Abstract
Background
The polyamines are a group of ubiquitous low-molecular–weight aliphatic molecules that play an essential role in various physiological functions of the mammalian CNS. Previous literature has indicated alterations in the expression of polyamine-related genes in the brains of individuals who died by suicide, including downregulation of spermidine/spermine N1-acetyltransferase, a key enzyme involved in polyamine catabolism. One such polyamine, agmatine, has been shown to act as an antidepressant in animal models of depressive-like behavior. However, agmatine concentrations have not been explored in postmortem human brain of individuals who died by suicide.
Methods
To measure agmatine in postmortem human brain tissue, we employed our previously published high-resolution capillary gas chromatography in combination with mass spectrometry method. Using this method, we analyzed agmatine levels in a total of 120 tissue samples from Brodmann areas 4, 11, and 44 of 40 male subjects comprising controls (n=13), individuals who died by suicide and met criteria for major depressive disorder (n=14), and subjects who died by suicide and did not meet criteria for major depressive disorder (n=13).
Results
Agmatine fell within the expected nanomolar range and was significantly reduced in the cortex of suicides, irrespective of meeting criteria for major depressive disorder compared with controls.
Conclusions
This is the first gas chromatography-mass spectrometry study to analyze agmatine concentrations in human postmortem brain of individuals who died by suicide. These results add to our mechanistic understanding of the role that the polyamine stress response pathway may play in the neurobiology of major depression and/or suicide.
Keywords: agmatine, high-resolution capillary gas chromatography-mass spectrometry, polyamines, suicide
Significance Statement
The polyamines are a group of molecules that play an essential role in various physiological functions of the mammalian CNS. Previous literature has indicated alterations in the expression of polyamine-related genes in the brains of individuals who died by suicide, including downregulation of spermidine/spermine N1-acetyltransferase (SAT1), a key enzyme involved in polyamine catabolism. In a previous report of ours, we showed evidence of elevated levels of the polyamines putrescine and spermidine in the cortex of depressed suicides compared with controls. Agmatine, another member of the polyaminergic system, has been extensively implicated as an antidepressant in preclinical models of depressive-like behavior. However, agmatine concentrations have not been explored in postmortem human brain of individuals who died by suicide. Here we describe such an investigation and reveal that agmatine levels are reduced in the cortex of suicides. The novel findings of this study further implicate a role of the polyaminergic system in the neurobiology of depression and suicide.
Introduction
The polyamines are a group of ubiquitous low-molecular–weight aliphatic molecules that include agmatine, ((4-aminobutyl) guanidine), putrescine (1,4-diaminobutane), spermidine (N-[3-aminopropyl]-1,4-diaminobutane), and spermine (N,N-bis[3-aminopropyl]-1,4-diaminobutane). In mammals, polyamines play essential roles in various physiological functions, including cell proliferation, signaling and apoptosis, immunity, neurotransmission, adult brain neurogenesis, and stress regulation at both the cellular and behavioral levels (Ramani et al., 2014). The majority of brain polyamines are stored in astrocytes and synaptic vesicles, allowing for their place in glial and neuronal communication. For instance, in the brain polyamines are synthesized in neurons, stored in synaptosomes, and released to modulate neurotransmission through ionotropic glutamate receptors such as N-methyl—aspartate (NMDA) receptors and alpha-amino-3-hydroxy-5-methylisoxazole-4-propionic acid (AMPA) receptors (as reviewed in Ramani et al., 2014).
Alterations in the expression of the polyamines and their metabolic enzymes have been found in many psychiatric illnesses, including schizophrenia (Liu et al., 2016), mood disorders (Limon et al., 2016), stress and anxiety (Karssen et al., 2007), addiction (Aricioglu et al., 2004), and suicidal behavior (Sequeira et al., 2006, 2007; Turecki, 2014).
Gene expression studies using postmortem human brain samples have consistently revealed dysregulation of the polyamine system in major depressive disorder (MDD) and suicide. The first study showed a significant decrease in gene and protein expression of spermidine/spermine N1-acetyltransferase (SAT1), a key enzyme responsible for polyamine catabolism, in Brodmann areas 4, 8/9, and 11 in both nondepressed suicides and MDD suicides compared with controls (Sequeira et al., 2006). This finding was subsequently confirmed by independent studies and different groups across various brain regions and sample populations (Sequeira et al., 2007; Klempan et al., 2009a, 2009b; Fiori et al., 2011; Pantazatos et al., 2015).
As such, there has been considerable interest in the polyaminergic system as a promising candidate for the treatment of CNS diseases, particularly depression. In fact, preclinical data indicate that agmatine specifically may act as an endogenous antidepressant compound. Zomkowski et al. (2002) were first to describe antidepressant-like effects of agmatine on mouse immobility during the forced swimming test and tail suspension test. Using the same behavioral measures of depressive-like behavior, Li et al. (2003) revealed an antidepressant-like effect produced by agmatine in both mice and rats. A significant number of follow-up studies have investigated the potential mechanisms through which agmatine exerts its antidepressant-like effects. One such mechanism includes blockade of NMDA receptors and inhibition of NOS (Zomkowski et al., 2002), which may thus result in protection against NMDA-induced cytotoxicity (Li et al., 2003). More recent evidence has also suggested that agmatine has fast-acting antidepressant properties comparable with those of the NMDA antagonist ketamine (Meylan et al., 2016; Neis et al., 2016a). Antidepressant-like effects of agmatine have also been shown to be mediated through an interaction with 5-HT(1A/1B) and 5-HT(2) receptors (Zomkowski et al., 2004; Jiang et al., 2008), μ- and δ-opioid receptors (Zomkowski et al., 2005), alpha2 receptors (Jiang et al., 2008), activation of monoaminergic systems (Neis et al., 2014), its effects on neurogenesis (Li et al., 2006), and activation of alpha-amino-3-hydroxy-5-methylisoxazole-4-propionic acid and TrkB receptors (Neis et al., 2016b). Most recently, agmatine was shown to attenuate chronic unpredictable mild stress-induced anxiety- and depressive-like behavior by inhibiting serum CORT and raising BDNF levels (Gawali et al., 2017). In extension to these findings, a clinical study by Shopsin (2013), albeit a small sample size, showed an antidepressant effect of exogenous agmatine on depressed subjects. Participants in the study achieved total illness remission without physical or behavioral side effects.
To our knowledge, agmatine concentrations have yet to be explored in postmortem human brain of suicides. The aim of the present study was to employ our previously published negative ion chemical ionization (NCI)-GC-MS method (Chen et al., 2010a) for quantitation of agmatine in postmortem human brain to investigate cortical brain concentrations of agmatine in suicides who met criteria for a diagnosis of MDD, suicides who did not, and controls.
Methods
Subjects and Diagnostic Procedures
Our sample consisted of 120 brain tissue samples from 40 male subjects of French-Canadian origin, including 13 control subjects (C), 14 subjects who died by suicide and met criteria for MDD (DS), and 13 subjects who died by suicide but did not meet criteria for MDD (NDS). We chose an only-male sample to avoid sex-related heterogeneity because sex-specific differences in levels of polyamines and their metabolic enzymes, as well as responses to polyamine exposure, have been reported (Ferioli et al., 1999; Gilad et al., 2002; Bastida et al., 2007; Barron et al., 2008). All subjects collected by the brain bank died suddenly without a prolonged agonal period. Controls died either in accidents or by natural death; cases and controls were matched for age, pH, and postmortem interval (PMI). Psychiatric diagnoses were obtained using the psychological autopsy method with the Structured Clinical Interview for DSM-IV Axis I, as described elsewhere (Dumais et al., 2005). Written informed consent was obtained for all subjects from next of kin. This study was approved by our local institutional review board. Postmortem brain tissues were obtained from the Douglas–Bell Canada Brain Bank (http://www.douglas.qc.ca/page/brain-bank) in which they were processed and dissected at 4°C and snap-frozen in liquid nitrogen before storage at −80°C following standard procedures (Bird and Vonsattel, 1993). Brain tissue dissection was performed following standard procedures and anatomical landmarks (Nolte, 2002; Mai et al., 2007). Specifically, we focused on Brodmann areas 4 (motor cortex), 11(orbital cortex), and 44 (prefrontal cortex); the former 2 were brain regions used in our original report indicating SAT1 differential expression in suicide (Sequeira et al., 2006) and another report showing elevated levels of putrescine and spermidine in suicide (Chen et al., 2010b). Brodmann area 44 was previously used to provide evidence that various epigenetic mechanisms contribute to the differential expression of polyaminergic genes in suicide (Fiori et al., 2011; Fiori et al., 2012; Gross et al., 2013; Lopez et al., 2014).
NCI-GC-MS Method for Analysis Agmatine
For effective extraction and quantitation of agmatine from a small quantity of postmortem human brain tissue, we used an NCI-GC-MS method previously developed in our laboratory (Chen et al., 2010a). Briefly, this method includes a negative chemical ionization GC-MS with a selected ion monitoring (SIM) technique. This allowed us to accurately measure agmatine to the level of 0.1 ng/mg of wet brain tissue level. 15N4-agmatine was used as the internal standard for endogenous agmatine, and an authentic agmatine compound was used as a standard for calibration. A selected ion chromatogram for agmatine, extracted from the cerebral cortex of a control brain and one from a suicide brain with spiked internal standard 15N4-agmatine is given in Supplementary Figure 1.
GC-MS Conditions
NCI was used in full scan, and SIM mode was carried out for all measurements with an Agilent HP6890/MSD5973N Chemstation system (Agilent Technologies. Inc. Santa Clara, CA). The methane gas (99.99%) was set at 2.0 mL/min and helium flow at 1.0 mL/min. The tuning compound used for NCI was perfluoro-5,8-dimethyl-3,6,9-trioxidodecane. An HP-5MS capillary column was used for the analysis. The following GC program was employed: 110 to 210°C at 30°C/min, hold at 210°C for 2 minutes, then increase to 320°C at 20°C/min and held for 4 minutes to bake out. MS conditions were: source 120°C, quadrupole 110°C, interface 260°C, injector 270°C. Full scan started at 5.45 minutes and ended at 15 minutes. The SIM ions monitored were m/z 496 (M-) and 476 (M-HF) for the 15N4-agmatine HFAA derivative and 492 (M-), and 472 (M-HF) for the 14N4-agmatine-HFAA derivative.
Data Analysis
GC-MS data analysis was carried out with Chemstation software (Agilent Technologies). Statistical analyses were performed using SPSS 22.0. Pearson correlations were used to assess relationships between sample characteristics such as age, pH, and PMI with agmatine concentrations. To determine if there was an effect of alcohol dependence, age, pH, or PMI on agmatine concentrations, we conducted a 2-way ANCOVA with group and alcohol dependence as fixed factors and age, pH, and PMI as covariates. All nonsignificant variables were then removed and the most parsimonious model was employed. Each brain area was analyzed separately, and the normality assumption for the residuals of each model was tested via the Shapiro Wilks test. Bonferroni posthoc comparisons were used to analyze agmatine differences between clinical groups (C, DS, and NDS).
Results
Clinical and Demographic Features
Supplementary Table 1 lists the demographic and clinical characteristics of the subjects included in this study according to groups. DS and NDS cases, as well as controls, had similar demographic characteristics, with no significant differences in age (F(2,37)=1.122, P=.336), PMI (F(2,37)=1.560, P=.224), or pH (F(2,37)=0.276, P=.761).
Analysis of Agmatine Levels
Representative mass spectra are shown in Supplementary Figure 1. Levels of agmatine were within the expected nanogram range. Agmatine concentrations did not significantly correlate with age, pH, or PMI in any brain region (see Supplementary Table 2).
A 2-way ANCOVA revealed a significant main effect of group (BA 4: F(2,31)=25.798, P=2.53×10−7; BA 11: F(2,31)=20.756, P=2.0×10−6; BA 44: F(1,31)=18.881, P=4.0×10−6) but no effect of alcohol dependence on agmatine concentrations. The interaction between group and alcohol dependence was not significant, nor were there significant effects of age, pH, or PMI in any region (see Supplementary Table 3). The residuals of each model were normally distributed.
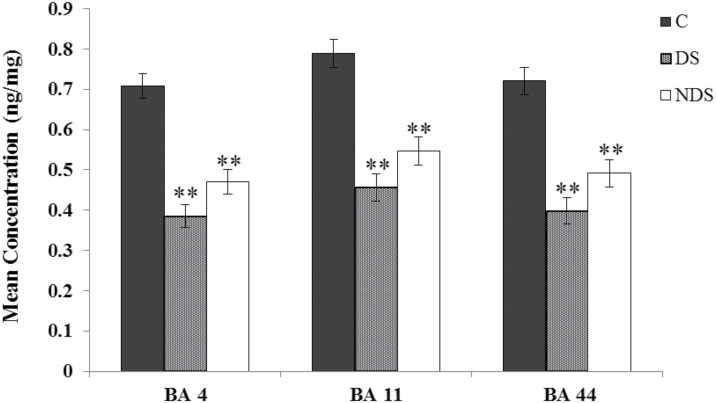
A 1-way ANOVA, including only group as a fixed factor, revealed a significant effect of group on agmatine levels in BA 4 (F(2,37)=32.529, P=7.04×10−9), BA 11 (F(2,37)=24.416, P=1.73×10−7), and BA 44 (F(2,37)=24.022, P=2.06×10−7). The residuals of each model were normally distributed. For all 3 brain regions, Bonferroni adjusted posthoc comparisons revealed a significant difference between DS and controls (P<.001) and between NDS and controls (P<.001). Comparisons between DS and NDS were not significant for BA 4 (P=.137), BA 11 (P=.220), and BA 44 (P=.179) (Figure 1).
Figure 1.
Agmatine concentrations in postmortem brain tissues by brain area and group. Concentrations (ng/mg)±SEM were obtained from controls (n=13), subjects who died by suicide and met criteria for MDD (n=14), and subjects who died by suicide but did not meet criteria for MDD (n=13) for Brodmann areas 4, 11, and 44. **P<.001.
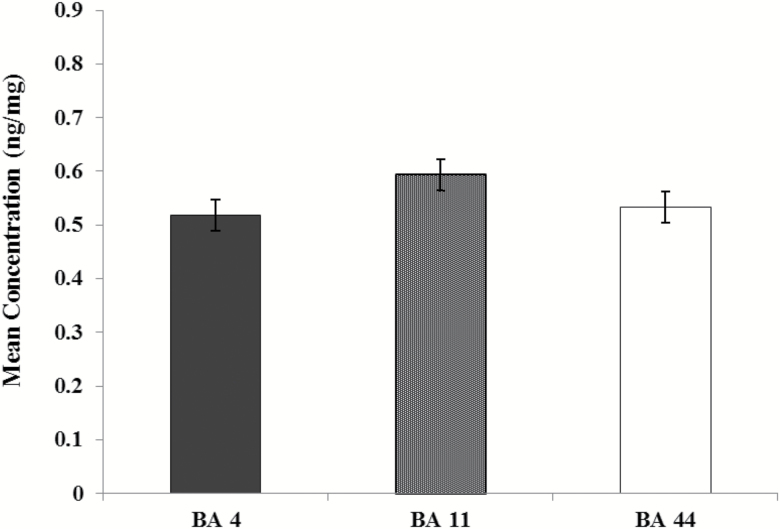
There was no overall effect of brain area on agmatine concentrations (F(2,117)=1.982, P=.142) (Figure 2).
Figure 2.
Overall agmatine concentrations in postmortem brain tissue by brain area. Concentrations (ng/mg)±SEM were determined by combing all groups and comparing across Brodmann areas 4 (n=40), 11 (n=40), and 44 (n=40).
Discussion
Using an NCI-GC-MS method specifically designed for measuring agmatine concentrations in postmortem brain tissues, we were able to successfully determine agmatine levels in tissue samples from 3 brain regions obtained from 40 male subjects. We found significant decreases in agmatine concentrations across all 3 Brodmann areas (B4, 11, 44), for both suicide groups, irrespective of MDD diagnosis, compared with controls. We did not find a significant difference in overall agmatine concentrations across brain areas.
Our present findings are in agreement with a previous study of ours using an overlapping sample and 2 of the same brain areas, which found elevated putrescine and spermidine levels in cortical tissue samples of suicides (Chen et al., 2010b).
Agmatine is inactivated through hydrolyzation, by agmatinase, to produce putrescine. Thus, elevated levels of putrescine may be due in part to over active biosynthesis from its agmatine precursor. One explanation for this finding is that agmatinase may be overexpressed and/or displays higher enzymatic activity in the brains of suicides. Agmatine would therefore be more efficiently converted to putrescine, thereby reducing its levels. This hypothesis is supported by immunohistochemical evidence of increased agmatinase expression in hippocampal interneurons of individuals with affective disorder, most of whom died by suicide (Bernstein et al., 2012). Agmatine has been shown to suppress ornithine decarboxylase (ODC) activity in numerous cell lines (Vargiu et al., 1999). Another interpretation is that less agmatine would result in less ODC suppression and therefore increased putrescine biosynthesis through its conventional pathway. Interestingly, we have previously shown increased expression of 2 antizymes, OAZ1 and OAZ2, which directly inhibit ODC activity and protein levels (Fiori et al., 2011). Translation of each antizyme is controlled by a unique frameshift that is induced by elevated polyamine levels (Mangold, 2005). Taking into account the presence of increased polyamine levels (Chen et al. (2010b), in conjunction with elevated antizyme mRNA levels, it seems likely that protein levels of antizyme are also increased as a potential regulatory response targeted at decreasing ODC activity in these brain regions. However, lower agmatine may impinge upon this natural negative feedback mechanism responsible for regulating ODC activity and downstream polyamine levels.
Taken together, our previous report of elevated putrescine and spermidine levels in the brain of suicides may be due in part to overactive biosynthesis of the former 2 polyamines and depletion of agmatine. Reduced SAT1 gene and protein expression may thus be a consequence of a compensatory mechanism intended to counter the overactive biosynthesis of putrescine and indicative of an improper polyamine stress response. Although we did not find a difference between MDD suicides and non-MDD suicides, it is reasonable to speculate that agmatine changes may associate with depressed mood. Whether meeting criteria for MDD or not, individuals who die by suicide do so while depressed and hopeless.
In contrast to our findings in postmortem tissue, increased plasma concentrations of agmatine have been reported in depressed subjects compared with controls (Halaris et al., 1999). While agmatine is able to cross the blood brain barrier (Piletz et al., 2003), the relationship between its concentrations in plasma vs brain is not yet clear in depression and/or suicide. Given that our reported findings in postmortem tissue are in the opposite direction as the aforementioned study, central concentrations may be inversely related to those in the periphery. Interestingly, increased plasma agmatine concentrations have also been reported in both plasma and postmortem brain samples from schizophrenic individuals (Uzbay et al., 2013; Liu et al., 2016). Therefore, a crucial avenue to explore would be to examine agmatine concentrations in both postmortem brain and plasma samples from individuals who died by suicide. Furthermore, understanding how agmatine concentrations vary in other Brodmann areas, which may not be related to mood, would help clarify whether dysregulation is region specific. This is, in fact, likely to be the case, as evidence for region-specific gene expression differences in suicide has been reported (Fiori et al., 2011). A final and equally important question to explore would be to tease apart the effects of depression vs suicide by investigating postmortem concentrations of agmatine in cortical samples of individuals who were depressed but did not die by suicide. Nevertheless, our previous findings of elevated putrescine and spermidine levels coupled with our current findings of reduced agmatine levels in the brains of individuals who died by suicide further implicate a role of the polyamine stress response in the neurobiology of depression and suicide.
Supplementary Materials
Supplementary data are available at International Journal of Neuropsychopharmacology (IJNPPY) online.
Funding
G.T. holds a Canada Research Chair (Tier 1) and a NARSAD Distinguished Investigator Award. He is supported by grants from the Canadian Institute of Health Research (CIHR) (FDN148374 and EGM141899), by the FRQS through the Quebec Network on Suicide, Mood Disorders and Related Disorders, and through an investigator-initiated research grant from Pfizer. D.A. is a Vanier Scholar funded by the CIHR.
Acknowledgments
We thank the Douglas Bell Canada Brain Bank for providing the human postmortem brain tissue for this study.
Statement of Interest
None
References
- Aricioglu F, Means A, Regunathan S(2004)Effect of agmatine on the development of morphine dependence in rats: potential role of camp system. Eur J Pharmacol 504:191–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barron S, Mulholland PJ, Littleton JM, Prendergast MA(2008)Age and gender differences in response to neonatal ethanol withdrawal and polyamine challenge in organotypic hippocampal cultures. Alcohol Clin Exp Res 32:929–936. [DOI] [PubMed] [Google Scholar]
- Bastida CM, Cremades A, Castells MT, López-Contreras AJ, López-García C, Sánchez-Mas J, Peñafiel R(2007)Sexual dimorphism of ornithine decarboxylase in the mouse adrenal: influence of polyamine deprivation on catecholamine and corticoid levels. Am J Physiol Endocrinol Metab 292:E1010–E1017. [DOI] [PubMed] [Google Scholar]
- Bernstein HG, Stich C, Jäger K, Dobrowolny H, Wick M, Steiner J, Veh R, Bogerts B, Laube G(2012)Agmatinase, an inactivator of the putative endogenous antidepressant agmatine, is strongly upregulated in hippocampal interneurons of subjects with mood disorders. Neuropharmacology 62:237–246. [DOI] [PubMed] [Google Scholar]
- Bird ED, Vonsattel JP(1993)The development of a brain bank. J Neural Transm Suppl 39:17–23. [PubMed] [Google Scholar]
- Chen GG, Turecki G, Mamer OA (2010a) A novel liquid-liquid extraction and stable isotope dilution NCI-GC-MS method for quantitation of agmatine in postmortem brain cortex. J Mass Spectrom 45:560–565. [DOI] [PubMed] [Google Scholar]
- Chen GG, Fiori LM, Moquin L, Gratton A, Mamer O, Mechawar N, Turecki G (2010b) Evidence of altered polyamine concentrations in cerebral cortex of suicide completers. Neuropsychopharmacology 35:1477–1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumais A, Lesage AD, Lalovic A, Séguin M, Tousignant M, Chawky N, Turecki G(2005)Is violent method of suicide a behavioral marker of lifetime aggression?Am J Psychiatry 162:1375–1378. [DOI] [PubMed] [Google Scholar]
- Ferioli ME, Pinotti O, Pirona L(1999)Gender-related differences in polyamine oxidase activity in rat tissues. Amino Acids 17:139–148. [DOI] [PubMed] [Google Scholar]
- Fiori LM, Bureau A, Labbe A, Croteau J, Noël S, Mérette C, Turecki G(2011)Global gene expression profiling of the polyamine system in suicide completers. Int J Neuropsychopharmacol 14:595–605. [DOI] [PubMed] [Google Scholar]
- Fiori LM, Gross JA, Turecki G(2012)Effects of histone modifications on increased expression of polyamine biosynthetic genes in suicide. Int J Neuropsychopharmacol 15:1161–1166. [DOI] [PubMed] [Google Scholar]
- Gawali NB, Bulani VD, Gursahani MS, Deshpande PS, Kothavade PS, Juvekar AR(2017)Agmatine attenuates chronic unpredictable mild stress-induced anxiety, depression-like behaviours and cognitive impairment by modulating nitrergic signalling pathway. Brain Res 1663:66–77. [DOI] [PubMed] [Google Scholar]
- Gilad VH, Halperin R, Chen-Levy Z, Gilad GM(2002)Cyclic changes of plasma spermine concentrations in women. Life Sci 72:135–141. [DOI] [PubMed] [Google Scholar]
- Gross JA, Fiori LM, Labonté B, Lopez JP, Turecki G(2013)Effects of promoter methylation on increased expression of polyamine biosynthetic genes in suicide. J Psychiatr Res 47:513–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halaris A, Zhu H, Feng Y, Piletz JE(1999)Plasma agmatine and platelet imidazoline receptors in depression. Ann N Y Acad Sci 881:445–451. [DOI] [PubMed] [Google Scholar]
- Jiang XZ, Li YF, Zhang YZ, Chen HX, Li J, Wang NP(2008)5-HT1A/1B receptors, alpha2-adrenoceptors and the post-receptor adenylate cyclase activation in the mice brain are involved in the antidepressant-like action of agmatine. Yao Xue Xue Bao 43:467–473. [PubMed] [Google Scholar]
- Karssen AM, Her S, Li JZ, Patel PD, Meng F, Bunney WE Jr, Jones EG, Watson SJ, Akil H, Myers RM, Schatzberg AF, Lyons DM(2007)Stress-induced changes in primate prefrontal profiles of gene expression. Mol Psychiatry 12:1089–1102. [DOI] [PubMed] [Google Scholar]
- Klempan TA, Sequeira A, Canetti L, Lalovic A, Ernst C, ffrench-Mullen J, Turecki G (2009a) Altered expression of genes involved in ATP biosynthesis and gabaergic neurotransmission in the ventral prefrontal cortex of suicides with and without major depression. Mol Psychiatry 14:175–189. [DOI] [PubMed] [Google Scholar]
- Klempan TA, Rujescu D, Mérette C, Himmelman C, Sequeira A, Canetti L, Fiori LM, Schneider B, Bureau A, Turecki G (2009b) Profiling brain expression of the spermidine/spermine n1-acetyltransferase 1 (SAT1) gene in suicide. Am J Med Genet B Neuropsychiatr Genet 150B:934–943. [DOI] [PubMed] [Google Scholar]
- Li YF, Gong ZH, Cao JB, Wang HL, Luo ZP, Li J(2003)Antidepressant-like effect of agmatine and its possible mechanism. Eur J Pharmacol 469:81–88. [DOI] [PubMed] [Google Scholar]
- Li YF, Chen HX, Liu Y, Zhang YZ, Liu YQ, Li J(2006)Agmatine increases proliferation of cultured hippocampal progenitor cells and hippocampal neurogenesis in chronically stressed mice. Acta Pharmacol Sin 27:1395–1400. [DOI] [PubMed] [Google Scholar]
- Limon A, Mamdani F, Hjelm BE, Vawter MP, Sequeira A(2016)Targets of polyamine dysregulation in major depression and suicide: activity-dependent feedback, excitability, and neurotransmission. Neurosci Biobehav Rev 66:80–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu P, Jing Y, Collie ND, Dean B, Bilkey DK, Zhang H(2016)Altered brain arginine metabolism in schizophrenia. Transl Psychiatry 6:e871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez JP, Fiori LM, Gross JA, Labonte B, Yerko V, Mechawar N, Turecki G(2014)Regulatory role of mirnas in polyamine gene expression in the prefrontal cortex of depressed suicide completers. Int J Neuropsychopharmacol 17:23–32. [DOI] [PubMed] [Google Scholar]
- Mai JK, Paxinos G, Voss T(2007)Atlas of the Human Brain. New York: Academic Press. [Google Scholar]
- Mangold U.(2005)The antizyme family: polyamines and beyond. IUBMB Life 57:671–676. [DOI] [PubMed] [Google Scholar]
- Meylan EM, Breuillaud L, Seredenina T, Magistretti PJ, Halfon O, Luthi-Carter R, Cardinaux JR(2016)Involvement of the agmatinergic system in the depressive-like phenotype of the crtc1 knockout mouse model of depression. Transl Psychiatry 6:e852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neis VB, Manosso LM, Moretti M, Freitas AE, Daufenbach J, Rodrigues AL(2014)Depressive-like behavior induced by tumor necrosis factor-α is abolished by agmatine administration. Behav Brain Res 261:336–344. [DOI] [PubMed] [Google Scholar]
- Neis VB, Bettio LEB, Moretti M, Rosa PB, Ribeiro CM, Freitas AE, Gonçalves FM, Leal RB, Rodrigues ALS (2016a) Acute agmatine administration, similar to ketamine, reverses depressive-like behavior induced by chronic unpredictable stress in mice. Pharmacol Biochem Behav 150–151:108–114. [DOI] [PubMed] [Google Scholar]
- Neis VB, Moretti M, Bettio LE, Ribeiro CM, Rosa PB, Gonçalves FM, Lopes MW, Leal RB, Rodrigues AL (2016b) Agmatine produces antidepressant-like effects by activating AMPA receptors and mtor signaling. Eur Neuropsychopharmacol 26:959–971. [DOI] [PubMed] [Google Scholar]
- Nolte J.(2002)The human brain: an introduction to its functional neuroanatomy. St Louis:Mosby. [Google Scholar]
- Pantazatos SP, Andrews SJ, Dunning-Broadbent J, Pang J, Huang YY, Arango V, Nagy PL, John Mann J(2015)Isoform-level brain expression profiling of the spermidine/spermine N1-acetyltransferase1 (SAT1) gene in major depression and suicide. Neurobiol Dis 79:123–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piletz JE, May PJ, Wang G, Zhu H(2003)Agmatine crosses the blood-brain barrier. Ann N Y Acad Sci 1009:64–74. [DOI] [PubMed] [Google Scholar]
- Ramani D, De Bandt JP, Cynober L(2014)Aliphatic polyamines in physiology and diseases. Clin Nutr 33:14–22. [DOI] [PubMed] [Google Scholar]
- Sequeira A, Gwadry FG, Ffrench-Mullen JM, Canetti L, Gingras Y, Casero RA Jr, Rouleau G, Benkelfat C, Turecki G(2006)Implication of SSAT by gene expression and genetic variation in suicide and major depression. Arch Gen Psychiatry 63:35–48. [DOI] [PubMed] [Google Scholar]
- Sequeira A, Klempan T, Canetti L, ffrench-Mullen J, Benkelfat C, Rouleau GA, Turecki G(2007)Patterns of gene expression in the limbic system of suicides with and without major depression. Mol Psychiatry 12:640–655. [DOI] [PubMed] [Google Scholar]
- Shopsin B.(2013)The clinical antidepressant effect of exogenous agmatine is not reversed by parachlorophenylalanine: a pilot study. Acta Neuropsychiatr 25:113–118. [DOI] [PubMed] [Google Scholar]
- Turecki G.(2014)The molecular bases of the suicidal brain. Nat Rev Neurosci 15:802–816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uzbay T, Goktalay G, Kayir H, Eker SS, Sarandol A, Oral S, Buyukuysal L, Ulusoy G, Kirli S(2013)Increased plasma agmatine levels in patients with schizophrenia. J Psychiatr Res 47:1054–1060. [DOI] [PubMed] [Google Scholar]
- Vargiu C, Cabella C, Belliardo S, Cravanzola C, Grillo MA, Colombatto S(1999)Agmatine modulates polyamine content in hepatocytes by inducing spermidine/spermine acetyltransferase. Eur J Biochem 259:933–938. [DOI] [PubMed] [Google Scholar]
- Zomkowski AD, Hammes L, Lin J, Calixto JB, Santos AR, Rodrigues AL(2002)Agmatine produces antidepressant-like effects in two models of depression in mice. Neuroreport 13:387–391. [DOI] [PubMed] [Google Scholar]
- Zomkowski AD, Rosa AO, Lin J, Santos AR, Calixto JB, Rodrigues AL(2004)Evidence for serotonin receptor subtypes involvement in agmatine antidepressant like-effect in the mouse forced swimming test. Brain Res 1023:253–263. [DOI] [PubMed] [Google Scholar]
- Zomkowski AD, Santos AR, Rodrigues AL(2005)Evidence for the involvement of the opioid system in the agmatine antidepressant-like effect in the forced swimming test. Neurosci Lett 381:279–283. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.