Abstract
Aims
Pre‐capillary pulmonary hypertension (PHpre‐cap) has a poor prognosis, especially when caused by pulmonary arterial hypertension (PAH) associated with systemic sclerosis (SSc‐PAH). Whether cardiac magnetic resonance (CMR)‐based quantification of atrial volumes in PHpre‐cap is beneficial in risk assessment is unknown. The aims were to investigate if (i) atrial volumes using CMR are associated with death or lung transplantation in PHpre‐cap, (ii) atrial volumes differ among four unmatched major PHpre‐cap subgroups, and (iii) atrial volumes differ between SSc‐PAH and idiopathic/familial PAH (IPAH/FPAH) when matched for pulmonary vascular resistance (PVR).
Methods and results
Seventy‐five PHpre‐cap patients (57 ± 19 years, 53 female, 43 de novo) with CMR and right heart catheterization were retrospectively included. Short‐axis stacks of cine images were analysed, and right and left atrial maximum (RAVmax and LAVmax) and minimum volume (RAVmin and LAVmin) were indexed for body surface area. Increased (mean + 2 SD) and reduced (mean – 2 SD) volumes were predefined from CMR normal values.
Transplantation‐free survival was lower in patients with increased RAVmax than in those with normal [hazard ratio (HR) = 2.1, 95% confidence interval (CI) 1.1–4.0] but did not differ between those with reduced LAVmax and normal (HR 2.0, 95% CI 0.8–5.1). RAVmax and RAVmin showed no differences among unmatched or matched groups (P = ns). When matched for PVR, LAVmax, LAVmin, and pulmonary artery wedge pressure were reduced in SSc‐PAH compared with IPAH/FPAH (95% CI 0.3–21.4, 95% CI 0.8–19.6, and 95% CI 2–7, respectively).
Conclusions
Patients with PHpre‐cap and increased right atrial volume measured with CMR had worse clinical outcome. When matched for PVR, left atrial volume was lower in SSc‐PAH than in IPAH/FPAH, consistent with left‐sided underfilling, indicating a potential differentiator between the groups.
Keywords: Pulmonary hypertension, Right atrial volume, Left atrial volume, Transplantation‐free survival, Cardiac magnetic resonance imaging
1. Introduction
Pre‐capillary pulmonary hypertension (PHpre‐cap) is a severe condition with poor prognosis.1 PHpre‐cap is characterized by elevated pulmonary arterial pressure due to increased pulmonary vascular resistance (PVR), which ultimately leads to right heart failure and premature death unless lung transplantation is performed.1 The causes of PHpre‐cap are heterogeneous with several underlying clinical subgroups: pulmonary arterial hypertension (PAH), PHpre‐cap due to lung disease, chronic thrombo‐embolic PHpre‐cap (CTEPH), and PHpre‐cap with unclear or multifactorial mechanisms.1 Survival rates differ among groups and even within groups with lowest survival in PAH associated with systemic sclerosis (SSc‐PAH).2, 3
Findings on echocardiography are indicative of PHpre‐cap.1, 4 To confirm the diagnosis, invasive right heart catheterization is required. Haemodynamic features differ among subgroups at the time of diagnosis.5, 6 Patients with idiopathic or familial PAH (IPAH/FPAH) often have higher mean pulmonary artery pressure than do SSc‐PAH and other connective tissue disease (CTD) PAH patients.6 On the other hand, SSc‐PAH patients have higher mortality even when IPAH/FPAH and SSc‐PAH have similar haemodynamic status at diagnosis.2, 6 The cardiac causes for the poor survival in SSc‐PAH are likely diverse and not fully clarified.
Cardiac magnetic resonance (CMR) is gold standard for time‐resolved volumetric cardiac assessment. While enlarged right atrial size measured by maximal end‐systolic area using two‐dimensional echocardiography has prognostic value for negative outcome in PHpre‐cap, the accuracy of echocardiographic right atrial volume (RAV) measurement has been questioned.7, 8 Only modest association of RAV with three‐dimensional methods and RA size from echocardiography has been described.9 Three‐dimensional echocardiography is advancing as a RAV metric for right atrial pressure estimation.10, 11, 12 Reduced left atrial volume (LAV) as a sign for underfilling of the left heart in PHpre‐cap has been suggested and could serve as an indicator of poor prognosis.13, 14 So far, a few studies have targeted the prognostic value of three‐dimensional‐assessed atrial volume in PHpre‐cap using CMR.12, 15 However, the long‐term performance of atrial volumes as prognostic factors for survival in PHpre‐cap is not fully elucidated.
The hypotheses tested in the present project are (i) CMR‐determined atrial volumes are associated with outcome in patients with PHpre‐cap and (ii) atrial volumes differ among PHpre‐cap subgroups. Therefore, we retrospectively examined (i) if CMR atrial volumes were outcome predictors for death or lung transplantation, (ii) if atrial volumes could differentiate among four major aetiologic PAH subgroups, and (iii) if RAV and LAV or survival differed between SSc‐PAH and IPAH/FPAH groups when matched for PVR.
2. Materials and methods
2.1. Study population
Patients with pulmonary hypertension (PH), who were examined with CMR between 2003 and 2015, were retrospectively identified at the Department of Clinical Physiology and Nuclear Imaging, Skåne University Hospital, Lund, Sweden. PHpre‐cap inclusion criteria were mean pulmonary arterial pressure ≥ 25 mmHg and pulmonary artery wedge pressure ≤ 15 mmHg at normal or reduced cardiac output.1 Exclusion criteria were clinically significant shunts or congenital heart disease, PH due to left heart disease with pulmonary artery wedge pressure > 15 mmHg or with PH due to idiopathic lung disease or hypoxia.1 Patients with chronic obstructive pulmonary disease (COPD) and emphysema defined as the cause to PH were not included. When there are slight signs of COPD, that by clinical decision was not defined as the primary cause to PH, COPD was listed as co‐morbidity. Patients with dataset lacking full atrial images in the short‐axis stack were excluded. In total, 75 patients examined between 2005 and 2015 fulfilled criteria and were included (Supporting Information, Data S1).
Patients were divided into subgroups as (i) IPAH/FPAH, (ii) SSc‐PAH, (iii) CTD‐PAH other than systemic sclerosis (SSc), and (iv) CTEPH. All patients had signed informed consent, and the regional ethics committee, Region Skåne, Sweden, approved the study (EPN Dnr 621/2004, EPN Dnr 2010/114, EPN Dnr 2010/248, and EPN Dnr 2011/777). The investigation conformed to the principles outlined in the Declaration of Helsinki. The Lund cohort contributing to the Swedish National PAH register and medical records was used for patient characteristics, lung transplantation, and vital status dates. Primary combined endpoint was defined as death or lung transplantation. Follow‐up time ended June 2016.
2.2. Cardiac magnetic resonance imaging
CMR was performed with 1.5 tesla magnetic resonance imaging scanners (Philips Achieva, Best, The Netherlands, and Siemens MAGNETOM Aera, Erlangen, Germany) and with a cardiac coil. ECG‐gated parallel short‐axis stack, long‐axis, and transversal cine steady‐state free precession images were acquired at end‐expiratory breath‐hold covering the whole heart. Typical image parameters for Philips were temporal resolution of 47 ms reconstructed to 30 time phases per cardiac cycle, 60° flip angle, 3 ms cycle repetition time, 1.4 ms echo time, and slice thickness 8 mm with no slice gap; and for Siemens, temporal resolution was 46 ms reconstructed to 25 time phases per cardiac cycle, 60° flip angle, 3 ms cycle repetition time, 1.4 ms echo time, and slice thickness 6 mm with 2 mm slice gap.
Image analysis was performed using freely available software Segment version 2.0 (http://segment.heiberg.se).16 RAV and LAV were measured by manual tracing of atrial endocardial maximal and minimal contour in short‐axis stack of images at ventricular end‐systole and end‐diastole, respectively. All CMR measures were indexed for body surface area to enable comparison among patients despite differences in body composition, and hence, all volumes described in this study are indexed volumes. Atrial appendages were included in the volumes. For RAV, the inferior and superior venae cavae as well as the coronary sinus were excluded. Lung veins were excluded from LAV. Right and left atrial maximal and minimal volume (RAVmax, RAVmin, LAVmax, and LAVmin) were calculated from the short‐axis stacks at both ventricular end‐systole and end‐diastole.
Increased RAVmax was predefined from previously reported normal values as mean + 2 standard deviations (SD).17 Therefore, RAVmax > 74 mL/m2 (54 + 2 × 10 mL/m2) was considered increased. Reduced LAVmax predefined as normal value mean – 2 SD resulting in LAVmax < 26 mL/m2 (39 − 2 × 6.7 mL/m2) was considered reduced.17 As increased LAVmax is an indicator of increased filling pressure and an established prognostic factor for poor outcome in various heart diseases, patients with increased LAVmax (mean + 2 SD) > 52 mL/m2 were included in a separate group in the Kaplan–Meier survival analysis.18, 19 In the compilation of normal values from CMR by Kawel‐Boehm et al., biplane, non‐indexed normal minimal values have been suggested.17 However, reference values for RAVmin and LAVmin have not previously been reported with a three‐dimensional volumetric assessment from CMR, therefore median in the specific group was used for defining increased and decreased minimal volume.17
The atrial indices of right to left maximal and minimal volumes (AImax and AImin) were calculated as RAVmax/LAVmax and RAVmin/LAVmin.20
2.3. Right heart catheterization
All patients recruited were referred for right heart catheterization on the basis of clinical indications. Right heart catheterization was performed at rest in the supine position with local anaesthesia, via an 8 French sheath inserted in the right internal jugular vein using a triple‐lumen 7.3 French balloon‐tipped Swan‐Ganz catheter. Pulsatile and mean right atrial pressures, pulmonary arterial pressures, and pulmonary artery wedge pressures were recorded. Cardiac output was calculated via thermodilution, with PVR expressed as (PAmean − wedgemean)/CO.
2.4. Statistics
Statistical analyses were performed in GraphPad Prism 7 and SPSS 24. Continuous data were expressed as mean ± SD; categorical data were expressed in absolute numbers and per cent. Kaplan–Meier curves were used for a transplantation‐free survival analysis. Data were analysed with the Cox regression analysis and entered into a univariate regression analysis. Measures with P < 0.1 were then included in a multivariate analysis of imaging measures. Colinearity was examined for atrial volumes, and a correlation of r > 0.8 was considered closely related, and both would therefore not be included in the multivariate Cox regression analysis. Distribution of co‐morbidities was investigated with Fisher's exact test. Comparisons among groups were performed using a log‐rank (Mantel–Cox) test. The Kruskal–Wallis test was used for comparison among PVR‐unmatched subgroups. For comparison of PVR‐matched SSc‐PAH and IPAH/FPAH subgroups, the Mann–Witney U‐test was performed. Hazard ratio (HR) and difference between matched groups were expressed with 95% confidence interval (95% CI). Correlations were examined with Spearman's correlation coefficient. Normal distribution was not assumed and was tested with histograms. Intraobserver and interobserver variability was tested in 14 patients with results expressed as intraclass correlation (ICC) and bias described as according to the Bland–Altman method with mean ± SD in per cent and volume in millilitre.21 A two‐sided P‐value of < 0.05 was considered to be of statistical significance.
3. Results
3.1. Patient characteristics
In total, 75 patients (age 57 ± 19 years, 53 female, 43 de novo) with PHpre‐cap fulfilled the criteria. The included patients were examined between 2005 and 2015. The selection process for including patients is shown in the Supporting Information. Clinical characteristics are shown in Table 1. When atrial volumes were compared in SSc‐PAH and IPAH/FPAH adjusted for PVR, 15 patients with SSc‐PAH [PVR 7.3 ± 3.2 Wood units (WU)] had complete data including invasive haemodynamics and were matched one to one with 15 patients with IPAH/FPAH (PVR 7.8 ± 3.1 WU). The median time between CMR and right heart catheterization was 2 days. The median follow‐up time was 2.3 years. The composite endpoint occurred in 36 patients, including 29 deaths and seven lung transplantations.
Table 1.
Patient characteristics
| All patients (n = 75) | IPAH/FPAH (n = 33) | SSc‐PAH (n = 20)a | CTD‐PAH (n = 13) | CTEPH (n = 9) | |
|---|---|---|---|---|---|
| Age (years) | 57 ± 19 | 52 ± 23 | 66 ± 11 | 59 ± 14 | 56 ± 18 |
| Females (n/%) | 53/71% | 24/73% | 15/75% | 8/61% | 6/67% |
| BSA (m2) | 1.84 ± 0.24 | 1.85 ± 0.25 | 1.81 ± 0.22 | 1.83 ± 0.17 | 1.90 ± 0.29 |
| NYHA class (1–4) | 3 (1–4) | 3 (1–3)b | 3 (2–4)c | 3 (2–4)d | 3 (2–3)e |
| NT‐proBNP (ng/L) | 2659 ± 2695 | 3008 ± 2776b | 2605 ± 2443f | 2621 ± 3114g | 946 ± 413e |
| CMR | |||||
| De novo | 43/57% | 20/61% | 10/50% | 8/62% | 5/56% |
| RVEDV (mL/m2) | 109 ± 32 | 118 ± 25 | 99 ± 27 | 105 ± 35 | 103 ± 45 |
| RVEF (%) | 37 ± 11 | 35 ± 11 | 39 ± 10 | 39 ± 12 | 39 ± 13 |
| LVEDV (mL/m2) | 64 ± 17 | 64 ± 18 | 62 ± 12 | 64 ± 11 | 65 ± 26 |
| LVEF (%) | 55 ± 9 | 54 ± 10 | 57 ± 7 | 56 ± 10 | 55 ± 11 |
| RHC | |||||
| Heart rate (b.p.m.) | 85 ± 15 | 84 ± 18 | 88 ± 13 | 81 ± 11 | 84 ± 13 |
| sNIBP (mmHg) | 126 ± 20 | 129 ± 22 | 118 ± 13 | 127 ± 22 | 131 ± 15 |
| dNIBP (mmHg) | 79 ± 13 | 82 ± 16 | 74 ± 7 | 77 ± 12 | 84 ± 11 |
| sPAP (mmHg) | 73 ± 18 | 79 ± 18 | 65 ± 15 | 70 ± 15 | 74 ± 17 |
| mPAP (mmHg) | 45 ± 11 | 50 ± 11 | 40 ± 9 | 44 ± 10 | 44 ± 9 |
| PAWP (mmHg) | 8 ± 4 | 8 ± 3 | 6 ± 3 | 8 ± 4 | 10 ± 3 |
| RAP (mmHg) | 7 ± 5 | 8 ± 5 | 6 ± 5 | 6 ± 6 | 7 ± 5 |
| CI (L/min/m2) | 2.6 ± 0.7 | 2.3 ± 0.6 | 2.9 ± 0.6 | 2.8 ± 0.9 | 2.5 ± 0.6 |
| PVR (WU) | 10 ± 8 | 13 ± 11 | 7 ± 3 | 8 ± 4 | 8 ± 4 |
| Co‐morbidity | |||||
| Diabetes | 15 | 8 | 3 | 2 | 2 |
| COPD/emphysema | 14 | 6 | 2 | 2 | 4 |
| IHD | 8 | 6 | 1 | 1 | 0 |
| Hypertension | 25 | 13 | 2 | 5 | 5 |
| Thyroid disease | 11 | 5 | 4 | 1 | 1 |
| Atrial fibrillation | 9 | 4 | 1 | 4 | 0 |
| Stroke | 4 | 3 | 0 | 1 | 0 |
| Medication | |||||
| PAH dedicated | 41/55% | 18/55% | 15/75% | 5/38% | 3/33% |
| ERA | 31/41% | 15/45% | 10/50% | 5/38% | 1/11% |
| PDE5I | 17/23% | 7/21% | 8/40% | 0 | 2/22% |
| Prostanoids | 4/5% | 1/3% | 3/15% | 0 | 0 |
| CCB | 20/36% | 5/15% | 9/45% | 5/38% | 1/11% |
| Diuretics | 37/49% | 18/55% | 8/40% | 8/62% | 3/33% |
| ACEI/ARB | 19/25% | 9/27% | 4/20% | 4/31% | 2/22% |
| BB | 17/23% | 11/33% | 0 | 5/38% | 1/11% |
ACEI/ARB, angiotensin‐converting enzyme inhibitor and angiotensin II receptor blocker; BB, beta‐blockers; BSA, body surface area; CCB, cardio‐selective calcium channel blockers; CI, cardiac index; CMR, cardiac magnetic resonance; COPD, chronic obstructive pulmonary disease; CTD‐PAH, patients with pulmonary arterial hypertension associated with connective tissue disease; CTEPH, patients with pulmonary hypertension due to chronic thrombo‐embolism; De novo, CMR performed at diagnosis; dNIBP, systemic non‐invasive diastolic blood pressure; ERA, endothelin receptor antagonists; IHD, ischaemic heart disease; IPAH/FPAH, patients with idiopathic or familial pulmonary arterial hypertension; LVEDV, left ventricular end‐diastolic volume; LVEF, left ventricular ejection fraction; mPAP, mean pulmonary arterial pressure; NT‐proBNP, N‐terminal pro‐brain natriuretic peptide; NYHA class, functional class in patients with heart failure according to the New York Heart Association; PAWP, pulmonary artery wedge pressure; PDE5I, phosphodiesterase type 5 inhibitors; Prostanoids, prostacyclin analogues and prostacyclin receptor agonists; PVR, pulmonary vascular resistance; RAP, mean right atrial pressure; RHC, right heart catheterization; RVEDV, right ventricular end‐diastolic volume; RVEF, right ventricular ejection fraction; sNIBP, systemic non‐invasive systolic blood pressure; sPAP, systolic pulmonary arterial pressure; SSc‐PAH, patients with pulmonary arterial hypertension due to systemic sclerosis; WU, Wood units.
Values expressed as mean ± SD or in median and range in parentheses. All volumes are indexed for body surface area. Co‐morbidities expressed in absolute numbers.
One patient was not investigated with RHC owing to contraindicating co‐morbidities, but diagnosis was confirmed by echocardiography.
n = 29.
n = 16.
n = 9.
n = 5.
n = 16.
n = 10.
3.2. Survival
RAVmax in all the 75 PHpre‐cap patients was 76 ± 36 mL/m2. Thirty‐four patients had an increased RAVmax > 74 mL/m2, and 41 patients had a normal or low RAVmax. Survival with increased RAVmax was shorter than that without (3.1 vs. 5.5 years, HR 2.1, 95% CI 1.1–4.0) (Figure 1 A). When comparing the prevalence of co‐morbidities (diabetes mellitus, COPD/emphysema, ischaemic heart disease, systemic hypertension, thyroid disease, atrial fibrillation, and stroke; Table 1) between patients with increased and normal RAVmax, atrial fibrillation was the only co‐morbidity to differ between the groups. Patients with increased RAVmax were more likely to suffer from atrial fibrillation than were patients with normal RAVmax (P = 0.009). When the Cox regression analysis was performed for co‐morbidities, stroke, atrial fibrillation, and thyroid disease had P < 0.1 for survival in a univariate analysis. These were included in a multivariate model with RAV, and only stroke (P = 0.008) and RAV (RAVmax P = 0.02, RAVmin P = 0.04) remained associated with survival.
Figure 1.
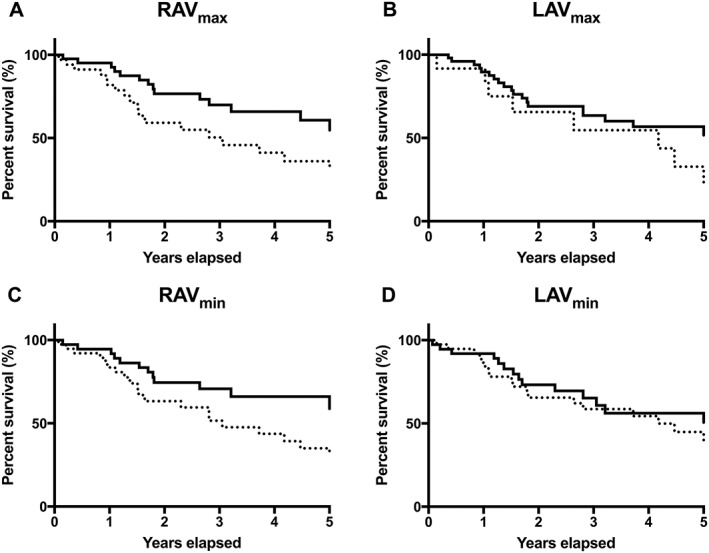
Transplantation‐free survival analyses of atrial volumes in pre‐capillary pulmonary hypertension. Kaplan–Meier transplantation‐free survival analysis of pre‐capillary pulmonary hypertension patients from timepoint of CMR. All volumes are indexed for body surface area. (A) Patients with normal right atrial maximal volume (RAVmax) (full line) compared with patients with increased RAVmax (dashed line). Hazard ratio (HR) for enlarged RAVmax was 2.1 (95% CI 1.1–4.0, P = 0.03). (B) Patients with normal left atrial maximal volume (LAVmax) (full) compared with patients with reduced LAVmax (dashed). HR for reduced LAVmax was 2.0 (95% CI 0.8–5.1, P = 0.07). (C) Patients with right atrial minimal volume (RAVmin) below median (full) compared with patients with RAVmin above median (dashed). HR for RAVmin above median was 2.3 (95% CI 1.2–4.3, P = 0.02). (D) Patients with left atrial minimal volume (LAVmin) above median (full) compared with patients with LAVmin below median (dashed). HR for LAVmin below median was 1.7 (95% CI 0.9–3.2, P = 0.12).
RAVmin in all PHpre‐cap patients was 53 ± 35 mL/m2. The survival analysis showed that patients with RAVmin above median had significantly shorter survival than had patients with RAVmin below median (HR 2.3, 95% CI 1.2–4.3) (Figure 1 C).
LAVmax in all PHpre‐cap patients was 41 ± 20 mL/m2. Twelve patients had reduced LAVmax < 26 mL/m2, 51 patients had normal LAVmax, and 12 patients had increased LAVmax. Survival with reduced LAVmax was shorter than that of patients with normal LAVmax, but not significantly (4.2 vs. 6.8 years, HR 2.0, 95% CI 0.8–5.1) (Figure 1 B). Survival for patients with increased LAVmax was 3.1 years compared with 6.8 years in patients with normal LAVmax (HR 1.6, 95% CI 0.6–4.3). The survival analysis showed no difference between patients with LAVmin below median and patients with LAVmin above median (HR 1.2, 95% CI 0.6–2.3) (Figure 1 D).
In a univariate regression analysis of CMR atrial and ventricular volumes in all 75 PHpre‐cap patients (Table 2), RAVmax and RAVmin were associated with an increased risk for transplantation or death (RAVmax HR 1.014, 95% CI 1.004–1.023 and RAVmin HR 1.013, 95% CI 1.004–1.023). No other CMR measure was found to be significantly associated with risk for transplantation or death (Table 2). RAVmax, RAVmin, and right ventricular ejection fraction (RVEF) (P = 0.005, P = 0.006, and P = 0.08, respectively) were included in a multivariate analysis. RAVmax and RAVmin showed a very strong correlation with each other (r = 0.96, P < 0.001) and were therefore analysed in relation to RVEF separately. In a multivariate analysis, RAVmax (HR 1.014, 95% CI 1.002–1.023) and RAVmin (HR 1.012, 95% CI 1.001–1.022) remained significant, but RVEF showed no significance (Table 2).
Table 2.
Cox regression analysis
| Measure (n = 75) | Volume | HR for Δ | 95% CI | P‐value |
|---|---|---|---|---|
| Univariate | ||||
| RVESV (mL/m2) | 71 ± 31 | 1.007 | 0.997–1.017 | 0.16 |
| RVEDV (mL/m2) | 109 ± 32 | 1.006 | 0.996–1.016 | 0.22 |
| RVSV (mL/m2) | 38 ± 9 | 0.995 | 0.964–1.027 | 0.76 |
| RVEF (%) | 37 ± 11 | 0.975 | 0.947–1.003 | 0.08 |
| RAVmax (mL/m2) | 76 ± 36 | 1.014 | 1.004–1.023 | 0.004 |
| RAVmin (mL/m2) | 53 ± 35 | 1.013 | 1.004–1.023 | 0.006 |
| LVESV (mL/m2) | 29 ± 13 | 0.991 | 0.964–1.019 | 0.52 |
| LVEDV (mL/m2) | 64 ± 17 | 0.985 | 0.963–1.007 | 0.17 |
| LVSV (mL/m2) | 35 ± 10 | 0.977 | 0.945–1.010 | 0.17 |
| LVEF (%) | 55 ± 9 | 0.997 | 0.969–1.027 | 0.86 |
| LAVmax(mL/m2) | 41 ± 20 | 1.005 | 0.987–1.024 | 0.60 |
| LAVmin (mL/m2) | 27 ± 20 | 1.009 | 0.993–1.025 | 0.29 |
| CI (L/min/m2)a | 2.4 ± 0.7 | 0.758 | 0.479–1.199 | 0.24 |
| Multivariate 1 | ||||
| RAVmax (mL/m2) | 76 ± 36 | 1.012 | 1.002–1.023 | 0.022 |
| RVEF (%) | 37 ± 11 | 0.993 | 0.961–1.026 | 0.686 |
| Multivariate 2 | ||||
| RAVmin (mL/m2) | 53 ± 35 | 1.012 | 1.001–1.022 | 0.029 |
| RVEF (%) | 37 ± 11 | 0.989 | 0.958–1.020 | 0.47 |
CI, cardiac index; LAVmax, left atrial maximal volume; LAVmin, left atrial minimal volume; LVEDV, left ventricular end‐diastolic volume; LVEF, left ventricular ejection fraction; LVESV, left ventricular end‐systolic volume; LVSV, left ventricular stroke volume; RAVmax, right atrial maximal volume; RAVmin, right atrial minimal volume; RVEDV, right ventricular end‐diastolic volume; RVEF, right ventricular ejection fraction; RVESV, right ventricular end‐systolic volume; RVSV, right ventricular stroke volume.
Univariate and multivariate Cox regression analyses with cardiac magnetic resonance examination as baseline. Values are expressed as mean ± SD. All volumes are indexed for body surface area. Correlations examined for multivariate analysis: RAVmax vs. RAVmin r = 0.96, RAVmax vs. RVEF r = −0.44, and RAVmin vs. RVEF r = −0.40. Bold emphasizes values with significant P‐values.
n = 74.
Survival in all PHpre‐cap patients was 5.0 years. In IPAH/FPAH, survival was 5.5 years, in SSc‐PAH 2.8 years, and in CTD‐PAH 6.2 years. Survival in CTEPH could not be calculated owing to the small sample size. No differences in survival time among the remaining groups were significant, when unmatched for PVR (P = 0.13).
3.3. Atrial volume comparison
Volumes within the aetiologic subgroups are shown in Table 3. There were no differences among aetiologic groups in RAVmax, RAVmin, LAVmax, LAVmin, AImax, or AImin (Table 3, Figure 2 ).
Table 3.
Results of atrial measures from cardiac magnetic resonance
| All patients (n = 75) | IPAH/FPAH (n = 33) | SSc‐PAH (n = 20) | CTD‐PAH (n = 13) | CTEPH (n = 9) | |
|---|---|---|---|---|---|
| RAVmax (mL/m2) | 76 ± 36 | 83 ± 32 | 70 ± 35 | 73 ± 45 | 69 ± 34 |
| RAVmin (mL/m2) | 53 ± 35 | 61 ± 33 | 46 ± 31 | 52 ± 42 | 45 ± 33 |
| LAVmax (mL/m2) | 41 ± 20 | 42 ± 22 | 36 ± 10 | 44 ± 25 | 42 ± 14 |
| LAVmin (mL/m2) | 27 ± 20 | 30 ± 24 | 20 ± 8 | 29 ± 22 | 27 ± 12 |
| AImax | 0.61 ± 0.30 | 0.55 ± 0.28 | 0.66 ± 0.35 | 0.64 ± 0.22 | 0.68 ± 0.30 |
| AImin | 0.60 ± 0.33 | 0.53 ± 0.30 | 0.63 ± 0.38 | 0.65 ± 0.28 | 0.60 ± 0.34 |
AImax, maximal atrial index; AImin, minimal atrial index; CTD‐PAH, patients with pulmonary arterial hypertension associated with connective tissue disease; CTEPH, patients with pulmonary hypertension due to chronic thrombo‐embolism; IPAH/FPAH, patients with idiopathic or familial pulmonary arterial hypertension; LAVmax, left atrial maximal volume; LAVmin, left atrial minimal volume; RAVmax, right atrial maximal volume; RAVmin, right atrial minimal volume; SSc‐PAH, patients with pulmonary arterial hypertension due to systemic sclerosis.
Values are expressed as mean ± SD. All volumes are indexed for body surface area.
Figure 2.
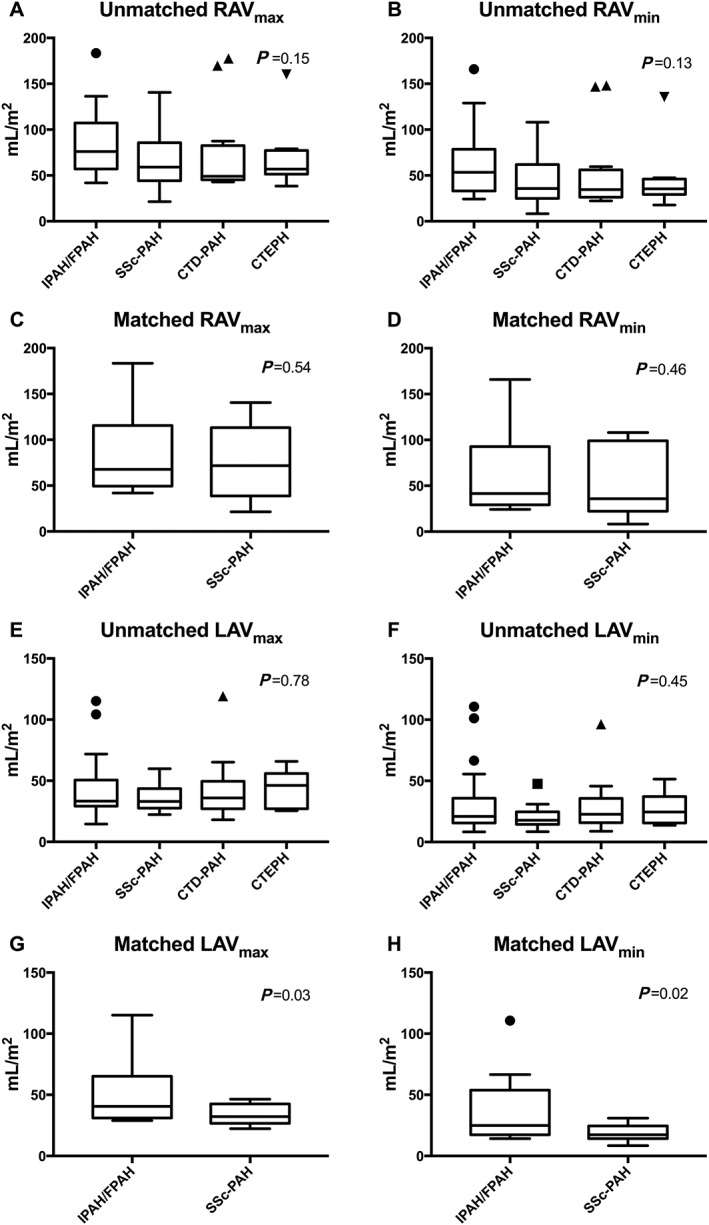
Comparison of atrial volumes, unmatched and matched for PVR. Tukey box plot showing comparison of atrial volumes among unmatched and matched subgroups of pre‐capillary pulmonary hypertension. All volumes are indexed for body surface area. (A–D) Right atrial volumes. (E–H) Left atrial volumes: left column, maximal volumes; right column, minimal volumes. (A, B, E, and F) Comparison among groups when unmatched for PVR. (C, D, G, and H) Comparison between groups when matched for PVR. RAVmax, right atrial maximal volume; RAVmin, right atrial minimal volume; IPAH/FPAH, idiopathic or familial pulmonary arterial hypertension; SSc‐PAH, pulmonary arterial hypertension associated with systemic sclerosis; CTD‐PAH, pulmonary arterial hypertension associated with connective tissue disorders; CTEPH, chronic thrombo‐embolic pulmonary hypertension; LAVmax, left atrial maximal volume; LAVmin, left atrial minimal volume; PVR, pulmonary vascular resistance.
Atrial measures with similar PVR in patients with SSc‐PAH (n = 15) matched to IPAH/FPAH (n = 15) are shown in Table 4. There was no difference between SSc‐PAH patients and IPAH/FPAH patients in RAVmax, RAVmin, or right atrial pressure (Table 4, Figure 2 C, 2 D).
Table 4.
Comparison of atrial measures with matched pulmonary vascular resistance
| IPAH/FPAH (n = 15) |
SSc‐PAH (n = 15) |
95% CI of the difference | P‐value | |
|---|---|---|---|---|
| PVR (WU) | 7.8 ± 3.0 | 7.3 ± 3.1 | −2.9 to 1.5 | 0.47 |
| RAP (mmHg) | 7.9 ± 5.3 | 6.1 ± 4.6 | −5 to 2 | 0.36 |
| PAWP (mmHg) | 9.4 ± 3.3a | 5.6 ± 2.7 | 2−7 | 0.004 |
| RAVmax(mL/m2) | 81.2 ± 38.7 | 72.9 ± 38.7 | −36.1 to 23.4 | 0.54 |
| RAVmin (mL/m2) | 60.0 ± 40.2 | 49.2 ± 34.4 | −29.8 to 11.0 | 0.46 |
| LAVmax (mL/m2) | 49.1 ± 22.2 | 34.4 ± 8.1 | 0.3−21.4 | 0.03 |
| LAVmin (mL/m2) | 35.2 ± 25.5 | 18.6 ± 5.9 | 0.8−19.6 | 0.02 |
| AImax | 0.67 ± 0.27 | 0.67 ± 0.40 | −0.31 to 0.23 | 0.80 |
| AImin | 0.64 ± 0.27 | 0.64 ± 0.42 | −0.30 to 0.28 | 0.57 |
| RVEDV (mL/m2) | 119.9 ± 24.9 | 103.3 ± 28.6 | −31 to 9 | 0.36 |
| RVESV (mL/m2) | 76.4 ± 30.7 | 65.5 ± 24.6 | −32 to 11 | 0.31 |
| LVEDV (mL/m2) | 68.7 ± 17.6 | 61.0 ± 13.1 | −20 to 5 | 0.26 |
| LVESV (mL/m2) | 32.8 ± 16.8 | 25.7 ± 6.5 | −11 to 2 | 0.20 |
| CI (L/min/m2) | 2.2 ± 0.7 | 2.6 ± 0.7 | −0.1 to 1.05 | 0.07 |
AImax, maximal atrial index; AImin, minimal atrial index; CI, cardiac index (computed from aortic flow; in one patient, CI was computed from left ventricular stroke volume); IPAH/FPAH, patients with idiopathic or familial pulmonary arterial hypertension; LAVmax, left atrial maximal volume; LAVmin, left atrial minimal volume; LVEDV, left ventricular end‐diastolic volume; LVESV, left ventricular end‐systolic volume; PAWP, pulmonary artery wedge pressure; PVR, pulmonary vascular resistance; RAP, mean right atrial pressure; RAVmax, right atrial maximal volume; RAVmin, right atrial minimal volume; RVEDV, right ventricular end‐diastolic volume; RVESV, right ventricular end‐systolic volume; SSc‐PAH, patients with pulmonary arterial hypertension due to systemic sclerosis; WU, Wood units.
Values are expressed as mean ± SD. All volumes are indexed for body surface area. Statistical comparison performed with Mann–Whitney U‐test. Bold emphasizes values with significant P‐values.
n = 14.
LAVmax and LAVmin were lower in SSc‐PAH than in IPAH/FPAH (95% CI 0.3–21.4 and 0.8–19.6) (Table 4). Also, pulmonary artery wedge pressure was lower in SSc‐PAH than in IPAH/FPAH, when matched for PVR (95% CI 2–7) (Table 4, Figure 2 G, 2 H).
AImax and AImin did not differ between SSc‐PAH and IPAH/FPAH (Table 4).
Survival was shorter in patients with SSc‐PAH (2.8 years) than in patients with IPAH/FPAH (5.7 years, HR 2.6, 95% CI 1.0–7.1), when matched for PVR.
3.4. Correlation with prognostic factors
RAVmax significantly correlated with invasively measured right atrial pressure and cardiac index as well as N‐terminal pro‐brain natriuretic peptide (NT‐proBNP) levels (Figure 3 ). RAVmax did not differ among groups of New York Heart Association (NYHA) class (P = 0.50). LAVmax did not correlate with right atrial pressure, cardiac index, or NT‐proBNP levels. However, a correlation was seen between LAVmax and cardiac index when excluding patients with atrial fibrillation from the analysis (Figure 3 ). LAVmax did not differ among groups of NYHA class (P = 0.91).
Figure 3.
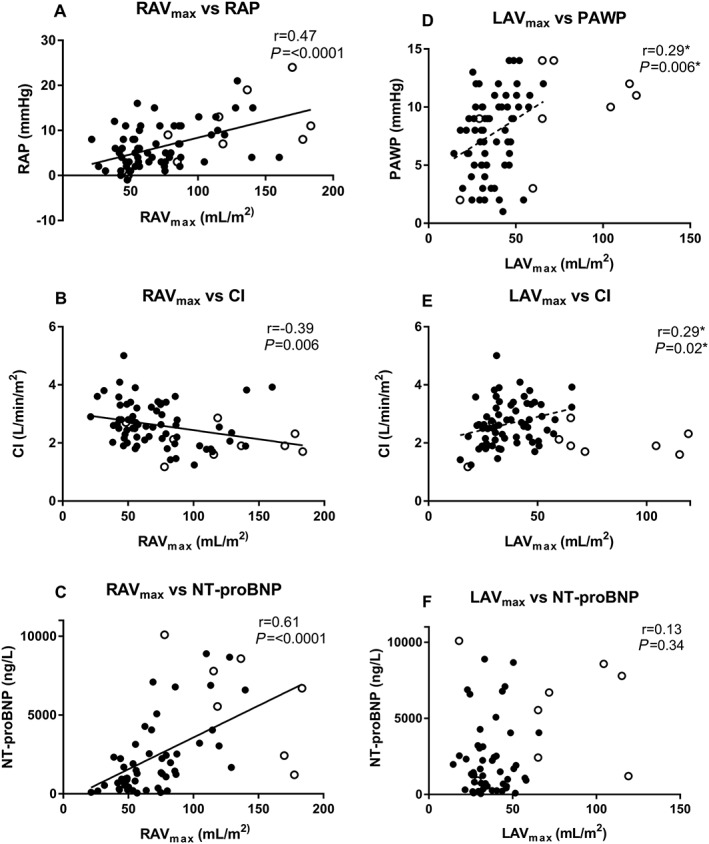
Atrial volumes in relation to right atrial pressure, cardiac index, NT‐proBNP, and pulmonary artery wedge pressure. Correlation of atrial maximal volumes indexed for body surface area with invasive right atrial pressure (A), cardiac index (B and E), NT‐proBNP (C and F), and pulmonary artery wedge pressure (D) expressed with Spearman's correlation coefficient (r). Filled circles indicate patients without atrial fibrillation; open circles, patients with atrial fibrillation; asterisks, patients with atrial fibrillation excluded from analysis. (A–C) Right atrial maximal volumes. (D–F) Left atrial maximal volumes. RAVmax, right atrial maximal volume; LAVmax, left atrial maximal volume; RAP, right atrial pressure; CI, cardiac index; NT‐proBNP, N‐terminal pro‐brain natriuretic peptide; PAWP, pulmonary artery wedge pressure.
3.5. Intraobserver and interobserver variability
ICC for intraobserver variability was 0.995 (95% CI 0.986–0.999) for RAVmax, 0.988 (95% CI 0.964–0.996) for RAVmin, 0.966 (95% CI 0.895–0.989) for LAVmax, and 0.938 (95% CI 0.808–0.980) for LAVmin. The bias for RAVmax was 3.6 ± 8.4% (2.5 ± 4.3 mL), for RAVmin 6.8 ± 17.5% (2.6 ± 5.5 mL), for LAVmax 1.3 ± 8.6% (0.3 ± 3.3 mL), and for LAVmin 1.5 ± 16.2 (0.7 ± 3.3 mL).
ICC for interobserver variability for RAVmax was 0.985 (95% CI 0.953–0.995, for RAVmin 0.976 (95% CI 0.926–0.992), for LAVmax 0.957 (95% CI 0.867–0.986), and for LAVmin 0.901 (95% CI 0.693–0.968). The bias for RAVmax was 14.0 ± 9.3% (10.6 ± 8.5 mL), for RAVmin 15.5 ± 15.2% (8.3 ± 9.3 mL), for LAVmax 7.5 ± 11.4% (3.1 ± 3.9 mL), and for LAVmin 6.3 ± 19.0% (1.6 ± 3.9 mL).
4. Discussion
This study shows that in patients with PHpre‐cap, an increased RAV was associated with worse clinical outcome. Also, there was no significant association between reduced LAV and survival in PHpre‐cap. Furthermore, there were no differences in RAV or LAV among unmatched PHpre‐cap subgroups. Lastly, when SSc‐PAH and IPAH/FPAH were matched for PVR, SSc‐PAH had reduced LAV, but RAV did not differ between the subgroups.
Our results of the association between RAV and clinical outcome are in concordance with previous studies with both two‐dimensional and three‐dimensional echocardiography in PHpre‐cap patients.12, 22 Patel et al. and Fukuda et al. have shown that the right atrial maximal areas or volumes are of importance for poor outcome, although the association with invasively measured right atrial pressure is only moderate.10, 11, 22 But echocardiography alone is insufficient for monitoring PHpre‐cap and detecting disease progression.5 For example, estimation of peak pulmonary systolic pressure by tricuspid regurgitant gradient is useful in early PHpre‐cap detection but underestimates severity of disease when cardiac output decreases in a later stage.1, 8 Therefore, evaluation of atrial volumes may be more appropriate to monitor disease progression.11, 12 Prognostic factors in PHpre‐cap from CMR have been focused on ventricular measures of which right ventricular end‐diastolic and stroke volumes as well as left ventricular end‐diastolic volume are associated with poor outcome.23 Our study showed that the association between survival and RAV also applies for CMR. Sato et al. showed that increased RAVmin, defined as above the median within the group, was associated with clinical worsening in PHpre‐cap such as hospitalization, death, or transplantation, but with no record of maximal RAVs and of relations to normal values.15 Darsaklis et al. have also targeted the subject with CMR‐assessed right atrial function in patients with PH, but in both pre‐capillary and post‐capillary PH (Groups 1–5),24 using single‐plane two‐dimensional method in the four‐chamber view for detecting RAVs from an area‐length method. They showed that decreased right atrial emptying fraction is associated with poor survival. Our study supports previous data and furthermore shows that full volumetric non‐approximative assessment with CMR is of relevance, when using a cut‐off value derived from normal values. To the best of our knowledge, our study is the first to use a cut‐off from normal values with three‐dimensional measures of atrial volume from CMR in PHpre‐cap patients. Our findings are further supported by the significant correlation of RAVmax to known prognostic markers such as right atrial pressure, cardiac index, and NT‐proBNP. A cut‐off from normal values is applicable to other studies instead of using the median within a specific study. This method could add prognostic information when performing CMR on PHpre‐cap patients in a clinical setting.
Our study was not designed to perform a comparison with well‐validated risk scores such as the REVEAL study,25 but aimed to test a simple risk stratification strategy including the atrial volumes from CMR. From echocardiography, outcome in PAH is associated with right atrial size alone as demonstrated by Bustamanta‐Labarta et al.26 and Raymond et al.27 and, furthermore, to the expanded right heart score including systolic blood pressure with right atrial area and right ventricular function as shown by Haddad et al.28 Even if our study was retrospective and focused on prevalent cases of patients with PAH, a large proportion of patients was investigated de novo and was treatment naïve. In our univariate regression analysis, we found an increased HR of 1% for transplantation or death for each increased millilitre per square metre of RAV. This increased HR remained in a multivariate analysis when adjusting for RVEF. RVEF has been suggested as the strongest predictor of mortality from CMR on meta‐analysis.29 However, former studies have seldom included RAV. Of note, in the present study, increased HR was not significantly shown for ventricular volumes. Our findings suggest that RAV may provide additive information to the ventricular volumes and the RVEF from CMR. In the newly published studies on risk assessment from the risk score of guidelines, right atrial area from CMR is used equivalent to echocardiographic cut‐off data.1, 30, 31, 32 The prognostic use of right atrial area is not supported in CMR studies but builds on echocardiographic data.1, 30, 31, 32 Our findings that RAVmin and RAVmax were associated with outcome support that these volumes are highly relevant measures, when performing CMR in PAH patients. Therefore, RAV using CMR can be a new variable in risk assessment of patients with PHpre‐cap.30 To include the newly suggested right heart score in a prospective CMR study and to design a prospective study where the atrial volume is followed in different treatment groups are possible approaches for further studies.28
Twelve patients in our study had reduced LAV. No significant association was found between reduced LAV and transplantation‐free survival in this study; however, the results indicate an increased HR, which could possibly be of significance in a larger study with more statistical power. The origin of the left ventricular dysfunction that occurs in PHpre‐cap is an issue of current debate. Underfilling of the left side related to reduced preload or reduced flow has been suggested, rather than a true diastolic dysfunction, which would result in enlarged LAV.33, 34, 35, 36 Increased LAV is a known correlate of left ventricular dysfunction and reflects increased left ventricular filling pressure.8, 18 In contrast, a small LAV could therefore reflect underfilling, as Marston et al. showed in CTEPH patients.13 Kopic et al. showed that left atrial pressure in pulmonary regurgitation is closely related to right ventricular dysfunction and decreased longitudinal pumping, suggesting that left‐sided underfilling originates from the right ventricle.37 Although LAV was not associated with outcome in our small retrospective study, the findings indicate that LAV may be associated with survival in a U‐shaped way with decreased survival in patients with both reduced and increased LAV compared with normal LAV. Normal values from three‐dimensional volumetric imaging for minimal LAV are wanted and could assist in deepening the now limited knowledge about left atrial haemodynamics in PHpre‐cap. Left‐sided underfilling and its pathophysiological significance merit further attention and should be investigated in a larger cohort.
In our study, we found smaller LAVs, lower left atrial pressure, and a reduced survival in patients with SSc‐PAH compared with IPAH/FPAH, when matched for PVR. This could reflect a higher degree of left‐sided underfilling in SSc‐PAH. Another explanation could be higher heart rate in SSc‐PAH (81 ± 13 b.p.m.) than in IPAH/FPAH (71 ± 12 b.p.m., P = 0.02). Higher heart rate consequently reduces diastolic filling time and reciprocally affects atrial filling. Altered diastolic filling time leads to the ventricle being not fully relaxed when contraction starts and consequently smaller end‐diastolic atrial volumes. Atrial index was the same in both groups, reflecting that RAV was also smaller in the SSc‐PAH group than in IPAH/FPAH; however, this difference was not statistically significant owing to the small sample size and the larger variation in RAV. Possible differences in right atrial indices should be investigated in a larger cohort. SSc is in itself a severe condition, and PAH is among the leading causes of mortality.38 SSc‐PAH has the poorest survival among subgroups of PHpre‐cap.2, 6 To characterize cardiac pathophysiological differences between SSc and other causes of PHpre‐cap would therefore be of particular interest for understanding the causes of this increased mortality, and atrial volumes could be a new approach. As haemodynamic status differs between the groups with higher mean pulmonary arterial pressure and PVR generally seen in IPAH/FPAH at diagnosis, matching the groups to be compared on the basis of (mean) pressure or resistance allows investigating group differences independent of haemodynamic status.6 Atrial measures in SSc have received limited attention. D'Andrea et al. showed that SSc patients without PAH compared with controls have impaired right atrial function, with impairment more evident in patients with higher pulmonary arterial pressure at exercise, suggesting that the right atrial function may be altered even before PAH diagnosis.39 To the best of our knowledge, the left atrium in SSc‐PAH has not been previously investigated with CMR. The present data on differences in both pressure and volume measures of the left atrium may represent a former undescribed pathophysiological difference between IPAH/FPAH and SSc‐PAH. This supports our hypothesis that left heart haemodynamics is of importance in PHpre‐cap and justifies future studies.
4.1. Limitations
This study was a single‐centre retrospective study of a rare condition. Numbers of recruited subjects limited the possibility of matching in larger groups for age and gender. But PHpre‐cap subgroups are not phenotypically similar in age and gender with CTD being more common in women than in men.6 This means that matching of gender and age remains a substantial challenge even in larger study populations.
For inclusion for comparison between SSc‐PAH and IPAH/FPAH, right heart catheterization had to be performed within 2 months of CMR. Non‐contemporaneousness of haemodynamic vs. CMR data acquisition allows for disease progression/regression and associated haemodynamic alterations. Nevertheless, the time between CMR and right heart catheterization was similar in subgroups with median time difference of 1 day in SSc‐PAH and 2 days in IPAH/FPAH. Of note, in the REVEAL study, 1 year survival did not differ between patients enrolled within 3 days of right heart catheterization and patients enrolled within 3 months of right heart catheterization.25 In the study by Haddad et al., there was an average time between CMR and diagnosis of 1.5 ± 1.5 years.28 Therefore, our time difference of median 2 days between right heart catheterization and CMR and with a majority of cases de novo could be considered well within the time spans of both the latter studies on risk stratification.25, 28
Nine patients had atrial fibrillation and accounted for most of the patients with increased LAVmax. By excluding these patients from the non‐decreased LAVmax group, atrial fibrillation as a confounder was minimized. Patients with atrial fibrillation were also presented separately in the correlation analysis.
Lastly, intraobserver variability bias was excellent with somewhat larger bias for interobserver variability. However, both intraobserver and interobserver variability had excellent ICC > 0.9, which suggests that the volumetric assessments are reliable.
5. Conclusions
Increased RAVs, but not LAVs, were associated with shorter transplantation‐free survival in PHpre‐cap. Our study shows, for the first time, that CMR‐based full volumetric RAV quantification can serve as a new prognostic indicator in PHpre‐cap. All PHpre‐cap atrial volumes behave as expected with no differences in atrial volumes among the four unmatched subgroups. However, when matched for PVR, LAVs were reduced in SSc‐PAH compared with IPAH/FPAH, despite similar haemodynamics. This apparent paradox of smaller LAV in SSc‐PAH, even though they still have worse prognosis, may be explained by a mechanism where the LAV is underfilled by different causes than PVR alone. This suggests that left‐sided underfilling may be a potential pathophysiological differentiator between these subgroups and that atrial volumes merit further investigation in PHpre‐cap.
Conflict of interest
H.A. is a shareholder of Imacor AB, Lund, Sweden. A.B., G.R., R.H., and E.O. declare no conflict of interest.
Funding
This work was supported by Skåne University Hospital, Region of Skåne, Southern Healthcare Region of Sweden and Lund University.
Supporting information
Data S1. Patient inclusion.
Acknowledgements
Great appreciation goes to Ann‐Helen Arvidsson, Christel Carlander, and Helle Puntervold for assistance with acquisition and collection of data.
Bredfelt, A. , Rådegran, G. , Hesselstrand, R. , Arheden, H. , and Ostenfeld, E. (2018) Increased right atrial volume measured with cardiac magnetic resonance is associated with worse clinical outcome in patients with pre‐capillary pulmonary hypertension. ESC Heart Failure, 5: 864–875. 10.1002/ehf2.12304.
References
- 1. Galie N, Humbert M, Vachiery JL, Gibbs S, Lang I, Torbicki A, Simonneau G, Peacock A, Vonk Noordegraaf A, Beghetti M, Ghofrani A, Gomez Sanchez MA, Hansmann G, Klepetko W, Lancellotti P, Matucci M, McDonagh T, Pierard LA, Trindade PT, Zompatori M, Hoeper M, ESC Scientific Document Group . 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Hear J 2016; 37: 67–119. [DOI] [PubMed] [Google Scholar]
- 2. Ruiz‐Cano MJ, Escribano P, Alonso R, Delgado J, Carreira P, Velazquez T, Sanchez MAG, Saenz de la Calzada C. Comparison of baseline characteristics and survival between patients with idiopathic and connective tissue disease‐related pulmonary arterial hypertension. J Heart Lung Transplant 2009; 28: 621–627. [DOI] [PubMed] [Google Scholar]
- 3. Condliffe R, Kiely DG, Peacock AJ, Corris PA, Gibbs JSR, Vrapi F, Das C, Elliot CA, Johnson M, DeSoyza J, Torpy C, Goldsmith K, Hodgkins D, Hughes RJ, Pepke‐Zaba J, Coghlan JG. Connective tissue disease‐associated pulmonary arterial hypertension in the modern treatment era. Am J Respir Crit Care Med 2009; 179: 151–157. [DOI] [PubMed] [Google Scholar]
- 4. Coghlan JG, Denton CP, Grunig E, Bonderman D, Distler O, Khanna D, Muller‐Ladner U, Pope JE, Vonk MC, Doelberg M, Chadha‐Boreham H, Heinzl H, Rosenberg DM, McLaughlin VV, Seibold JR. Evidence‐based detection of pulmonary arterial hypertension in systemic sclerosis: the DETECT study. Ann Rheum Dis 2014; 73: 1340–1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Farber HW, Foreman AJ, Miller DP, McGoon MD. REVEAL Registry: correlation of right heart catheterization and echocardiography in patients with pulmonary arterial hypertension. Congest Hear Fail 2011; 17: 56–64. [DOI] [PubMed] [Google Scholar]
- 6. Chung L, Liu J, Parsons L, Hassoun PM, McGoon M, Badesch DB, Miller DP, Nicolls MR, Zamanian RT. Characterization of connective tissue disease‐associated pulmonary arterial hypertension from REVEAL: identifying systemic sclerosis as a unique phenotype. Chest 2010; 138: 1383–1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Austin C, Alassas K, Burger C, Safford R, Pagan R, Duello K, Kumar P, Zeiger T, Shapiro B. Echocardiographic assessment of estimated right atrial pressure and size predicts mortality in pulmonary arterial hypertension. Chest 2015; 147: 198–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lang RM, Badano LP, Mor‐Avi V, Afilalo J, Armstrong A, Ernande L, Flachskampf FA, Foster E, Goldstein SA, Kuznetsova T, Lancellotti P, Muraru D, Picard MH, Rietzschel ER, Rudski L, Spencer KT, Tsang W, Voigt JU. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Hear J Cardiovasc Imaging 2015; 16: 233–270. [DOI] [PubMed] [Google Scholar]
- 9. Ramanathan R, Anumandla AK, Haramati LB, Spevack DM, Godelman A, Jain VR, Kazam J, Burton WB, Levsky JM. Evaluation of the cardiac chambers on axial CT: comparison with echocardiography. J Comput Assist Tomogr 2014; 38: 53–60. [DOI] [PubMed] [Google Scholar]
- 10. Patel AR, Alsheikh‐Ali AA, Mukherjee J, Evangelista A, Quraini D, Ordway LJ, Kuvin JT, Denofrio D, Pandian NG. 3D echocardiography to evaluate right atrial pressure in acutely decompensated heart failure correlation with invasive hemodynamics. JACC Cardiovasc Imaging 2011; 4: 938–945. [DOI] [PubMed] [Google Scholar]
- 11. Ostenfeld E, Werther‐Evaldsson A, Engblom H, Ingvarsson A, Roijer A, Meurling C, Holm J, Radegran G, Carlsson M. Discriminatory ability of right atrial volumes with two‐ and three‐dimensional echocardiography to detect elevated right atrial pressure in pulmonary hypertension. Clin Physiol Funct Imaging 2018; 38: 192–199. [DOI] [PubMed] [Google Scholar]
- 12. Grapsa J, Gibbs JS, Cabrita IZ, Watson GF, Pavlopoulos H, Dawson D, Gin‐Sing W, Howard LS, Nihoyannopoulos P. The association of clinical outcome with right atrial and ventricular remodelling in patients with pulmonary arterial hypertension: study with real‐time three‐dimensional echocardiography. Eur Hear J Cardiovasc Imaging 2012; 13: 666–672. [DOI] [PubMed] [Google Scholar]
- 13. Marston NA, Auger WR, Madani MM, Kimura BJ, Strachan GM, Raisinghani AB, DeMaria AN, Blanchard DG. Assessment of left atrial volume before and after pulmonary thromboendarterectomy in chronic thromboembolic pulmonary hypertension. Cardiovasc Ultrasound 2014; 12: 32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Motoji Y, Tanaka H, Fukuda Y, Sano H, Ryo K, Imanishi J, Miyoshi T, Sawa T, Mochizuki Y, Matsumoto K, Emoto N, Hirata K. Interdependence of right ventricular systolic function and left ventricular filling and its association with outcome for patients with pulmonary hypertension. Int J Cardiovasc Imaging 2015; 31: 691–698. [DOI] [PubMed] [Google Scholar]
- 15. Sato T, Tsujino I, Ohira H, Oyama‐Manabe N, Ito YM, Yamada A, Ikeda D, Watanabe T, Nishimura M. Right atrial volume and reservoir function are novel independent predictors of clinical worsening in patients with pulmonary hypertension. J Heart Lung Transplant 2015; 34: 414–423. [DOI] [PubMed] [Google Scholar]
- 16. Heiberg E, Sjogren J, Ugander M, Carlsson M, Engblom H, Arheden H. Design and validation of Segment—freely available software for cardiovascular image analysis. BMC Med Imaging 2010; 10: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kawel‐Boehm N, Maceira A, Valsangiacomo‐Buechel ER, Vogel‐Claussen J, Turkbey EB, Williams R, Plein S, Tee M, Eng J, Bluemke DA. Normal values for cardiovascular magnetic resonance in adults and children. J Cardiovasc Magn Reson 2015; 17: 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Tamura H, Watanabe T, Nishiyama S, Sasaki S, Arimoto T, Takahashi H, Shishido T, Miyashita T, Miyamoto T, Nitobe J, Hirono O, Kubota I. Increased left atrial volume index predicts a poor prognosis in patients with heart failure. J Card Fail 2011; 17: 210–216. [DOI] [PubMed] [Google Scholar]
- 19. Nagueh SF, Appleton CP, Gillebert TC, Marino PN, Oh JK, Smiseth OA, Waggoner AD, Flachskampf FA, Pellikka PA, Evangelisa A. Recommendations for the evaluation of left ventricular diastolic function by echocardiography. Eur J Echocardiogr 2009; 10: 165–193. [DOI] [PubMed] [Google Scholar]
- 20. Huis In't Veld AE, Van Vliet AG, Spruijt OA, Handoko ML, Marcus JT, Noordegraaf AV, Bogaard H‐J. CTA‐derived left to right atrial size ratio distinguishes between pulmonary hypertension due to heart failure and idiopathic pulmonary arterial hypertension. Int J Cardiol 2016; 223: 723–728. [DOI] [PubMed] [Google Scholar]
- 21. Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet (London, England) 1986; 1: 307–310. [PubMed] [Google Scholar]
- 22. Fukuda Y, Tanaka H, Motoji Y, Ryo K, Sawa T, Imanishi J, Miyoshi T, Mochizuki Y, Tatsumi K, Matsumoto K, Shinke T, Emoto N, Hirata K‐I. Utility of combining assessment of right ventricular function and right atrial remodeling as a prognostic factor for patients with pulmonary hypertension. Int J Cardiovasc Imaging 2014; 30: 1269–1277. [DOI] [PubMed] [Google Scholar]
- 23. van Wolferen SA, Marcus JT, Boonstra A, Marques KMJ, Bronzwaer JGF, Spreeuwenberg MD, Postmus PE, Vonk‐Noordegraaf A. Prognostic value of right ventricular mass, volume, and function in idiopathic pulmonary arterial hypertension. Eur Heart J 2007; 28: 1250–1257. [DOI] [PubMed] [Google Scholar]
- 24. Darsaklis K, Dickson ME, Cornwell W 3rd, Ayers CR, Torres F, Chin KM, Matulevicius S. Right atrial emptying fraction non‐invasively predicts mortality in pulmonary hypertension. Int J Cardiovasc Imaging 2016; 32: 1121–1130. [DOI] [PubMed] [Google Scholar]
- 25. Benza RL, Gomberg‐Maitland M, Miller DP, Frost A, Frantz RP, Foreman AJ, Badesch DB, McGoon MD. The REVEAL Registry risk score calculator in patients newly diagnosed with pulmonary arterial hypertension. Chest 2012; 141: 354–362. [DOI] [PubMed] [Google Scholar]
- 26. Bustamante‐Labarta M, Perrone S, De La Fuente RL, Stutzbach P, De La Hoz RP, Torino A, Favaloro R. Right atrial size and tricuspid regurgitation severity predict mortality or transplantation in primary pulmonary hypertension. J Am Soc Echocardiogr 2002; 15: 1160–1164. [DOI] [PubMed] [Google Scholar]
- 27. Raymond RJ, Hinderliter AL, Willis PW, Ralph D, Caldwell EJ, Williams W, Ettinger NA, Hill NS, Summer WR, de Boisblanc B, Schwartz T, Koch G, Clayton LM, Jobsis MM, Crow JW, Long W. Echocardiographic predictors of adverse outcomes in primary pulmonary hypertension. J Am Coll Cardiol 2002; 39: 1214–1219. [DOI] [PubMed] [Google Scholar]
- 28. Haddad F, Spruijt OA, Denault AY, Mercier O, Brunner N, Furman D, Fadel E, Bogaard HJ, Schnittger I, Vrtovec B, Wu JC, de Jesus Perez V, Vonk‐Noordegraaf A, Zamanian RT. Right heart score for predicting outcome in idiopathic, familial, or drug‐ and toxin‐associated pulmonary arterial hypertension. JACC Cardiovasc Imaging 2015; 8: 627–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Baggen VJM, Leiner T, Post MC, van Dijk AP, Roos‐Hesselink JW, Boersma E, Habets J, Sieswerda GT. Cardiac magnetic resonance findings predicting mortality in patients with pulmonary arterial hypertension: a systematic review and meta‐analysis. Eur Radiol 2016; 26: 3771–3780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kylhammar D, Kjellstrom B, Hjalmarsson C, Jansson K, Nisell M, Soderberg S, Wikstrom G, Radegran G. A comprehensive risk stratification at early follow‐up determines prognosis in pulmonary arterial hypertension. Eur Heart J 2017. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 31. Hoeper MM, Kramer T, Pan Z, Eichstaedt CA, Spiesshoefer J, Benjamin N, Olsson KM, Meyer K, Vizza CD, Vonk‐Noordegraaf A, Distler O, Opitz C, Gibbs JSR, Delcroix M, Ghofrani HA, Huscher D, Pittrow D, Rosenkranz S, Grunig E. Mortality in pulmonary arterial hypertension: prediction by the 2015 European pulmonary hypertension guidelines risk stratification model. Eur Respir J 2017; 50: 1700740. [DOI] [PubMed] [Google Scholar]
- 32. Boucly A, Weatherald J, Savale L, Jaïs X, Cottin V, Prevot G, Picard F, de Groote P, Jevnikar M, Bergot E, Chaouat A, Chabanne C, Bourdin A, Parent F, Montani D, Simonneau G, Humbert M, Sitbon O. Risk assessment, prognosis and guideline implementation in pulmonary arterial hypertension. Eur Respir J 2017; 50; pii: 1700889. [DOI] [PubMed] [Google Scholar]
- 33. Gurudevan SV, Malouf PJ, Auger WR, Waltman TJ, Madani M, Raisinghani AB, DeMaria AN, Blanchard DG. Abnormal left ventricular diastolic filling in chronic thromboembolic pulmonary hypertension: true diastolic dysfunction or left ventricular underfilling? J Am Coll Cardiol 2007; 49: 1334–1339. [DOI] [PubMed] [Google Scholar]
- 34. Marcus JT, Vonk Noordegraaf A, Roeleveld RJ, Postmus PE, Heethaar RM, Van Rossum AC, Boonstra A. Impaired left ventricular filling due to right ventricular pressure overload in primary pulmonary hypertension: noninvasive monitoring using MRI. Chest 2001; 119: 1761–1765. [DOI] [PubMed] [Google Scholar]
- 35. Gan C, Lankhaar JW, Marcus JT, Westerhof N, Marques KM, Bronzwaer JG, Boonstra A, Postmus PE, Vonk‐Noordegraaf A. Impaired left ventricular filling due to right‐to‐left ventricular interaction in patients with pulmonary arterial hypertension. Am J Physiol Hear Circ Physiol 2006; 290: H1528–H1533. [DOI] [PubMed] [Google Scholar]
- 36. Manders E, Bogaard HJ, Handoko ML, van de Veerdonk MC, Keogh A, Westerhof N, Stienen GJ, Dos Remedios CG, Humbert M, Dorfmuller P, Fadel E, Guignabert C, van der Velden J, Vonk‐Noordegraaf A, de Man FS, Ottenheijm CA. Contractile dysfunction of left ventricular cardiomyocytes in patients with pulmonary arterial hypertension. J Am Coll Cardiol 2014; 64: 28–37. [DOI] [PubMed] [Google Scholar]
- 37. Kopic S, Stephensen SS, Heiberg E, Arheden H, Bonhoeffer P, Ersboll M, Vejlstrup N, Sondergaard L, Carlsson M. Isolated pulmonary regurgitation causes decreased right ventricular longitudinal function and compensatory increased septal pumping in a porcine model. Acta Physiol (Oxf) 2017; 221: 163–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Steen VD, Medsger TA. Changes in causes of death in systemic sclerosis, 1972–2002. Ann Rheum Dis 2007; 66: 940–944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. D'Andrea A, D'Alto M, Di Maio M, Vettori S, Benjamin N, Cocchia R, Argiento P, Romeo E, Di Marco G, Russo MG, Valentini G, Calabro R, Bossone E, Grunig E. Right atrial morphology and function in patients with systemic sclerosis compared to healthy controls: a two‐dimensional strain study. Clin Rheumatol 2016; 35: 1733–1742. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1. Patient inclusion.


