Abstract
Aims
Heart failure (HF) is a multifactorial disease. Current treatments target only a fraction of the putative pathophysiological pathways. In patients with HF, reduced cardiac output and congestion cause increased gut wall permeability. It has been suggested that leakage of microbial products is detrimental to the heart, at least partly through activation of systemic inflammatory pathways, which again could promote gut leakage. Whether manipulating the gut microbiota can improve cardiac function in patients with HF remains unknown. We aim to evaluate the effect of drugs targeting the gut microbiota on left ventricular function, quality of life, and functional capacity, as well as on markers of gut leakage and inflammation, in stable patients with HF with reduced ejection fraction.
Methods and results
GutHeart is a randomized, open‐label, controlled trial. Four centres will randomize 150 patients with stable HF and a left ventricular ejection fraction <40% to receive the antibiotic rifaximin, the probiotic yeast Saccharomyces boulardii (ATCC 74012), or no treatment (control) in a 1:1:1 fashion. Treatment will last for 3 months. The primary endpoint is baseline‐adjusted left ventricular ejection fraction as measured by echocardiography after 3 months. A further follow‐up 6 months after randomization will be undertaken.
Conclusions
This trial is likely to give new insights into important disease processes involving the gut microbiota in HF patients, hereby leading to new potential therapeutic strategies to prevent and down‐regulate the inflammation seen in these patients.
Keywords: Heart failure, Gut microbiota, Remodelling, Randomized controlled trial, Study design, Microbial translocation
Introduction
Heart failure (HF) is an important cause of morbidity and mortality worldwide. The last decades have seen significant progress in the treatment of HF,1 but the mortality and morbidity remain high, suggesting that important pathogenic mechanisms remain at least partly unmodified.2 Structural, cellular, and molecular processes in the myocardium, referred to as remodelling, characterize the development of HF. Left ventricular (LV) remodelling enables the preservation of cardiac output in the face of reduced myocardial contractility. However, over time, this process turns maladaptive, leading to a progressive decrease in LV function.3 Inflammatory and metabolic mechanisms may play an important role in the development and progression of chronic HF, and these interacting processes may contribute to the shift from adaptive to maladaptive remodelling.4
The gastrointestinal tract contains a dynamic microbial community with a combined genome exceeding the human genome by two orders of magnitude.5 It provides a large and complex cache of potential triggers, enhancers, and inhibitors of inflammatory and metabolic pathways. The composition of the microbiota determines the type and number of molecules that challenge the mucosal barrier and interact with the immune system within the gut wall and systemically. As an example, the gut microbial composition in patients with inflammatory bowel disease differs from that of healthy control subjects, potentially contributing to intestinal inflammation. Vice versa, genetic susceptibility factors in patients with inflammatory bowel disease may shape the microbial community composition, potentially in a pro‐inflammatory direction.6 Notably, the gut microbiota seems to be of importance not only for intestinal inflammation but also for systemic inflammatory and metabolic disorders like Type 2 diabetes7 and obesity.8 It may also play a role in atherosclerosis by interfering with inflammatory and metabolic pathways that at least partly involve the metabolism of certain nutrients containing carnitine (e.g. red meat) or phosphatidyl choline (e.g. dairy products and egg).9 Hence, the gut microbiota has been proposed as a cardio‐metabolic target for intervention.10
In patients with HF, the decreased cardiac output and congestion contribute to ischaemia and oedema of the gut wall. Consequently, structural and functional changes may cause increased gut permeability or even secondary inflammation. Sandek et al.11 reported a 210% increase in large intestine permeability measured by sucralose excretion. The severity of HF symptoms seems to correlate well with the magnitude of permeability, the amount of pathogenic bacteria, and secondary inflammation.12
Several studies have shown that low‐grade leakage of microbial products, such as lipopolysaccharide (LPS), occurs across the gut wall.13, 14 This may cause systemic inflammation by activating toll‐like receptor 4 on cells of the innate immune system,15 which again may promote gut leakage in the gastrointestinal tract. Lipopolysaccharide‐induced toll‐like receptor 4 activation induces the release of inflammatory cytokines like tumour necrosis factor, which could act as a suppressor of cardiac function via several pathways, including reduced mitochondrial activity, altered calcium homeostasis, and impaired β‐adrenergic signalling in cardiomyocytes.16 Other inflammatory cytokines, like interleukin (IL)‐1 and IL‐6, may also promote myocardial dysfunction.16
We recently showed that the microbiota‐dependent marker trimethylamine N‐oxide was associated with outcome in patients with chronic HF.17 Other investigators have shown that gut decontamination with antibiotics reduces intestinal levels of LPS, monocyte expression of the LPS co‐receptor CD14, and the production of IL‐6, IL‐1β, and tumour necrosis factor.18 Selective gut decontamination favourably affects post‐operative outcome in patients undergoing cardiac surgery.19 Recently, our co‐investigators in Brazil published a pilot trial showing that manipulation of the gut microbiota could promote LV functional improvement in patients with HF.20 Figure 1 shows some of the putative mechanisms involved in the interaction between the cardiovascular system and the gut. However, our knowledge of the interaction between the gut microbiota, systemic inflammatory and metabolic disturbances, and myocardial function in patients with HF remains limited, and other mechanisms than gut leakage could be involved in the translation of a disturbed gut microbiota into activation of systemic inflammatory and metabolic pathways.
Figure 1.
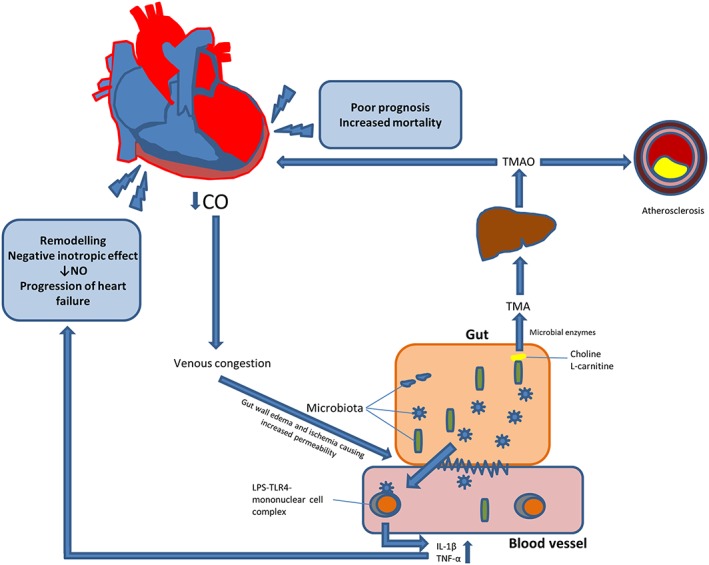
Putative mechanisms involved in the interaction between the gut and the cardiovascular system. IL, interleukin; LPS, lipopolysaccharide; TLR4, toll‐like receptor 4; TMA, trimethylamine; TMAO, trimethylamine N‐oxide; TNF, tumour necrosis factor.
Design
Study rationale and objectives
While most studies on inflammation in HF have focused on downstream mediators of inflammation and tissue damage, the present study will address the gut microbiota as a potential upstream trigger of inflammatory activation. In addition to promoting inflammation, an altered gut microbiota may play a key pathogenic role in HF through the induction of metabolic disturbances.
We postulate that the gut microbiota is altered in patients with HF and that the gut microbiota in these patients contributes to low‐grade systemic inflammation as well as metabolic disturbances. Furthermore, we assume that interventions with probiotics or non‐absorbable antibiotics will attenuate these inflammatory and metabolic disturbances and favourably affect cardiac function. We aim to investigate whether treatment with the non‐absorbable antibiotic rifaximin or the probiotic yeast Saccharomyces boulardii (ATCC 74012), on top of recommended treatment for HF, improves LV ejection fraction (LVEF), quality of life, and exercise capacity and reduces systemic inflammation in patients with HF and reduced ejection fraction.
Outline
The GutHeart trial is a Phase II, randomized, open‐label, controlled trial conducted at three centres in Norway and one in Brazil. The trial has been registered at ClinicalTrials.gov (NCT02637167, www.clinicaltrials.gov).
Eligibility
The inclusion and exclusion criteria are presented in Table 1. We include patients who have stable, symptomatic HF and who are not likely to improve further from recommended treatment. Prior to enrolment, we require at least 3 months optimal pharmacological treatment for HF and at least 6 months with cardiac resynchronization therapy, if indicated. Exclusion criteria include concomitant diseases that we assume affect the composition of the gut microbiota substantially and antibiotic treatment within the last 3 months. We also exclude patients who take probiotic drugs or over‐the‐counter probiotic substances on a regular basis.
Table 1.
Inclusion and exclusion criteria
| Inclusion criteria |
| Between 18 and 75 years of age |
| Symptomatic heart failure, NYHA Class II–III |
| Left ventricular ejection fraction <40% on echocardiography |
| Receiving optimal treatment for heart failure for at least 3 months |
| Haemoglobin >100 g/L |
| Estimated glomerular filtration rate ≥30 mL/min |
| Alanine aminotransferase <150 U/L |
| Signed informed consent |
| Acceptable acoustic windows for echocardiographic assessment |
| Exclusion criteria |
| Treatment with antibiotics or probiotics within 12 weeks prior to randomization |
| History of hypersensitivity to rifaximin or other rifamycin‐derived antimicrobial agents, or any of the components of Xifaxan® |
| History of hypersensitivity to S. boulardii, yeast, or any of the components of Precosa® |
| Polypharmacy with increased risk for interactions, i.e. an extensive list of medications (e.g. 10 drugs or more) that may influence with the patient safety or compromise the study results |
| Malignancy of any cause, excluding basal cell carcinoma of the skin, which has not been curatively treated >5 years ago, or where there has been relapse within the last 5 years |
| Acute coronary syndrome within 12 weeks prior to randomization |
| Impaired liver function classified as Child–Pugh B or C |
| Ongoing infection, including gastrointestinal infection |
| Inflammatory bowel disease |
| Bowel obstruction |
| Active myocarditis, including Chagas disease |
| Severe, primary valvular heart disease |
| Atrial fibrillation with ventricular rate >100/min |
| Initiation of cardiac resynchronization therapy within 6 months prior to randomization |
| Any other, severe co‐morbid disease that must be expected to significantly reduce the efficacy of the interventional products, survival, or compliance |
| Ongoing treatment with immunosuppressive drugs |
| Ongoing treatment with rifamycins other than rifaximin |
| Central venous catheter |
| Pregnancy or planned pregnancy |
| Nursing |
| Poor compliance |
| Any reason why, in the opinion of the investigator, the patient should not participate |
NYHA, New York Heart Association.
Procedures
Figure 2 is a flow chart depicting patient recruitment, randomization, study drug allocation, and follow‐up. We recruit patients at the cardiology departments at four centres: Oslo University Hospital, Rikshopitalet (Oslo, Norway), Oslo University Hospital, Ullevål (Oslo, Norway), Nordlandssykehuset (Bodø, Norway), and Instituto Nacional de Cardiologia (Rio de Janeiro, Brazil).
Figure 2.

Study design. EF, ejection fraction; NYHA, New York Heart Association; QoL, quality of life.
After verification of eligibility, the investigators obtain written, informed consent from all participants. The patients then undergo study‐specific procedures, including clinical examination, echocardiography, a 6 min walk test, a quality of life questionnaire (Minnesota Living with Heart Failure Questionnaire), and blood sampling for measuring safety and efficacy. At inclusion, all participants are asked to complete a comprehensive food frequency questionnaire. They are asked to refrain from probiotics and probiotic foods during the study period, except from small amounts of foods that are a part of their usual diet, such as the occasional yoghurt. All drugs taken by the patient, including over‐the‐counter drugs and herbal medicines, are registered at inclusion and throughout the study period. We measure the height and weight of all study participants.
Randomization and allocation to treatment arm are performed on the online platform Viedoc™ (PCG Solutions, Uppsala, Sweden). The randomization list was generated using STATA 13™ (StatCorpLP, College Station, TX, USA). The patients are assigned a unique patient identification number and are randomized to one of three open‐label treatment arms.
Faecal samples are provided at inclusion and after 3 and 6 months. We apply the Spinstool® preservation solution that stabilizes DNA for 72 h at room temperature and register the time from collection to freezer for all patients. After DNA extraction, V3–V4 region of the 16S rRNA gene will be amplified. Libraries will be submitted to the Norwegian Sequencing Centre (Oslo, Norway) for Illumina MiSeq sequencing (San Diego, CA, USA). The microbiota will be characterized with regard to α‐diversity and β‐diversity and relative abundance of individual taxa, with correction for multiple comparisons.
An extensive biobank will be established per accepted common sampling and processing protocols at three study visits. Samples will be frozen and create the basis for secondary biochemical endpoints.
Endpoints
Primary endpoint
The primary endpoint of this study is the baseline‐adjusted LVEF as measured by echocardiography after 3 months of intervention. The trial is powered to detect a 5% point increase in either intervention arm compared with the control group.
Secondary endpoints
The secondary endpoints will assess differences between either of the treatment arms and the control group after 3 months' intervention and after another 3 months' follow‐up, regarding (i) the gut microbiota composition, (ii) microbiota‐related metabolites, (iii) parameters of cardiac function other than LVEF, (iv) inflammatory and anti‐inflammatory mediators in plasma, serum, circulating leucocytes, and peripheral blood mononuclear cells, (v) health‐related quality of life, (vi) functional capacity, (vii) endothelial function measured by asymmetric dimethylarginine, the l‐arginine/asymmetric dimethylarginine ratio, and Endo‐PAT®, and (viii) safety. Endpoints are detailed in Table 2.
Table 2.
Endpoints
| Primary endpoint |
|
| Secondary endpoints |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
CRP, C‐reactive protein; IL, interleukin; LPS, lipopolysaccharide; LVEF, left ventricular ejection fraction; NT‐proBNP, N‐terminal pro‐brain natriuretic peptide; TMAO, trimethylamine N‐oxide; TNF, tumour necrosis factor.
The trial is a proof‐of‐concept study regarding the effect of altering the gut microbiota on cardiac function in patients with HF. In addition to assessing differences between either treatment arm and the control arm, we also plan to perform secondary analyses as to whether altering the microbiota through both treatments will affect the respective endpoints (i.e. whether there is a difference in the baseline‐adjusted efficacy measures between the control arm and the two treatment arms combined) and whether there are differences between the two treatment arms.
Statistical considerations
To observe a difference in LVEF of 5 percentage points between patients in either active drug arm compared with controls, with an α of 5% and power of 80%, we will need approximately 37 patients in each arm. The calculation is based on a presumed standard deviation of LVEF of 7.5 percentage points. To increase the chance of attaining significant differences in secondary endpoints and to allow for dropout, we aim to include 50 patients in each arm. No interim analysis is planned, unless there are a substantially increased number of serious adverse events in either of the intervention arms, as judged by the data monitoring board.
The analyses will follow intention‐to‐treat principles. The primary endpoint: baseline‐adjusted LVEF at the end of treatment (i.e. 3 months after start of treatment) will be analysed by analysis of covariance, the statistical null hypothesis being that LVEF does not differ between either of the two treatment arms and the control arm. Secondary, continuous efficacy variables will also be assessed by analysis of covariance. If necessary, values will be log‐transformed to meet the assumptions of the test. Ordinal categorical variables, such as New York Heart Association functional class, will be analysed using ordinal logistic regression, whereas count variables will be assessed by Poisson regression. We intend to perform exploratory, secondary endpoint analyses according to the per‐protocol principle, where compliance above 80% throughout the treatment period will be regarded as protocol adherence. Exploratory, secondary analyses will be made for efficacy variables stratified by country (i.e. separate analyses for Norwegian and Brazilian patients). Safety analyses will include tabulation of type and frequency of all adverse events. Any serious adverse events will be reported with comprehensive narratives.
Discussion
We have devised the GutHeart trial to assess an interplay hitherto largely overlooked in medicine: the gut–heart axis (Figure 1 ). Our assumption is that by manipulating the bacterial composition of the gut content, we might be able to improve the inflammatory and metabolic environment for the cardiovascular system, thereby promoting cardiac healing and adaptive remodelling. We have chosen to test this hypothesis in a randomized controlled trial with the non‐absorbable antibiotic rifaximin, the probiotic yeast S. boulardii, or no treatment (control) on top of recommended treatment for HF. Saccharomyces boulardii was used in the aforementioned pilot study conducted by our co‐investigators in Brazil, who found that treatment reduced markers of inflammation and improved LVEF in patients with HF. However, these results should be interpreted with caution because of the small sample size. We found it feasible and scientifically important to investigate the same drug in a larger and well‐powered study.
Saccharomyces boulardii is a yeast, and the risk of fungaemia must be recognized. In the clinical setting, this probiotic is used in different long‐term treatment regimens such as in recurrent Clostridium difficile infections. The safety data on S. boulardii are mostly derived from case reports. Typical risk factors for fungaemia are the use of central venous catheters, enteral or parenteral nutrition, being treated in an intensive care unit, and immunosuppression. In a meta‐analysis by Shen et al.21 investigating the use of probiotics in C. difficile infections, 538 patients received S. boulardii. No fungaemia was reported. To our knowledge, only limited data exist on the use of S. boulardii in HF populations; thus, we will remain vigilant in reporting adverse events.21 Rifaximin was chosen for its local effects in the gut and its low absorption. It is a non‐absorbable antibiotic with minimal systemic effects. It has a broad microbial range and is indicated for treatment in a variety of clinical conditions such as travellers' diarrhoea, hepatic encephalopathy, and irritable bowel disease with diarrhoea. Clostridium difficile infections have been reported as a direct consequence of treatment with rifaximin. This is an uncommon but potentially serious adverse effect. Kimer et al.22 published a systemic review in 2014, including 19 RCTs and 1370 patients who were randomized to rifaximin or placebo in the treatment of hepatic encephalopathy. The length of treatment ranged from 5 to 180 days. None of the trials found a difference between the rifaximin and the control groups regarding serious adverse events. There were only two trials, comprising all together 203 patients, where treatment with rifaximin was continued for >3 months with doses of 1.1 g or above. In these groups, only two cases of C. difficile infections were reported, suggesting an incidence rate of 0.1%. Given the current state of knowledge, the large disease burden posed by HF, and the favourable safety profile of the investigational drugs, we find that randomizing patients to different treatments is justified. Gut microbiota manipulation remains a promising, but far from proven, treatment option in patients with HF.
Our trial will be the first to produce solid evidence regarding the effect of manipulating the gut microbiota in patients with HF. Moreover, the observed association between gut microbiota and systemic inflammatory disorders does not necessarily imply causality. Hence, proof‐of‐concept studies like the GutHeart trial are needed to establish a causal relationship.
Left ventricular ejection fraction is a strong predictor of all‐cause mortality and cardiovascular death,23 whereas an increase is associated with a reduced risk of cardiac death.24 Left ventricular ejection fraction thus serves as a natural surrogate endpoint in interventional studies in patients with systolic HF.25 Echocardiography is a readily available, safe, and versatile imaging tool, which can be applied without concern in patients with cardiac devices. When assessing LVEF, the main limitation is image quality. To limit the impact of poor acoustic settings, we demand that all eligible patients have acceptable acoustic windows.
The study is powered to reveal a difference of 5 percentage point increase in either intervention group compared with control. An inter‐observer variability for LVEF assessed by two‐dimensional echocardiography of ±7% and a test–retest reliability of ±5% has previously been reported.26, 27 To minimize inter‐observer differences, all images will be analysed at the European Association of Cardiovascular Imaging accredited echocardiography core lab at the Department of Cardiology at Oslo University Hospital, Rikshospitalet.
The study will expand our knowledge about the mechanisms behind the inflammatory and dysregulated metabolic states in chronic HF. We will assess biomarkers of microbial translocation, a potential inducer of inflammation, and markers of platelet and endothelial cell activation, potential resultants of an inflammatory response. We will also perform in‐depth studies on metabolic disturbances consequence of disturbed gut microbiota composition. In addition to measuring circulating proteins and gene expression, we will perform analyses in peripheral blood cells to evaluate regulatory pathways.
Whereas focus has often been directed at the association between the gut microbiota and systemic inflammation, less is known about the effects the gut microbiota exerts on the regulation of metabolic pathways. An important goal of the GutHeart trial will be to elucidate the gut–heart metabolic axis. We hypothesize that interventions targeting the gut microbiota will alter its composition to reduce microbiota‐specific as well as systemic inflammation and to exert favourable effects on metabolic pathways. In turn, these changes may result in improved cardiac function as measured by echocardiography and also improvement in functional capacity and quality of life. These studies will give insight not only into how gut microbiota interact with the systemic inflammatory and metabolic pathways but also into how could it potentially delineate novel pathogenic pathways that are of importance for progression of HF.
Trial status
This study was initiated in March 2016 and is currently recruiting patients. As of 19 September 2017, 75 patients have been enrolled. We expect patient recruitment to be complete by the third quarter of 2018. We expect the trial to be completed and results to be available in 2019. Demographic data for the first 30 patients are presented in Table 3.
Table 3.
Baseline characteristics of the first 30 patients
| Age (years) ± SD | 60 ± 7 |
| Male gender—n (%) | 25 (83) |
| Body mass index (kg/m2) | 29 ± 6 |
| Systolic blood pressure (mmHg) | 118 ± 18 |
| Diastolic blood pressure (mmHg) | 75 ± 12 |
| Heart rate (b.p.m.) | 67 ± 11 |
| Atrial fibrillation/flutter—n (%) | 10 (33) |
| NYHA Class II/III—n (%) | 21 (70)/9 (30) |
| Medical history | |
| Smokers—n (%) | 16 (53) |
| History of hypertension—n (%) | 12 (40) |
| Diabetes mellitus—n (%) | 7 (23) |
| Implantable cardioverter defibrillator—n (%) | 18 (60) |
| Cardiac resynchronization therapy—n (%) | 7 (23) |
| Laboratory values | |
| Haemoglobin (g/dL) | 14.3 ± 1.3 |
| Creatinine (μmol/L) | 104 ± 33 |
| N‐terminal pro‐brain natriuretic peptide (pg/mL) | 837.2 (IQR 334.1–1584) |
| Echocardiography | |
| Left ventricular ejection fraction (%) | 29 ± 5 |
| 6 min walk test | |
| Distance (m) | 480 ± 157 |
| Peak heart rate (b.p.m.) | 96 ± 18 |
IQR, interquartile range; NYHA, New York Heart Association; SD, standard deviation.
Data are given as n (%), mean ± SD, or median (IQR) as appropriate.
The baseline characteristics are comparable with those of patients in recently published HF trials.28 A median N‐terminal pro‐brain natriuretic peptide of 837.2 pg/mL (interquartile range 334.1–1584.0) despite a mean LVEF of just 29 ± 5% testifies to the clinical stability of the patients and is consistent with optimal evidenced‐based treatment at baseline.
Ethical perspectives
The GutHeart trial is designed to assess the effect of a therapeutic intervention in a prevalent disease that imposes a large burden on individual patients as well as society. The trial is conducted according to Good Clinical Practice guidelines. Based on previous trials and drug pharmacodynamics, we do not expect a substantial number of drug‐related severe adverse events. The trial complies with the Declaration of Helsinki. The regional ethics committees as and the relevant competent authorities have approved the trial. All patients provide written informed consent before enrolment and randomization.
Conclusions
The GutHeart trial is likely to lead to increased understanding of the interactions between gut microbiota, inflammation, metabolic disturbances, and cardiac function. Potentially, our results will provide the rationale for new therapeutic strategies in patients with HF. The findings could also be of relevance for other disorders where there may be interactions between gut microbiota on the one hand and inflammation and metabolic pathways on the other. Such disorders include atherosclerosis and related metabolic disorders (e.g. obesity, diabetes, and liver steatosis) as well as autoimmune and autoinflammatory diseases.
Conflict of interest
None declared.
Funding and study management
The GutHeart trial is an investigator‐initiated study supported by unrestricted grants provided by the Norwegian Health Association and Stein Erik Hagen's Foundation for Clinical Heart Research. Alfasigma and Biocodex have provided the investigational medicinal products used in this trial. Biocodex has also provided an unrestricted lump sum for the implementation of the study. The funding sources have had no role in the design of the study; neither will they participate in the implementation of the trial, in the analyses of the results, or in the decision to publish. The trial is the result of a multidisciplinary and international collaboration, to which experts in the fields of gastroenterology, inflammation, and cardiology have contributed. The investigators take sole responsibility for the integrity of the data, the writing of the manuscript, and the dissemination of the results.
A Trial Steering Committee oversees the progress of the trial. An independent Data Monitoring and Safety Committee is responsible for the regular monitoring of trial data, and it will give advice on whether the accumulated safety data, together with the results from other relevant research, necessitate premature termination of the trial.
Acknowledgements
We thank the Norwegian Health Association and Stein Erik Hagen's Foundation for Clinical Heart Research for funding this project. The investigational medicinal products were kindly provided by Alfasigma and Biocodex.
Mayerhofer, C. C. K. , Awoyemi, A. O. , Moscavitch, S. D. , Lappegård, K. T. , Hov, J. R. , Aukrust, P. , Hovland, A. , Lorenzo, A. , Halvorsen, S. , Seljeflot, I. , Gullestad, L. , Trøseid, M. , and Broch, K. (2018) Design of the GutHeart—targeting gut microbiota to treat heart failure—trial: a Phase II, randomized clinical trial. ESC Heart Failure, 5: 977–984. 10.1002/ehf2.12332.
References
- 1. McMurray JJ. Improving outcomes in heart failure: a personal perspective. Eur Heart J 2015; 36: 3467–3470. [DOI] [PubMed] [Google Scholar]
- 2. Stewart S, MacIntyre K, Hole DJ, Capewell S, McMurray JJ. More ‘malignant’ than cancer? Five‐year survival following a first admission for heart failure. Eur J Heart Fail 2001; 3: 315–322. [DOI] [PubMed] [Google Scholar]
- 3. Shimizu I, Minamino T. Physiological and pathological cardiac hypertrophy. J Mol Cell Cardiol 2016; 97: 245–262. [DOI] [PubMed] [Google Scholar]
- 4. Wrigley BJ, Lip GY, Shantsila E. The role of monocytes and inflammation in the pathophysiology of heart failure. Eur J Heart Fail 2011; 13: 1161–1171. [DOI] [PubMed] [Google Scholar]
- 5. Li J, Jia H, Cai X, Zhong H, Feng Q, Sunagawa S, Arumugam M, Kultima JR, Prifti E, Nielsen T, Juncker AS, Manichanh C, Chen B, Zhang W, Levenez F, Wang J, Xu X, Xiao L, Liang S, Zhang D, Zhang Z, Chen W, Zhao H, Al‐Aama JY, Edris S, Yang H, Wang J, Hansen T, Nielsen HB, Brunak S, Kristiansen K, Guarner F, Pedersen O, Doré J, Ehrlich SD, Consortium MHIT, Bork P, Wang J, MetaHIT Consortium . An integrated catalog of reference genes in the human gut microbiome. Nat Biotechnol 2014; 32: 834–841. [DOI] [PubMed] [Google Scholar]
- 6. Forbes JD, Van Domselaar G, Bernstein CN. The gut microbiota in immune‐mediated inflammatory diseases. Front Microbiol 2016; 7: 1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Burcelin R, Serino M, Chabo C, Blasco‐Baque V, Amar J. Gut microbiota and diabetes: from pathogenesis to therapeutic perspective. Acta Diabetol 2011; 48: 257–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Compare D, Rocco A, Sanduzzi Zamparelli M, Nardone G. The gut bacteria‐driven obesity development. Dig Dis 2016; 34: 221–229. [DOI] [PubMed] [Google Scholar]
- 9. Backhed F. Meat‐metabolizing bacteria in atherosclerosis. Nat Med 2013; 19: 533–534. [DOI] [PubMed] [Google Scholar]
- 10. Vinje S, Stroes E, Nieuwdorp M, Hazen SL. The gut microbiome as novel cardio‐metabolic target: the time has come. Eur Heart J 2014; 35: 883–887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sandek A, Bauditz J, Swidsinski A, Buhner S, Weber‐Eibel J, Von Haehling S, Schroedl W, Karhausen T, Doehner W, Rauchhaus M, Poole‐Wilson P, Volk HD, Lochs H, Anker SD. Altered intestinal function in patients with chronic heart failure. J Am Coll Cardiol 2007; 50: 1561–1569. [DOI] [PubMed] [Google Scholar]
- 12. Pasini E, Aquilani R, Testa C, Baiardi P, Angioletti S, Boschi F, Verri M, Dioguardi F. Pathogenic gut flora in patients with chronic heart failure. JACC Heart Fail 2016; 4: 220–227. [DOI] [PubMed] [Google Scholar]
- 13. Vors C, Pineau G, Drai J, Meugnier E, Pesenti S, Laville M, Laugerette F, Malpuech‐Brugère C, Vidal H, Michalski MC. Postprandial endotoxemia linked with chylomicrons and lipopolysaccharides handling in obese versus lean men: a lipid dose‐effect trial. J Clin Endocrinol Metab 2015; 100: 3427–3435. [DOI] [PubMed] [Google Scholar]
- 14. Neal MD, Leaphart C, Levy R, Prince J, Billiar TR, Watkins S, Li J, Cetin S, Ford H, Schreiber A, Hackam DJ. Enterocyte TLR4 mediates phagocytosis and translocation of bacteria across the intestinal barrier. J Immunol 2006; 176: 3070–3079. [DOI] [PubMed] [Google Scholar]
- 15. Charalambous BM, Stephens RC, Feavers IM, Montgomery HE. Role of bacterial endotoxin in chronic heart failure: the gut of the matter. Shock 2007; 28: 15–23. [DOI] [PubMed] [Google Scholar]
- 16. Anker SD, von Haehling S. Inflammatory mediators in chronic heart failure: an overview. Heart 2004; 90: 464–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Troseid M, Ueland T, Hov JR, Van Saene HK, Ieven MM, Bosmans JM, Schuerwegh A, Bridts CH, Wuyts F, Stevens WJ, Anker SD, Rauchhaus M, Vrints CJ. Microbiota‐dependent metabolite trimethylamine‐N‐oxide is associated with disease severity and survival of patients with chronic heart failure. J Intern Med 2015; 277: 717–726. [DOI] [PubMed] [Google Scholar]
- 18. Conraads VM, Jorens PG, De Clerck LS, van Saene HK, Ieven MM, Bosmans JM, Schuerwegh A, Bridts CH, Wuyts F, Stevens WJ, Anker SD, Rauchhaus M, Vrints CJ. Selective intestinal decontamination in advanced chronic heart failure: a pilot trial. Eur J Heart Fail 2004; 6: 483–491. [DOI] [PubMed] [Google Scholar]
- 19. Fox MA, Peterson S, Fabri BM, van Saene HK. Selective decontamination of the digestive tract in cardiac surgical patients. Crit Care Med 1991; 19: 1486–1490. [DOI] [PubMed] [Google Scholar]
- 20. Costanza AC, Moscavitch SD, Faria Neto HC, Mesquita ET. Probiotic therapy with Saccharomyces boulardii for heart failure patients: a randomized, double‐blind, placebo‐controlled pilot trial. Int J Cardiol 2015; 179: 348–350. [DOI] [PubMed] [Google Scholar]
- 21. Shen NT, Maw A, Tmanova LL, Pino A, Ancy K, Crawford CV, Simon MS, Evans AT. Timely use of probiotics in hospitalized adults prevents Clostridium difficile infection: a systematic review with meta‐regression analysis. Gastroenterology 2017; 152; 1889‐900, e9. [DOI] [PubMed] [Google Scholar]
- 22. Kimer N, Krag A, Moller S, Bendtsen F, Gluud LL. Systematic review with meta‐analysis: the effects of rifaximin in hepatic encephalopathy. Aliment Pharmacol Ther 2014; 40: 123–132. [DOI] [PubMed] [Google Scholar]
- 23. McMurray JJ, Packer M, Desai AS, Gong J, Lefkowitz M, Rizkala AR, Rouleau JL, Shi VC, Solomon SD, Swedberg K, Zile MR, PARADIGM‐HF Committees Investigators . Baseline characteristics and treatment of patients in prospective comparison of ARNI with ACEI to determine impact on global mortality and morbidity in heart failure trial (PARADIGM‐HF). Eur J Heart Fail 2014; 16: 817–825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Meta‐analysis Global Group in Chronic Heart Failure . The survival of patients with heart failure with preserved or reduced left ventricular ejection fraction: an individual patient data meta‐analysis. Eur Heart J 2012; 33: 1750–1757. [DOI] [PubMed] [Google Scholar]
- 25. Solomon SD, Anavekar N, Skali H, McMurray J, Swedberg K, Yusuf S, Granger CB, Michelson EL, Wang D, Pocock S, Pfeffer MA, Candesartan in Heart Failure Reduction in Mortality (CHARM) Investigators . Influence of ejection fraction on cardiovascular outcomes in a broad spectrum of heart failure patients. Circulation 2005; 112: 3738–3744. [DOI] [PubMed] [Google Scholar]
- 26. Himelman RB, Cassidy MM, Landzberg JS, Schiller NB. Reproducibility of quantitative two‐dimensional echocardiography. Am Heart J 1988; 115: 425–431. [DOI] [PubMed] [Google Scholar]
- 27. Galderisi M, Henein MY, D'Hooge J, Sicari R, Badano LP, Zamorano JL, Roelandt JR, European Association of Echocardiography . Recommendations of the European Association of Echocardiography: how to use echo‐Doppler in clinical trials: different modalities for different purposes. Eur J Echocardiogr 2011; 12: 339–353. [DOI] [PubMed] [Google Scholar]
- 28. McMurray JJ, Anand IS, Diaz R, Maggioni AP, O'Connor C, Pfeffer MA, Solomon SD, Tendera M, van Veldhuisen DJ, Albizem M, Cheng S, Scarlata D, Swedberg K, Young JB, RED‐HF Committees Investigators . Baseline characteristics of patients in the Reduction of Events with Darbepoetin alfa in Heart Failure trial (RED‐HF). Eur J Heart Fail 2013; 15: 334–341. [DOI] [PMC free article] [PubMed] [Google Scholar]


