Abstract
Aims
The primary aim of the TRIAGE‐HF trial was to correlate cardiac implantable electronic device‐generated heart failure risk status (HFRS) with signs, symptoms, and patient behaviours classically associated with worsening heart failure (HF).
Methods and results
TRIAGE‐HF enrolled 100 subjects with systolic HF implanted with a Medtronic high‐performance device and followed up at three Canadian HF centres. Study follow‐up was up to 8 months. The HFRS assigned each subject's overall risk of HF hospitalization in the next 30 days and also highlighted abnormal device parameters contributing to a patient's risk status at the time of remote data transmission. Subjects with a high HFRS were contacted by telephone to assess symptoms, and compliance with prescribed therapies, nutrition, and exercise. Clinician‐assessed risk and HFRS‐calculated risk were correlated at both study baseline and exit. Twenty‐four high HFRS occurrences were observed among 100 subjects. Device parameters associated with increased risk of HF hospitalization included OptiVol index (n = 20), followed by low patient activity (n = 18) and elevated night heart rate (n = 12). High HFRS was associated with symptoms of worsening HF in 63% of cases (n = 15) increasing to 83% of cases (n = 20) when non‐compliance with pharmacological therapies and lifestyle was considered.
Conclusions
TRIAGE‐HF is the first study to provide prospective data on the distribution of abnormal device parameters contributing to high HFRS. High HFRS has good predictive accuracy for patient‐reported signs, symptoms, and behaviours associated with worsening HF status. As such, HFRS may be a useful tool for ambulatory HF monitoring to improve both patient‐centred and health system level outcomes.
Keywords: Implantable device diagnostics, Heart failure, Remote monitoring, Risk scores
Introduction
Heart failure (HF) is associated with significant morbidity and mortality.1 This complex clinical syndrome is punctuated by periods of decompensation,2 which in turn drives health care utilization (HCU)3 and negatively impacts quality of life (QoL).4 As such, in recent years, there has been growing interest in identifying novel strategies for early detection of disease progression to mitigate an individual patient's risk of hospitalization. Remote monitoring of HF patients utilizing existing implantable cardiac devices is one approach that has been evaluated in this context. However, the results from clinical trials and meta‐analyses have been variable.5, 6, 7, 8, 9, 10
The seemingly disparate findings across a number of studies are likely influenced by key factors including the number and types of sensors used, the methodology of assessing risk as a point estimate in time rather than as a dynamic variable, the complex topography of HF decompensation, and the disconnect between acquisition of diagnostic data and implementation of appropriate therapeutic actions.11 It is also important to highlight that evaluation of available HF remote monitoring technologies has almost exclusively focused on population health and health system outcomes with very little emphasis on valuing the patient experience of disease. As such, a more fulsome understanding of the correlation between clinical status and behaviours of HF self‐efficacy with device diagnostic data is needed.8
Heart failure risk status (HFRS) is a validated dynamic HF risk prediction tool available on Medtronic cardiac resynchronization therapy device with defibrillation capability (CRT‐D) and implantable cardioverter defibrillator (ICD) devices, which integrates diagnostic data to generate a patient‐specific assessment of low, medium, or high risk for HF hospitalization (HFH) in the next 30 days.8, 12 The primary objective of the TRIAGE‐HF trial was to correlate high HFRS with signs, symptoms, and patient behaviours associated with worsening HF.
Methods
One hundred subjects implanted with either a CRT‐D or ICD device were enrolled at three centres in Canada. The study was reviewed and approved by the ethics committee at each of the participating centres (NCT 01798797). All study subjects were required to have a device capable of wireless telemetry to allow for communication with CareLink (Medtronic Plc., MN, USA) in the absence of patient involvement. Subjects were followed up for a total study duration of 8 months. Subjects who underwent a system modification at any time during the study period were exited from the study.
The HFRS feature integrates all device diagnostic data to generate a low, medium, or high HFRS during each data transmission episode. Specific details and validation of the HFRS algorithm have been published previously.8, 12, 13 In brief, device diagnostic parameters including impedance/OptiVol (Medtronic Plc., MN, USA), patient activity, night heart rate (NHR), heart rate variability (HRV), percent CRT pacing, atrial tachycardia/atrial fibrillation (AT/AF) burden, ventricular rate during AT/AF (VRAF), and detected arrhythmia episodes/therapy delivered are integrated using a Bayesian belief network (BBN) approach to compute a numeric score ranging from 0 to 1. Each parameter is categorized into normal and abnormal ranges by drawing one or more cut‐offs before they are input into the BBN model.
Briefly, the measurement scheme and cut‐offs for various parameters were as follows. The parameters are segmented such that a lower level (i.e. Level 1) signifies normal values and higher levels signify increasingly abnormal values. Impedance (Z) is measured intrathoracically across the right ventricular (RV) coil and device by injecting a small current pulse (I) and measuring the developed voltage (V; Z = I/V). The OptiVol index is computed as accumulation of the difference between the daily and reference thoracic impedance and is segmented into four levels (Level 1: <30 Ω·days; Level 2: 30 to <60 Ω·days; Level 3: 60–100 Ω·days; Level 4: >100 Ω·days). All other parameters described latter are segmented into two levels. Activity is measured by a single‐axis accelerometer in the device and is reported as active minutes per day (Level 1: >60 min/day; Level 2: ≤60 min/day or decreasing trend). Heart‐rate‐related parameters such as NHR, HRV, AF burden, and VRAF are derived from atrial and ventricular electrograms acquired by the device at a resolution of 10 ms. NHR is the average heart rate between midnight and 4 a.m. and is a measure of resting heart rate (Level 1: 55–85 b.p.m.; Level 2: ≥85 b.p.m. or ≤55 b.p.m. or increasing trend). HRV is measured as the standard deviation of sinus rhythm intervals during a 24 h period (Level 1: >60 ms; Level 2: ≤ 60 ms or decreasing trend). AF burden is measured as total duration of fast atrial rate during a 24 h period associated with atrio‐ventricular conduction ratio ≥ 2:1 (Level 1: <60 min/day; Level 2: ≥60 min/day). VRAF is the average ventricular rate during AF over 24 h duration and considered abnormal (Level 2) when ventricular rate ≥ 90 b.p.m. and AF burden ≥6 h/day.
A risk score < 0.054 is categorized as low HFRS, 0.054–0.20 as medium HFRS, and ≥0.20 as high HFRS. A low HFRS is associated with an HFH rate of 0.6% in the next 30 days, a medium HFRS is associated with an HFH rate of 1.3%, and a high HFRS is associated with an HFH rate of 6.8%. Thus, a high HFRS is associated with a 10‐fold increase in an individual patient's risk of HFH in the next 30 days, whereas a medium HFRS score, compared with a low HFRS score, confers a doubling of that individual's risk of HFH in the next 30 days.
Figure 1 shows a representative HFRS‐generated HF management report. The first component of the report (Figure 1 A) shows the monthly risk status, which prognosticates an individual patient's risk of an HF event in the next 30 days. The second element of the report (Figure 1 B) shows detailed trends for individual components of the HFRS score. The topmost trend shows the daily risk status, which, in turn, is used to derive the monthly risk status shown in Figure 1 A based on data from the 30 days prior to transmission. HFRS dynamically varies over time as device parameters change. Thus, a given patient can have low, medium, or high HFRS at different time points depending on the device parameters.
Figure 1.
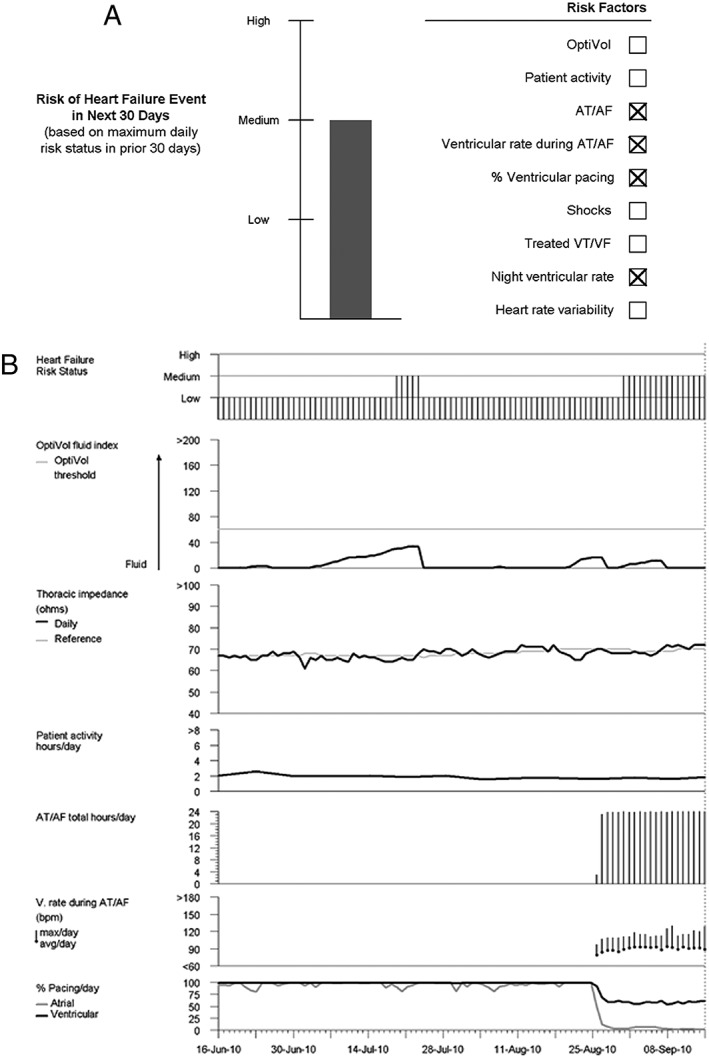
Example of a heart failure management report showing heart failure risk status‐reported risk status. The first component of the report shows future 30 day risk for a patient at medium risk (A) including device parameters contributing to that risk. The second component of the report (B) shows trends in various device parameters. Daily risk status, which is dynamic and used to derive future 30 day risk status, is shown at the top of (B). AT/AF, atrial tachycardia/atrial fibrillation.
After informed consent was obtained, enrolling physicians were required to assign each subject with a below‐average, average, or above‐average probability of experiencing an HF event in the next 90 days on the basis of their clinical judgement at the time of study enrolment and again at study completion. Clinical judgement included factors such as patient history, symptom status, physical exam findings, and available laboratory investigations at the time of evaluation. Other measures of HF severity performed at baseline and study completion included QoL assessment using the Minnesota Living with Heart Failure (MLWHF) instrument, natriuretic peptide levels, 6 min hall walk (6MHW), and determination of New York Heart Association (NYHA) functional class.
To enable a real‐world assessment of the HFRS feature's performance and to ensure seamless integration into clinic workflow, the TRIAGE‐HF protocol did not recommend a minimum transmission frequency. However, whenever a CareLink transmission with high‐risk status was received, the study protocol mandated a phone call to the subject within the following 24 business hours. Transmissions with low‐risk status required no patient contact. For medium‐risk status transmissions, the decision to contact, or not to contact, the subject was left to the discretion of the health care provider team. All subjects were contacted every 2 months to assess for any scheduled or unscheduled HCU, which was defined as any hospitalization, urgent care or emergency room visit, visit to health care provider for HF treatment, and unscheduled office visit or phone call initiated by the subject complaining of signs/symptoms of worsening HF.
The primary objective of the TRIAGE‐HF study was to correlate high HFRS with signs, symptoms, and behaviours associated with worsening HF. During each phone call, subjects were evaluated for signs and symptoms of worsening HF including weight gain, dyspnoea, peripheral oedema, abdominal bloating, nocturnal cough, fatigue, palpitations, and dizziness/light‐headedness. Similarly, subjects were evaluated for non‐compliance related to prescribed medications, nutrition (e.g. adherence to low salt consumption), and exercise.
Additional objectives of the TRIAGE‐HF study were (i) to evaluate signs, symptoms, and behaviours associated with worsening HF in those for whom high HFRS was driven primarily by OptiVol and in those with a medium‐risk status transmission who were contacted by telephone; (ii) to determine the correlation between physician‐assessed 90 day risk of HFH and HFRS‐calculated risk burden at the time of study enrolment—HFRS risk burden was calculated as sum of HFRS numeric scores for 30 days prior to enrolment; and (iii) to measure change in MLWHF score over duration of the study as an indicator of the potential efficacy of HFRS‐guided management on QoL.
Although the TRIAGE‐HF study protocol did not require that any therapeutic actions be taken in those with medium or high HFRS at the time of telephone contact, any actions that did occur were recorded and tabulated as a tertiary study outcome.
All continuous variables were analysed as mean ± SD and all categorical variables as percentages. The HCU rates were analysed as events per month with the 95% confidence interval estimated using the normal approximation to the Poisson distribution. A Kruskal–Wallis test was used to compare HFRS risk burden. A paired t‐test was used to evaluate the change in MLWHF score. Statistical analyses were performed using SAS, version 9.4 (SAS Institute, Cary, North Carolina).
Results
Patient demographics
Table 1 summarizes clinical and demographic data for all 100 subjects included in the TRIAGE‐HF study. Sixty‐nine (69%) subjects had a CRT‐D device, and the remaining 31% of subjects had either a single‐chamber (VR) or dual‐chamber (DR) ICD device. Consistent with guidelines for device implantation, a majority (82%) of patients had NYHA Class II–III functional status. The mean ejection fraction was 31%. Subjects were well managed with HF guideline‐recommended pharmacological therapies.
Table 1.
Patient demographics for 100 patients enrolled in the study
| Gender (n, %) | |
| Male | 78 (78%) |
| Age (mean ± SD) | 66.9 ± 11.0 years |
| Type of device implanted (n, %) | |
| CRT‐D | 69 (69%) |
| ICD DR | 20 (20%) |
| ICD VR | 11 (11%) |
| LVEF (mean ± SD)a | 31.1 ± 12.3% |
| NYHA (n, %) | |
| Class I | 16 (16%) |
| Class II | 50 (50%) |
| Class III | 32 (32%) |
| Class IV | 0 (0%) |
| Not available | 2 (2%) |
| History of atrial fibrillation | 44 (44%) |
| BNP (n = 74), pg/mL | 367.8 ± 459.9 |
| NT‐pro‐BNP (n = 24), pg/mL | 2018.75 ± 1839.82 |
| History of ventricular arrhythmia | 30 (30%) |
| Type II diabetes | 41 (41%) |
| COPD | 17 (17%) |
| Sleep apnoea | 16 (16%) |
| Hypertension | 64 (64%) |
| Drug use | |
| Beta‐blockers | 95 (95%) |
| ACE inhibitors | 56 (56%) |
| Angiotensin II receptor blocker | 28 (28%) |
| Mineralocorticoid antagonist | 49 (49%) |
| Diuretic | 81 (81%) |
| Nitrate | 17 (17%) |
ACE, angiotensin‐converting enzyme; COPD, chronic obstructive pulmonary disease; CRT‐D, cardiac resynchronization therapy device with defibrillation capability; ICD DR, Dual chamber implantable cardioverter defibrillator; ICD VR, single chamber implantable cardioverter defibrillator; LVEF, left ventricular ejection fraction; NT‐pro‐BNP, N‐terminal pro‐BNP; NYHA, New York Heart Association.
Data available in 97 patients.
Eighty‐seven (87%) subjects completed the entire 8 month study period. Five (5%) subjects exited because of system modification, three (3%) died, and the remaining five (5%) subjects withdrew for various other reasons or were lost to follow‐up.
Correlation of HF risk status to signs, symptoms, and behaviours associated with worsening heart failure
A total of 648 CareLink transmissions were received from 100 patients over the study duration (mean ± SD of 6.5 ± 4.9 per patient). There were a total of 24 high‐risk transmissions in 16 unique subjects, which resulted in subject phone contact during the study duration (because HFRS is dynamically varying, a given subject can have more than one high HFRS). Table 2 summarizes the association between HFRS and signs/symptoms of worsening HF as well as non‐compliance for all high‐risk transmissions. In 83% of subject contacts (n = 20 in 13 unique subjects), signs, symptoms, or behaviours associated with worsening HF were uncovered. If non‐compliance were to be excluded, worsening signs/symptoms of HF were reported in 15 (63%) of the telephone interviews. In a large subset of high HFRS transmissions (20/24), OptiVol exceeded the threshold of 60 Ω·days and hence was a contributing factor. The percentage of phone interviews found to have worsening signs/symptoms or non‐compliance in this subset was 85% (Table 2).
Table 2.
Association between risk and worsening heart failure signs/symptoms and non‐compliance
| Risk status | n (number resulting in patient contact) | Worsening HF signs/symptoms or non‐compliance | Worsening HF signs/symptoms only |
|---|---|---|---|
| High | 24 | 20 (83%) | 15 (63%) |
| High (with OptiVol as contributing factor) | 20 | 17 (85%) | 13 (65%) |
| Medium (subset resulting in patient contact) | 31 | 29 (94%) | 25 (81%) |
HF, heart failure.
Thirty‐one medium‐risk transmissions resulted in subject contact at the health care providers' discretion. This was a small subset (8.4%) of the total (n = 368) medium‐risk transmissions that occurred over the study's duration. Among these 31 medium‐risk transmissions, 29 phone contacts uncovered at least one sign, symptom, or behaviour associated with worsening HF. With the exclusion of non‐compliance, 25 of the phone interviews revealed at least one sign/symptom of worsening HF.
Types of symptoms and non‐compliance behaviours revealed during patient contact
Table 3 summarizes the specific signs and symptom of worsening HF and types of non‐compliance described by subjects who experienced a medium‐risk or high‐risk transmission. For those transmissions in the high HFRS category, regardless of whether OptiVol was present as a contributing factor, dyspnoea was the most commonly reported symptom occurring in ~50% of the cases. Other prevalent symptoms included worsening fatigue and peripheral oedema. Among the medium‐risk transmissions, worsening fatigue was the most common reported symptom followed by dyspnoea, dizziness/light‐headedness, and abdominal oedema.
Table 3.
Worsening heart failure signs/symptoms and non‐compliance findings for various risk statuses
| Worsening HF sign/symptom, non‐compliance | Risk status | ||
|---|---|---|---|
| High (n = 24) | High with OptiVol as contributing factor (n = 20) | Medium (subset resulting in patient contact; n = 31) | |
| Dyspnoea | 13 (54%) | 11 (55%) | 16 (52%) |
| Peripheral oedema | 6 (25%) | 5 (25%) | 5 (16%) |
| Abdominal oedema | 3 (13%) | 2 (10%) | 9 (29%) |
| Nocturnal coughing | 4 (17%) | 4 (20%) | 4 (13%) |
| Fatigue | 10 (42%) | 8 (40%) | 19 (61%) |
| Heart palpitations | 4 (17%) | 4 (20%) | 4 (13%) |
| Dizziness/light‐headedness | 5 (21%) | 3 (15%) | 9 (29%) |
| Medication non‐compliance | 0 (0%) | 0 (0%) | 1 (3%) |
| Dietary non‐compliance | 3 (13%) | 3 (15%) | 10 (32%) |
| Exercise non‐compliance | 13 (54%) | 10 (50%) | 20 (65%) |
HF, heart failure.
In general, participants were compliant with medications, and only one subject with a medium‐risk transmission was non‐compliant with prescribed pharmacological therapies. By contrast, non‐compliance with exercise was quite common ranging from ~50% for high HFRS transmissions to 65% for medium‐risk transmissions. Dietary non‐compliance ranged from 13% to 15% for the high HFRS transmissions and was 32% among the medium HFRS transmissions.
Device‐specific diagnostic parameters contributing to heart failure risk status
Table 4 summarizes device parameters that drove risk assignment among the medium and high HFRS transmissions. OptiVol was the most common contributing factor in medium‐risk and high‐risk categories followed by declining activity, elevated NHR, and abnormal HRV as common contributing factors in the high‐risk category. In the medium‐risk category, elevated NHR, declining activity, and presence of AF were the most common contributing factors after OptiVol. Regardless of which device diagnostic parameter was contributing to the assignment of risk status, the overall frequency of each parameter, with the exception of shocks, was less in medium‐risk transmissions compared with those with high‐risk transmissions (e.g. although HRV was present in ~30% of high‐risk status cases, it was present in only 6% of medium‐risk status cases).
Table 4.
Device diagnostics‐based contributing factors for the various risk statuses
| Device diagnostics based contributing factor | Risk status | ||
|---|---|---|---|
| High (n = 24) | High with OptiVol as contributing factor (n = 20) | Medium (subset resulting in patient contact; n = 31) | |
| OptiVol | 20 (83%) | 20 (100%) | 18 (58%) |
| NHR | 12 (50%) | 8 (40%) | 8 (26%) |
| Activity | 18 (75%) | 14 (70%) | 6 (19%) |
| HRV | 8 (33%) | 6 (30%) | 2 (6%) |
| Shock | 0 (0%) | 0 (0%) | 2 (6%) |
| %VP | 6 (25%) | 4 (20%) | 2 (6%) |
| AF | 5 (21%) | 5 (25%) | 4 (13%) |
AF, atrial fibrillation; HRV, heart rate variability; NHR, night heart rate; %VP, percent ventricular pacing.
Correlation between physician‐assessed risk and heart failure risk status‐derived risk burden
At both study entry and exit, HFRS‐estimated risk burden correlated well with health care providers' clinical assessment of the individual subject's risk of HFH in the next 90 days (Figure 2 , P‐value < 0.05 for baseline and exit visit).
Figure 2.
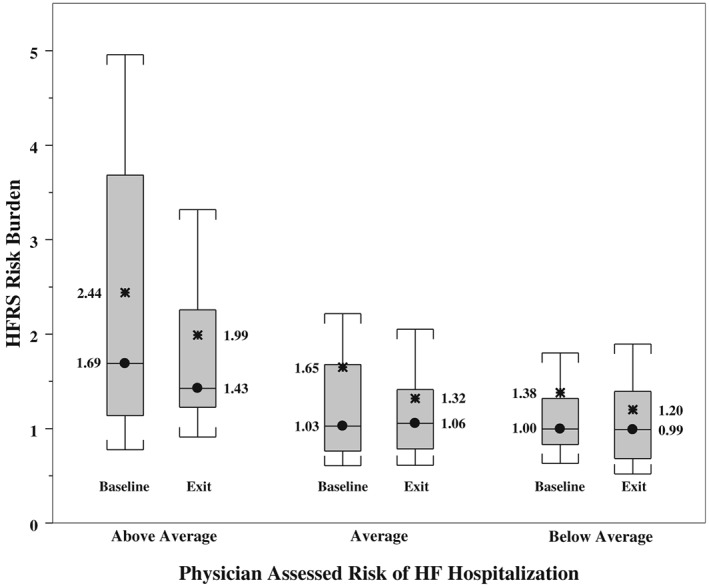
Box plot highlighting the relationship between heart failure risk status (HFRS)‐derived risk burden and physician‐assessed risk of heart failure (HF) hospitalization at patient enrolment and exit. The circle represents the median. The asterisks represent the average values.
Actions taken in response to heart failure risk status‐prompted patient contact
For the high‐risk transmissions, actions emerged in 13 (54%) of cases whereas actions were taken in 24 (6.5% of all and 77% of contacted) medium‐risk cases. Medication changes occurred in four (17%) of the high‐risk transmissions and 12 (3.3% of all and 39% of contacted) medium‐risk transmissions. Request for an additional subject‐initiated manual transmission occurred in 11 (46%) highs and 13 (3.5% of all and 42% contacted) mediums. No patients with high‐risk transmissions and two patients with medium‐risk transmissions were directed to the emergency department/hospital. Three patients were instructed to visit their primary care physician for non‐HF‐related reasons.
Health care utilization
A total of 96 HCU events in 45 subjects occurred during the study period. Among these, 26 HCU events were adjudicated by the investigator to be HF related. A total of 28 all‐cause hospitalizations occurred during the study duration, and 13 of these were adjudicated to be HF‐related hospitalizations.
Quality of life and other measures of heart failure severity
Eighty‐eight subjects completed the MLWHF questionnaire at both baseline and study exit. For these individuals, the baseline score was 32.8 ± 21.0 and improved to 30.0 ± 21.6, a mean improvement of −2.8 ± 20.1. However, this improvement did not reach statistical significance (P = 0.19). Sixty subjects completed 6MHW at both baseline and study exit. For these subjects, the baseline 6MHW was 323 ± 115 and declined to 295 ± 116 at study exit (P = 0.01). BNP/N‐terminal pro‐BNP and NYHA at exit were not different from the baseline values shown in Table 1.
Discussion
TRIAGE‐HF was designed to be a real‐life pragmatic evaluation of a novel HF tool with the a priori intention to compare HFRS with patient‐centric parameters of HF decompensation. Signs/symptoms of HF and non‐compliance with prescribed therapies were identified in 83–85% of patients with high HFRS. This suggests that high HFRS strongly correlates with signs, symptoms, and patient behaviours associated with worsening HF.
Among patients with medium HFRS, this number was 8%, although it rose to 94% when only patients who were contacted were considered.
All‐cause and HF‐related hospitalization rates in TRIAGE‐HF were 0.42% and 0.20% per patient‐year, respectively. These are consistent with event rates observed in previous device studies and the expected event rate based on our trial design.5, 14 HFRS alerts were not linked to protocol‐mandated actions, as the trial was designed to explore the association between this novel integrated diagnostic tool with symptoms, signs, and behaviours which characterize HF decompensation. Any protocol‐mandated therapeutic maneuver would have impacted our ability to evaluate this association. Although optimizing treatment was not the goal of TRIAGE‐HF and despite excellent baseline medical therapy in the study cohort, high and contacted medium HFRS triggered actions in 54% and 77% of patients, respectively. Moreover, clinicians were able to target their intervention on the basis of the HFRS component that was primarily driving risk assessment (e.g. intrathoracic impedance, heart rate, and arrhythmia), and patients at medium risk appeared to receive even more intensive intervention to minimize risk of progressing to high‐risk status.
Existing HF diagnostic algorithms have not been designed or utilized to corroborate disease decompensation at the individual patient level but rather have leveraged our understanding of HF pathophysiology to identify opportunities for early intervention. TRIAGE‐HF, therefore, is the first trial to look at the correlation between device diagnostics and the patient experience of disease, which is a fundamental pillar of quality health care. Moreover, longitudinal measurement, with dynamic baseline adjustment using individual patients as their own control, more accurately captures the patient journey. Quality of life showed a trend towards improvement in TRIAGE‐HF, a finding consistent across other trials of remote monitoring.15, 16, 17
Cowie and colleagues,8 in their validation study of HFRS, hypothesized that this integrated diagnostic tool could be used to guide HF practitioners on when to collect additional clinical information that in turn could guide care. TRIAGE‐HF has now demonstrated a strong correlation between HFRS and clinical status in a prospective setting, confirming the value of this approach. Moreover, HFRS is consistent with the health care provider's assessment of an individual patient's risk of HFH further capitalizing on the utility of this tool. As such, HFRS can enhance clinical capacity and streamline workflow through timely triage and appropriate risk stratification of those patients who would benefit from early and more intensive HF care.
Limitations
TRIAGE‐HF only enrolled subjects with a Medtronic CRT‐D/ICD device, and therefore extrapolation of the study findings to a broader HF population cannot be made; specifically, the utility of HFRS in less well medically treated subjects, in those with preserved or mid‐range ejection fraction, and in those with devices for bradycardia support remains unclear.
As OptiVol is derived from impedance measured across a pathway between RV coil to can (i.e. device‐can implanted in the left pectoral region), any fluid accumulation that does not fall in this path will not be detected by OptiVol (e.g. abdominal fluid accumulation as can occur during right HF18). Thus, HFRS may not be sensitive to all types of HF worsening equally. However, it has been proposed that mechanism for some HF decompensations may be fluid re‐distribution from abdominal and splanchnic spaces into vascular space giving rise to increase pre‐load.19 OptiVol, and thus HFRS, should be equally sensitive to such fluid re‐distribution‐initiated episodes of worsening HF.
Per protocol, assessment of symptom status and compliance with prescribed therapies/behaviours was only mandated in those subjects with high HFRS, whereas the decision to contact subjects with medium HFRS was left to the investigator and subjects at low HFRS were not contacted at all. As a result, we do not know the symptom status and level of compliance with self‐efficacy behaviours in the latter groups. Moreover, as the study was intended to be pragmatic with no minimum transmission frequency, it is possible that HFRS and subject clinical status may have fluctuated significantly between transmissions, and this was not captured as part of the study.
TRIAGE‐HF was an observational study with no requirement for a prescribed action in response to an individual subject's reported HFRS. As such, HFRS must not be considered an HF intervention but rather a disease management tool that correlates with an individual's symptoms and behaviours, thus allowing the clinician to individualize their therapeutic approach.
Conclusions
TRIAGE‐HF is the first‐ever study to provide prospective data on the distribution of abnormal device parameters contributing to high HFRS for this novel HF management tool. In the TRIAGE‐HF study, a high HFRS had good predictive accuracy for signs, symptoms, and behaviours associated with HF decompensation. HFRS risk burden correlated well with clinician assessment of a subject's overall risk of HFH. This suggests that HFRS may be a reliable, efficient, and patient‐centred alternative to in‐person clinical assessment providing convenience to both patients and care providers.
Conflict of interest
S.A.V. received research grants and honoraria from Medtronic. V.S. is an employee of Medtronic Plc. M.M. is an employee of Medtronic Plc. J.K. is an employee of Medtronic Plc. B.T. has no disclosures. S.Z. received research grants and honoraria from Medtronic.
Funding
Medtronic of Canada.
Virani, S. A. , Sharma, V. , McCann, M. , Koehler, J. , Tsang, B. , and Zieroth, S. (2018) Prospective evaluation of integrated device diagnostics for heart failure management: results of the TRIAGE‐HF study. ESC Heart Failure, 5: 809–817. 10.1002/ehf2.12309.
References
- 1. Lloyd‐Jones D, Adams R, Carnethon M, De Simone G, Ferguson TB, Flegal K, Ford E, Furie K, Go A, Greenlund K, Haase N, Hailpern S, Ho M, Howard V, Kissela B, Kittner S, Lackland D, Lisabeth L, Marelli A, McDermott M, Meigs J, Mozaffarian D, Nichol G, O'Donnell C, Roger V, Rosamond W, Sacco R, Sorlie P, Stafford R, Steinberger J, Thom T, Wasserthiel‐Smoller S, Wong N, Wylie‐Rosett J, Hong Y. Heart disease and stroke statistics—2009 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation 2009; 119: e21–e181. [DOI] [PubMed] [Google Scholar]
- 2. Gheorghiade M, De Luca L, Fonarow GC, Filippatos G, Metra M, Francis GS. Pathophysiologic targets in the early phase of acute heart failure syndromes. Am J Cardiol 2005; 96: 11G–17G. [DOI] [PubMed] [Google Scholar]
- 3. Klersy C, Boriani G, De Silvestri A, Mairesse GH, Braunschweig F, Scotti V, Balduini A, Cowie MR, Leyva F. Effect of telemonitoring of cardiac implantable electronic devices on healthcare utilization: a meta‐analysis of randomized controlled trials in patients with heart failure. Eur J Heart Fail 2016; 18: 195–204. [DOI] [PubMed] [Google Scholar]
- 4. Nieminen MS, Dickstein K, Fonseca C, Serrano JM, Parissis J, Fedele F, Wikstrom G, Agostoni P, Atar S, Baholli L, Brito D, Colet JC, Edes I, Gomez Mesa JE, Gorjup V, Garza EH, Gonzalez Juanatey JR, Karanovic N, Karavidas A, Katsytadze I, Kivikko M, Matskeplishvili S, Merkely B, Morandi F, Novoa A, Oliva F, Ostadal P, Pereira‐Barretto A, Pollesello P, Rudiger A, Schwinger RH, Wieser M, Yavelov I, Zymlinski R. The patient perspective: quality of life in advanced heart failure with frequent hospitalisations. Int J Cardiol 2015; 191: 256–264. [DOI] [PubMed] [Google Scholar]
- 5. van Veldhuisen DJ, Braunschweig F, Conraads V, Ford I, Cowie MR, Jondeau G, Kautzner J, Aguilera RM, Lunati M, Yu CM, Gerritse B, Borggrefe M. Intrathoracic impedance monitoring, audible patient alerts, and outcome in patients with heart failure. Circulation 2011; 124: 1719–1726. [DOI] [PubMed] [Google Scholar]
- 6. Bohm M, Drexler H, Oswald H, Rybak K, Bosch R, Butter C, Klein G, Gerritse B, Monteiro J, Israel C, Bimmel D, Kaab S, Huegl B, Brachmann J. Fluid status telemedicine alerts for heart failure: a randomized controlled trial. Eur Heart J 2016; 37: 3154–3163. [DOI] [PubMed] [Google Scholar]
- 7. Boriani G, Da Costa A, Quesada A, Ricci RP, Favale S, Boscolo G, Clementy N, Amori V, Mangoni di SSL, Burri H. Effects of remote monitoring on clinical outcomes and use of healthcare resources in heart failure patients with biventricular defibrillators: results of the MORE‐CARE multicentre randomized controlled trial. Eur J Heart Fail 2017; 19: 416–425. [DOI] [PubMed] [Google Scholar]
- 8. Cowie MR, Sarkar S, Koehler J, Whellan DJ, Crossley GH, Tang WH, Abraham WT, Sharma V, Santini M. Development and validation of an integrated diagnostic algorithm derived from parameters monitored in implantable devices for identifying patients at risk for heart failure hospitalization in an ambulatory setting. Eur Heart J 2013; 34: 2472–2480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sharma V, Rathman LD, Small RS, Whellan DJ, Koehler J, Warman E, Abraham WT. Stratifying patients at the risk of heart failure hospitalization using existing device diagnostic thresholds. Heart Lung J Crit Care 2015; 44: 129–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Dierckx R, Houben R, Goethals M, Verstreken S, Bartunek J, Saeys R, De Proft M, Boel E, Vanderheyden M. Integration of remote monitoring of device diagnostic parameters into a multidisciplinary heart failure management program. Int J Cardiol 2014; 172: 606–607. [DOI] [PubMed] [Google Scholar]
- 11. Hawkins NM, Virani SA, Sperrin M, Buchan IE, McMurray JJ, Krahn AD. Predicting heart failure decompensation using cardiac implantable electronic devices: a review of practices and challenges. Eur J Heart Fail 2016; 18: 977–986. [DOI] [PubMed] [Google Scholar]
- 12. Burri H, da Costa A, Quesada A, Ricci RP, Favale S, Clementy N, Boscolo G, Villalobos FS, Mangoni di SSL, Sharma V, Boriani G. Risk stratification of cardiovascular and heart failure hospitalizations using integrated device diagnostics in patients with a cardiac resynchronization therapy defibrillator. Europace 2017; 20: e69–e77. [DOI] [PubMed] [Google Scholar]
- 13. Gula LJ, Wells GA, Yee R, Koehler J, Sarkar S, Sharma V, Skanes AC, Sapp JL, Redfearn DP, Manlucu J, Tang AS. A novel algorithm to assess risk of heart failure exacerbation using ICD diagnostics: validation from RAFT. Heart Rhythm 2014; 11: 1626–1631. [DOI] [PubMed] [Google Scholar]
- 14. Conraads VM, Tavazzi L, Santini M, Oliva F, Gerritse B, Yu CM, Cowie MR. Sensitivity and positive predictive value of implantable intrathoracic impedance monitoring as a predictor of heart failure hospitalizations: the SENSE‐HF trial. Eur Heart J 2011; 32: 2266–2273. [DOI] [PubMed] [Google Scholar]
- 15. Landolina M, Perego GB, Lunati M, Curnis A, Guenzati G, Vicentini A, Parati G, Borghi G, Zanaboni P, Valsecchi S, Marzegalli M. Remote monitoring reduces healthcare use and improves quality of care in heart failure patients with implantable defibrillators: the evolution of management strategies of heart failure patients with implantable defibrillators (EVOLVO) study. Circulation 2012; 125: 2985–2992. [DOI] [PubMed] [Google Scholar]
- 16. Lim PC, Lee AS, Chua KC, Lim ET, Chong DT, Tan BY, Ho KL, Teo WS, Ching CK. Remote monitoring of patients with cardiac implantable electronic devices: a Southeast Asian, single‐centre pilot study. Singapore Med J 2016; 57: 372–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hindricks G, Elsner C, Piorkowski C, Taborsky M, Geller JC, Schumacher B, Bytesnik J, Kottkamp H. Quarterly vs. yearly clinical follow‐up of remotely monitored recipients of prophylactic implantable cardioverter‐defibrillators: results of the REFORM trial. Eur Heart J 2014; 35: 98–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sundaram V, Fang JC. Gastrointestinal and liver issues in heart failure. Circulation 2016; 133: 1696–1703. [DOI] [PubMed] [Google Scholar]
- 19. Cotter G, Metra M, Milo‐Cotter O, Dittrich HC, Gheorghiade M. Fluid overload in acute heart failure—re‐distribution and other mechanisms beyond fluid accumulation. Eur J Heart Fail 2008; 10: 165–169. [DOI] [PubMed] [Google Scholar]


