Abstract
Aims
Left atrium (LA) dilation is associated with adverse cardiovascular (CV) outcomes. Blood stasis, thrombus formation and atrial fibrillation may occur, especially in heart failure (HF) patients. It is not known whether preventive antithrombotic treatment may decrease the incidence of CV events in HF patients with LA enlargement.
We investigated the relationship between LA enlargement and CV outcomes in HF patients and the effect of different antithrombotic treatments.
Methods and results
Two‐dimensional echocardiography with LA volume index (LAVi) measurement was performed in 1148 patients with systolic HF from the Warfarin versus Aspirin in Reduced Ejection Fraction (WARCEF) trial. Patients were randomized to warfarin or aspirin and followed for 3.4 ± 1.7 years. While the primary aim of the trial was a composite of ischaemic stroke, death, and intracerebral haemorrhage, the present report focuses on the individual CV events, whose incidence was compared across different LAVi and treatment subgroups.
After adjustment for demographics and clinical covariates, moderate or severe LA enlargement was significantly associated with total death (hazard ratio 1.6 and 2.7, respectively), CV death (HR 1.7 and 3.3), and HF hospitalization (HR 2.3 and 2.6) but not myocardial infarction (HR 1.0 and 1.4) or ischaemic stroke (1.1 and 1.5). The increased risk was observed in both patients treated with warfarin or aspirin. In warfarin‐treated patients, a time in therapeutic range >60% was associated with lower event rates, and an interaction between LAVi and time in therapeutic range was observed for death (P = 0.034).
Conclusions
In patients with systolic HF, moderate or severe LA enlargement is associated with death and HF hospitalization despite treatment with antithrombotic medications. The possibility that achieving a more consistent therapeutic level of anticoagulation may decrease the risk of death requires further investigation.
Keywords: Heart failure, Echocardiography, Left atrium, Anticoagulants, Aspirin
Introduction
In the past decades, an enlargement of the left atrium (LA) has been shown to be associated with unfavourable cardiovascular (CV) outcomes. LA enlargement has been associated with increased risk of death,1, 2 stroke,2, 3, 4 heart failure (HF),5, 6 and development of atrial fibrillation.7, 8, 9 These associations have been established in the general population as well as in patients with CV diseases. In patients with HF, LA size has been shown to be a powerful predictor of outcome, providing additional prognostic information to that provided by left ventricular (LV) systolic and diastolic function.10 LA volume (LAV), the most accurate echocardiographic measure of LA size, has been shown to be independently associated with mortality in individuals with suspected HF from the community,11 inversely associated with transplant‐free survival in patients with dilated cardiomyopathy,12 and predictive of outcome in patients undergoing cardiac resynchronization therapy.13
The mechanisms underlying the association between LA size and outcome are not entirely clear. Because the LA dilates in response to multiple stimuli (mitral valve disease, arterial hypertension, and any condition increasing the LV filling pressures),14, 15, 16 LA size may be regarded as an indicator of the combined effect of these conditions over time, a circumstance that may explain in part its association with CV outcomes. However, LA enlargement also predisposes to conditions that may directly affect the risk of CV events, especially those of embolic origin such as ischaemic stroke. LA enlargement is associated with blood stasis and is a strong risk factor for the development of atrial fibrillation,7, 8, 9 both conditions that are associated with hypercoagulability and are especially frequent in HF. Therefore, the use of systemic anticoagulation in HF patients with LA enlargement could theoretically decrease in them the risk of embolic complications.
In the Warfarin versus Aspirin in Reduced Cardiac Ejection Fraction (WARCEF) trial, warfarin treatment reduced the risk of ischaemic stroke in patients with systolic HF in sinus rhythm, although the benefit was offset by an increased frequency of major haemorrhage. No significant difference between warfarin and aspirin treatment was observed for other CV outcomes.17 In the present report, we analyse the relationship between LA size and CV events in the WARCEF cohort and the effect on it of treatment with warfarin or aspirin.
Methods
Study patients
The details of the WARCEF trial enrollment have been previously published. Briefly, from October 2002 to January 2010, a total of 2305 patients were enrolled in the trial (1119 in the USA and Canada and 1186 in Europe and Argentina) at 168 centres in 11 countries. Eligible patients were 18 years of age or older and had normal sinus rhythm, no contraindication to warfarin therapy, and a left ventricular ejection fraction (LVEF) of 35% or less as assessed by quantitative echocardiography (or a wall motion index of ≤1.2) or by radionuclide or contrast angiography within 3 months before randomization.
Patients in any New York Heart Association (NYHA) functional classes were eligible, but patients in NYHA Class I could account for no more than 20% of the total number of patients undergoing randomization. Patients who had a clear indication for warfarin or aspirin were not eligible. Patients were also ineligible if they had a condition that conferred a high risk of cardiac embolism, such as atrial fibrillation, a mechanical cardiac valve, endocarditis, or an intracardiac mobile or pedunculated thrombus. Planned treatment with a beta‐blocker, an angiotensin‐converting enzyme inhibitor, or an angiotensin‐receptor blocker or hydralazine and nitrates was also a reason for ineligibility, whereas current treatment with those medications was allowed.
The study complies with the Declaration of Helsinki. Patients provided informed consent, and the study was approved by the international review boards and ethics boards of participating centres.
Study medication
Patients were randomized to antithrombotic treatment with either adjusted dose‐warfarin with target international normalized ratio (INR) of 2.75 (acceptable range between 2.0 and 3.5) or aspirin 325 mg daily in a double‐blind, double‐dummy design. The statistical analysis centre fabricated clinically plausible INR results for patients in the aspirin group and provided these results to the sites, along with the actual INR results for the patients in the warfarin group, so that all the patients were treated as if they were receiving active warfarin.
Treatment for HF was continued and titrated as clinically indicated, and individual medications usage was recorded.
Left atrium volume determination
All echocardiograms were reinterpreted, blinded to treatment assignment, at a core echocardiography laboratory to confirm the accuracy of LVEF assessment.18 LAV was then measured by Simpson's biplane rule and indexed by body surface area. The LAV index (LAVi) thus obtained was divided into four categories (normal, mildly, moderately, or severely dilated) according to the guidelines of the American Society of Echocardiography.19 The present analysis is based on the 1148 patients who had the echocardiographic views for LAV measurement available. A comparison between patients with and without LAVi information is provided in Supporting Information, Table S1 .
Follow‐up and outcome events
Follow‐up was performed monthly by telephone or in person at the time the blood was obtained for determination of the INR, to assess adherence to the study drug and to regulate INR values. A follow‐up assessment in person was also conducted quarterly for a clinical evaluation and annually for a detailed examination.
While the primary outcome of the trial was the time to the first event in a composite end point of ischaemic stroke, intracerebral haemorrhage, or death from any cause, the present report focuses on individual CV outcomes, which were defined as follows.
Stroke was defined as a clinically relevant new lesion detected on computed tomography or magnetic resonance imaging or, in its absence, clinical findings consistent with clinical stroke and lasting over 24 h.
The diagnosis of myocardial infarction (MI) was based on two of the following: (i) typical cardiac pain or its equivalent; (ii) electrocardiogram evidence of acute MI; or (iii) positive cardiac biomarkers.
Sudden death was defined as (i) death witnessed or occurring within 15 min of observed collapse or new cardiac symptoms, without preceding other modes of death, or (ii) death unwitnessed but known to have occurred in the prior 72 h in the absence of other modes of death or (iii) patient resuscitated from cardiac arrest and dying within 24 h or prior to discharge, in case neurologic function was not restored.
Cardiovascular death included sudden death, documented ventricular tachycardia or fibrillation, documented bradyarrhythmia, MI, and circulatory failure.
Hospitalizations for HF during the follow‐up were defined as admissions with typical symptoms; IV diuretics, vasodilator, or inotropic therapy; and at least 24 h of hospital stay.
Statistical analysis
Baseline demographics, clinical characteristics, and various outcome events by LAV categories were compared using ANOVA F‐tests for continuous variables, χ2 tests for categorical variables, and log‐rank tests for time‐to‐event outcomes. Univariable and multivariable Cox models were used to assess the independent effect of demographic and clinical variables on the outcome events of interest. Missing values for basic covariates were imputed using means for continuous variables and modal values for categorical variables.
The incidence rates of outcome events stratified by LAVi categories were reported. The association between LAVi category and each outcome was first assessed with univariable Cox models and then with adjustment for other patient covariates, including incident atrial fibrillation.
We further investigated the risk of events stratified by type of antithrombotic treatment (aspirin or warfarin). Cox models were used to evaluate the association between LAVi category and outcome events separately in patients treated with aspirin or warfarin and also to assess the interaction of LAVi and treatment type.
We used the same approach to evaluate the possibility that the interaction between LAVi and warfarin treatment on outcome risk might be mediated by differences in time in therapeutic range (TTR). Cox models were used to evaluate the association between LAVi category and outcome events separately in patients with TTR ≤60% or >60% and also to assess the interaction of LAVi and TTR.
Results
Patients with and without LAVi information available differed for some demographic and clinical variables but not for frequency of outcome events (Supporting Information, Table S1 ). The demographics and clinical characteristics of the study cohort by LAVi category are illustrated in Table 1. LAVi categories differed by cohort geographic location and race–ethnicity distribution. Indices of HF severity (NYHA classification; LVEF; and use of diuretics, aldosterone blockers, and beta‐blockers) were also significantly different across categories of LAVi, along with peripheral vascular disease and indices of renal function.
Table 1.
Patient demographics and clinical characteristics by LAVi category
| LAVi (mL/m2) | P‐value* | ||||
|---|---|---|---|---|---|
| Reference range (≤34) | Mildly abnormal (35–41) | Moderately abnormal (42–48) | Severely abnormal (≥49) | ||
| (n = 465) | (n = 225) | (n = 187) | (n = 271) | ||
| Location | 0.026 | ||||
| Argentina | 20/465 (4.3) | 6/225 (2.7) | 5/187 (2.7) | 6/271 (2.2) | — |
| Europe | 264/465 (56.8) | 117/225 (52.0) | 106/187 (56.7) | 180/271 (66.4) | — |
| North America | 181/465 (38.9) | 102/225 (45.3) | 76/187 (40.6) | 85/271 (31.4) | — |
| Patient characteristics | |||||
| Age, years | 61.2 ± 11.0 | 60.1 ± 11.7 | 60.0 ± 11.2 | 60.9 ± 12.2 | 0.510 |
| Male sex | 369/465 (79.4) | 173/225 (76.9) | 155/187 (82.9) | 220/271 (81.2) | 0.445 |
| Race or ethnic group | 0.026 | ||||
| Non‐Hispanic White | 374/465 (80.4) | 163/225 (72.4) | 147/187 (78.6) | 217/271 (80.1) | — |
| Non‐Hispanic Black | 44/465 (9.5) | 43/225 (19.1) | 23/187 (12.3) | 38/271 (14.0) | — |
| Hispanic | 31/465 (6.7) | 15/225 (6.7) | 11/187 (5.9) | 8/271 (3.0) | — |
| Other | 16/465 (3.4) | 4/225 (1.8) | 6/187 (3.2) | 8/271 (3.0) | — |
| Height, cm | 171.9 ± 9.1 | 172.3 ± 9.2 | 171.5 ± 9.6 | 171.3 ± 8.7 | 0.659 |
| Weight, kg | 83.6 ± 18.9 | 86.9 ± 17.9 | 85.9 ± 19.2 | 86.0 ± 19.3 | 0.112 |
| Body mass index | 28.2 ± 5.8 | 29.2 ± 5.3 | 29.1 ± 5.6 | 29.2 ± 6.2 | 0.050 |
| Educational level | 0.888 | ||||
| <High school | 226/464 (48.7) | 103/225 (45.8) | 83/185 (44.9) | 121/271 (44.6) | — |
| High school graduate or some college | 167/464 (36.0) | 90/225 (40.0) | 72/185 (38.9) | 110/271 (40.6) | — |
| College graduate or postgraduate | 71/464 (15.3) | 32/225 (14.2) | 30/185 (16.2) | 40/271 (14.8) | — |
| Alcohol consumption | 0.072 | ||||
| Current, >2 oz/day | 129/465 (27.7) | 45/225 (20.0) | 42/187 (22.5) | 53/271 (19.6) | — |
| Previous, >2 oz/day | 96/465 (20.6) | 44/225 (19.6) | 35/187 (18.7) | 49/271 (18.1) | — |
| Never | 240/465 (51.6) | 136/225 (60.4) | 110/187 (58.8) | 169/271 (62.4) | — |
| Smoking status | 0.153 | ||||
| Current smoker | 90/464 (19.4) | 33/225 (14.7) | 30/186 (16.1) | 41/271 (15.1) | — |
| Former smoker | 254/464 (54.7) | 121/225 (53.8) | 91/186 (48.9) | 139/271 (51.3) | — |
| Never smoker | 120/464 (25.9) | 71/225 (31.6) | 65/186 (34.9) | 91/271 (33.6) | — |
| Clinical characteristics | |||||
| Heart rate, beats/min | 71.8 ± 11.3 | 72.6 ± 12.3 | 73.2 ± 13.3 | 72.2 ± 12.7 | 0.588 |
| Systolic blood pressure, mmHg | 124.2 ± 17.6 | 126.7 ± 20.1 | 123.8 ± 17.5 | 122.5 ± 17.3 | 0.074 |
| NYHA classification | 0.009 | ||||
| I | 74/465 (15.9) | 26/225 (11.6) | 27/187 (14.4) | 28/271 (10.3) | — |
| II | 266/465 (57.2) | 129/225 (57.3) | 93/187 (49.7) | 143/271 (52.8) | — |
| III | 119/465 (25.6) | 64/225 (28.4) | 64/187 (34.2) | 100/271 (36.9) | — |
| IV | 6/465 (1.3) | 6/225 (2.7) | 3/187 (1.6) | 0/271 (0.0) | — |
| LV ejection fraction, % | 25.6 ± 8.1 | 24.8 ± 7.0 | 23.4 ± 6.9 | 23.0 ± 7.3 | <0.001 |
| Distance covered on 6 min walk, m | 367.8 ± 143.8 | 334.4 ± 139.4 | 357.1 ± 150.0 | 360.6 ± 137.5 | 0.053 |
| Pacemaker or defibrillator | 121/465 (26.0) | 45/225 (20.0) | 39/187 (20.9) | 72/269 (26.8) | 0.164 |
| Medical comorbidities | |||||
| Atrial fibrillation | 12/465 (2.6) | 13/225 (5.8) | 9/187 (4.8) | 13/269 (4.8) | 0.176 |
| Diabetes mellitus | 140/465 (30.1) | 76/225 (33.8) | 57/187 (30.5) | 78/269 (29.0) | 0.694 |
| Hypertension | 273/454 (60.1) | 143/219 (65.3) | 104/183 (56.8) | 167/264 (63.3) | 0.295 |
| Ischaemic cardiomyopathy | 207/465 (44.5) | 84/224 (37.5) | 77/187 (41.2) | 135/269 (50.2) | 0.034 |
| Myocardial infarction | 231/465 (49.7) | 104/225 (46.2) | 84/187 (44.9) | 131/269 (48.7) | 0.666 |
| Peripheral vascular disease | 36/465 (7.7) | 38/225 (16.9) | 11/187 (5.9) | 40/271 (14.8) | <0.001 |
| Prior stroke or TIA | 62/465 (13.3) | 37/225 (16.4) | 16/187 (8.6) | 28/269 (10.4) | 0.065 |
| Blood chemistry | |||||
| Creatinine, mg/dL | 1.1 ± 0.3 | 1.2 ± 0.4 | 1.1 ± 0.3 | 1.2 ± 0.3 | 0.030 |
| eGFR | 69.0 ± 20.6 | 69.0 ± 21.1 | 71.7 ± 20.5 | 66.2 ± 21.4 | 0.050 |
| Haemoglobin, g/dL | 14.1 ± 1.4 | 14.0 ± 1.5 | 14.1 ± 1.6 | 13.9 ± 1.7 | 0.343 |
| Haematocrit, % | 41.8 ± 4.0 | 41.4 ± 4.6 | 41.9 ± 4.8 | 41.8 ± 4.8 | 0.640 |
| Sodium, mEq/L | 139.6 ± 3.3 | 140.1 ± 3.2 | 139.7 ± 3.2 | 139.4 ± 3.4 | 0.157 |
| White blood cell count, ×109/L | 7.6 ± 2.1 | 7.3 ± 2.1 | 7.4 ± 2.1 | 7.3 ± 1.9 | 0.094 |
| Medications | |||||
| Aspirin or other antiplatelet agent | 269/380 (70.8) | 128/176 (72.7) | 99/138 (71.7) | 140/210 (66.7) | 0.571 |
| Warfarin or other oral anticoagulant | 28/465 (6.0) | 24/225 (10.7) | 17/187 (9.1) | 29/271 (10.7) | 0.079 |
| ACE inhibitor or ARB | 463/465 (99.6) | 221/225 (98.2) | 186/187 (99.5) | 262/268 (97.8) | 0.079 |
| Beta‐blocker | 412/465 (88.6) | 210/225 (93.3) | 159/187 (85.0) | 255/269 (94.8) | 0.001 |
| Aldosterone blocker | 148/292 (50.7) | 88/141 (62.4) | 67/113 (59.3) | 104/170 (61.2) | 0.049 |
| Nitrate | 100/464 (21.6) | 64/225 (28.4) | 42/187 (22.5) | 72/269 (26.8) | 0.155 |
| Calcium‐channel blocker | 41/465 (8.8) | 25/225 (11.1) | 13/187 (7.0) | 24/268 (9.0) | 0.531 |
| Diuretic | 350/465 (75.3) | 194/225 (86.2) | 161/187 (86.1) | 243/269 (90.3) | <0.001 |
| Statin | 292/360 (81.1) | 142/170 (83.5) | 103/126 (81.7) | 162/209 (77.5) | 0.499 |
ACE, angiotensin‐converting enzyme; ARB, angiotensin receptor blocker; eGFR, estimated glomerular filtration rate; LAVi, left atrial volume index; LV, left ventricular; NYHA, New York Heart Association; TIA, transient ischaemic attack.
Denominators may vary due to missing information.
P‐values were calculated using ANOVA F‐test for continuous variables and χ2 test for categorical variables.
In the 1148 patients of the LAVi cohort, the mean follow‐up time was 3.4 ± 1.7 years, and the total follow‐up time was 3846 patient‐years. Overall, 179 patients (15.6%) had CV death, 105 patients (9.1%) had sudden death, 28 patients (2.4%) had an MI, 36 (3.1%) suffered an ischaemic stroke, and 229 patients (20.0%) experienced an HF hospitalization. Incident atrial fibrillation was observed in 127 patients (11.1%; 13.3% in the aspirin group and 8.8% in the warfarin group; P = 0.017).
Left atrial volume index and outcomes
The incidence rates and adjusted hazard ratios (HRs) of outcome events stratified by LAVi category, after adjustment for pertinent covariates, are reported in Figure 1 . Moderately or severely dilated LAVi was significantly associated with total death [HR 1.6, 95% confidence interval (CI) 1.1 to 2.4 and HR 2.7, CI 2.0 to 3.7, respectively], CV death (HR 1.7, CI 1.1 to 2.8 and HR 3.3, CI 2.2 to 4.9, respectively), and HF hospitalization (HR 2.3, CI 1.5 to 3.3 and HR 2.6, CI 1.8 to 3.6, respectively) but not with MI (HR 1.0, CI 0.3 to 3.3 and HR 1.4, CI 0.5 to 4.0, respectively) or ischaemic stroke (HR 1.5, CI 0.6 to 3.8 and HR 0.8, CI 0.3 to 2.1, respectively).
Figure 1.
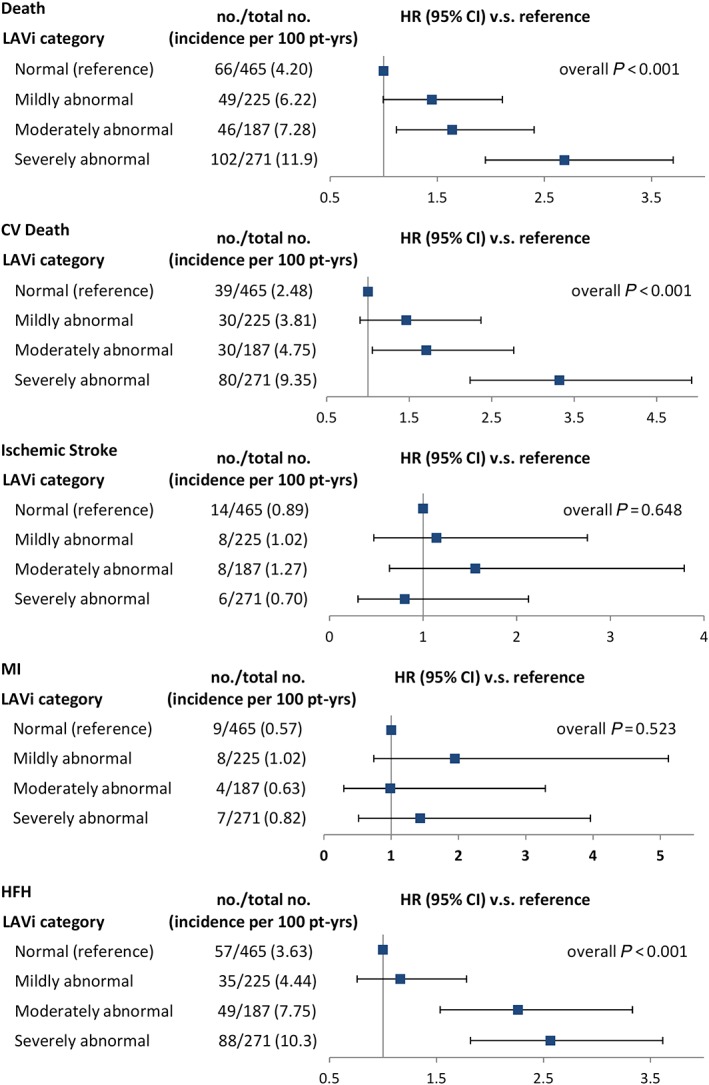
Outcome incidence rates by category of left atrial volume index (LAVi). CI, confidence interval; CV, cardiovascular; HFH, heart failure hospitalization; HR, hazard ratio; MI, myocardial infarction.
Effect of antithrombotic treatment
Figure 2 shows the outcome incidence rates by type of antithrombotic treatment (aspirin or warfarin), stratified by LAVi category. Incidence rates were similar between aspirin‐treated and warfarin‐treated patients. The deleterious effect on the risk of death of a larger LAVi tended to be stronger in warfarin‐treated than in aspirin‐treated patients, but no significant interaction between LAVi and treatment type was detected (P = 0.604). No significant interaction was observed for any of the other outcomes as well.
Figure 2.
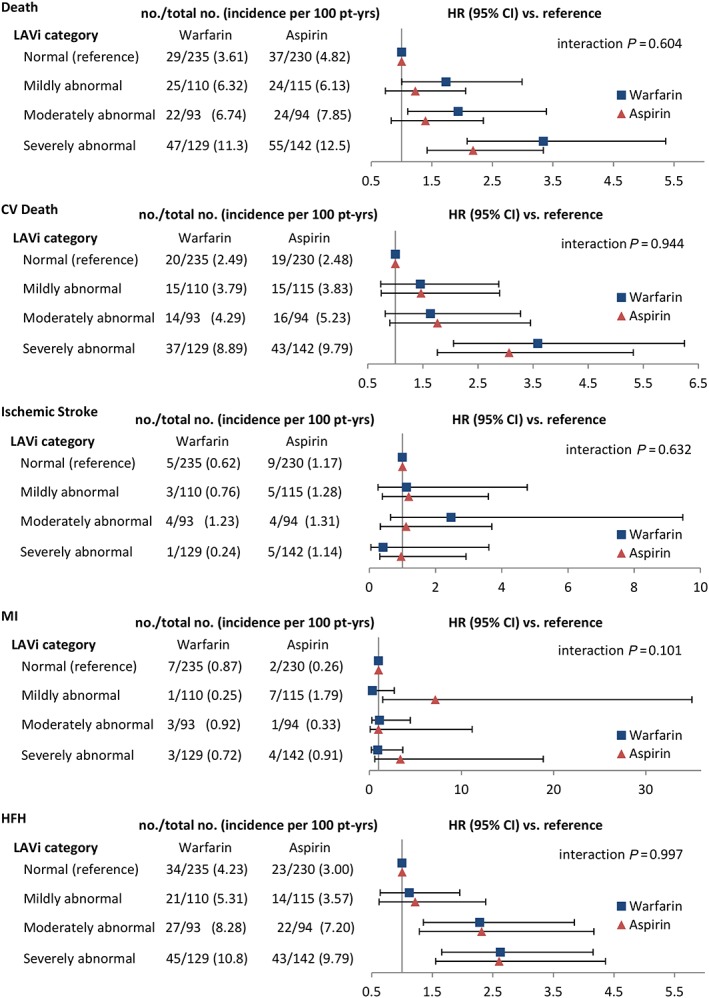
Outcome incidence rates by antithrombotic treatment type, stratified by left atrial volume index (LAVi) category. Abbreviations are as in Figure 1 .
In warfarin‐treated patients, TTR was evaluated as possibly affecting the relationship between LAVi and outcomes. Although a relationship between moderately or severely dilated LA and death was observed both in patients with TTR >60% or ≤60%, a significant interaction between TTR and LAVi category was observed (P = 0.034; Supporting Information, Table S2 ). A trend towards a similar interaction was observed for CV death (P = 0.058), whereas no interaction was observed for other outcomes.
Discussion
In the present report, we describe how LA enlargement, especially when of moderate or severe degree, remained significantly associated with death and HF hospitalization in a cohort of patients with systolic HF in sinus rhythm who were treated with currently recommended HF regimen, including antithrombotic medications. On the other hand, no significant association was observed between LA enlargement and ischaemic stroke or MI.
The role of LA enlargement as a predictor of CV events and death is well documented in the general population and in patients with CV disease. The most accredited explanation for this observation is that LA enlargement, rather than directly affecting the risk of CV events, may represent an indicator of the combined effect over time of various conditions (such as hypertension, diabetes, mitral valve disease, and any condition increasing LV filling pressures)3, 14, 15, 16 some of which are in themselves powerful CV risk factors. However, LA enlargement may also contribute to CV events in a more direct fashion, by increasing the propensity for intra‐atrial blood stasis and therefore thrombus formation or by facilitating the development of atrial fibrillation, an established source for thrombo‐embolic events. These mechanisms may be even more likely to occur in systolic HF, a condition that is known to be associated in itself with blood hypercoagulability20 and risk of atrial fibrillation development.7, 8, 9 As a consequence, LA enlargement in HF might be associated with an increased frequency of events of embolic origin, which would make the use of antithrombotic drugs, and especially systemic anticoagulation, an appealing preventive choice. In a previous meta‐analysis on 1157 patients with HF, LA area was also associated with mortality, but the antithrombotic treatment was not reported.10 Our present observation of a persisting increase in CV risk associated with LA enlargement despite treatment with antithrombotic medications seems to suggest that LA enlargement may in fact be a risk indicator, rather than an actual embolic source, in patients with systolic HF. These results were observed after adjustment for incident atrial fibrillation, which allowed a better assessment of the risk of embolic events directly related to LA enlargement, rather than to the more frequent development of atrial fibrillation secondary to it. Moreover, warfarin treatment did not seem to decrease the risk of events in comparison with aspirin treatment, as would have been conceivable in the case of an embolic mechanism being frequently at play. An alternative explanation of this latter finding might be that an adequate TTR was not uniformly achieved in warfarin‐treated patients, thus conceivably reducing the treatment effect on embolic events. An adequate TTR (>60%) was achieved in only 53.3% of patients, which may have diluted the effect of warfarin treatment on the results. A significant interaction between LAVi and TTR on the risk of death was indeed observed, and a non‐significant trend was observed for CV death. This observation raises the question of whether achieving a better TTR might have amplified the observed treatment differences; also, it opens the field to the possibility that the use of novel oral anticoagulants, such as factor X inhibitors, might achieve a more consistent anticoagulation level and affect the association between LA enlargement and CV events to a greater extent than what was observed for warfarin in WARCEF. Testing novel anticoagulants in this setting, by analogy with what is known about their effect on thrombo‐embolic event in patients with atrial fibrillation, may provide new insights on preventing CV events in patients with systolic HF and LA enlargement.
Surprisingly, no significant association was observed between LA enlargement and events of possibly embolic mechanism, such as MI and, especially, ischaemic stroke. This lack of association may have been driven in part by the low number of such events, which confirms how these adverse outcomes are infrequent in systolic HF patients in sinus rhythm treated with currently recommended medical regimen, including an antithrombotic medication. Also, no difference in efficacy was observed in this regard between warfarin and aspirin. In the main results of WARCEF, warfarin significantly reduced the risk of ischaemic stroke compared with aspirin, although the benefit was offset by an increase in major haemorrhagic events.17 The lack of a similar beneficial effect of warfarin when LA enlargement is factored into the results seems to again suggest that most strokes in patients with moderate or severe LA enlargement may not have originated from an embolic mechanism; however, given the overall small number of strokes, this consideration should be regarded as hypothesis‐generating rather than as a firm conclusion. It should also be noted that 13.3% of patients in the aspirin‐treated group developed atrial fibrillation during follow‐up and were switched to warfarin treatment, which may have reduced the difference in treatment effect between warfarin and aspirin in the intention‐to‐treat analysis.
Our study has some limitations. Approximately half of the original WARCEF cohort had adequate information on LAVi. This smaller sample size may have decreased the ability to detect significant associations between LA enlargement and low‐frequency events such as ischaemic stroke and MI. On the other hand, the central interpretation of the echocardiograms assured the standardization of the assessment of LAVi. As per WARCEF protocol, only patients with systolic HF (LVEF <35%) were included in the study; therefore, the relationship between LA size and outcomes in patient with diastolic HF could not be investigated. Finally, the study represents a post hoc analysis involving multiple comparisons from a trial that was not originally designed to evaluate the relationship between LA size and outcome; therefore, its results should be regarded as exploratory.
In conclusion, LA enlargement, especially when of moderate or severe degree, remains associated with death and HF hospitalization in patients with systolic HF treated with currently recommended medical regimen and antithrombotic medications. However, our data provide initial evidence that treatment with systemic anticoagulation may decrease mortality in patients with moderate or severe LA enlargement, provided an adequate level of anticoagulation is achieved. The possibility that maintaining a consistent therapeutic level of anticoagulation, whether by higher TTR during warfarin treatment or by using newer oral anticoagulants, may prevent a fraction of CV events in patients with LA enlargement remains to be tested.
Conflict of interest
S.H. reports receiving payment from AGA Medical (now St. Jude Medical) for his work as a member of a data and safety monitoring board and consulting fees from Boehringer Ingelheim. B.L. reports receiving consulting fees from United Healthcare. Dr J.R.T. reports receiving research grants or consulting fees from Amgen, Bayer, Cardio3 Biosciences, Cytokinetics, Mast Therapeutics, Medtronic, Novartis, St. Jude, and Trevena. A.J.L. reports receiving grant support from Boehringer Ingelheim and BMS Pfizer, lecture fees from Boehringer Ingelheim, and fees for the development of educational presentations from the American College of Cardiology. R.L.S. reports institutional research support from NIH, AHA/ASA, McKnight Foundation, and Boehringer Ingelheim. S.D.A. reports receiving consulting fees from Vifor Pharma, Bayer, Janssen, Novartis, Relypsa, ZS Pharma, and Thermo Fisher; grant support from Vifor Pharma and Abbott Vascular; and lecture fees from Vifor Pharma, Novartis, and Stealth Peptides. P.P. reports receiving consulting fees from Bayer, Boehringer Ingelheim, Coridea, Corthera, Johnson & Johnson, Pfizer, Respicardia, and Vifor Pharma; grant support from Vifor Pharma on behalf of himself and his institution; and lecture fees from Abbott, Boehringer Ingelheim, Merck Serono, Pfizer, Respicardia, Sanofi‐Aventis, Servier, and Vifor Pharma. G.Y.H.L. reports consultancy for Bayer/Janssen, BMS/Pfizer, Biotronik, Medtronic, Boehringer Ingelheim, Novartis, Verseon, and Daiichi Sankyo; speaker for Bayer, BMS/Pfizer, Medtronic, Boehringer Ingelheim, and Daiichi Sankyo; no fees are received personally. The other authors report no conflicts.
Funding
This work was supported by the National Institute of Neurological Disorders and Stroke (NINDS; grant numbers U01‐NS‐043975 to S.H. and U01‐NS‐039143 to J.L.P.T.).
Supporting information
Table S1. Comparison of patients with and without left atrial volume index (LAVi) information available.
Table S2. Effect of Time in Therapeutic Range (TTR) on the relationship between LAVi and outcomes in warfarin‐treated patients.
Di Tullio, M. R. , Qian, M. , Thompson, J. L. P. , Labovitz, A. J. , Mann, D. L. , Sacco, R. L. , Pullicino, P. M. , Freudenberger, R. S. , Teerlink, J. R. , Graham, S. , Lip, G. Y. H. , Levin, B. , Mohr, J. P. , Buchsbaum, R. , Estol, C. J. , Lok, D. J. , Ponikowski, P. , Anker, S. D. , Homma, S. , and for the WARCEF Investigators (2018) Left atrial volume and cardiovascular outcomes in systolic heart failure: effect of antithrombotic treatment. ESC Heart Failure, 5: 800–808. 10.1002/ehf2.12331.
References
- 1. Gardin JM, McClelland R, Kitzman D, Lima JA, Bommer W, Klopfenstein HS, Wong ND, Smith VE, Gottdiener J. M‐mode echocardiographic predictors of six‐ to seven‐year incidence of coronary heart disease, stroke, congestive heart failure, and mortality in an elderly cohort (the Cardiovascular Health Study). Am J Cardiol 2001; 87: 1051–1057. [DOI] [PubMed] [Google Scholar]
- 2. Benjamin EJ, D'Agostino RB, Belanger AJ, Wolf PA, Levy D. Left atrial size and the risk of stroke and death. The Framingham Heart Study. Circulation 1995; 92: 835–841. [DOI] [PubMed] [Google Scholar]
- 3. Di Tullio MR, Sacco RL, Sciacca RR, Homma S. Left atrial size and the risk of ischaemic stroke in an ethnically mixed population. Stroke 1999; 30: 2019–2024. [DOI] [PubMed] [Google Scholar]
- 4. Barnes ME, Miyasaka Y, Seward JB, Gersh BJ, Rosales AG, Bailey KR, Petty GW, Wiebers DO, Tsang TS. Left atrial volume in the prediction of first ischemic stroke in an elderly cohort without atrial fibrillation. Mayo Clin Proc 2004; 79: 1008–1014. [DOI] [PubMed] [Google Scholar]
- 5. Gottdiener JS, Kitzman DW, Aurigemma GP, Arnold AM, Manolio TA. Left atrial volume, geometry, and function in systolic and diastolic heart failure of persons > or =65 years of age (The Cardiovascular Health Study). Am J Cardiol 2006; 97: 83–89. [DOI] [PubMed] [Google Scholar]
- 6. Takemoto Y, Barnes ME, Seward JB, Lester SJ, Appleton CA, Gersh BJ, Bailey KR, Tsang TS. Usefulness of left atrial volume in predicting first congestive heart failure in patients > or = 65 years of age with well‐preserved left ventricular systolic function. Am J Cardiol 2005; 96: 832–836. [DOI] [PubMed] [Google Scholar]
- 7. Vaziri SM, Larson MG, Benjamin EJ, Levy D. Echocardiographic predictors of nonrheumatic atrial fibrillation. The Framingham Heart Study. Circulation 1994; 89: 724–730. [DOI] [PubMed] [Google Scholar]
- 8. Psaty BM, Manolio TA, Kuller LH, Kronmal RA, Cushman M, Fried LP, White R, Furberg CD, Rautaharju PM. Incidence of and risk factors for atrial fibrillation in older adults. Circulation 1997; 96: 2455–2461. [DOI] [PubMed] [Google Scholar]
- 9. Tsang TS, Gersh BJ, Appleton CP, Tajik AJ, Barnes ME, Bailey KR, Oh JK, Leibson C, Montgomery SC, Seward JB. Left ventricular diastolic dysfunction as a predictor of the first diagnosed nonvalvular atrial fibrillation in 840 elderly men and women. J Am Coll Cardiol 2002; 40: 1636–1644. [DOI] [PubMed] [Google Scholar]
- 10. Rossi A, Temporelli PL, Quintana M, Dini FL, Ghio S, Hillis GS, Klein AL, Marsan NA, Prior DL, Yu CM, Poppe KK, Doughty RN, Whalley GA, MeRGE Heart Failure Collaborators . Independent relationship of left atrial size and mortality in patients with heart failure: an individual patient meta‐analysis of longitudinal data (MeRGE Heart Failure). Eur J Heart Fail 2009; 11: 929–936. [DOI] [PubMed] [Google Scholar]
- 11. Lim TK, Dwivedi G, Hayat S, Majumdar S, Senior R. Independent value of left atrial volume index for the prediction of mortality in patients with suspected heart failure referred from the community. Heart 2009; 95: 1172–1178. [DOI] [PubMed] [Google Scholar]
- 12. Gulati A, Ismail TF, Jabbour A, Ismail NA, Morarji K, Ali A, Raza S, Khwaja J, Brown TD, Liodakis E, Baksi AJ, Shakur R, Guha K, Roughton M, Wage R, Cook SA, Alpendurada F, Assomull RG, Mohiaddin RH, Cowie MR, Pennell DJ, Prasad SK. Clinical utility and prognostic value of left atrial volume assessment by cardiovascular magnetic resonance in non‐ischaemic dilated cardiomyopathy. Eur J Heart Fail 2013; 15: 660–670. [DOI] [PubMed] [Google Scholar]
- 13. Kuperstein R, Goldenberg I, Moss AJ, Solomon S, Bourgoun M, Shah A, McNitt S, Zareba W, Klempfner R. Left atrial volume and the benefit of cardiac resynchronization therapy in the MADIT‐CRT trial. Circ Heart Fail 2014; 7: 154–160. [DOI] [PubMed] [Google Scholar]
- 14. Abhayaratna WP, Seward JB, Appleton CP, Douglas PS, Oh JK, Tajik AJ, Tsang TS. Left atrial size: physiologic determinants and clinical applications. J Am Coll Cardiol 2006; 47: 2357–2363. [DOI] [PubMed] [Google Scholar]
- 15. Tsang TS, Barnes ME, Gersh BJ, Bailey KR, Seward JB. Left atrial volume as a morphophysiologic expression of left ventricular diastolic dysfunction and relation to cardiovascular risk burden. Am J Cardiol 2002; 90: 1284–1289. [DOI] [PubMed] [Google Scholar]
- 16. Pritchett AM, Mahoney DW, Jacobsen SJ, Rodeheffer RJ, Karon BL, Redfield MM. Diastolic dysfunction and left atrial volume: a population‐based study. J Am Coll Cardiol 2005; 45: 87–92. [DOI] [PubMed] [Google Scholar]
- 17. Homma S, Thompson JL, Pullicino PM, Levin B, Freudenberger RS, Teerlink JR, Ammon SE, Graham S, Sacco RL, Mann DL, Mohr JP, Massie BM, Labovitz AJ, Anker SD, Lok DJ, Ponikowski P, Estol CJ, Lip GY, Di Tullio MR, Sanford AR, Mejia V, Gabriel AP, del Valle ML, Buchsbaum R. Warfarin and aspirin in patients with heart failure and sinus rhythm. N Engl J Med 2012; 366: 1859–1869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Di Tullio MR, Qian M, Thompson JL, Labovitz AJ, Mann DL, Sacco RL, Pullicino PM, Freudenberger RS, Teerlink JR, Graham S, Lip GY, Levin B, Mohr JP, Buchsbaum R, Estol CJ, Lok DJ, Ponikowski P, Anker SD, Homma S. Left ventricular ejection fraction and risk of stroke and cardiac events in heart failure: data from the Warfarin Versus Aspirin in Reduced Ejection Fraction Trial. Stroke 2016; 47: 2031–2037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lang RM, Badano LP, Mor‐Avi V, Afilalo J, Armstrong A, Ernande L, Flachskampf FA, Foster E, Goldstein SA, Kuznetsova T, Lancellotti P, Muraru D, Picard MH, Rietzschel ER, Rudski L, Spencer KT, Tsang W, Voigt JU. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging 2015; 16: 233–270. [DOI] [PubMed] [Google Scholar]
- 20. Lip GY, Gibbs CR. Does heart failure confer a hypercoagulable state? Virchow's triad revisited. J Am Coll Cardiol 1999; 33: 1424–1426. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Comparison of patients with and without left atrial volume index (LAVi) information available.
Table S2. Effect of Time in Therapeutic Range (TTR) on the relationship between LAVi and outcomes in warfarin‐treated patients.


