Abstract
Aims
Functional mitral regurgitation is complicating end‐stage heart failure and potential heart transplantation by increasing pulmonary artery pressures. The aim of the present study was to investigate feasibility and haemodynamic effects of percutaneous mitral valve edge‐to‐edge repair using the MitraClip™ device in patients with end‐stage heart failure awaiting heart transplantation.
Methods and results
In this retrospective study, we identified nine patients suffering from end‐stage heart failure listed for heart transplantation in whom moderate–severe or severe functional mitral regurgitation was recognized and treated with percutaneous mitral valve edge‐to‐edge repair. Twenty‐two patients listed for heart transplantation and presenting with moderate–severe or severe functional mitral regurgitation treated in the pre‐MitraClip™ era served as controls. Patients were analysed at two separate time points: MitraClip™ group: pre‐procedure and post‐procedure (follow‐up: 215 ± 53 days) and control group: study entry with recognition of moderate–severe or severe functional mitral regurgitation (follow‐up: 197 ± 47 days). Percutaneous mitral valve edge‐to‐edge repair with the MitraClip™ was feasible and safe in our high‐risk end‐stage heart failure population. The intervention resulted in significant reduction of mitral regurgitation (grade 3.0 [0.5] to 1.5 [0.5]; P = 0.009), left atrial diameter (51 mm [16] to 49 mm [4]; follow‐up MitraClip™ vs. control group P = 0.0497), pulmonary artery pressures (sPA 50 mmHg [15] to 45 mmHg [10]; P = 0.02; mPA 34 mmHg [8] to 30 mmHg [10]; P = 0.02), and New York Heart Association class (3.5 [1.0] to 3.0 [0.5]; P = 0.01) and improved mixed‐venous oxygen saturation (57% [11] to 55% [7]; follow‐up MitraClip™ vs. control group P = 0.02). No changes in the control group were observed.
Conclusions
MitraClip™ implantation as ‘bridge‐to‐transplant’ strategy in patients with end‐stage heart failure and severe functional mitral regurgitation awaiting heart transplantation is feasible and appears to result in favourable haemodynamic effects.
Keywords: End‐stage heart failure, Heart transplantation, Bridge to transplant, Mitral regurgitation, Endovascular mitral valve repair, MitraClip™
Introduction
Percutaneous repair of mitral valves in patients with moderate–severe or severe mitral regurgitation and high surgical risk has evolved as a suitable alternative to surgical intervention.1, 2, 3, 4, 5, 6, 7, 8 Currently, the most widely used device for percutaneous mitral valve edge‐to‐edge repair (PMVR) is the MitraClip™ system (Abbott Vascular Devices, Santa Clara, CA, USA).6, 8, 9, 10, 11, 12
In end‐stage heart failure patients awaiting heart transplantation, moderate–severe or severe functional mitral regurgitation contributes to morbidity and mortality by increasing pulmonary artery pressure and resistance, which are critical predictors of right ventricular failure post‐transplantation and associated with immediate graft failure.13, 14, 15, 16, 17, 18 In this regard, a fixed pulmonary artery resistance greater than 5 Wood units or a transpulmonary gradient exceeding 15 mmHg is associated with increased mortality and thus generally considered a contraindication to isolated heart transplantation.19 However, the use of left ventricular assist devices in end‐stage heart failure unloads the left ventricle and can reduce elevated pulmonary artery pressures in many patients, as such these changes can be reversible.20, 21 Because of lack of organ donors and thus prolonged waiting times for heart transplantation, the correction of moderate–severe or severe functional mitral regurgitation and its potential impact on pulmonary artery pressure could be critical for outcome.
In this study, we investigated the feasibility, efficacy, and safety of percutaneous MitraClip™ implantation in end‐stage heart failure patients. In addition, haemodynamic effects of PMVR were studied including pulmonary artery pressures, as heart transplant candidates undergo repetitive right heart catheterizations within the clinical routine. In an as‐treated analysis, MitraClip™ patients were compared with a historical non‐MitraClip™ control group.
Methods
Our retrospective study conforms with the principles outlined in the Declaration of Helsinki. All patients were informed about specific risks and alternatives of MitraClip™ therapy and gave informed written consent to the procedure. The study protocol was in accordance with the local ethics committee.
Patient selection
All patients included in our study suffered from end‐stage heart failure [New York Heart Association (NYHA) III or IV, left ventricular ejection fraction < 30%] and concomitant moderate–severe or severe mitral regurgitation and were listed for heart transplantation at Eurotransplant international foundation. All patients were considered as high‐risk surgical candidates because of end‐stage heart failure. Only patients with suitable mitral valve anatomy for PMVR using the MitraClip™ device were included.
Assessment of mitral regurgitation
Mitral regurgitation was initially characterized using transthoracic echocardiography and transoesophageal echocardiography by experienced investigators unaware of the study and according to current recommendations.22 Because the prevalence of eccentric mitral regurgitation in the study population was high, the method of the proximal isovelocity surface area for grading mitral regurgitation was not employed. Instead, mitral regurgitation was graded in a semiquantitative manner with colour Doppler, added to the assessment of the width of the vena contracta. This was similarly used for the post‐implant grading of mitral regurgitation. Furthermore, left ventricular angiogram and invasive haemodynamic measurements of pulmonary artery pressure and left atrial pressure were used to quantify mitral regurgitation severity.
MitraClip™ group
Between September 2009 and beginning of March 2015, more than 300 patients had been treated with a MitraClip™ device at our institution. In a retrospective analysis, we identified nine patients during this period who fulfilled the previously mentioned inclusion criteria. In these patients, successful percutaneous mitral valve edge‐to‐edge repair using the MitraClip™ device was performed. Only patients with severely reduced left ventricular ejection fraction and moderate–severe or severe mitral regurgitation despite optimized pharmacological and device‐based (implantable cardioverter defibrillator and/or cardiac resynchronization therapy) heart failure therapy had been considered for MitraClip™ implantation. The main reason for performing PMVR in the identified end‐stage heart failure patients listed for heart transplantation was prolongation of a clinically stable status on regular urgency status ‘T’, thus potentially increasing the chance of reaching the goal of heart transplantation. Other concomitant reasons comprised symptom relief and patient recompensation after cardiac deterioration due to heart failure and volume overload.
Control group
To enable a comparison of outcome data with comparably diseased however conservatively managed patients, a historical patient cohort of the pre‐MitraClip™ era was employed. Twenty‐two patients listed for heart transplantation presenting with moderate–severe or severe functional mitral regurgitation were retrospectively identified and served as control group. Those patients were treated in our institution in the pre‐MitraClip™ era; thus, PMVR was not an option in those cases.
Table 1 summarizes patient characteristics of both groups.
Table 1.
Baseline patient characteristics of the control group (n = 22) and the MitraClip™ group (n = 9)
| Control | MitraClip™ | P value | |
|---|---|---|---|
| n | 22 | 9 | |
| Sex (male/female) | 16/6 | 8/1 | ns |
| Age (years) | 57.8 (4.4) | 59.0 (9.0) | ns |
| BMI | 25.8 (4.7) | 22.0 (5.2) | ns |
| NYHA class | 3.0 (0.5) | 3.5 (1.0) | ns |
| Functional MR (%) | 22/22 (100) | 9/9 (100) | ns |
| MR grade | 2.5 (0.5) | 3.0 (0.5) | ns |
| Logistic EuroSCORE (%) | 17.4 (11.5) | 17.5 (16.7) | ns |
| 6 min walk test (m) | 372 (104) | 330 (86) | ns |
| NT‐proBNP (ng/L) | 5102 (5960) | 7312 (6587) | ns |
| Ejection fraction (%) | 15 (10) | 20 (5) | ns |
| LA diameter (mm) | 52.5 (9.5) | 51.0 (16.0) | ns |
| LVEDD (mm) | 68.0 (16.3) | 67.0 (12.8) | ns |
| Cardiac index (L/min/m2) | 1.7 (0.6) | 2.1 (0.6) | ns |
| Systolic pulmonary artery pressure (mmHg) | 55.0 (17.5) | 50.0 (15.0) | ns |
| Mean pulmonary artery pressure (mmHg) | 40.0 (9.3) | 34.0 (8.0) | ns |
| Pulmonary artery resistance (dyn·s/cm5) | 245.5 (93.8) | 217.0 (145.0) | ns |
| Mixed venous oxygen saturation (%) | 49.0 (14.8) | 57.0 (11.0) | ns |
| Pulmonary capillary wedge pressure (mmHg) | 29.0 (8.3) | 23.0 (9.0) | ns |
| Significant CAD (%) | 11/22 (50) | 5/9 (56) | ns |
| Implantable cardioverter defibrillator (%) | 19/22 (86) | 8/9 (89) | ns |
| Cardiac resynchronization therapy (%) | 15/22 (68) | 4/9 (44) | ns |
| Atrial fibrillation (%) | 10/22 (45) | 6/9 (67) | ns |
| Prior stroke (%) | 4/22 (18) | 2/9 (22) | ns |
| Renal insufficiency (creatinine > 1.3 mg/dL) (%) | 11/22 (50) | 4/9 (44) | ns |
| Diabetes mellitus (%) | 5/22 (23) | 1/9 (11) | ns |
BMI, body mass index; CAD, coronary artery disease, median ± interquartile range; LA, left atrial; LVEDD, left ventricular end‐diastolic diameter; MR, mitral regurgitation; ns, not significant; NT‐proBNP, N‐terminal pro brain natriuretic peptide; NYHA, New York Heart Association.
MitraClip™ implantation procedure
All procedures were performed under general anaesthesia—monitored by a cardiac anaesthesiologist and transoesophageal echocardiography control as published previously.12 MitraClip™ was deployed as previously published.8, 12 Intraprocedural anticoagulation with heparin was adjusted to an activated clotting time of 250–300 s. All patients received prophylactic antibiotic therapy for 3 days after PMVR.
Access site closure was achieved by applying one ProGlide SH closure device (Abbott Vascular, Abbott Park, IL, USA) using the pre‐closure technique as described before.23 Patients were transferred to our intensive care or advanced heart failure unit after the procedure (for at least 24 h).
Initial evaluation and follow‐up
Patients were analysed at two separate time points. In the MitraClip™ group, patients were analysed pre‐procedure (study entry) and during follow‐up, and in the control group, study entry was defined with recognition of moderate–severe or severe functional mitral regurgitation. For follow‐up the latest available time point before study end (03/2015) or before heart transplantation, ventricular assist‐device implantation or death was chosen. In the MitraClip™ group, the follow‐up was 215 ± 53 days post‐initial haemodynamic evaluation and 185 ± 53 days post‐MitraClip™ implantation. In the control group, the follow‐up was 212 ± 45 days post‐initial haemodynamic evaluation. Patients were assessed before the procedure and followed post‐procedure in the clinical routine including required right heart catheters as indicated for heart transplant candidates with regular urgency status ‘T’. During these visits, patients additionally received blood analyses including N‐terminal pro brain natriuretic peptide and a transthoracic echocardiography. New York Heart Association class was assessed during each visit. Repetitive right heart catheterizations (every 6 months for heart transplant candidates in regular urgency status ‘T’) are necessary to monitor pulmonary artery pressures and resistance and mixed‐venous oxygen saturation. Cardiac index was determined according to the Fick principle. In addition, event‐free survival was analysed. An event was defined as the composite endpoint of death, high‐urgency listing for heart transplantation, and ventricular assist device implantation.
Statistics
Data are presented as medians with interquartile ranges. Because of the small sample size, a non‐normal distribution was assumed for every parameter. A Mann–Whitney U test was used for unpaired analyses and a Wilcoxon test for paired analyses. Fisher's exact test was applied in case of two nominal variables. Event‐free survival was analysed with a log‐rank test. A P‐value of less than 0.05 was considered statistically significant. For statistical analysis, GraphPad Prism™ (GraphPad, La Jolla, CA, USA) software was used.
Results
Baseline characteristics
Table 1 summarizes patient characteristics of both groups. Patients in both groups had end‐stage heart failure and were all listed for heart transplantation at Eurotransplant International Foundation. Both groups were comparable concerning baseline characteristics including ejection fraction, NYHA class, mitral regurgitation grade, haemodynamic measures, and co‐morbidities (Table 1). With respect to concomitant heart failure therapies, the groups were comparable. Because of advanced disease, five of 22 patients in the control group and one of nine in the MitraClip™ group were on intravenous dobutamine therapy at study inclusion (non‐significant difference).
Percutaneous mitral valve edge‐to‐edge repair as ‘bridge to transplant’ using the MitraClip™ system
Patients in the MitraClip™ group had reduced mitral regurgitation and symptoms (NYHA class) at follow‐up, while controls remained unchanged (Figure 1 A). In all patients of the MitraClip™ group, at least one grade reduction of mitral regurgitation was achieved during implantation. In three patients, one MitraClip™ was implanted, and in six patients, two MitraClips™ were deployed. Mitral regurgitation was reduced from Grade 2.8 ± 0.1 pre‐MitraClip™ (study entry) to Grade 1.6 ± 0.1 post‐MitraClip™ (follow‐up) (Figure 1 B).
Figure 1.
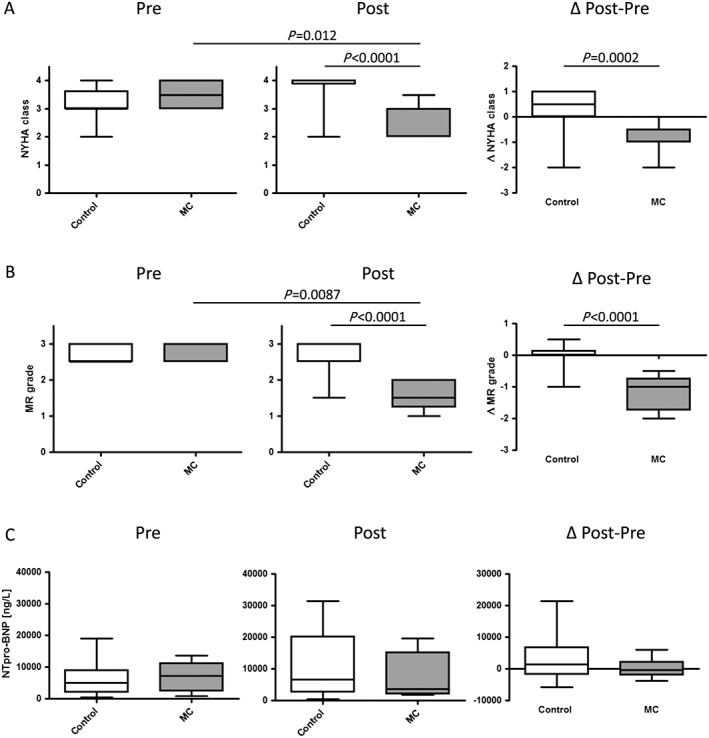
MitraClip™ implantation for percutaneous mitral valve edge‐to‐edge repair in patients awaiting heart transplantation reduces symptoms and mitral regurgitation. (A) New York Heart Association (NYHA) class. (B) Mitral regurgitation (MR) grade as determined by transthoracic or transoesophageal echocardiography. (C) N‐terminal pro brain natriuretic peptide (NT‐proBNP in ng/L). Left panel absolute values; right panel difference (Δ) pre (study entry) and post (follow‐up) evaluation. Median ± interquartile range. Control, control group; MC, MitraClip™ group.
Functional effects of MitraClip™
Cardiac biomarker N‐terminal pro brain natriuretic peptide did not change significantly in both groups (Figure 1 C). Ejection fraction minimally decreased in both groups with no evident differences (Figure 2 A). Left atrial diameter was not reduced in controls at follow‐up (Figure 2 B). Interestingly, left atrial diameter was significantly smaller in the MitraClip™ group compared with the control group at follow‐up (post) (Figure 2 B). Left ventricular end‐diastolic diameter remained similar in both groups at follow‐up (Figure 2 C).
Figure 2.
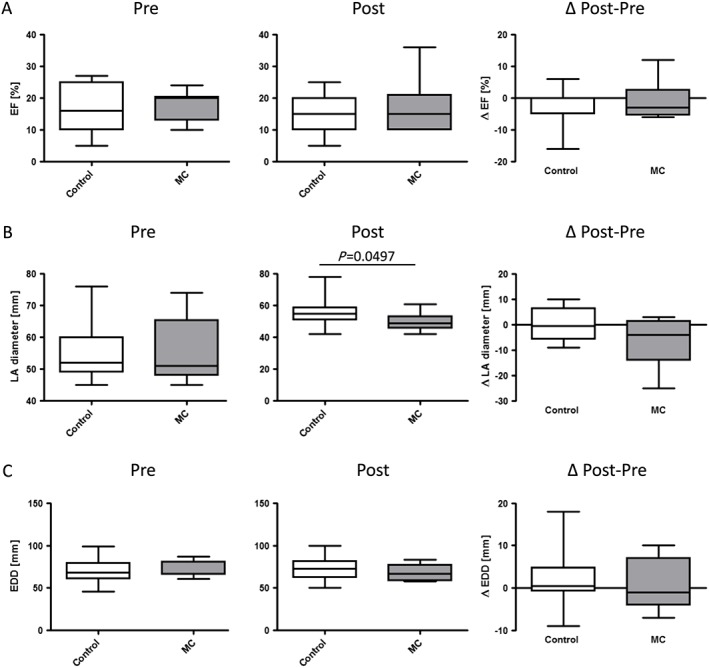
Echocardiographic assessment. (A) Ejection fraction (EF in %). (B) Left atrial (LA) diameter (in mm). (C) Left ventricular end‐diastolic diameter (EDD) (in mm). Left panel absolute values; right panel difference (Δ) pre (study entry) and post (follow‐up) evaluation. Median ± interquartile range. Control, control group; MC, MitraClip™ group.
An interesting finding is inotropic dependency, which was not different between groups at study inclusion. However, at follow‐up, 15 of 22 in the control group and two of nine in the MitraClip™ group were dependent on dobutamine (P = 0.0439).
Haemodynamic effects of MitraClip™
Haemodynamics remained stable in the control group at follow‐up with respect to cardiac index, mixed‐venous oxygen saturation, pulmonary artery pressures and resistance, and pulmonary capillary wedge pressure (Figure 3 A–G). On the contrary, in MitraClip™‐treated patients, systolic and mean pulmonary artery pressures significantly declined; the diastolic pulmonary artery pressure showed a trend towards reduction (Figure 3 C–E). Interestingly, diastolic pulmonary artery pressure was significantly lower in the MitraClip™ group compared with the control group at follow‐up (post). Changes in pulmonary artery pressures (Δ follow‐up to study entry) showed a trend towards reduction in the MitraClip™ group (Figure 3 C–E). In line with these findings, a trend towards reduced pulmonary capillary wedge pressure was found in the MitraClip™ compared with the control group at follow‐up (post).
Figure 3.
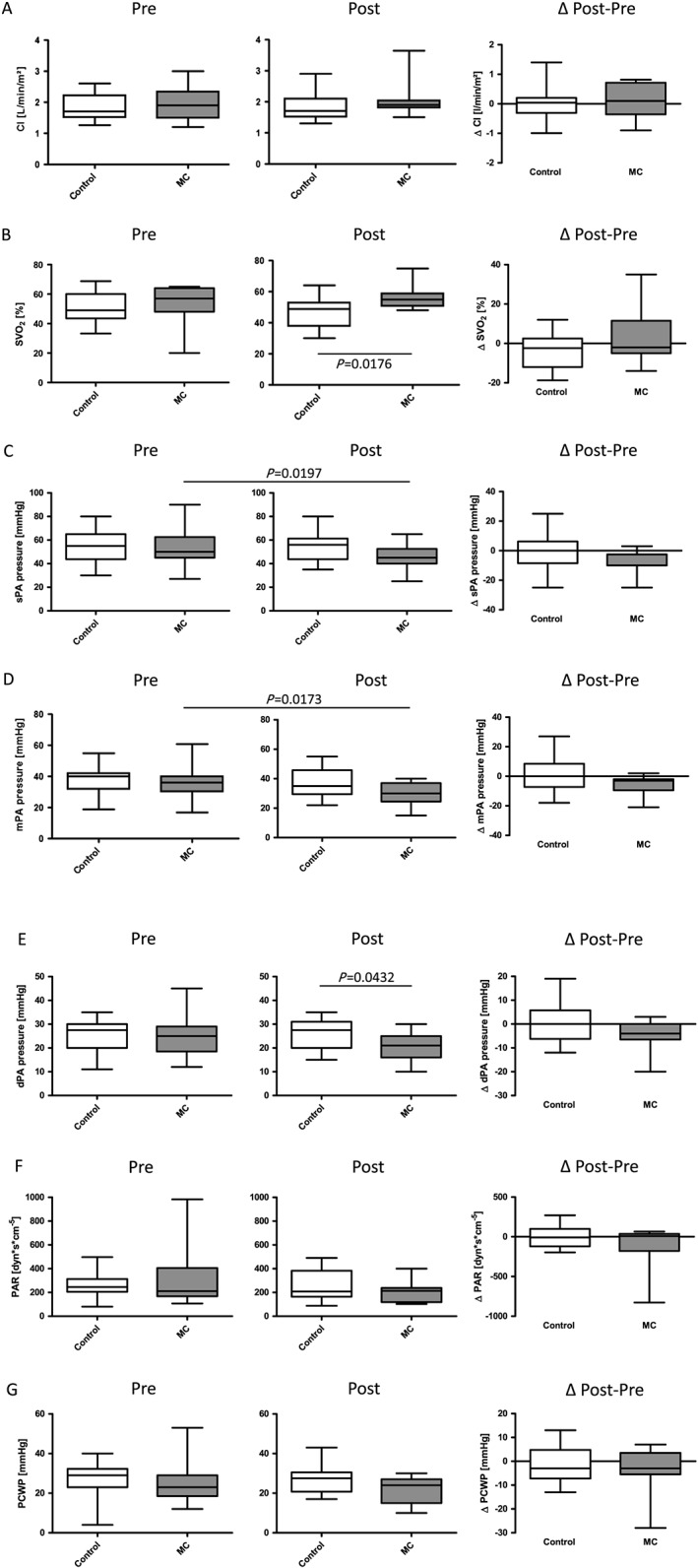
Haemodynamic assessment of percutaneous mitral valve edge‐to‐edge repair with MitraClip™ in patients awaiting heart transplantation. (A) Cardiac index (CI in L/min/m2). (B) Mixed‐venous oxygen saturation (SVO2 in %). (C) Systolic (s), (D) diastolic (d), and (E) mean (m) pulmonary artery (PA) pressure (in mmHg). (F) Pulmonary vascular resistance (in dyn·s/cm5). (G) Pulmonary capillary wedge pressure (PCWP in mmHg). Left panel absolute values; right panel difference (Δ) pre (study entry) and post (follow‐up) evaluation. Median ± interquartile range. Control, control group; MC, MitraClip™ group.
Mixed venous oxygen saturation at follow‐up (post) was significantly higher in the MitraClip™‐treated patients compared with the control group (Figure 3 B). No differences in cardiac index measurements were found (Figure 3 A).
Event‐free survival
Interestingly, patients successfully treated with a MitraClip™ had a slightly improved event‐free survival compared with the control group, albeit not significant (χ 2 = 1.233, P = 0.2669) (Figure 4 ). In addition, two patients in the MitraClip™ group were successfully heart transplanted from normal transplant status because of waiting time and not via high urgency. Both patients are still alive as this manuscript was composed.
Figure 4.
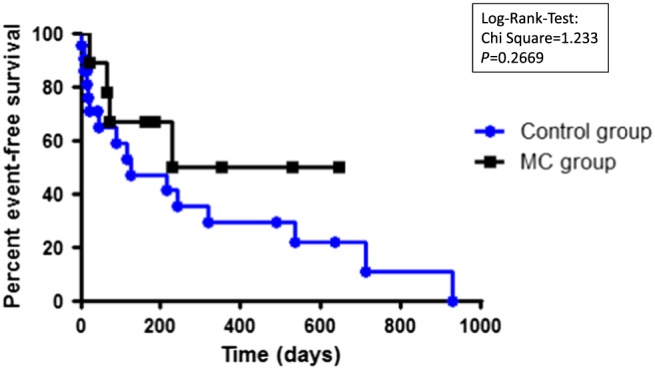
Event‐free survival in the MitraClip™ group (MC) and in the control group (Control). An event was defined as the composite endpoint of death, high‐urgency heart transplant listing, and ventricular‐assist‐device implantation. Log‐rank test.
Major adverse events within 30 days
No patient died during the MitraClip™ procedure, and all patients survived 30 days post‐intervention. Within 30 days, no device embolization, thrombus embolization, stroke, myocardial infarction, bleeding, infection, or pericardial effusion occurred.
Discussion
Heart failure is the leading disease causing hospitalization in Germany and a major cause of death in Germany and the Western world.24, 25 Accordingly, many patients with advanced disease states and end‐stage heart failure require suitable treatment. Gold standard for management of end‐stage heart failure is heart transplantation.24 However, because of organ shortage, waiting times increase.26 Because of heart failure and co‐morbidity, these end‐stage heart failure patients might finally not reach the goal of heart transplantation. A significant and frequent co‐morbidity in these patients is moderate–severe or severe mitral regurgitation because of mitral ring dilatation, termed as functional mitral regurgitation.13, 14, 15, 16 Moderate–severe or severe mitral regurgitation leads to increases in pulmonary artery pressures, thus contributing to dyspnoea, exercise limitations, and mortality in end‐stage heart failure.13, 14, 15, 16 In addition, an increase in pulmonary artery pressures is a risk factor for mortality after orthotropic heart transplantation.19
Management of advanced and terminal heart failure patients with moderate–severe or severe functional mitral regurgitation is complex. Surgical mitral valve repair was evaluated in a prospective trial in congestive heart failure patients with moderate–severe or severe mitral regurgitation. In this trial, surgical mitral valve reconstruction was not associated with an overall survival benefit.27 A novel alternative therapeutic approach is endovascular mitral valve repair.1, 2, 3, 4, 5, 6, 7, 8 The most commonly used device is the MitraClip™.6, 8, 9, 10, 11, 12 A prospective randomized controlled trial showed similar survival compared with surgical mitral valve repair or replacement in functional or degenerative mitral regurgitation, but higher re‐operation rates after MitraClip™ implantation because of recurrent mitral regurgitation.8 However, patients with end‐stage and terminal heart failure were excluded from this study. As such, the safety and effectiveness of MitraClip™ therapy in patients with end‐stage and terminal heart failure is less predictable.
This is to our knowledge the first analysis of a series of end‐stage heart failure patients with moderate–severe or severe functional mitral regurgitation and MitraClip™ implantation as ‘bridge‐to‐transplant’ strategy. First, our data suggest that MitraClip™ therapy is feasible and safe in end‐stage heart failure patients. After successful MitraClip™ implantation, a significant reduction in mitral regurgitation was achieved. In addition, NYHA class improved significantly. Successful MitraClip™ deployment resulted in significant reduction in systolic and mean pulmonary artery pressures. As increased pulmonary artery pressures favour dyspnoea, the clinical improvement is likely to be attributed to the percutaneous mitral valve edge‐to‐edge repair with the MitraClip™ system. Furthermore, mixed‐venous oxygen saturation significantly increased in the MitraClip™ group compared with the control group at follow‐up, pointing towards improved cardiac function. However, no differences in cardiac index measurements were found. This discrepancy might be explained by the transseptal puncture (24F) during MitraClip™ implantation with creation of an atrial left to right shunt potentially negatively impacting cardiac index assessment. This potentially applies to all patients in the MitraClip™ group. Unfortunately, we do not know in these patients if the shunt was still open at follow‐up. In light of significantly more inotrope (dobutamine)‐dependent patients in the control group at follow‐up compared with the MitraClip™ group, the improvements in pulmonary artery pressures and mixed‐venous oxygen saturations after PMVR are considerable. Overall, these results are in concordance with previous studies focusing on MitraClip™ therapy for severe functional mitral regurgitation in heart failure patients.28, 29, 30, 31 Contrary to other studies on MitraClip™ in heart failure patients,12, 28, 31 left ventricular dimensions and thus left ventricular remodelling were not altered after successful MitraClip™ implantation in our retrospective study. Most likely, because of end‐stage heart failure observed in our patients, sole reduction of mitral regurgitation did not allow reversal of left ventricular dilatation.
Thus, MitraClip™ implantation might be an option in end‐stage heart failure patients listed for heart transplantation and concomitant moderate–severe or severe functional mitral regurgitation with subsequent elevation of pulmonary artery pressures. MitraClip™ implantation could result in an enhanced or stabilized clinical status and thus possibly quality of life while awaiting heart transplant presumably by significant reduction of pulmonary artery pressures. As such, the chance of reaching heart transplantation in a suitable clinical status increases. Event‐free survival was poor in both groups reflecting the frailty of our included patients. Interestingly, a slight trend towards improved event‐free survival was observed in patients successfully treated with the MitraClip™ device. In addition, as opposed to surgical mitral valve approaches, no sternotomy or lateral (mini‐)thoracotomy is required for MitraClip™ implantation, which renders subsequent heart transplantation less complicated.
Limitations of the study
It is a retrospective trial. Overall, the sample size of our present study is rather small. However, this is a rather selected patient cohort with high morbidity and mortality, and these data are helpful in developing the confidence of using the MitraClip™ device in end‐stage heart failure patients awaiting heart transplantation in the future.
In addition, assessment of cardiac index is hampered by transseptal puncture with creation of an atrial left to right shunt and is consequently potentially misleading. As such, no significant differences were found at follow‐up, despite improved mixed‐venous oxygen saturation in the MitraClip™ group compared with the controls. Furthermore, haemodynamic measurements might certainly be influenced by inotropic therapy. However, the fact that more patients in the control group at follow‐up were on pharmacological inotropic support even strengthens potential improvements in the MitraClip™ group.
The nearly complete lack of data in the field of interventional mitral valve repair as ‘bridge‐to‐transplant’ strategy justifies this pilot study providing the groundwork for a prospective study.
Conclusions
Heart transplantation is the ‘gold standard’ in end‐stage heart failure treatment. However, because of organ donor shortage and increased waiting times, co‐morbidities such as mitral regurgitation and resulting pulmonary hypertension contribute to morbidity and mortality, and patients might not reach life‐saving heart transplantation. The results of our current study suggest that percutaneous mitral valve edge‐to‐edge repair with the MitraClip™ is feasible and safe in a high‐risk advanced and terminal heart failure population. Reduction of mitral regurgitation results in symptom relief and favourable impact on pulmonary artery pressures and increase in mixed‐venous oxygen saturation compared with the control group. As such, MitraClip™ implantation holds potential, and our study justifies further prospective evaluation.
Conflict of interest
N.A.G., S.T.P., R.B., E.C., and P.W.J.R. are investigators in the RESHAPE 1 and RESHAPE 2 trial. S.T.P. received research grants from Abbott Germany. S.T.P. and P.W.J.R. received speaker honoraria from Abbott Germany. The authors report no relationships that could be construed as a conflict of interest.
Funding
None.
Acknowledgement
We thank Lorenz Uhlmann, MSc (Institute for Medical Biometry and Medical Informatics, University of Heidelberg, Heidelberg, Germany), for statistical advice and review.
Geis, N. A. , Pleger, S. T. , Bekeredjian, R. , Chorianopoulos, E. , Kreusser, M. M. , Frankenstein, L. , Ruhparwar, A. , Katus, H. A. , and Raake, P. W. J. (2018) Haemodynamic effects of percutaneous mitral valve edge‐to‐edge repair in patients with end‐stage heart failure awaiting heart transplantation. ESC Heart Failure, 5: 892–901. 10.1002/ehf2.12313.
References
- 1. Schofer J, Siminiak T, Haude M, Herrman JP, Vainer J, Wu JC, Levy WC, Mauri L, Feldman T, Kwong RY, Kaye DM, Duffy SJ, Tübler T, Degen H, Brandt MC, Van Bibber R, Goldberg S, Reuter DG, Hoppe UC. Percutaneous mitral annuloplasty for functional mitral regurgitation: results of the CARILLON Mitral Annuloplasty Device European Union Study. Circulation 2009; 120: 326–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Stone GW , Adams DH, Abraham WT, Kappetein AP, Généreux P, Vranckx P, Mehran R, Kuck K‐H, Leon MB, Piazza N, Head SJ, Filippatos G, Vahanian AS, Mitral Valve Academic Research Consortium (MVARC) . Clinical trial design principles and endpoint definitions for transcatheter mitral valve repair and replacement: part 2: endpoint definitions: a consensus document from the Mitral Valve Academic Research Consortium. Eur Heart J 2015; 36: 1878–1891. [DOI] [PubMed] [Google Scholar]
- 3. Fukamachi K. Percutaneous and off‐pump treatments for functional mitral regurgitation. J Artif Organs 2008; 11: 12–18. [DOI] [PubMed] [Google Scholar]
- 4. Feldman T, Cilingiroglu M. Percutaneous leaflet repair and annuloplasty for mitral regurgitation. J Am Coll Cardiol 2011; 57: 529–537. [DOI] [PubMed] [Google Scholar]
- 5. Goldberg SL, Feldman T. Percutaneous mitral valve interventions: overview of new approaches. Curr Cardiol Rep 2010; 12: 404–412. [DOI] [PubMed] [Google Scholar]
- 6. Feldman T, Kar S, Elmariah S, Smart SC, Trento A, Siegel RJ, Apruzzese P, Fail P, Rinaldi MJ, Smalling RW, Hermiller JB, Heimansohn D, Gray WA, Grayburn PA, Mack MJ, Lim DS, Ailawadi G, Herrmann HC, Acker MA, Silvestry FE, Foster E, Wang A, Glower DD, Mauri L, EVEREST II Investigators . Randomized comparison of percutaneous repair and surgery for mitral regurgitation: 5‐year results of EVEREST II. J Am Coll Cardiol 2015; 66: 2844–2854. [DOI] [PubMed] [Google Scholar]
- 7. Piazza N, Bonan R. Transcatheter mitral valve repair for functional mitral regurgitation: coronary sinus approach. J Interv Cardiol 2007; 20: 495–508. [DOI] [PubMed] [Google Scholar]
- 8. Feldman T, Foster E, Glower DD, Glower DG, Kar S, Rinaldi MJ, Fail PS, Smalling RW, Siegel R, Rose GA, Engeron E, Loghin C, Trento A, Skipper ER, Fudge T, Letsou GV, Massaro JM, Mauri L, EVEREST II Investigators . Percutaneous repair or surgery for mitral regurgitation. N Engl J Med 2011; 364: 1395–1406. [DOI] [PubMed] [Google Scholar]
- 9. Feldman T, Kar S, Rinaldi M, Fail P, Hermiller J, Smalling R, Whitlow PL, Gray W, Low R, Herrmann HC, Lim S, Foster E, Glower D, EVEREST Investigators . Percutaneous mitral repair with the MitraClip system: safety and midterm durability in the initial EVEREST (Endovascular Valve Edge‐to‐Edge REpair Study) cohort. J Am Coll Cardiol 2009; 54: 686–694. [DOI] [PubMed] [Google Scholar]
- 10. Glower DD, Kar S, Trento A, Lim DS, Bajwa T, Quesada R, Whitlow PL, Rinaldi MJ, Grayburn P, Mack MJ, Mauri L, McCarthy PM, Feldman T. Percutaneous mitral valve repair for mitral regurgitation in high‐risk patients: results of the EVEREST II study. J Am Coll Cardiol 2014; 64: 172–181. [DOI] [PubMed] [Google Scholar]
- 11. Mauri L, Foster E, Glower DD, Apruzzese P, Massaro JM, Herrmann HC, Hermiller J, Gray W, Wang A, Pedersen WR, Bajwa T, Lasala J, Low R, Grayburn P, Feldman T, EVEREST II Investigators . 4‐year results of a randomized controlled trial of percutaneous repair versus surgery for mitral regurgitation. J Am Coll Cardiol 2013; 62: 317–328. [DOI] [PubMed] [Google Scholar]
- 12. Pleger ST, Schulz‐Schönhagen M, Geis N, Mereles D, Chorianopoulos E, Antaredja M, Lewening M, Katus HA, Bekeredjian R. One year clinical efficacy and reverse cardiac remodelling in patients with severe mitral regurgitation and reduced ejection fraction after MitraClip implantation. Eur J Heart Fail 2013; 15: 919–927. [DOI] [PubMed] [Google Scholar]
- 13. Patel JB, Borgeson DD, Barnes ME, Rihal CS, Daly RC, Redfield MM. Mitral regurgitation in patients with advanced systolic heart failure. J Card Fail 2004; 10: 285–291. [DOI] [PubMed] [Google Scholar]
- 14. Capodanno D, Adamo M, Barbanti M, Giannini C, Laudisa ML, Cannata S, Curello S, Immè S, Maffeo D, Bedogni F, Petronio AS, Ettori F, Tamburino C, Grasso C, GRASP‐IT Investigators . Predictors of clinical outcomes after edge‐to‐edge percutaneous mitral valve repair. Am Heart J 2015; 170: 187–195. [DOI] [PubMed] [Google Scholar]
- 15. Rossi A, Dini FL, Faggiano P, Agricola E, Cicoira M, Frattini S, Simioniuc A, Gullace M, Ghio S, Enriquez‐Sarano M, Temporelli PL. Independent prognostic value of functional mitral regurgitation in patients with heart failure. A quantitative analysis of 1256 patients with ischaemic and non‐ischaemic dilated cardiomyopathy. Heart BMJ 2011. Oct; 97: 1675–1680. [DOI] [PubMed] [Google Scholar]
- 16. Trichon BH, Felker GM, Shaw LK, Cabell CH, O'Connor CM. Relation of frequency and severity of mitral regurgitation to survival among patients with left ventricular systolic dysfunction and heart failure. Am J Cardiol 2003; 91: 538–543. [DOI] [PubMed] [Google Scholar]
- 17. Murali S, Kormos RL, Uretsky BF, Schechter D, Reddy PS, Denys BG, Armitage JM, Hardesty RL, Griffith BP. Preoperative pulmonary hemodynamics and early mortality after orthotopic cardiac transplantation: the Pittsburgh experience. Am Heart J 1993; 126: 896–904. [DOI] [PubMed] [Google Scholar]
- 18. Chen JM, Levin HR, Michler RE, Prusmack CJ, Rose EA, Aaronson KD. Reevaluating the significance of pulmonary hypertension before cardiac transplantation: determination of optimal thresholds and quantification of the effect of reversibility on perioperative mortality. J Thorac Cardiovasc Surg 1997; 114: 627–634. [DOI] [PubMed] [Google Scholar]
- 19. Lindelöw B, Andersson B, Waagstein F, Bergh CH. High and low pulmonary vascular resistance in heart transplant candidates. A 5‐year follow‐up after heart transplantation shows continuous reduction in resistance and no difference in complication rate. Eur Heart J 1999; 20: 148–156. [DOI] [PubMed] [Google Scholar]
- 20. Zimpfer D, Zrunek P, Sandner S, Schima H, Grimm M, Zuckermann A, Wolner E, Wieselthaler G. Post‐transplant survival after lowering fixed pulmonary hypertension using left ventricular assist devices. Eur J Cardiothorac Surg 2007; 31: 698–702. [DOI] [PubMed] [Google Scholar]
- 21. Etz CD, Welp HA, Tjan TDT, Hoffmeier A, Weigang E, Scheld HH, Schmid C. Medically refractory pulmonary hypertension: treatment with nonpulsatile left ventricular assist devices. Ann Thorac Surg 2007; 83: 1697–1705. [DOI] [PubMed] [Google Scholar]
- 22. Zoghbi WA, Enriquez‐Sarano M, Foster E, Grayburn PA, Kraft CD, Levine RA, Nihoyannopoulos P, Otto CM, Quinones MA, Rakowski H, Stewart WJ, Waggoner A, Weissman NJ, American Society of Echocardiography . Recommendations for evaluation of the severity of native valvular regurgitation with two‐dimensional and Doppler echocardiography. J Am Soc Echocardiogr: official publication of the American Society of Echocardiography 2003; 16: 777–802. [DOI] [PubMed] [Google Scholar]
- 23. Geis NA, Pleger ST, Chorianopoulos E, Müller OJ, Katus HA, Bekeredjian R. Feasibility and clinical benefit of a suture‐mediated closure device for femoral vein access after percutaneous edge‐to‐edge mitral valve repair. EuroIntervention 2015. 10: 1346–1353. [DOI] [PubMed] [Google Scholar]
- 24. McMurray JJV , Adamopoulos S, Anker SD, Auricchio A, Böhm M, Dickstein K, Falk V, Filippatos G, Fonseca C, Gomez‐Sanchez MA, Jaarsma T, Køber L, Lip GYH, Maggioni AP, Parkhomenko A, Pieske BM, Popescu BA, Rønnevik PK, Rutten FH, Schwitter J, Seferovic P, Stepinska J, Trindade PT, Voors AA, Zannad F, Zeiher A, ESC Committee for Practice Guidelines . ESC guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: the Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology developed in collaboration with the Heart Failure Association (HFA) of the ESC. Eur Heart J 2012; 33: 1787–1847. [DOI] [PubMed] [Google Scholar]
- 25. Neumann T, Biermann J, Erbel R, Neumann A, Wasem J, Ertl G, Dietz R. Heart failure: the commonest reason for hospital admission in Germany: medical and economic perspectives. Dtsch Arztebl Int 2009; 106: 269–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Smits JM, de Vries E, De Pauw M, Zuckermann A, Rahmel A, Meiser B, Laufer G, Reichenspurner H, Strueber M. Is it time for a cardiac allocation score? First results from the Eurotransplant pilot study on a survival benefit‐based heart allocation. J Heart Lung Transplant 2013; 32: 873–880. [DOI] [PubMed] [Google Scholar]
- 27. Wu AH, Aaronson KD, Bolling SF, Pagani FD, Welch K, Koelling TM. Impact of mitral valve annuloplasty on mortality risk in patients with mitral regurgitation and left ventricular systolic dysfunction. J Am Coll Cardiol 2005; 45: 381–387. [DOI] [PubMed] [Google Scholar]
- 28. Franzen O, van der Heyden J, Baldus S, Schlüter M, Schillinger W, Butter C, Hoffmann R, Corti R, Pedrazzini G, Swaans MJ, Neuss M, Rudolph V, Sürder D, Grünenfelder J, Eulenburg C, Reichenspurner H, Meinertz T, Auricchio A. MitraClip® therapy in patients with end‐stage systolic heart failure. Eur J Heart Fail 2011; 13: 569–576. [DOI] [PubMed] [Google Scholar]
- 29. Baldus S, Schillinger W, Franzen O, Bekeredjian R, Sievert H, Schofer J, Kuck K‐H, Konorza T, Möllmann H, Hehrlein C, Ouarrak T, Senges J, Meinertz T, German Transcatheter Mitral Valve Interventions (TRAMI) Investigators . MitraClip therapy in daily clinical practice: initial results from the German transcatheter mitral valve interventions (TRAMI) registry. Eur J Heart Fail 2012; 14: 1050–1055. [DOI] [PubMed] [Google Scholar]
- 30. Kienemund J, Kuck K‐H, Frerker C. Cardiac resynchronisation therapy or MitraClip® implantation for patients with severe mitral regurgitation and left bundle branch block? Arrhythm Electrophysiol Rev 2014; 3: 190–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Auricchio A, Schillinger W, Meyer S, Maisano F, Hoffmann R, Ussia GP, Pedrazzini GB, van der Heyden J, Fratini S, Klersy C, Komtebedde J, Franzen O, PERMIT‐CARE Investigators . Correction of mitral regurgitation in nonresponders to cardiac resynchronization therapy by MitraClip improves symptoms and promotes reverse remodeling. J Am Coll Cardiol 2011; 58: 2183–2189. [DOI] [PubMed] [Google Scholar]


