Abstract
Background
Pharmacological profiles of new psychoactive substances can be established rapidly in vitro and provide information on potential psychoactive effects in humans. The present study investigated whether specific in vitro monoamine transporter and receptor interactions can predict effective psychoactive doses in humans.
Methods
We correlated previously assessed in vitro data of stimulants and psychedelics with human doses that are reported on the Internet and in books.
Results
For stimulants, dopamine and norepinephrine transporter inhibition potency was positively correlated with human doses, whereas serotonin transporter inhibition potency was inversely correlated with human doses. Serotonin 5-hydroxytryptamine-2A (5-HT2A) and 5-HT2C receptor affinity was significantly correlated with psychedelic doses, but 5-HT1A receptor affinity and 5-HT2A and 5-HT2B receptor activation potency were not.
Conclusions
The rapid assessment of in vitro pharmacological profiles of new psychoactive substances can help to predict psychoactive doses and effects in humans and facilitate the appropriate scheduling of new psychoactive substances.
Keywords: new psychoactive substance, stimulants, psychedelics, receptor, transporter
Introduction
The unprecedented proliferation of new psychoactive substances (NPSs) over the last decade has introduced a variety of substance classes to recreational drug users worldwide. The Internet plays a major role in the distribution of such compounds and in acquiring information about their effects and reported subjective effective doses in substance users. From 2011 to 2017, we assessed the monoamine transporter and receptor interaction profiles of more than 100 NPSs and related classic amphetamine-type and psychedelic drugs of abuse using the same in vitro assays and procedures in our laboratory (Simmler et al., 2013; Simmler et al., 2014a, 2014b; Rickli et al., 2015a, 2015b, 2015c, 2016; Luethi et al., 2018a, 2018b, 2018c, 2018d). The compounds that we investigated can predominantly be classified as stimulants or psychedelics based on their pharmacological and reported psychoactive effect profiles. Stimulants exert their pharmacological effects mainly by interacting with transmembrane monoamine transporters (i.e., norepinephrine [NE], dopamine [DA], and serotonin [5-hydroxytryptamine (5-HT)] transporters [NET, DAT, and SERT, respectively]), either as inhibitors or as transporter substrates that mediate the non-exocytotic release of neurotransmitters (Rothman and Baumann, 2003). Psychedelics mediate their mind-altering effects by interacting with 5-HT receptors, mainly 5-HT2A receptor agonism (Nichols, 2016; Liechti, 2017). The present study investigated whether (1) in vitro monoamine transporter inhibition potencies and (2) in vitro serotonin receptor binding and activation can be used to predict human doses of stimulants and psychedelics, respectively, that are reported on online drug information websites and in books.
Methods
Drugs
The present study included drugs for which we previously investigated and published in vitro pharmacological profiles using identical assays and procedures in our laboratory (Simmler et al., 2013; Simmler et al., 2014a, 2014b; Rickli et al., 2015a, 2015b, 2015c, 2016; Luethi et al., 2018a, 2018b, 2018c, 2018d). These drugs could be categorized as either psychostimulants or psychedelics based on their chemical structure and reported pharmacological effects. Substances that predominantly inhibited monoamine transporters were classified as stimulants. Substances that most potently bound to 5-HT2 receptors were pharmacologically classified as psychedelics. Five aminoindanes, 8 benzofurans, 28 cathinones, 3 piperazines, 10 piperidines, and 6 other NPSs were categorized as psychostimulants. One benzodifuran, 1 ergoline, and 7 tryptamines were categorized as psychedelics. The class of phenethylamines was further divided into 15 stimulant phenethylamines (amphetamine-type substances) and 36 psychedelic phenethylamines (ring-substituted phenethylamines, including 2C drugs and their methoxybenzyl [NBOMe] analogs). The stimulants are listed in supplementary Table 1. The psychedelics are listed in supplementary Table 2.
Dose Estimates
Dose estimates for human psychoactive doses were based on information that is found on the websites erowid.org, psychonautwiki.org, and tripsit.me (accessed December 17, 2017) and in published books and other publications (Shulgin and Shulgin, 1995, 1997; Simmler et al., 2013; Trachsel et al., 2013). The average midrange of the common dose range that is reported on the websites or in the books was taken as the dose estimate. Unless stated otherwise, oral doses of the racemic mixtures were used for this study.
Monoamine Transporter Inhibition
Norepinephrine, DA, and 5-HT uptake inhibition was assessed in human embryonic kidney 293 cells that were transfected with the human NET, DAT, or SERT as previously described in detail (Luethi et al., 2018c). Briefly, the cells were suspended in buffer and incubated with the drugs for 10 minutes before [3H]-NE, [3H]-DA, or [3H]-5-HT at a final concentration of 5 nM was added for an additional 10 minutes to initiate uptake transport. The cells were then separated from the uptake buffer by centrifugation through silicone oil. The centrifugation tubes were frozen in liquid nitrogen, and the cell pellet was cut into scintillation vials that contained lysis buffer. Scintillation fluid was added, and uptake was quantified by liquid scintillation counting. Transporter inhibitors (10 μM nisoxetine for the NET, 10 μM mazindol for the DAT, and 10 μM fluoxetine for the SERT) were added to assess nonspecific monoamine uptake. Monoamine uptake data were fit by nonlinear regression to variable-slope sigmoidal dose-response curves, and IC50 values were determined using Prism 7.0a software (GraphPad).
5-HT Receptor Binding Affinities
Radioligand binding affinities for 5-HT receptors were assessed as previously described in detail (Luethi et al., 2018d). Briefly, membrane preparations overexpressing the respective human receptors were incubated for 30 minutes (5-HT1A and 5-HT2A receptors) or 2 hours (5-HT2C receptor) with radiolabeled selective ligands at concentrations equal to Kd, and ligand displacement by the compounds was measured. Specific binding of the radioligand to the target receptor was defined as the difference between total binding and nonspecific binding that was determined in the presence of competitors. The following radioligands and competitors, respectively, were used: 1.39 nM [3H]8-hydroxy-2-(di-n-propylamine)tetralin and 10 μM pindolol (5-HT1A receptor), 0.45 nM [3H]ketanserin and 10 μM spiperone (5-HT2A receptor), and 1.6 nM [3H]mesulgerine and 10 μM mianserin (5-HT2C receptor).
Activity at the 5-HT2A Receptor
Activity at the 5-HT2A receptor was assessed as previously described in detail (Luethi et al., 2018a). Briefly, NIH-3T3 cells expressing the human 5-HT2A receptor were incubated in buffer for 1 hour at 37°C before 100 μL of dye solution (fluorescence imaging plate reader [FLIPR] calcium 5 assay kit; Molecular Devices) was added to each well, and the plates were again incubated for 1 hour at 37°C. The plates were then placed in a FLIPR, and 25 μL of the test drugs that were diluted in buffer was added online. The increase in fluorescence was measured for 51 s. EC50 values were derived from the concentration-response curves using nonlinear regression.
Activity at the 5-HT2B Receptor
Activity at the 5-HT2B receptor was assessed as previously described in detail (Luethi et al., 2018a). Briefly, human embryonic kidney 293 cells that expressed the human 5-HT2B receptor were incubated in growth medium overnight. The growth medium was then removed by snap inversion, and 100 μL of the calcium indicator Fluo-4 solution (Molecular Probes) was added to each well. The plates were incubated for 45 minutes at 31°C. The Fluo-4 solution was then removed by snap inversion, and 100 μL of Fluo-4 solution was added a second time for 45 minutes at 31°C. The cells were washed using an EMBLA cell washer, and 100 μL of assay buffer was added. The plates were then placed in a FLIPR, and 25 μL of the test substances that were diluted in buffer was added online. The increase in fluorescence was measured for 51 seconds. EC50 values were derived from the concentration-response curves using nonlinear regression.
Statistical Correlation
Mean estimated dose values were correlated with previously published mean IC50 values for the monoamine transporter inhibition of stimulants and the mean serotonin receptor affinity (Ki) and receptor activation (EC50) values of psychedelics. The Spearman rank-order correlation coefficient (rs) was computed using Prism 7.0a software (GraphPad). Values of P<.05 (2-tailed) were considered statistically significant. Multiple regression analysis was conducted to assess the relative contribution of different predictors to the dose estimate using Statistica 12 software (StatSoft) after logarithmic transformation of the data.
Results
Based on the reported information, dose estimates could be made for 54 of 75 stimulants and 35 of 45 psychedelics. The doses apply to the oral route of administration if not indicated otherwise (supplementary Tables 1 and 2). References for the information sources of the pharmacological data and for the dose estimates for each substance are listed in supplementary Tables 1 (stimulants) and 2 (psychedelics).
Stimulants
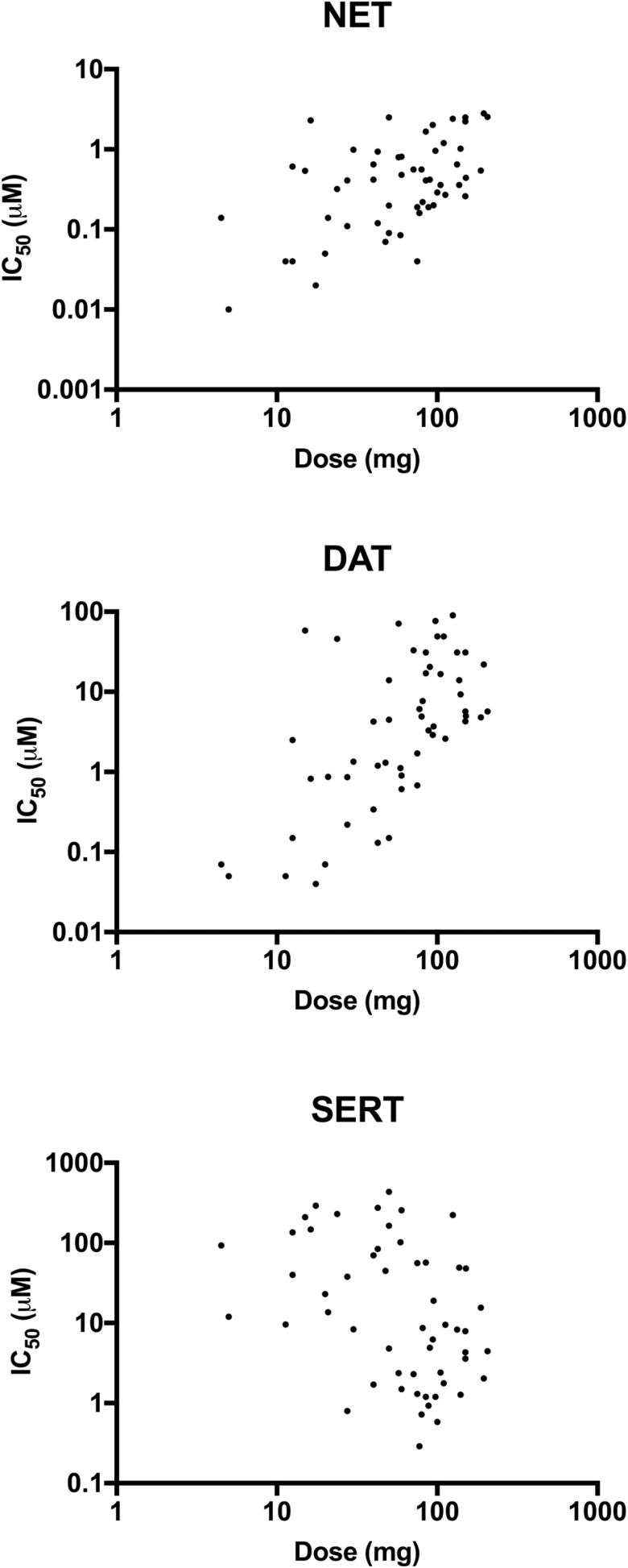
Correlations between transporter inhibition potencies (mean IC50 values) of stimulants and their mean dose estimates are shown in Figure 1. Inhibition potency values of the NET and DAT were significantly correlated with the human dose estimates (rs = 0.48, P < .001, n = 54, and rs = 0.60, P < .001, n = 54, respectively). Furthermore, the NET and DAT inhibition potencies were significantly intercorrelated (rs = 0.61, P < .001, n = 74). In contrast, the inhibition potency values of the SERT were inversely correlated with the dose estimates (rs = -0.41, P < .01, n = 54) and inversely intercorrelated with DAT inhibition (rs = 0.26, P < .05, n = 73) but not NET inhibition. When DAT and NET inhibition was used as the predictor within a multiple regression analysis to predict the dose, DAT inhibition and NET inhibition alone were significant predictors (R = 0.55, P < .001, and R = 0.51, P < .001, respectively) when entered alone, but adding NET to DAT inhibition only minimally and nonsignificantly increased the overall prediction (multiple R = 0.59, P < .001). However, SERT inhibition was inversely correlated with dose when analyzed alone (R = 0.36, P < .01) and relevantly and significantly increased the overall prediction when it was added to NET and DAT inhibition (multiple R = 0.63, P < .001, n = 54).
Figure 1.
Correlations between dose estimates of stimulants and their transporter inhibition potencies (mean IC50 values).
Psychedelics
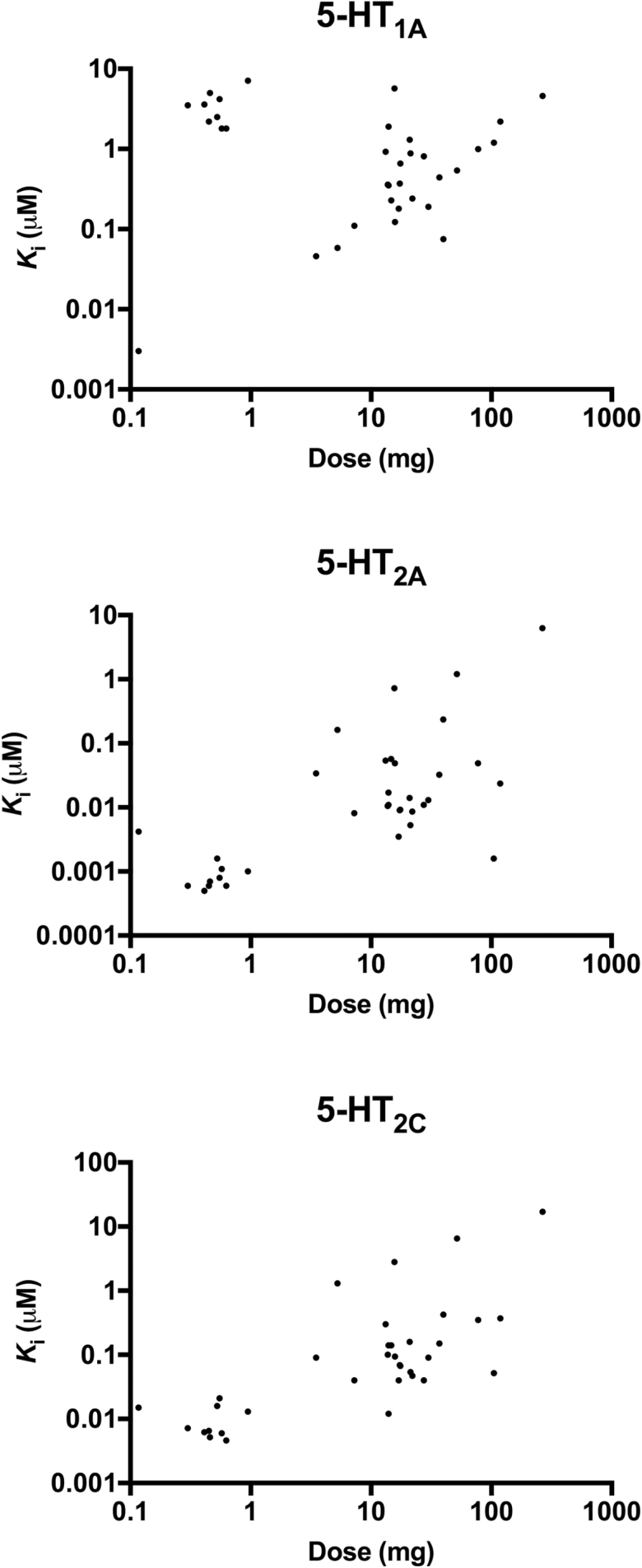
Correlations between 5-HT receptor affinities (mean Ki values) and their dose estimates are shown in Figure 2. Reported human doses for psychedelics were significantly correlated with 5-HT2A and 5-HT2C receptor binding (rs = 0.62, P < .001, n = 35, and rs = 0.69, P < .001, n = 35, respectively) but not with 5-HT1A receptor binding (rs = -0.18, P = .3, n = 35). The 5-HT2A and 5-HT2C affinity values were significantly intercorrelated (rs = 0.90, P < .001, n = 45), and the 5-HT1A and 5-HT2A affinity values were inversely intercorrelated (-0.32, P < .05, n = 45). No correlation was found between 5-HT1A receptor binding and 5-HT2c receptor binding.
Figure 2.
Correlations between dose estimates of psychedelics and their serotonin 5-HT receptor affinities (mean Ki values).
5-HT2A receptor activation potencies (mean EC50 values) did not correlate with reported human doses (rs = -0.08, P = .6, n = 35). Four substances did not activate the 5-HT2B receptor in the investigated concentration range, and these substances thus could not be included in the statistical analysis. The 5-HT2B receptor activation of the remaining psychedelics did not correlate with the dose estimates (rs = 0.25, P = .2, n = 31).
Discussion
Stimulants
In the present study, we found that both NET and DAT inhibition potencies were correlated highly significantly with human doses that are reportedly used across a larger set of psychoactive, mostly amphetamine-type stimulants. In contrast, SERT inhibition potency was inversely correlated with human doses, a finding that is consistent with the notion that serotonergic activity is inversely linked to the drug abuse liability of amphetamine-type substances (Ritz et al., 1987; Kuhar et al., 1991; Wee et al., 2005; Wee and Woolverton, 2006). We also found a significant intercorrelation between NET and DAT inhibition potencies across substances, which is unsurprising given their similarity (i.e., high amino acid sequence similarity [Andersen et al., 2015]) and the ability of both transporters to transport NE and DA across the cell membrane (Gu et al., 1994). The present data are consistent with a small previous study that reported that oral doses of 5 classic amphetamine-type stimulants used in clinical studies correlated with their NE-releasing potencies, although no significant correlation was shown for DA release (Rothman et al., 2001). In another study, Iversen and colleagues found no correlation between uptake inhibition potency and doses of stimulant drugs producing subjective effects (Iversen et al., 2013). The lack of correlation may relate to the small number of compounds tested.
We previously showed that DAT and NET inhibition potency but not SERT inhibition potency (IC50 values) were correlated with psychotropic effective doses within a subset of substances that were also included in the present analysis (Simmler et al., 2013). Altogether, the present study showed that DAT and NET inhibition potency values that are defined in vitro can be used to estimate whether a novel substance is psychoactive in humans, and the dose can be predicted when other known substances are co-analyzed as references. This finding has important implications because it indicates that relatively fast and simple in vitro measures are useful for legally scheduling novel substances as psychoactive and thus as illegal NPSs. Both the DAT and NET may serve as predictors of the human dose, whereas SERT inhibition potency can be used as an additional indicator, predicting lower clinical potency of the substance. Furthermore, the DAT/SERT inhibition ratio, which is defined as 1/DAT IC50: 1/SERT IC50 (Baumann et al., 2012), is a marker of the reinforcing effects and abuse liability of a substance (Baumann et al., 2000). Compounds with higher SERT vs DAT inhibition potency are typically associated with 3,4-methylenedioxymethamphetamine-like entactogenic effects, whereas drugs with high DAT vs SERT inhibition potency exert amphetamine-type psychostimulant effects and pose a higher risk for addiction (Simmler et al., 2013, 2014a; Liechti, 2014; Suyama et al., 2016).
Psychedelics
We showed that the doses of psychedelics were correlated with 5-HT2A receptor affinity (Ki values) but not with receptor activation potency in the calcium release assay used to determine EC50 values. 5-HT2A receptor activation is assumed to mediate the mind-altering effects of psychedelics (Glennon et al., 1984; Titeler et al., 1988) and such effects can be blocked by 5-HT2A receptor antagonists, such as ketanserin (Preller et al., 2017). All of the psychedelics that were included in our study were receptor agonists, and the correlation with receptor binding but not activation might be explained by higher sensitivity of the ligand-binding assays compared with the receptor activation assay. There are different 5-HT2A receptor activation assays, and the potencies for inducing calcium release in the assay that was used in the present study may not reflect the same pathway or mechanism that mediates the subjective effects of hallucinogens in humans. In fact, others have also reported that high-affinity agonist binding did not correlate well with the receptor activation of 5-HT2 receptors (Roth et al., 1997; Acuña-Castillo et al., 2002). Despite the lack of utility for predicting doses, the determination of 5-HT2A receptor activity remains crucial for determining whether a NPS has receptor agonist properties and may thus be classified as a psychedelic or whether it is an antagonist that only binds to the receptor. The present study showed that 5-HT2A receptor binding allows an estimate of the dose at which the substance is psychoactive in humans. Besides the correlation of the dose estimates for psychedelics with 5-HT2A receptor affinities, we also found a correlation with 5-HT2C receptor affinities. Today, it is widely accepted that 5-HT2A receptor activation is crucial for the action of psychedelics (Preller et al., 2017); the role of 5-HT2C receptor activation, however, remains enigmatic. As all known psychedelics are both 5-HT2A and 5-HT2C agonists, a contribution of 5-HT2C activation to psychedelic effects cannot be excluded (reviewed in Nichols, 2004, 2016).
Limitations
The outcomes of the present analysis highly depended on the types of substances that were included and may be different for other sets of psychoactive compounds. Although valid pharmacological data were used, the dose estimates were mainly derived from user reports. No controlled studies are currently available for most NPSs, but doses for some of the substances included in the present analysis are available from clinical studies. These doses were comparable to the reported recreational doses. Doses derived from clinical studies are available for mephedrone (200 mg; Papaseit et al., 2016), 3,4-methylenedioxymethamphetamine (100–125 mg; Tancer and Johanson, 2003; Papaseit et al., 2016; Vizeli and Liechti, 2017); MDAI (3 mg/kg; V. Auwärter et al., personal communication); cathinone (0.5 base mg/kg; Brenneisen et al., 1990); 4-fluoroamphetamine (100 mg; K. Kuypers et al., personal communication); D-amphetamine (15–40 mg; Martin et al., 1971; Brauer and de Wit, 1996; Dolder et al., 2017b); methamphetamine (15–30 mg; Martin et al., 1971; Gouzoulis-Mayfrank et al., 1999); MDEA (2 mg/kg; Gouzoulis-Mayfrank et al., 1999); BZP (100 mg; Lin et al., 2011); mCPP (0.5–0.75 mg/kg; Tancer and Johanson, 2003); methylphenidate (40–60 mg; Schmid et al., 2014); cocaine (48–96 mg; Volkow et al., 2000); diclofensine (50 mg; Funke et al., 1986); LSD (0.1 mg; Dolder et al., 2017a); 2C-B (20 mg; Gonzalez et al., 2015); mescaline sulfate (500 mg; Hermle et al., 1992); and psilocin/psilocybin (5–20 mg; Studerus et al., 2012). Therefore, even though the dose estimates of the current study were not derived from clinical studies, they are in accordance with the available clinical data.
Not accounted for in the in vitro assays were in vivo factors (e.g., bioavailability, route of administration, distribution, and brain penetration), which may influence clinical potency.
Conclusion
The present study found that in vitro pharmacological profiles of substances that interact with monoaminergic systems allow the characterization of substances as stimulants or psychedelics and may be used to predict human psychoactive doses. For stimulants, potent DAT and NET inhibition was associated with lower pharmacological doses in humans. In contrast, higher SERT inhibition potency was an additional indicator of lower stimulant potency and higher human doses. The potency of psychedelics was best predicted by 5-HT2A and 5-HT2C binding affinity. In contrast, the calcium mobilization assay used to determine 5-HT2A receptor activation potency did not predict the clinical potency of psychedelics. However, it is a necessity to determine whether a drug is a 5-HT2A agonist and therefore likely a psychedelic in humans.
Funding
This work was supported by the Federal Office of Public Health (grant no. 16.921318).
Supplementary Material
Acknowledgments
The authors thank Michael Arends for text editing.
Statement of Interest
None.
References
- Acuña-Castillo C, Villalobos C, Moya PR, Sáez P, Cassels BK, Huidobro-Toro JP(2002)Differences in potency and efficacy of a series of phenylisopropylamine/phenylethylamine pairs at 5-HT(2A) and 5-HT(2C) receptors. Br J Pharmacol 136:510–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen J, Ringsted KB, Bang-Andersen B, Strømgaard K, Kristensen AS(2015)Binding site residues control inhibitor selectivity in the human norepinephrine transporter but not in the human dopamine transporter. Sci Rep 5:15650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann MH, Ayestas MA, Dersch CM, Brockington A, Rice KC, Rothman RB(2000)Effects of phentermine and fenfluramine on extracellular dopamine and serotonin in rat nucleus accumbens: therapeutic implications. Synapse 36:102–113. [DOI] [PubMed] [Google Scholar]
- Baumann MH, Ayestas MA Jr, Partilla JS, Sink JR, Shulgin AT, Daley PF, Brandt SD, Rothman RB, Ruoho AE, Cozzi NV(2012)The designer methcathinone analogs, mephedrone and methylone, are substrates for monoamine transporters in brain tissue. Neuropsychopharmacology 37:1192–1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brauer LH, de Wit H(1996)Subjective responses to d-amphetamine alone and after pimozide pretreatment in normal, healthy volunteers. Biol Psychiatry 39:26–32. [DOI] [PubMed] [Google Scholar]
- Brenneisen R, Fisch HU, Koelbing U, Geisshüsler S, Kalix P(1990)Amphetamine-like effects in humans of the khat alkaloid cathinone. Br J Clin Pharmacol 30:825–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolder PC, Schmid Y, Steuer AE, Kraemer T, Rentsch KM, Hammann F, Liechti ME (2017a) Pharmacokinetics and pharmacodynamics of lysergic acid diethylamide in healthy subjects. Clin Pharmacokinet 56:1219–1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolder PC, Strajhar P, Vizeli P, Hammann F, Odermatt A, Liechti ME (2017b) Pharmacokinetics and pharmacodynamics of lisdexamfetamine compared with D-amphetamine in healthy subjects. Front Pharmacol 8:617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funke HJ, Holtmann W, Ismail S, Jansen W, Leonhardt KF, Muth H, Omer LM, O’Connolly M, Ramm H(1986)Double-blind comparison of diclofensine with nomifensine in outpatients with dysphoric mood. Pharmacopsychiatry 19:120–123. [DOI] [PubMed] [Google Scholar]
- Glennon RA, Titeler M, McKenney JD(1984)Evidence for 5-HT2 involvement in the mechanism of action of hallucinogenic agents. Life Sci 35:2505–2511. [DOI] [PubMed] [Google Scholar]
- González D, Torrens M, Farré M(2015)Acute effects of the novel psychoactive drug 2C-B on emotions. Biomed Res Int 2015:643878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gouzoulis-Mayfrank E, Schreckenberger M, Sabri O, Arning C, Thelen B, Spitzer M, Kovar KA, Hermle L, Büll U, Sass H(1999)Neurometabolic effects of psilocybin, 3,4-methylenedioxyethylamphetamine (MDE) and d-methamphetamine in healthy volunteers. A double-blind, placebo-controlled PET study with [18F]FDG. Neuropsychopharmacology 20:565–581. [DOI] [PubMed] [Google Scholar]
- Gu H, Wall SC, Rudnick G(1994)Stable expression of biogenic amine transporters reveals differences in inhibitor sensitivity, kinetics, and ion dependence. J Biol Chem 269:7124–7130. [PubMed] [Google Scholar]
- Hermle L, Fünfgeld M, Oepen G, Botsch H, Borchardt D, Gouzoulis E, Fehrenbach RA, Spitzer M(1992)Mescaline-induced psychopathological, neuropsychological, and neurometabolic effects in normal subjects: experimental psychosis as a tool for psychiatric research. Biol Psychiatry 32:976–991. [DOI] [PubMed] [Google Scholar]
- Iversen L, Gibbons S, Treble R, Setola V, Huang XP, Roth BL(2013)Neurochemical profiles of some novel psychoactive substances. Eur J Pharmacol 700:147–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhar MJ, Ritz MC, Boja JW(1991)The dopamine hypothesis of the reinforcing properties of cocaine. Trends Neurosci 14:299–302. [DOI] [PubMed] [Google Scholar]
- Liechti ME.(2014)Novel psychoactive substances (designer drugs): overview and pharmacology of modulators of monoamine signalling. Swiss Med Weekly 144:w14043. [DOI] [PubMed] [Google Scholar]
- Liechti ME.(2017)Modern clinical research on LSD. Neuropsychopharmacology 42:2114–2127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin JC, Jan RK, Lee H, Jensen MA, Kydd RR, Russell BR(2011)Determining the subjective and physiological effects of BZP combined with TFMPP in human males. Psychopharmacology (Berl) 214:761–768. [DOI] [PubMed] [Google Scholar]
- Luethi D, Hoener MC, Liechti ME (2018a) Effects of the new psychoactive substances diclofensine, diphenidine, and methoxphenidine on monoaminergic systems. Eur J Pharmacol 819:242–247. [DOI] [PubMed] [Google Scholar]
- Luethi D, Kaeser PJ, Brandt SD, Krähenbühl S, Hoener MC, Liechti ME (2018b) Pharmacological profile of methylphenidate-based designer drugs. Neuropharmacology 134:133–140. [DOI] [PubMed] [Google Scholar]
- Luethi D, Kolaczynska KE, Docci L, Krähenbühl S, Hoener MC, Liechti ME (2018c) Pharmacological profile of mephedrone analogs and related new psychoactive substances. Neuropharmacology 134:4–12. [DOI] [PubMed] [Google Scholar]
- Luethi D, Trachsel D, Hoener MC, Liechti ME (2018d) Monoamine receptor interaction profiles of 4-thio-substituted phenethylamines (2C-T drugs). Neuropharmacology 134:141–148. [DOI] [PubMed] [Google Scholar]
- Martin WR, Sloan JW, Sapira JD, Jasinski DR(1971)Physiologic, subjective, and behavioral effects of amphetamine, methamphetamine, ephedrine, phenmetrazine, and methylphenidate in man. Clin Pharmacol Ther 12:245–258. [DOI] [PubMed] [Google Scholar]
- Nichols DE.(2004)Hallucinogens. Pharmacol Ther 101:131–181. [DOI] [PubMed] [Google Scholar]
- Nichols DE.(2016)Psychedelics. Pharmacol Rev 68:264–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papaseit E, Pérez-Mañá C, Mateus JA, Pujadas M, Fonseca F, Torrens M, Olesti E, de la Torre R, Farré M(2016)Human pharmacology of mephedrone in comparison with MDMA. Neuropsychopharmacology 41:2704–2713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preller KH, Herdener M, Pokorny T, Planzer A, Kraehenmann R, Stämpfli P, Liechti ME, Seifritz E, Vollenweider FX(2017)The fabric of meaning and subjective effects in LSD-induced states depend on serotonin 2A receptor activation. Curr Biol 27:451–457. [DOI] [PubMed] [Google Scholar]
- Rickli A, Hoener MC, Liechti ME (2015a) Monoamine transporter and receptor interaction profiles of novel psychoactive substances: para-halogenated amphetamines and pyrovalerone cathinones. Eur Neuropsychopharmacol 25:365–376. [DOI] [PubMed] [Google Scholar]
- Rickli A, Kopf S, Hoener MC, Liechti ME (2015b) Pharmacological profile of novel psychoactive benzofurans. Br J Pharmacol 172:3412–3425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rickli A, Luethi D, Reinisch J, Buchy D, Hoener MC, Liechti ME (2015c) Receptor interaction profiles of novel N-2-methoxybenzyl (nbome) derivatives of 2,5-dimethoxy-substituted phenethylamines (2C drugs). Neuropharmacology 99:546–553. [DOI] [PubMed] [Google Scholar]
- Rickli A, Moning OD, Hoener MC, Liechti ME(2016)Receptor interaction profiles of novel psychoactive tryptamines compared with classic hallucinogens. Eur Neuropsychopharmacol 26:1327–1337. [DOI] [PubMed] [Google Scholar]
- Ritz MC, Lamb RJ, Goldberg SR, Kuhar MJ(1987)Cocaine receptors on dopamine transporters are related to self-administration of cocaine. Science 237:1219–1223. [DOI] [PubMed] [Google Scholar]
- Roth BL, Choudhary MS, Khan N, Uluer AZ(1997)High-affinity agonist binding is not sufficient for agonist efficacy at 5-hydroxytryptamine2A receptors: evidence in favor of a modified ternary complex model. J Pharmacol Exp Ther 280:576–583. [PubMed] [Google Scholar]
- Rothman RB, Baumann MH(2003)Monoamine transporters and psychostimulant drugs. Eur J Pharmacol 479:23–40. [DOI] [PubMed] [Google Scholar]
- Rothman RB, Baumann MH, Dersch CM, Romero DV, Rice KC, Carroll FI, Partilla JS(2001)Amphetamine-type central nervous system stimulants release norepinephrine more potently than they release dopamine and serotonin. Synapse 39:32–41. [DOI] [PubMed] [Google Scholar]
- Schmid Y, Hysek CM, Simmler LD, Crockett MJ, Quednow BB, Liechti ME(2014)Differential effects of MDMA and methylphenidate on social cognition. J Psychopharmacol 28:847–856. [DOI] [PubMed] [Google Scholar]
- Shulgin A, Shulgin A(1995)PIHKAL: a chemical love story. Berkley, CA: Transform Press. [Google Scholar]
- Shulgin A, Shulgin A(1997)TIHKAL: the continuation. Berkley, CA: Transform Press. [Google Scholar]
- Simmler LD, Buser TA, Donzelli M, Schramm Y, Dieu LH, Huwyler J, Chaboz S, Hoener MC, Liechti ME(2013)Pharmacological characterization of designer cathinones in vitro. Br J Pharmacol 168:458–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmler LD, Rickli A, Hoener MC, Liechti ME (2014a) Monoamine transporter and receptor interaction profiles of a new series of designer cathinones. Neuropharmacology 79:152–160. [DOI] [PubMed] [Google Scholar]
- Simmler LD, Rickli A, Schramm Y, Hoener MC, Liechti ME (2014b) Pharmacological profiles of aminoindanes, piperazines, and pipradrol derivatives. Biochem Pharmacol 88:237–244. [DOI] [PubMed] [Google Scholar]
- Studerus E, Gamma A, Kometer M, Vollenweider FX(2012)Prediction of psilocybin response in healthy volunteers. PLoS One 7:e30800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suyama JA, Sakloth F, Kolanos R, Glennon RA, Lazenka MF, Negus SS, Banks ML(2016)Abuse-related neurochemical effects of para-substituted methcathinone analogs in rats: microdialysis studies of nucleus accumbens dopamine and serotonin. J Pharmacol Exp Ther 356:182–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tancer M, Johanson CE(2003)Reinforcing, subjective, and physiological effects of MDMA in humans: a comparison with d-amphetamine and mcpp. Drug Alcohol Depend 72:33–44. [DOI] [PubMed] [Google Scholar]
- Titeler M, Lyon RA, Glennon RA(1988)Radioligand binding evidence implicates the brain 5-HT2 receptor as a site of action for LSD and phenylisopropylamine hallucinogens. Psychopharmacology (Berl) 94:213–216. [DOI] [PubMed] [Google Scholar]
- Trachsel D, Lehmann D, Enzensperger C(2013)Phenethylamine: von der struktur zur funktion. Solothurn, Switzerland: Nachtschatten Verlag. [Google Scholar]
- Vizeli P, Liechti ME(2017)Safety pharmacology of acute MDMA administration in healthy subjects. J Psychopharmacol 31:576–588. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fischman MW, Foltin R, Fowler JS, Franceschi D, Franceschi M, Logan J, Gatley SJ, Wong C, Ding YS, Hitzemann R, Pappas N(2000)Effects of route of administration on cocaine induced dopamine transporter blockade in the human brain. Life Sci 67:1507–1515. [DOI] [PubMed] [Google Scholar]
- Wee S, Anderson KG, Baumann MH, Rothman RB, Blough BE, Woolverton WL(2005)Relationship between the serotonergic activity and reinforcing effects of a series of amphetamine analogs. J Pharmacol Exp Ther 313:848–854. [DOI] [PubMed] [Google Scholar]
- Wee S, Woolverton WL(2006)Self-administration of mixtures of fenfluramine and amphetamine by rhesus monkeys. Pharmacol Biochem Behav 84:337–343. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.