm). The vertical dotted line shows the threshold value for pulmonary congestion on pulmonary artery wedge pressure (>15 mmHg) that serves as reference method. Hence, the diagnosis is false positive in upper left red area, true positive in upper right green area, true negative in lower left green area, and false negative in lower right red area.
Chest X‐ray
There was no significant difference in PAWP (P = 0.79) between patients that were reported to have pulmonary congestion on CXR compared with those that were reported not to have pulmonary congestion (Figure 3 D and Supporting Information, Table S1 ). CXR was false negative in 22 patients. CXR showed a poor chance‐adjusted agreement (κ = 0.12) compared with the V/P SPECT Expert Reader 2, which was the one that was most consistent with PAWP findings. The sensitivity, specificity, PPV, NPV, and accuracy for CXR were 27%, 75%, 67%, 35%, and 43%, respectively. In the clinical setting, V/P SPECT outperformed CXR in every measure except for specificity (Figure 4 B).
Discussion
The most important finding of this study is that the non‐invasive technique of V/P SPECT can be used to diagnose and quantify pulmonary congestion in patients with left HF that is being considered for orthotopic heart transplant. Further, if an elevated PAWP is present in a patient with severe HF, then V/P SPECT is more accurate than CXR in the diagnosis of pulmonary congestion. Lastly, quantitative tomographic V/P scintigraphy can allow comparison of the degree of pulmonary congestion broadly across the entirety of patients with severe left HF, including both those with and those without PH.11 No similar findings have been published previously in the clinical literature.
In the present study, V/P SPECT is for the first time validated as a tool to diagnose and quantify pulmonary congestion in HF using RHC as reference standard. The idea of using nuclear medicine methods to assess pulmonary congestion was previously explored in the 1980s,12, 13, 14 and we revisited the idea in 2008 in a study using V/P SPECT for the first time.8 This earlier study showed that V/P SPECT could be used to diagnose pulmonary congestion with a high PPV of 88% using the presence of HF in medical records as reference. In the current study, we have used PAWP, which is regarded as a better criterion standard for pulmonary congestion in HF. With PAWP from RHC as reference method to confirm pulmonary congestion, the average PPV for the expert readers was 85% and 81% for the clinical nuclear medicine reports, which is in line with our earlier V/P SPECT study.
While PAWP normally is ≤12 mmHg, this study employed PAWP > 15 mmHg as the threshold value for pulmonary congestion, as this is the threshold value used to classify PH patients as having post‐capillary PH.11 The two expert readers falsely assessed five and three patients with a PAWP ≤ 15, respectively, as having pulmonary congestion. All patients classified as having pulmonary congestion by the expert readers, however, did have a PAWP above normal (i.e. >12 mmHg). This indicates that changes in pulmonary perfusion pattern appear early and can be seen already when PAWP rises just above its normal range. If PAWP > 12 mmHg had been used as the threshold value, then a sensitivity of 86%, a specificity of 90%, a PPV of 97%, and an NPV of 64% would result for the expert reader.
While V/P SPECT correctly identified 93% (26 out of 28) of the patients with post‐capillary PH and pulmonary oedema, CXR missed 71% (20 out of 28) of them. Hence, CXR misses many patients with pulmonary congestion that could be diagnosed non‐invasively with V/P SPECT. The current study also shows that the assessment of pulmonary congestion with V/P SPECT is feasible to implement in a clinical setting in the nuclear medicine department with a diagnostic performance that is in good agreement with expert readers.
This study also investigated the performance of user‐independent perfusion gradients derived from V/P SPECT images in the diagnosis and grading of pulmonary congestion. The automatic perfusion gradients correctly identified all patients with a PAWP > 15 mmHg, except two. The perfusion gradients are, however, highly sensitive, with eight false‐positive patients when the 15 mmHg threshold value was used. The majority of these patients, however, had a PAWP above normal (i.e. >12 mmHg). Our conclusion is that a positive perfusion gradient could be used as an aid to draw the physicians' attention to visually assess whether pulmonary congestion is present or not and also be helpful to quantify the degree of pulmonary congestion.
The finding that many HF patients have an altered pulmonary perfusion pattern and signs of pulmonary congestion already when PAWP rises above 12 mmHg can have a clinical impact as this may affect pulmonary gas exchange and the patient's condition negatively. Ventilation/perfusion single‐photon emission computed tomography provides a non‐invasive tool to assess these pathological changes earlier and follow the response to treatment. This could improve patient care. Further studies are, however, necessary.
Presently, CXR is the standard non‐invasive method to assess pulmonary congestion,1, 11 but several studies have shown that the sensitivity of CXR for detecting pulmonary congestion is as low as 38–48%.3, 4 It is also stated in PH guidelines that a normal CXR can never rule out pulmonary congestion.11 Some authors have suggested that the limited sensitivity of CXR can be explained by compensatory mechanisms, triggered by high left heart pressures, that compensate for the fluid shift that has occurred which in turn would mask radiographical evidence of deranged haemodynamics.4 The present results do not support this theory because the redistribution of pulmonary perfusion associated with pulmonary congestion is still seen on V/P SPECT. In this study, CXR had a sensitivity of 27% in patients with severe HF and, although the specificity was 75%, the NPV was only 35%. Chest X‐ray has many benefits and is highly available, but the results of this study, together with those of others, lead to the question if CXR should still be used to exclude pulmonary congestion in HF.
Today, V/P SPECT is mentioned in PH and HF guidelines as a method to evaluate chronic thrombo‐embolic PH. This study shows that, in addition, V/P SPECT can be used as a tool to diagnose and quantify pulmonary congestion in HF patients with an accuracy that is considerably higher than that of CXR. Although not as available as CXR, V/P SPECT is a feasible alternative, especially because the introduction of SPECT and new ventilation tracers (i.e. Technegas) has lowered the number of non‐diagnostic studies to <3%, even in the presence of obstructive lung disease.6, 7, 15
A limitation of this study is that the interval between examinations was >72 h in 11 patients, which might affect the comparisons. The difference in diagnostic performance is, however, small if these 11 patients were excluded and, if anything, shows a trend towards higher diagnostic accuracy of the examination when compared with RHC. We have, therefore chosen to report the data from the whole population. Another limitation is that perfusion gradients can be affected by the presence of obstructive lung disease, especially emphysema, which was seen in two patients. Therefore, an adjustment of the current algorithm could be beneficial, and this work is in progress. Combined SPECT/CT systems are becoming common. Adding a CT to the V/P SPECT examination exposes the patient to additional radiation and has little impact on the assessment of pulmonary congestion. A CT could, however, help in the assessment of ventilation/perfusion defects in the presence of obstructive lung disease.
Conclusions
Ventilation/perfusion single‐photon emission computed tomography can be used as a non‐invasive method to diagnose and quantify pulmonary congestion in patients with HF and is more accurate than CXR in diagnosing pulmonary congestion in the clinical setting.
Conflict of interest
J.J., M.A.‐M., G.R., M.B., and H.A. declare no conflicts of interest related to the submitted work.
Outside the submitted work, H.A. is a shareholder in Imacor AB, and M.B. has, during the last 3 years, received research grants from GE, Lebugu, and Cyclomedica.
Funding
This study was funded with grants from the Swedish Heart and Lung Foundation, the Medical Faculty of Lund University (ALF) in Sweden, and the Region of Scania in Sweden.
Supporting information
Table S1. Hemodynamic data, pulmonary hypertension classification and results from CXR and V/P SPECT of the 46 included patients mean pulmonary artery pressure (mPAP), mean right atrial pressure (mRAP), mean pulmonary arterial wedge pressure (mPAWP), pulmonary vascular resistance (PVR), transpulmonary gradient (TPG), diastolic pulmonary vascular pressure gradient (DPG), Cardiac index (CI), isolated post‐capillary pulmonary hypertension (Ipc‐PH), combined post‐capillary pulmonary hypertension (Cpc‐PH).
Acknowledgements
We would like to thank our colleagues at the Department of Clinical Physiology and Nuclear Medicine and the Department of Cardiology.
Jögi, J. , Al‐Mashat, M. , Rådegran, G. , Bajc, M. , and Arheden, H. (2018) Diagnosing and grading heart failure with tomographic perfusion lung scintigraphy: validation with right heart catheterization. ESC Heart Failure, 5: 902–910. 10.1002/ehf2.12317.
References
- 1. McMurray JJ, Adamopoulos S, Anker SD, Auricchio A, Böhm M, Dickstein K, Falk V, Filippatos G, Fonseca C, Gomez‐Sanchez MA, Jaarsma T, Køber L, Lip GY, Maggioni AP, Parkhomenko A, Pieske BM, Popescu BA, Rønnevik PK, Rutten FH, Schwitter J, Seferovic P, Stepinska J, Trindade PT, Voors AA, Zannad F, Zeiher A, ESC Committee for Practice Guidelines . ESC guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: the Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC. Eur Heart J 2012; 33: 1787–1847. [DOI] [PubMed] [Google Scholar]
- 2. Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr, Drazner MH, Fonarow GC, Geraci SA, Horwich T, Januzzi JL, Johnson MR, Kasper EK, Levy WC, Masoudi FA, McBride PE, McMurray JJ, Mitchell JE, Peterson PN, Riegel B, Sam F, Stevenson LW, Tang WH, Tsai EJ, Wilkoff BL. 2013 ACCF/AHA guideline for the management of heart failure: executive summary: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation 2013; 128: 1810–1852. [DOI] [PubMed] [Google Scholar]
- 3. Costanzo WE, Fein SA. The role of the chest X‐ray in the evaluation of chronic severe heart failure: things are not always as they appear. Clin Cardiol 1988; 11: 486–488. [DOI] [PubMed] [Google Scholar]
- 4. Kataoka H, Takada S. The role of thoracic ultrasonography for evaluation of patients with decompensated chronic heart failure. J Am Coll Cardiol 2000; 35: 1638–1646. [DOI] [PubMed] [Google Scholar]
- 5. Platz E, Jhund PS, Campbell RT, McMurray JJ. Assessment and prevalence of pulmonary oedema in contemporary acute heart failure trials: a systematic review. Eur J Heart Fail 2015; 17: 906–916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bajc M, Neilly JB, Miniati M, Schuemichen C, Meignan M, Jonson B, EANM Committee . EANM guidelines for ventilation/perfusion scintigraphy: part 1. Pulmonary imaging with ventilation/perfusion single photon emission tomography. Eur J Nucl Med Mol Imaging 2009; 36: 1356–1370. [DOI] [PubMed] [Google Scholar]
- 7. Jögi J, Ekberg M, Jonson B, Bozovic G, Bajc M. Ventilation/perfusion SPECT in chronic obstructive pulmonary disease: an evaluation by reference to symptoms, spirometric lung function and emphysema, as assessed with HRCT. Eur J Nucl Med Mol Imaging 2011; 38: 1344–1352. [DOI] [PubMed] [Google Scholar]
- 8. Jögi J, Palmer J, Jonson B, Bajc M. Heart failure diagnostics based on ventilation/perfusion single photon emission computed tomography pattern and quantitative perfusion gradients. Nucl Med Commun 2008; 29: 666–673. [DOI] [PubMed] [Google Scholar]
- 9. Galie N, Hoeper MM, Humbert M, Torbicki A, Vachiery JL, Barbera JA, Beghetti M, Corris P, Gaine S, Gibbs JS, Gomez‐Sanchez MA, Jondeau G, Klepetko W, Opitz C, Peacock A, Rubin L, Zellweger M, Simonneau G, ESC Committee for Practice Guidelines (CPG) . Guidelines for the diagnosis and treatment of pulmonary hypertension: the Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS), endorsed by the International Society of Heart and Lung Transplantation (ISHLT). Eur Heart J 2009; 30: 2493–2537. [DOI] [PubMed] [Google Scholar]
- 10. Palmer J, Bitzen U, Jonson B, Bajc M. Comprehensive ventilation/perfusion SPECT. J Nuc Med 2001; 42: 1288–1294. [PubMed] [Google Scholar]
- 11. Galie N, Humbert M, Vachiery JL, Gibbs S, Lang I, Torbicki A, Simonneau G, Peacock A, Vonk Noordegraaf A, Beghetti M, Ghofrani A, Gomez Sanchez MA, Hansmann G, Klepetko W, Lancellotti P, Matucci M, McDonagh T, Pierard LA, Trindade PT, Zompatori M, Hoeper M, ESC Scientific Document Group . 2015 ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension: the Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Heart J 2016; 37: 67–119.26320113 [Google Scholar]
- 12. Friedman WF, Braunwald E. Alterations in regional pulmonary blood flow in mitral valve disease studied by radioisotope scanning. A simple nontraumatic technique for estimation of left atrial pressure. Circulation 1966; 34: 363–376. [DOI] [PubMed] [Google Scholar]
- 13. Gilday DL, James AE Jr. Lung scan patterns in pulmonary embolism versus those in congestive heart failure and emphysema. Am J Roentgenol Radium Ther Nucl Med 1972; 115: 739–750. [DOI] [PubMed] [Google Scholar]
- 14. Pistolesi M, Miniati M, Bonsignore M, Andreotti F, Ricco GD, Marini C, Rindi M, Biagini A, Milne ENC, Giuntini C. Factors affecting regional pulmonary blood flow in chronic ischemic heart disease. J Thorac Imaging 1988; 3: 65–72. [DOI] [PubMed] [Google Scholar]
- 15. Jögi J, Jonson B, Ekberg M, Bajc M. Ventilation–perfusion SPECT with 99mTc‐DTPA versus Technegas: a head‐to‐head study in obstructive and nonobstructive disease. J Nud Med 2010; 51: 735–741. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Hemodynamic data, pulmonary hypertension classification and results from CXR and V/P SPECT of the 46 included patients mean pulmonary artery pressure (mPAP), mean right atrial pressure (mRAP), mean pulmonary arterial wedge pressure (mPAWP), pulmonary vascular resistance (PVR), transpulmonary gradient (TPG), diastolic pulmonary vascular pressure gradient (DPG), Cardiac index (CI), isolated post‐capillary pulmonary hypertension (Ipc‐PH), combined post‐capillary pulmonary hypertension (Cpc‐PH).


