Abstract
Constitutive receptor activity/inverse agonism and functional selectivity/biased agonism are 2 concepts in contemporary pharmacology that have major implications for the use of drugs in medicine and research as well as for the processes of new drug development. Traditional receptor theory postulated that receptors in a population are quiescent unless activated by a ligand. Within this framework ligands could act as agonists with various degrees of intrinsic efficacy, or as antagonists with zero intrinsic efficacy. We now know that receptors can be active without an activating ligand and thus display “constitutive” activity. As a result, a new class of ligand was discovered that can reduce the constitutive activity of a receptor. These ligands produce the opposite effect of an agonist and are called inverse agonists. The second topic discussed is functional selectivity, also commonly referred to as biased agonism. Traditional receptor theory also posited that intrinsic efficacy is a single drug property independent of the system in which the drug acts. However, we now know that a drug, acting at a single receptor subtype, can have multiple intrinsic efficacies that differ depending on which of the multiple responses coupled to a receptor is measured. Thus, a drug can be simultaneously an agonist, an antagonist, and an inverse agonist acting at the same receptor. This means that drugs have an additional level of selectivity (signaling selectivity or “functional selectivity”) beyond the traditional receptor selectivity. Both inverse agonism and functional selectivity need to be considered when drugs are used as medicines or as research tools.
Keywords: constitutive receptor activity, inverse agonism, functional selectivity, biased agonism, pharmacology, G protein coupled receptor, signaling, drug development
Introduction
It is difficult to overestimate the importance of pharmacology for medicine and research. In medicine, drugs are essential components of a physician’s toolbox to treat disease. In fact, drugs have been used as medicines to treat disease since the beginning of recorded history (Leake, 1975; Wadud et al., 2007). In research, drugs are used to perturb physiological and cellular systems to gain understanding of how these systems function. In response to experimental evidence accumulated over the past 20 to 30 years, major changes in our traditional understanding of drug-receptor interactions have occurred. This article highlights 2 of the major changes to pharmacology: inverse agonism and functional selectivity.
In the Beginning…
Traditional drug-receptor theory developed gradually over a period from about 1935 to 1965 from the outstanding work by some pioneers of pharmacology: Clark, Ariëns, Stephenson, and Furchgott (Kenakin, 2013). The concepts of drug binding and action developed by these scientists have been the guiding force behind drug development for close to 50 years. Of the contributions made by these investigators, the most important with respect to drug development were that drugs had 2 properties, affinity and intrinsic efficacy. Affinity is the property of a drug that describes its ability to bind to a receptor. Affinity is a drug property that is a constant and is unique for each drug-receptor pair, as it is dependent on both the structures of the drug and the receptor. Numerically, affinity is the reciprocal of the equilibrium dissociation constant (1/KD) and represents the concentration of drug needed to occupy 50% of the receptor population. KD values of a drug can be measured in a variety of ways, including with binding (saturation, competition, kinetic) and functional assays (e.g., Furchgott method; Furchgott, 1966). Differential affinity for different receptor subtypes allows for receptor selectivity, which is generally regarded as a good thing (Note: Although selectivity (a “magic bullet”) is generally considered to be a valuable drug property, there is an argument to be made for non-selectivity (a “magic shotgun”) for therapeutics (Roth et al., 2004), especially for neuropsychiatric diseases, such as schizophrenia, which, because of redundancy inherent in neural circuitry, may require action at multiple systems for therapeutic effects.) because adverse effects are often attributed to off-target drug actions. A typical goal of medicinal chemistry is to improve selectivity by modifying compounds to increase affinity for a target receptor and decrease affinity for off-target receptors.
Although affinity gets a drug to a receptor, it does not dictate what functional consequences result from the drug-receptor interaction. Intrinsic efficacy, on the other hand, originally defined by Furchgott (1966), is the drug property that describes the effect a drug has on receptor activity that can lead to a change in cellular activity. Like affinity, intrinsic efficacy is a constant that is dependent on both the structures of the drug and the receptor and thus is unique for each drug-receptor pair. However, unlike affinity, intrinsic efficacy is a dimensionless term that cannot be measured directly. Therefore, relative measures are required whereby the intrinsic efficacy of a test drug relative to that of a standard or reference drug is obtained (Clarke and Bond, 1998; Kenakin, 2009).
In traditional receptor theory, the magnitude of response that a drug produces is due to the intrinsic efficacy of the drug, the fraction of the receptor population occupied by the drug (defined by the concentration of drug used and the drug’s affinity value), the total receptor density, and the efficiency with which the cell converts the activated receptors into a response. Thus, the cellular response to a specified concentration of a drug is composed of both system-dependent properties (receptor density and efficiency of receptor-effector coupling) and system-independent, drug-dependent properties (affinity and intrinsic efficacy). Although affinity and intrinsic efficacy are both drug-dependent properties, they are separate and can be individually manipulated by medicinal chemists for drug development. High affinity is generally valued in a drug, whereas intrinsic efficacy can be increased or decreased as desired for therapeutics by changing drug structure. Indeed, the Nobel Prize was awarded to Sir James Black for demonstrating that modifications to an agonist could decrease intrinsic efficacy, ultimately leading to a drug with zero intrinsic efficacy (an antagonist) without reducing affinity (Black, 1989). Importantly for drug development, because affinity and intrinsic efficacy are constants for each drug-receptor pair, it was possible to assess both drug properties in one system (often a cellular system with the target receptor expressed artificially in a clonal cell line), measure one cellular response amenable to high-throughput screening (often intracellular calcium mobilization), and extrapolate drug action to therapeutically relevant systems. This ability to extrapolate drug action obtained from a simple and high-throughput system to human physiology and behavior has been the foundation for drug development for over 50 years.
Within the framework of traditional receptor theory, drugs can behave as agonists or antagonists. Agonists are drugs with both affinity (they bind to the target receptor) and intrinsic efficacy (they change receptor activity to produce a response). Antagonists have affinity but zero intrinsic efficacy; therefore they bind to the target receptor but do not produce a response. By virtue of occupying a fraction of the receptor population (defined by the affinity of the antagonist), an antagonist reduces the probability of occupancy by an agonist. Thus, the presence of an antagonist will reduce receptor occupancy by an agonist with a corresponding reduction in response. However, by increasing the concentration of the agonist, the probability of receptor occupancy by the agonist increases, and thus the inhibitory/blocking effect of the antagonist can be surmounted. As intrinsic efficacy differs with drug structure, agonists can have different intrinsic efficacies and consequently be characterized as full or partial agonists. A full agonist typically produces the maximal response a system is capable of, whereas a partial agonist produces a submaximal response. Although it is clear that the intrinsic efficacy of a partial agonist is less than that of a full agonist, full agonists can also differ in intrinsic efficacy. Due to saturation of postreceptor signaling mechanisms, an agonist can produce a maximum response without occupancy of the entire receptor population (Note: This phenomenon of being capable of producing a maximal response without occupancy of 100% of the receptor population is sometimes referred to as “spare receptors” or “receptor reserve”. It should also be noted that these terms are misnomers in that all receptors participate in the generation of a response even if not all are needed for production of a maximal response (i.e. there are no “spare” receptors)). For example, one agonist may produce a maximal response through occupancy of 75% of the receptor population. However, a different agonist with a greater intrinsic efficacy may produce the same maximal response but require occupancy of only 25% of the receptor population. The former has lower intrinsic efficacy than the latter.
As mentioned above, the tenets of traditional receptor theory have guided the development of drugs for the past 50 years; however, there is now abundant experimental evidence to suggest that this theory needs revision. Further, this need for revision is underscored by the severe reduction in new drugs emerging from the drug discovery pipeline (Pammolli et al., 2011; Mullane et al., 2014; Scannell and Bosley, 2016), especially drugs for treatment of psychiatric disorders (Millan et al., 2015). In fact, some have gone so far as to call the new drug shortage a crisis as drug development expenditures have increased markedly, whereas the number of new drugs reaching the clinic has plummeted (Filmore et al., 2004; Pammolli et al., 2011). Below, we discuss 2 of the major changes to traditional receptor theory, constitutive receptor activity and inverse agonism and functional selectivity, which should be considered in today’s drug development process.
Constitutive Activity and Inverse Agonism
What Is Constitutive Receptor Activity?
Traditional receptor theory is based on the predicate that receptors in a population are quiescent unless acted on by a ligand that possesses both affinity and intrinsic efficacy (i.e., an agonist). However, we now know that receptor proteins can spontaneously adopt an “active” conformation capable of regulating cellular signaling systems in the absence of an agonist. The first demonstration of spontaneous or “constitutive” activity of receptors was published by Cerione et al. (1984), who showed that reconstitution of purified ß2-adrenergic receptors from guinea pig lung, along with purified Gαs from human erythrocytes, into phospholipid vesicles resulted in increased GTPase activity of the Gαs in the absence ligand. Somewhat later, Costa and Herz (1989) showed that delta opioid receptors, expressed naturally by NG108-15 neuroblastoma cells, constitutively activated Gi proteins in a membrane preparation in the absence of agonist. Although originally met with considerable skepticism due to concerns about the presence of endogenous agonist in the preparations, it is now accepted that most, if not all, receptors can signal in the absence of an agonist.
The magnitude of the constitutive activity of a receptor system in a cell can be quantified by constructing a receptor density-response curve. This can be done by transfecting different quantities of cDNA for a receptor into a cell and measuring the increase in basal level of response as a function of receptor density. In an elegant series of experiments exploring the molecular basis for the difference in constitutive activity of the human bradykinin (BK) B1 vs the BK B2 receptor, the Leeb-Lundberg group (Fathy et al., 1999; Leeb-Lundberg et al., 2001) showed that the slope of the curve for phospholipase C activity vs receptor density was 20-fold greater for the BK B1 receptor than the BK B2 receptor (0.58 vs 0.03, respectively). Using a similar procedure, the INI isoform of the human 5-HT2C receptor has 2 times the activity of the VNI isoform that results from RNA editing and differs from the INI isoform by only 1 amino acid in the second intracellular loop. Changes in all 3 of the RNA-editing sites to form the VGV isoform results in a 60-fold reduction in constitutive activity (Berg et al., 2008).
The magnitude of constitutive receptor activity is dependent on 2 factors: the ease with which a receptor protein can isomerize from an inactive to an active conformation (conformational flexibility) and the efficiency of receptor-effector coupling in the cell. The ease of receptor isomerization is based on the number and strength of stabilizing intramolecular forces (hydrogen bonds, electrostatic interactions, etc.), which are dependent on receptor structure and therefore differ for different receptors. The fewer of these intramolecular constraints, the more likely it is that a receptor will spontaneously adopt an active conformation capable of signaling in the absence of a ligand. For receptors with a high isomerization efficiency, the fraction of receptors in a population that are active at any point is time will be larger than for receptors with lower isomerization efficiency. The magnitude of signaling in a cell is in turn dependent on the number of receptors in an active conformation. Protein conformational flexibility can be assessed in relative terms by measuring the rate of denaturation at elevated temperature. It has been shown that receptors with high constitutive activity denature more readily at elevated temperature (Gether et al., 1997; Samama et al., 1997; Alewijnse et al., 2000; Ahn et al., 2013).
In addition to receptor isomerization, receptor-effector coupling efficiency (Note: The cellular process whereby activated receptors lead to a cellular response) also contributes to constitutive signaling by active receptors in a cell. Receptor-effector coupling efficiency is strongly influenced by the phenotype of the cell in which the receptor is expressed. It is well known that the magnitude of constitutive receptor signaling is directly related to receptor density, as is agonist-dependent signaling. Although for a particular receptor the fraction of the receptor population that is in an active conformation may be a constant (defined by the isomerization efficiency), cells that have a high level of receptor expression will have more active receptors. Since cellular signaling is dependent on the number of active receptors in a population, cells with a high density of receptors will have a correspondingly high level of constitutive signaling (Berg et al., 1999, 2008; Leeb-Lundberg et al., 2001). Thus, for receptors with low isomerization capacity (low fraction of active receptors), when expressed by cells at low density, the signal produced by the few active receptors in the population may be too low to be measured. However, in cells where receptor expression is high, the number of active receptors is high (as are the number of inactive receptors), leading to a more measurable signal.
In addition to receptor density, other cellular factors, such as levels of expression of signaling molecules and signaling regulators, influence receptor-effector coupling efficiency and thereby can enhance or depress constitutive receptor signaling. For example, in HEK cells, ligand-independent signaling of muscarinic receptors is very low but is dramatically increased when Gαq levels are increased (Burstein et al., 1997). Similarly, coexpression of Gαq increases constitutive activity of a variety of other receptors (Weiner et al., 2001). In HEK cells expressing a modified 5-HT1A receptor, expression of a regulator of G protein signaling protein to increase G protein GTPase activity augmented constitutive receptor activity by about 4-fold, and the magnitude of the regulator of G protein signaling effect was dependent on the type of G protein involved (Welsby et al., 2002). The presence of the Homer 3 scaffold protein has been shown to reduce the constitutive activity of metabotropic glutamate receptors in cerebellar granule cells (Ango et al., 2001). These studies indicate that the constitutive activity observed by a particular receptor varies depending on the type and quantity of signaling molecules and regulators expressed by cell.
What Is Inverse Agonism?
The discovery that receptors could signal in the absence of an activating ligand (agonist) led to the co-discovery that there were ligands that could reduce this constitutive receptor activity. In their experiments with wild-type, endogenously expressed delta opioid receptors in membranes of NG108-15 neuroblastoma cells, Costa and Herz (1989) found that many ligands previously characterized as antagonists decreased constitutive receptor-stimulated GTPase activity. Since their effect was opposite to that of agonists, such ligands were named “inverse” agonists. As agonists have intrinsic efficacy (the ability to increase the activity of a receptor), inverse agonists are said to have negative intrinsic efficacy (the ability to decrease the activity of a receptor). Just as agonist intrinsic efficacy for a receptor varies with the structure of the agonist (resulting in strong agonists and weaker [partial] agonists), inverse agonists also have different degrees of negative intrinsic efficacy, resulting in strong and weak (partial) inverse agonists.
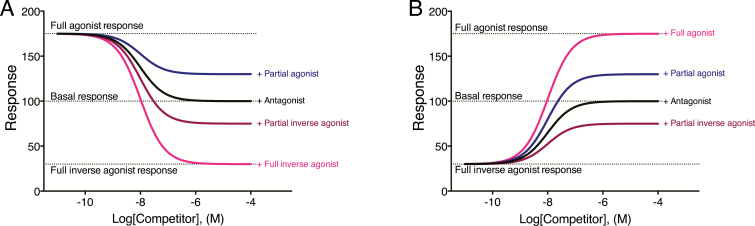
Due to the principle of mutual exclusivity (only 1 ligand can occupy the receptor at a time), agonist activity can be reduced by competition for occupancy of a receptor by a ligand of lower intrinsic efficacy (a partial agonist, antagonist, or inverse agonist). Similarly, the response to an inverse agonist can be reduced by a ligand with higher intrinsic efficacy (an antagonist, an inverse agonist with weaker negative intrinsic efficacy, or an agonist). As illustrated in Figure 1, the effect of a competitor for receptor occupancy, be it an agonist, antagonist, or inverse agonist, will be to bring the response of the test ligand to that commensurate with the intrinsic efficacy of the competitor.
Figure 1.
Simulated concentration-response curves to competitors with different intrinsic efficacies on the response to a full agonist (A) or a full inverse agonist (B). (A) Occupancy of the receptor by the full inverse agonist produces 175 units of response. Competition with a ligand of lower intrinsic efficacy reduces the response of the full agonist such that when occupancy of the receptor has been fully replaced by the competitor, the response remaining is due to the competitor and is dependent on the maximal response produced by the competitor. (B) Occupancy of the receptor by the full inverse agonist reduces the basal response (arbitrarily denoted here as 100 units) to 30 units. As a result of competition produced by ligands with higher intrinsic efficacy, the response of the full inverse agonist is reduced to become commensurate to the efficacy of the competitor. Note, the response elicited by the full inverse agonist is not zero, as there remains constitutive activity of signaling molecules (e.g., G proteins) and effectors in the system capable of producing 30 units of response in the absence of constitutive receptor activity.
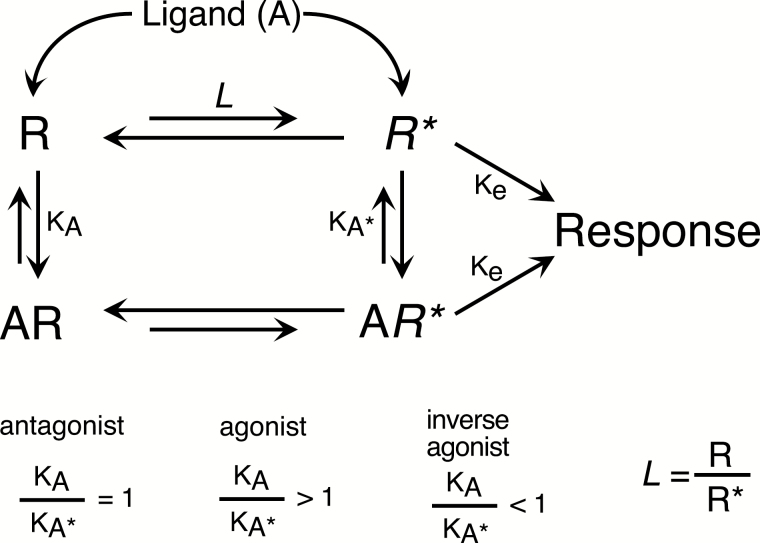
The discovery of constitutive receptor activity and inverse agonism led to the application of 2-state models, originally developed for ligand-gated ion channels (Del Castillo and Katz, 1957), to accommodate activity of a receptor in the absence of an activating ligand (Figure 2) (Leff, 1995). Within this framework, receptors in the absence of a ligand exist in equilibrium between 2 conformations (states): an inactive conformation (often denoted as “R”) that does not signal and an active conformation (often denoted as “R*”) that can regulate cellular signaling systems. The ratio of active to inactive receptors (R*/R) in population is defined by the isomerization efficiency, in turn dependent on the number and strength of intramolecular constraints. A ligand will have affinity (and thus bind to) both R (KA) and R* (KA*) and depending on relative differences in affinities, ligand binding will alter the equilibrium between R and R*, enriching or depleting R or R* depending on the relative affinities of the ligand (Figure 2). Thus, a ligand with higher affinity for R* than R (KA*<KA) will enrich the fraction of active receptors (R*) and deplete the inactive (R) fraction. The increase in the quantity of R* results in an increase in signaling. Such a ligand with KA*<KA is an agonist, and the intrinsic efficacy of the agonist is directly related to magnitude of the ratio KA/KA*. Conversely, a ligand with KA<KA* will enrich the R population at the expense of R*, thus decreasing the number of active receptors and decreasing signaling thereby behaving as an inverse agonist. Similar to that of agonists, the magnitude of the intrinsic efficacy of an inverse agonist is also related (inversely) to the magnitude of KA/KA*. In this 2-state model, an antagonist is a ligand that binds with equal affinity to R and R* (KA=KA*) and thus does not alter the equilibrium between R and R*. Since the quantity of R* does not change following binding of an antagonist, there is no change in the ongoing receptor-mediated response. However, because the antagonist does occupy the receptor population, it will hinder occupancy by an agonist or an inverse agonist, thereby reducing the change in response level caused by the agonist or inverse agonist. Note that within this model, the molecular basis for intrinsic efficacy is the magnitude of the difference between KA and KA*. The further the KA/KA* ratio is from unity, the larger the intrinsic efficacy (positive or negative).
Figure 2.
Two-state model of receptor function. In this model, receptors in a population exist in equilibrium between an inactive conformation (R) and an active conformation (R*). The proportion of receptors in the active conformation is defined by an allosteric transition constant (L), which is based on the number and strength on intramolecular stabilizing contacts and thus is dependent on the receptor protein structure. The magnitude of response is dependent on the quantity of active receptors, and the efficiency of receptor-effector coupling (Ke). Ligands (A) have affinity for both R (1/KA) and R* (1/KA*). Depending on the relative affinities for R vs R*, a ligand can act as an agonist, and inverse agonist or an antagonist. Ligands with higher affinity for R* than R (KA/KA*>1) will enrich the population of active receptors (and deplete the population of inactive receptors), leading to increased response, thereby acting as agonists. Conversely, a ligand with higher affinity for R over R* will enrich the population of receptors in the inactive conformation, depleting the population of active conformation receptors and thereby reducing the ongoing response acting as inverse agonists. The efficacy of agonists and inverse agonists is dependent on how far removed the KA/KA* ratio is from unity. Ligands with equal affinity for R and R* will not alter the quantity of active receptors and thus not change the ongoing level of responsiveness. However, the presence of an antagonist that can occupy the receptor population will reduce the likelihood of receptor occupancy by other ligands.
As Costa and Herz (1989) discovered with delta opioid receptor “antagonists,” many drugs previously characterized as antagonists are now known to be inverse agonists (Greasley and Clapham, 2006). It is sometimes difficult to observe the inverse agonist activity of a ligand, as this depends not only on the ligand’s negative intrinsic efficacy value (the strength of the inverse agonist – the magnitude of the KA*/KA ratio) but also on the degree of constitutive activity of the receptor system in which the ligand is tested. This in turn is dependent not only on the isomerization efficiency of the receptor but of the efficiency of receptor-effector coupling, as described above. Thus, to determine whether a drug has inverse agonist properties (KA<KA*), it is important to use a test system where there is measurable constitutive receptor activity (often accomplished using cells with a high density of receptor expression (Note: Sometimes high receptor expression in cells in culture is viewed as non-physiological. However, it is important to note that receptor expression, especially in neurons and skeletal muscle, can be very high due to clustering of receptors in specific regions of a cell (e.g. post-synaptic density)). In a system with measurable constitutive activity, a drug with inverse agonist properties will reduce the receptor-mediated response. However, if constitutive receptor activity is low, a drug with inverse agonist properties will behave as a simple competitive antagonist (Berg et al., 1999). It is also important to note that since agonism and inverse agonism are dependent on cell phenotype, the behavior of a ligand can appear to be different in different systems. It has been well known for many years that a partial agonist can behave as a simple antagonist when tested in a system with low receptor-effector coupling efficiency. Kenakin and Beek (1980) demonstrated that the ß1-adrenergic receptor agonist, prenalterol, behaved as full agonist (compared with isoproterenol), a partial agonist, or antagonist, in different tissues.
Why Are Constitutive Receptor Activity and Inverse Agonism Important?
The discovery of constitutive receptor activity and inverse agonism has added a new dimension to the pharmacology toolbox. In addition to ligands that increase receptor activity (full and partial agonists) and ligands that block occupancy of the receptor by agonists (antagonists), we now have ligands that can reduce receptor activity (partial and full inverse agonists). Thus, with these tools, pharmacologists have a greater degree of control over receptor function, and it is expected that this will translate into better treatment of disease.
Drugs characterized as antagonists constitute a large part of the pharmacopeia (Hauser et al., 2017; Wacker et al., 2017). However, most drugs previously characterized as antagonists instead have inverse agonist properties (Kenakin, 2004; Bond and Ijzerman, 2006). Based on current multi-state models of receptor function, it is predicted that the prevalence of antagonists (a drug, with equal affinity for all receptor conformations, that does not alter the distribution of receptor conformations) is rather rare. This suggests that inverse agonism may play a large role in therapeutics. In fact, the inverse agonist properties of 5-HT2A receptor ligands, previously characterized as antagonists, appears to be important in the therapeutic mechanism of action of antipsychotic drugs (Meltzer and Roth, 2013). However, as discussed above, in the absence of constitutive receptor activity, inverse agonists behave as antagonists. Since constitutive receptor activity differs in different brain regions and perhaps also with physiological state, the questions as to the role of constitutive receptor activity in (patho)physiological functions and whether inverse agonism is responsible for the therapeutic effects of drugs previously characterized as antagonists are difficult to answer and are still being investigated.
Although discovered more than 40 years ago, we still do not fully understand the roles of constitutive receptor activity or inverse agonism in the regulation of physiological functions or disease. As with any pharmacological tool, it is important to consider the properties of the drug (e.g., receptor selectivity, affinity, intrinsic efficacy) being used to treat a disease or to study the function of an organ system to properly interpret experimental observations.
Physiological Relevance
There are many examples where drugs with inverse agonist properties (as assessed using an in vitro test system) have been found to produce effects when given in vivo. Frequently, such effects are attributed to inverse agonism; however, caution must be used when making this interpretation as the behavior of a putative inverse agonist in vivo is almost always complicated by the presence of an endogenous agonist. As described above, in the absence of constitutive receptor activity, an inverse agonist will behave as a simple competitive antagonist. If there is an ongoing, basal or tonic level of response due to the actions of an endogenous agonist, a simple competitive antagonist can reduce the response to the agonist. This type of response by a drug with inverse agonist properties could be misinterpreted as due to reduction of constitutive receptor activity (inverse agonism) when in fact it is due to reduction of agonist-induced receptor activity. Just because a drug has inverse agonist properties does not mean that all responses produced by the drug are due to inverse agonism. As described above, constitutive receptor activity is dependent in part on the system under investigation (receptor density, receptor-effector coupling efficiency); thus, a drug with inverse agonist properties may act as an inverse agonist in some tissues and as a competitive antagonist in others depending on the degree of constitutive receptor activity and the activity of an endogenous agonist.
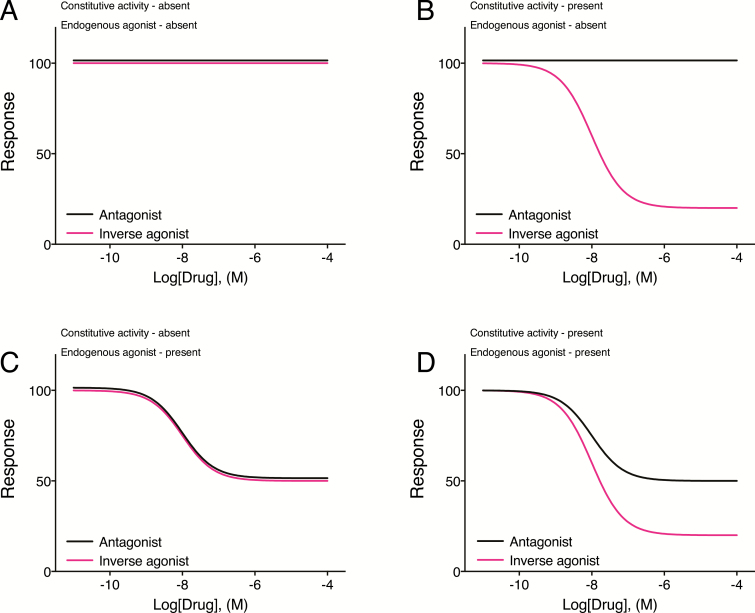
Although the presence of an endogenous agonist in vivo can confound interpretation of drug action, it is possible to distinguish between reduction of constitutive receptor activity (inverse agonism) and reduction of agonist-induced receptor activity (antagonism). As illustrated in Figure 3C, in the absence of constitutive receptor activity, all drugs that block the effect of an endogenous agonist (antagonists and inverse agonists) will have the same maximal response that is dependent on the degree of endogenous agonist tone. In a system in which there is constitutive receptor activity, without endogenous agonist tone (Figure 3B), the inverse agonist will produce a response, but the antagonist will not. When there is both constitutive receptor activity and endogenous agonist activity (Figure 3D), both the inverse agonist and the antagonist will produce a response, but the maximal effect of the inverse agonist will be greater than that of the antagonist (the inverse agonist blocks both constitutive and agonist-dependent receptor activity). Thus, when there is constitutive receptor activity present, inverse agonists and antagonists will have different maximal effects. Importantly, when there is an inverse agonist effect, an antagonist should reduce the inverse agonist component. As an example, Morisset et al., (2000) found that the effect of inverse agonist, ciproxifan and FUB 465 to increase histamine neuron activity in vivo (agonists decrease activity) was blocked by the antagonist, proxyfan, which by itself was without effect. Similarly, a serotonin type 2C inverse agonist, SB 206553, injected into the rat medial prefrontal cortex decreased morphine-induced dopamine release in the nucleus accumbens. This effect did not occur when SB 242084, a serotonin type 2C antagonist, was injected, but SB 242084 blocked the effect of SB 206553 suggesting that the inverse agonist properties of SB 206553, acting to inhibit serotonin type 2C receptor constitutive activity, were responsible for reducing morphine-induced dopamine release (Leggio et al., 2009).
Figure 3.
Effects of antagonists and inverse agonists in systems with or without constitutive receptor activity and endogenous agonist tone. (A) In a system where there is no endogenous agonist action and no constitutive receptor activity, application of inverse agonists or antagonists will not alter the basal level of response. (B) When there is constitutive receptor activity but no endogenous agonist action, an antagonist will not alter the basal level of response. By reducing constitutive receptor activity, an inverse agonist will reduce the basal response. (C) In a system with only endogenous agonist tone (no constitutive receptor activity), both antagonists and inverse agonists will reduce the ongoing agonist-dependent response equally as both will reduce receptor occupancy by the agonist. (D) When there is both constitutive receptor activity and action of an endogenous agonist, an antagonist will reduce the component of the response that is due to the endogenous agonist. An inverse agonist will reduce both the endogenous agonist component, but also will reduce constitutive receptor activity; therefore, the effect of the inverse agonist will be greater than that of the antagonist.
It is sometimes possible to remove a confound due to the presence of an endogenous agonist through the use of neurotoxins to deplete the endogenous agonist or with autoreceptor agonists to inhibit the release of the endogenous agonist. For example, depletion of neuronal serotonin by administration of the serotonin neurotoxin, 5,7-dihydroxytryptamine, did not change the effect of the serotonin type 2C receptor inverse agonist, SB 206553, to increase dopamine release in the striatum or nucleus accumbens (De Deurwaerdère et al., 2004). In the same study, activation of serotonergic autoreceptors with 8-dhydroxy-2-dipropylaminotetralin, to reduce firing activity of and release of serotonin from serotonergic neurons, also did not alter the effect of SB 206553. Together, these experiments demonstrate that in this system there was little endogenous serotonergic tone and the inverse agonist effect of SB 206553 therefore was not due to blockade of serotonin effects.
It is important to emphasize that the magnitude of constitutive receptor activity, and thus an inverse agonist effect, is dependent not only on the characteristics of the receptor (isomerization efficiency) but also on receptor-effector coupling efficiency, which in turn is dependent on the phenotype of the cell in which the receptor is expressed. Thus, it is expected that constitutive receptor activity and the magnitude of an effect of an inverse agonist will differ in different brain regions. Using the inverse agonist, SB 206553, Navailles et al (2006) demonstrated that constitutive activity of the serotonin type 2C receptor differed between the nucleus accumbens and the ventral tegmental area. Consequently, a drug with inverse agonist properties may behave as a strong inverse agonist, a weak inverse agonist, and as an antagonist in different brain regions depending on the intrinsic efficacy of the drug and the magnitude of constitutive receptor activity in the different cells of the brain.
Just as agonist activation of receptors can lead to desensitization, so too can constitutive receptor activity. In the absence of an agonist, even low levels of prolonged constitutive activity can result in receptor systems that exist in a state of constitutive desensitization, where responsiveness to agonist stimulation is reduced (Barak et al., 2003). Constitutive desensitization was first discovered using receptors that were mutated to artificially increase constitutive activity (Note: Receptor mutations can either decrease the energy barrier for a receptor to adopt an active conformation (increase isomerization efficiency) or can increase receptor-effector coupling efficiency). A constitutively active mutant of the ß2-adrenergic receptor was shown to be constitutively phosphorylated by G protein receptor kinase and downregulated compared to the wild-type receptor (Pei et al., 1994). Moreover, reduction of constitutive activity by prolonged treatment of an α1B-adrenoceptor constitutively active mutant with an inverse agonist, followed by washout, increased expression and responsiveness to an agonist (Lee et al., 1997). Presumably, constitutive receptor activity results in activation of desensitization mechanisms that cause downregulation of receptors. Cessation of this constitutive activity toward desensitization mechanisms by treatment with an inverse agonist stops receptor downregulation, resulting in increased receptor expression and enhanced responsiveness to agonist stimulation (Milligan and Bond, 1997) (Note: It is important to note that ligands characterized as having inverse agonist properties at constitutively active mutant receptors, may not necessarily have those same properties when examined in systems with constitutive activity of wild-type (non-mutated) receptors).
Constitutive desensitization can also occur for nonmutated, wild-type receptors. When the wild-type 5-HT2C receptor was expressed at a density for which no constitutive activity toward the canonical signaling pathway, Gq-mediated phospholipase C (PLC) could be detected, and where ligands with inverse agonist properties behaved as simple competitive antagonists, prolonged treatment (>4 hours) with those ligands enhanced the 5-HT2C agonist-stimulated PLC response by 2-fold (Berg et al., 1999). In this case, the mechanism for the inverse agonist-induced enhancement of agonist response was not due to increased receptor expression but to increased expression (due to decreased degradation) of Gq. Notably, prolonged 5-HT2C inverse agonist treatment also increased the responsiveness to agonist of an endogenous purinergic receptor that is coexpressed in the cells. This suggests that low-level constitutive receptor activity can cause both homologous (5-HT2C) and heterologous (purinergic) desensitization and indicates that inverse agonist treatment targeted to one receptor system can lead to enhancement of responsiveness of a different receptor system expressed by a cell. This effect (inverse agonist-induced heterologous sensitization) could be an interesting strategy for drug development. Whereas prolonged treatment with an inverse agonist can increase responsiveness to an agonist acting at the same receptor, this effect is not realized until the inverse agonist is removed from the system (rebound hyper-responsiveness). The continued presence of the inverse agonist blocks the receptor from occupancy by an agonist. However, if inverse agonist treatment leads to heterologous sensitization, the enhancement of responsiveness to the heterologous agonist will continue in the presence of the inverse agonist.
Prolonged treatment with inverse agonists can result in development of apparent “tolerance.” For example, the efficacy of histamine H2 “antagonists,” like cimetidine and ranitidine, for treatment of ulcers and gastroesophageal reflux decays with time (Nwokolo et al., 1990; Komazawa et al., 2003). This tolerance is likely due to the inverse agonist properties of these drugs, which results in upregulation of receptor density or increases in signaling efficiency due to inhibition of constitutive desensitization (Smit et al., 1996). Pure antagonists, such as burimamide, would not be expected to have this effect. Thus, although effects of inverse agonists can be greater than those of antagonists when constitutive receptor activity is present, there can be drawbacks with prolonged use.
Constitutive desensitization may also be operative for receptor systems in vivo. Mu and delta opioid receptors are expressed by peripheral pain-sensing neurons (nociceptors) in rats; however, application of opioid agonists to these neurons in vivo does not produce antinociception (Rowan et al., 2009; Stein and Zollner, 2009; Berg et al., 2011; Sullivan et al., 2015b; Sullivan et al., 2017). Similarly, mu and delta opioid receptor agonists do not inhibit adenylyl cyclase activity in these neurons in primary culture (Patwardhan et al., 2005; Berg et al., 2007; Berg et al., 2011, 2012; Sullivan et al., 2015b, 2017). However, prolonged treatment (90 minutes) with the inverse agonist, naloxone (Raehal et al., 2005b; Wang et al., 2007; Connor and Traynor, 2010), in vivo promoted antinociceptive responses to opioid agonists and in culture promoted inhibition of adenylyl cyclase activity (Sullivan et al., 2016). The effect of naloxone to promote opioid receptor responsiveness was not mimicked by the antagonist, 6ß-naltrexol, and 6ß-naltrexol blocked the effect of naloxone. Knockdown of ß-arrestin-2 expression with siRNA in cultured peripheral sensory neurons also promoted responsiveness to opioid agonists, suggesting that the lack of responsiveness of the mu and delta opioid receptor systems to agonist for antinociceptive signaling is due to constitutive desensitization likely mediated by ß-arrestin-2.
Therapeutic Relevance
Arguably, the most obvious use of inverse agonists for therapeutics is to treat diseases that are caused by enhanced constitutive receptor activity. Indeed, there are several diseases that result from mutations in receptors that increase constitutive activity (e.g., autosomal dominant hypocalcemia [calcium-sensing receptor], Jansen’s metaphyseal chondrodysplasia [parathyroid hormone receptor Type 1], spontaneous ovarian hyperstimulation syndrome [follicle-stimulating hormone receptor], familial male-limited precocious puberty [luteinizing hormone/chorionic gonadotropin receptor], nonautoimmune hyperthyroidism [thyroid-stimulating hormone receptor], and retinitis pigmentosa [rhodopsin]) (for reviews, see de Ligt et al., 2000; Parnot et al., 2002; Milligan, 2003; Smit et al., 2007). For these diseases, it makes sense that inverse agonist-mediated reduction in constitutive receptor activity (in addition to blocking the endogenous agonist) would be more efficacious than an antagonist that would just reduce receptor activation by the endogenous agonist. However, it is curious that none of these diseases are currently treated with inverse agonists. Perhaps because of the relative rarity of these diseases, development of inverse agonists for pharmacotherapy is not a priority.
Constitutive receptor activity has also been linked to cancer. Mutations in receptors that increase constitutive activity have been found to occur in diverse types of cancers and may participate in tumor growth and metastasis (Allen et al., 1991; Li et al., 2005; Dorsam and Gutkind, 2007; Audigier et al., 2013; Liu et al., 2014; Zhao et al., 2015; Xu et al., 2018). For example, certain mutations in the thyrotrophin stimulating hormone (TSH) receptor increase constitutive activity (Parma et al 1993) toward adenylyl cyclase. Activation of the TSH receptor not only increases thyroid hormone production and secretion, but also stimulates growth and proliferation of thyrocytes (Vassart and Dumont, 1992; Postiglione et al., 2002). Notably, activating TSH receptor mutations appears to be the cause of some thyroid cancers with corresponding hyperthyroidism (Grob et al., 2014; Kyrilli et al., 2017; Mon et al., 2018). Moreover, many cancers are known to overexpress receptors, which, as discussed above, can result in enhanced constitutive activity that may play a role in cancer progression and metastasis (Li et al., 2005; Dorsam and Gutkind, 2007; Moody et al., 2016; Insel et al., 2018; Xu et al., 2018). It would seem to be a worthwhile effort to explore the therapeutic potential of inverse agonists in cancer treatment.
Currently there is only one medication that purports to have therapeutic efficacy due to inverse agonism. The FDA has recently approved pimavanserin (Nuplazid, previously known as ACP-103) as a serotonin type 2A receptor inverse agonist to treat psychosis associated with Parkinson’s disease (Cummings et al., 2014). The basis for the inference that the therapeutic efficacy of pimavanserin is due to inverse agonism stems from in vitro characterization of the drug in test systems optimized to enhance serotonin type 2A receptor constitutive receptor activity. Because constitutive receptor activity, and therefore inverse agonist efficacy, is dependent on the phenotype of the cell in which the receptor is expressed, observation of inverse agonism in an in vitro system does not mean that the therapeutic efficacy of pimavanserin is due to inverse agonism (see the commentary by Nutt et al., 2017).
Although there is considerable discussion about the potential therapeutic benefit of inverse agonists for many diseases, their clinical utility is still unrealized. Optimism that inverse agonists have therapeutic utility stems from the findings that most, if not all, G protein coupled receptors can display constitutive receptor activity and that many (most?) clinically useful drugs previously characterized as antagonists, in fact, have inverse agonist properties when evaluated with appropriate test systems (Bond and Ijzerman, 2006; Greasley and Clapham, 2006; Parra and Bond, 2007; Khilnani and Khilnani, 2011). For example, almost all of the atypical antipsychotic drugs previously thought to be antagonists have been shown to have inverse agonist activity at serotonin Type 2 receptors (Herrick-Davis et al., 2000; Sullivan et al., 2015a; Meltzer, 2017), reinforcing the notion that inverse agonism may be important for therapeutic efficacy of these drugs. It is important to note that for many diseases, especially for those that stem from disorders of the CNS (schizophrenia, affective disorders, autism, etc.), the underlying pathology is not known and treatment is often symptomatic. Consequently, the roles that constitutive receptor activity and inverse agonism play in the etiology and treatment of a disease, respectively, are also not clear, and we are left with inferring mechanisms that underlie therapeutic efficacy from correlations with drug properties assigned from tests using in vitro systems. As mentioned above, since constitutive receptor activity and inverse agonism are dependent on the phenotype of the cells in which the receptor is expressed, predictions about the nature of an effect in vivo from in vitro studies can be incorrect. Unfortunately, studies to directly test the hypothesis that inverse agonism is responsible for therapeutic efficacy are unethical, as they would require using a known antagonist, without efficacy on its own, to block the therapeutic effect of the inverse agonist. Until we learn more about the etiology of neuropsychiatric diseases and the mechanisms by which our current drugs alleviate symptoms, conclusions about the therapeutic utility of inverse agonism vs antagonism will remain speculative.
Functional Selectivity / Biased Agonism
What Is Functional Selectivity?
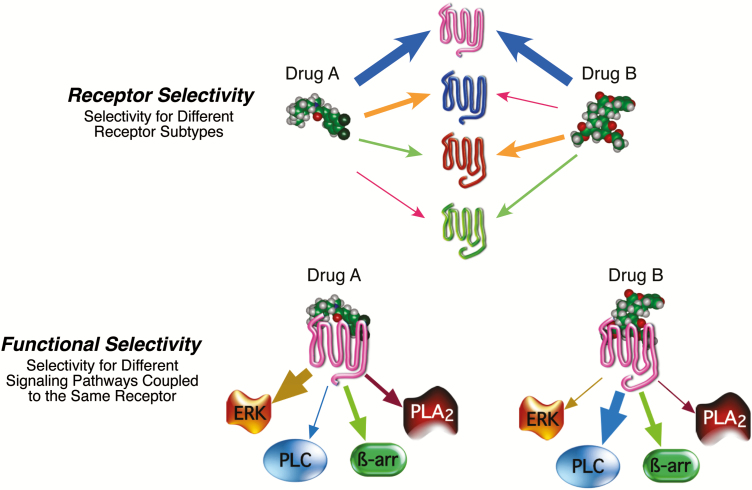
Since the mid-1980s, experimental evidence has been accumulating to indicate that the notion of intrinsic efficacy as a system-independent drug constant is overly simplistic. Numerous studies have shown that rank order of drug efficacy (or potency order, inasmuch as potency is influenced by efficacy) differs for drugs acting at a single receptor subtype depending on the cellular response that was measured (for reviews, see Urban et al., 2007; Kenakin and Miller, 2010; Kenakin, 2013; Shonberg et al., 2014; Zhou and Bohn, 2014; Rankovic et al., 2016; Kenakin, 2017; Michel and Charlton, 2018). In 1993, Spengler et al. (1993) reported that the potency of PACAP1-27 was greater than that of PACAP1-38 for stimulation of adenylyl cyclase activity via the PACAP type 1 receptor expressed in LLC PK1 cells. However, the potency of these 2 agonists was reversed when stimulation of phospholipase C was measured. Berg et al. (1998) demonstrated that the rank order of efficacy of serotonin type 2C agonists to activate phospholipase C was TFMPP=quipazine>bufotenin>DOI>LSD, whereas the efficacy order to activate phospholipase A2 was bufotenin=DOI>quipazine=TFMPP>LSD, even when both responses were measured simultaneously in the same cells. Such agonist behavior is inconsistent with traditional receptor theory and suggests that intrinsic efficacy is not system independent but is in fact dependent on the cellular response measured. Consequently, depending on how many effectors are regulated by a given receptor, a drug can have multiple intrinsic efficacies. Thus, not only can a drug have receptor selectivity, but drugs that act at a single receptor subtype can have selectivity for the cellular signaling pathways the receptor regulates (Figure 4).
Figure 4.
Drug selectivity. (Top) Receptor selectivity is based on differential affinity for different receptor subtypes. Affinity of drugs A and B is reflected by the thickness of the arrows. As shown, drugs A and B have high affinity for the magenta colored receptor and low affinity for the green colored receptor. (Bottom) Functional selectivity is based on differential efficacy of a drug to regulate the activity of various signaling pathways coupled to a single receptor subtype. Signaling selectivity is illustrated as thickness of the arrows between the drug-activated receptor and the cellular signaling pathway. As shown, the selectivity profile for drug A is ERK>ß-arrestin>PLA2>PLC, whereas that for drug B is PLC>ß-arrestin>PLA2>ERK. If PLC signaling led to a therapeutic benefit and/or ERK signaling led to an adverse effect, drug B would be the preferred drug. Abbreviations: ß-arr, ß-arrestin; ERK, extracellular signal-regulated kinase; PLA2, phospholipase A2; PLC, phospholipase C.
Although functional selectivity of ligands for different G protein subtypes has been demonstrated (McLaughlin et al., 2005; Mukhopadhyay and Howlett, 2005; M’Kadmi et al., 2015; Reinartz et al., 2015), G protein coupled receptors regulate cell function via a variety of different transducing molecules, in addition to G proteins (Hall et al., 1999; Premont and Hall, 2002; Hermans, 2003; Bockaert et al., 2004; Rajagopal et al., 2005; Walther and Ferguson, 2015). Perhaps the best studied, non-G protein transducing molecule is ß-arrestin (Shenoy and Lefkowitz, 2005; Gurevich and Gurevich, 2014; Smith and Rajagopal, 2016; Cahill et al., 2017; Peterson and Luttrell, 2017) and, as discussed below, numerous studies have demonstrated ligand bias toward or away from ß-arrestin. Although many papers seem to suggest that the only 2 signaling pathways worth considering are G protein and ß-arrestin, it is important to keep in mind that there are many other signaling pathways that can influence cell function and ligand bias, therefore all signaling pathways should be considered when characterizing a ligand’s efficacy.
Not only can the intrinsic efficacies of drugs differ quantitatively between responses, qualitative differences have also been observed. For example, the serotonin type 2C receptor ligand, SB 242084 is a strong inverse agonist for the phospholipase A2 pathway, but is a weak agonist for the phospholipase C pathway in CHO cells (De Deurwaerdère et al., 2004). The prototypical kappa opioid receptor antagonist norbinaltorphimine acts as an antagonist for Gi-mediated responses in HEK cells and peripheral pain-sensing neurons but is an agonist for activation of c-Jun N-terminal kinase (Bruchas et al., 2007; Melief et al., 2011; Jamshidi et al., 2016). Thus, a drug acting at the same receptor can be an agonist, an inverse agonist, and an antagonist at the same time, depending on the response measured. Over the years, response-dependent drug intrinsic efficacy has been referred to with a variety of names, including “agonist-directed trafficking of receptor stimulus,” “differential engagement,” and “stimulus trafficking.” Today, the terms “functional selectivity” or “biased agonism” are most commonly used. Importantly, because a drug can have multiple, response-dependent intrinsic efficacies, predictions of drug efficacy in vivo from characterization using a single response in a model system may be incorrect if the cellular mechanism that mediates the in vivo response is not the same as that in the model system.
As multiple intrinsic efficacies cannot be accommodated by traditional receptor theory or the 2-state model of receptor function, models that incorporate multi-active receptor conformational states are necessary to explain ligand functional selectivity (Leff et al., 1997; Kenakin and Miller, 2010). In these models, receptors in a population are in equilibrium between an inactive and 2 or more active conformations that are each capable of regulating the activity of different cellular effector pathways (Figure 5). Not only do these multi-active state models allow for ligands to have different response-dependent intrinsic efficacies, but they also allow for response-dependent constitutive activity based on differential isomerization constants for the various receptor conformations and differential receptor conformation-effector coupling efficiencies. Notably, these models predict that inverse agonist efficacy can also be response dependent, depending on the magnitude of constitutive activity toward a particular signaling pathway and the differential affinity values of a ligand for each of the receptor conformational states (i.e., the magnitude of negative intrinsic efficacy for the pathway).
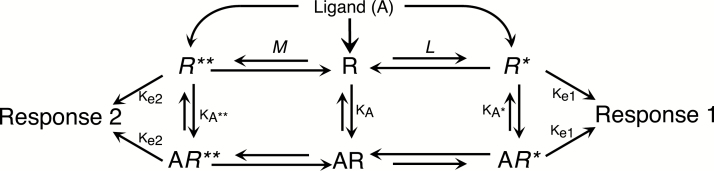
Figure 5.
Three-state model of receptor function. The simplest multi-active state model of receptor function is the 3-state model, where receptors in a population can adopt either an inactive conformation (R), or 1 of 2 active conformation (R* and R**). As in the 2-state model described in Figure 2, the active receptor conformations, R* and R** are in equilibrium with the inactive conformation (R), as defined by the allosteric transition constants, L and M. The magnitude of response is dependent on the quantity of receptors in an active conformation and the efficiency of receptor-effector coupling (Ke). Thus, the magnitude of constitutive activity can differ for Response 1 vs Response 2, either because L and M are different or Ke1 and Ke2 differ, or both. Ligands have affinity for all 3 receptor conformational states (KA, KA*, and KA**), and ligand efficacy is dependent on the differential affinity values for the 3 conformations. With this model, it is possible that a ligand with disproportionately high affinity for R* vs R and R** could act as a strong agonist for Response 1 (due to enrichment of the R* population), however act as an inverse agonist for Response 2 due to depletion of R**. Thus the same ligand could be simultaneously both an agonist and an inverse agonist, acting via the same receptor. It is important to keep in mind that this model is a pronounced oversimplification on many levels. It is likely that receptors can adopt many more than 3 conformations. Moreover, although this model depicts Response 1 being controlled by R* and Response 2 controlled by R**, it is certainly possible that each active conformation could regulate both responses with different Ke (e.g., Ke1a and Ke1b) values. Also, the model as presented shows that for R* to transition to the R** conformation, it must first become R. This need not happen as it is possible that R* could directly transition to R**. Although likely oversimplified (e.g., Ke associated with R*(*) need not equal Ke associated with AR*(*)), this model was able to account for the behavior of 5-HT2C agonists to differentially regulate PLC and PLA2 signaling (Berg et al., 1998).
It is well-known that small changes in ligand structure can result in large changes in receptor selectivity (consider that the difference between norepinephrine and dopamine is a single hydroxyl group). Similarly, small changes in ligand structure can result in large changes in functional selectivity profiles (Shonberg et al., 2014). Risperidone and its active metabolite, paliperidone, are atypical antipsychotic drugs that differ by a single hydroxyl group and have marked differences in functional selectivity signaling profiles at several receptors (Clarke et al., 2013). Moya et al. (2007) examined functional selectivity profiles for a series of phenethylamine and phenylisopropylamine derivatives at human 5-HT2A and 5-HT2C receptors and found that subtle changes in ligand structure resulted in pronounced difference in cellular signaling profiles. These data highlight the need for careful ligand structure-functional selectivity relationship studies to improve our understanding of the molecular mechanisms that underlie functional selectivity (Chen et al., 2012; Shonberg et al., 2013; Szabo et al., 2014; White et al., 2014; Chang et al., 2015; Lovell et al., 2015; Baltos et al., 2016; Manglik et al., 2016; Männel et al., 2017; Aurelio et al., 2018; Chun et al., 2018).
Why Is Functional Selectivity Important?
In 1947, the discovery by Ahlquist that receptor subtypes existed and that drugs could selectively target these receptor subtypes was a major advance in pharmacotherapy (Ahlquist, 1948). Development of drugs with high affinity for a particular target receptor subtype and low affinity for off-target receptor subtypes resulted in more effective drugs with reduced incidence of adverse effects. As presented above, ligands can have more selectivity than that afforded by differential affinity for different receptor subtypes. Selectivity of ligands for cellular signaling pathways (i.e., functional selectivity or signaling bias) may herald a similar substantial advance for pharmacotherapy. Just as off-target receptors can mediate adverse effects, so too can cellular signaling pathways that are regulated by a drug but that are not part of the therapeutic effect. For example, activation of the mu opioid receptor produces relief of pain via activation of Gi proteins; however, concomitant activation of ß-arrestin not only reduces the analgesic effect but appears to mediate adverse effects, such as constipation and respiratory depression (Bohn et al., 1999; Raehal et al., 2005a). Development of ligands that are not only selective for specific receptors, but also selective for regulation of specific cellular signaling pathways, is expected to improve the therapeutic index of drugs.
With the premise that selectivity is an important pharmacological property for therapeutics (Note: Although selectivity for a specific receptor subtype may be of importance to limit adverse effects mediated by off-target actions of drugs, there is strong evidence that targeting multiple receptors may be important in therapeutic efficacy (Musk, 2004; Roth et al., 2004; Bianchi, 2010).), the discovery that drugs can have functional selectivity warrants changes in how drugs are developed. Typically, drug discovery efforts (Hughes et al., 2011) consist of initially identifying a target (e.g., a receptor) and a lead compound followed by medicinal chemistry efforts to increase potency and selectivity and refine efficacy. Receptor subtype-selective drugs are then screened for efficacy by measuring a convenient and high-throughput cellular effector pathway, such as intracellular calcium mobilization. The rationale for the use of a single response to characterize efficacy stems from traditional receptor theory (see above) where intrinsic efficacy was believed to be a constant for each drug-receptor pair and independent of the response measured. Compounds with the desired efficacy properties (agonist, partial agonist, antagonist) then move forward through the preclinical drug development process. However, as discussed above, we now know that intrinsic efficacy is not response-independent and that drugs can have multiple intrinsic efficacies. We also know that more and more drugs are failing in clinical studies due to poor efficacy (Arrowsmith and Miller, 2013). While such failures in expensive clinical trials may stem from inadequate preclinical models of disease, it is also possible that the wrong signaling pathway was used to characterize the drug in the first place.
For some diseases, a cellular signaling pathway to be targeted is known. For example, increases in cellular cAMP cause relaxation of smooth muscle of the bronchi and of the vasculature. Consequently, it makes sense to screen for drugs to treat asthma or hypertension where smooth muscle relaxation is the desired therapeutic response using measurement of cAMP in smooth muscle cells in culture. However, for many diseases, especially neuropsychiatric diseases, the signaling pathway(s) responsible for therapeutic effect is not known. This of course makes it difficult to develop an in vitro model system to obtain drug efficacy values that are predictive of therapeutic efficacy. Unfortunately, it may be quite a long time before we understand neural circuitry and receptor systems in the CNS sufficiently well to identify receptors and intracellular signaling pathways to model with in vitro screening assays. However, it may be possible to use currently known therapeutically efficacious drugs to identify desirable receptor signaling profiles to use as in vitro models. Presumably, therapeutically efficacious drugs regulate a cadre of receptors and cellular signaling pathways coupled to those receptors in a manner that results in therapeutic efficacy. Each of these drugs may also regulate receptors and signaling pathways that are not involved in therapeutic efficacy but that could be responsible for adverse effects. Perhaps by identifying the receptors and signaling pathways that therapeutically efficacious drugs have in common, a profile, or a fingerprint, of receptor affinity (selectivity) and response-dependent intrinsic efficacies can be identified that can be used as templates to screen new drugs for promising compounds or for structure-functional selectivity studies to modify and improve existing drugs. Similarly, it may be possible to identify receptors and cellular signaling activity fingerprints that are associated with an adverse effect by comparing the fingerprints of drugs that have that adverse effect in common (e.g., weight gain associated with some antipsychotic drugs). This fingerprint could then be used as a template to screen against drugs that match.
Physiological Relevance
There are many examples where ligands with different functional selectivity profiles, as identified in cell culture models, have differential effects in vivo (for reviews, see Urban et al., 2007; Kenakin, 2013; Zhou and Bohn, 2014; Galandrin et al., 2016; Michel and Charlton, 2018). However, as with pimavanserin and inverse agonism discussed above, the vast majority of these studies have examined in vivo effects of functionally selective ligands that were characterized as such with in vitro test systems utilizing heterologous expression (e.g., HEK cells heterologously expressing the receptor of interest) that differ substantially from those that mediate the physiological response. Because drug effects are dependent on the cellular expression of receptors and signaling proteins, functional selectivity profiles of ligands also differ with cell phenotype (Luttrell, et al., 2015; Costa-Neto et al., 2016; Shonberg et al., 2014; Kenakin, 2013), and therefore profiles of signaling bias defined in 1 cell type may not predict those in another cell. For example, differences in the expression levels of ß3-adrenergic receptors can change the predominant signaling pathway from stimulation of adenylyl cyclase activity to activation of p38 MAPK resulting in changes in the functional selectivity profiles of agonists (Sato et al., 2007). In addition, changes in the expression level of Gαs reverses the potency order of calcitonin receptor type 2 agonists (Watson et al., 2000).
Moreover, although not well studied for functional selectivity, it would also be expected that cell physiological state, which influences both cell phenotype (proteins expressed) and function of cellular signaling (e.g., desensitization, super-sensitization, etc.), would also impact the functional selectivity profile of ligands. For example, in rats made tolerant to morphine, the potency of morphine to stimulate extracellular signal-regulated kinase (ERK) in the ventrolateral periaqueductal gray is enhanced, whereas antinociceptive signaling is reduced (Macey et al., 2015). Similarly, due to differences in agonist-induced rapid desensitization between the phospholipase C (PLC) and PLA2 signaling pathways, the cellular response to activation of the 5-HT2C receptor by 5-HT is markedly different (Stout et al., 2002). Moreover, there are numerous examples of signaling cross-talk between different receptor systems that influence the signaling responses to activation of a receptor (Cordeaux and Hill, 2002; Hur and Kim, 2002; Werry et al., 2003; Grammatopoulos, 2017). Signaling pathway-dependent changes in response to changes in cell physiological state are especially important for the prediction of drug effects in diseased states when drugs are characterized in normal, nondiseased cells/tissues.
Ideally, it would be best to characterize functional selectivity profiles of drugs in vitro using the same cells that mediate a physiological response in vivo. For example, sensory neurons that detect noxious stimuli in the periphery and transmit pain signals to the brain can be studied both in culture and in the animal. In cultured sensory neurons, the kappa opioid receptor agonist, Salvinorin A, inhibits adenylyl cyclase activity and activates c-Jun N-terminal kinase (JNK). When applied directly to sensory nerve endings in the rat hindpaw, the Salvinorin A dose-response curve for antinociception has an inverted “U” , in which the descending phase is mediated by activation of JNK. A change to the Salvinorin A structure to form EOM-Salvinorin B, abolishes activation of JNK, without interfering with inhibition of adenylyl cyclase in peripheral sensory neurons cultures and results in a monotonic dose-response antinociceptive curve in vivo (Jamshidi et al., 2015).
Although it is not always possible to evaluate drug properties in the cells/tissue of interest, in some cases it may be possible to make reasonable predictions of functionally selective drug effects in vivo from those assessed in vitro. This is the case when signaling pathways that lead to a response in vivo are known and are present in the cell model system used for in vitro assessment. For example, the antinociceptive effect of the mu opioid receptor agonist, DAMGO, is enhanced in ß-arrestin-2 knockout mice (Bohn et al., 1999), and there is evidence that some of the adverse effects associated with mu opioid receptor activation (e.g., respiratory depression, constipation) are mediated by ß-arrestin-2 (Raehal et al., 2005a). Consequently, mu opioid ligands that are biased away from ß-arrestin-2 would be expected to have augmented antinociceptive efficacy and reduced adverse effects. In an elegant study, Manglik et al (2016) identified PZM27 from a virtual library of over 3 million compounds using computational docking and structure-based optimization methods. In comparison to morphine, PZM27 displayed high potency for activation of Gi but minimal activity toward coupling to ß-arrestin-2 in cell culture models expressing the mu opioid receptor. When tested in vivo and compared with morphine, PZM27 had increased efficacy for antinociception in the hot-plate assay (but interestingly, was ineffective in the tail-flick assay), lower efficacy for constipation, and did not produce respiratory depression or conditioned place preference (an indicator of rewarding properties), as expected of a functional selectivity ligand with bias toward Gi and away from ß-arrestin-2.
Although it may be possible to extrapolate functional selectivity profiles identified in vitro to in vivo effects when signaling systems responsible for in vivo actions are known, caution is advised because the roles of cell phenotype and physiological state on signaling systems may result in incorrect interpretation of drug actions. For systems where it is not possible to study cells that participate in the physiological response in vitro, it is important to take steps to ensure that the signaling pathways involved in fact participate in the physiological responses measured using knock-out/in strategies or pharmacological inhibitors/activators. However, even when the physiologically relevant cell population is available for study, it may not be possible to effectively model the diseased state. Since disease can change both cell phenotype and physiological state, functional selectivity fingerprints of drugs obtained in nondiseased cells may not be useful in predicting therapeutic efficacy. As such, it has been suggested that exemplar molecules identified from in vitro screens be advanced into therapeutically relevant systems as soon as possible to test for translation (Kenakin, 2012, 2017, 2018).
Therapeutic Relevance
There is high optimism that ligand functional selectivity will prove to be of immense value for pharmacotherapy in a manner similar to the value obtained from the knowledge that drugs have selectivity for different receptor subtypes (Mailman, 2007; Luttrell, 2014; Shonberg et al., 2014; Violin et al., 2014; Luttrell et al., 2015; Kenakin, 2017, 2018; Michel and Charlton, 2018). The recognition (realization) that ligands have the ability to be functionally selective has also opened the door for reassessment of drug action on old targets and on targets that may have been deemed unsuitable, perhaps due to production of a serious adverse effect. In effect, ligand functional selectivity allows for additional “shots on goal” for previously discarded targets. However, the concept is still in its infancy and there have been few clinical studies with functionally selective drugs.
The first functionally selective drug to be tested in humans was a ß-arrestin-biased agonist for the angiotensin II Type 1 receptor. TRV120027 was developed by Trevena, Inc. for the treatment of acute heart failure (Boerrigter et al., 2011). In vitro, TRV120027 competitively antagonized angiotensin II G protein signaling (as do other clinically used drugs, such as losartan) but stimulated ß-arrestin signaling (Violin et al., 2010). As ß-arrestin signaling had been shown to improve cardiac myocyte contractility in vitro (Rajagopal et al., 2006), it was predicted that TRV120027 would be a better drug than existing angiotensin II Type 1 receptor antagonists for the treatment of acute heart failure by not only decreasing peripheral resistance (antagonizing the vasoconstrictive actions of angiotensin II) but also by increasing cardiac performance. Unfortunately, in phase II clinical trials of hospitalized patients in heart failure, TRV120027 failed to demonstrate improvement over placebo. The failure of TRV120027 to provide therapeutic benefit highlights the caution that must be used when drug characteristics are obtained using surrogate in vitro models that may not faithfully recapitulate the phenotype and physiological status (e.g., disease) of the target cells in vivo.
The antipsychotic activity of aripiprazole (Abilify) has been attributed to its functional selectivity at dopamine D2 receptors (de Bartolomeis et al., 2015; Tuplin and Holahan, 2017). Aripiprazole was originally identified as a low-efficacy agonist (partial agonist) at dopamine D2 receptors (Burris et al., 2002; Cosi et al., 2006). However, on the basis of a rather complex pharmacological profile of action at various subpopulations of dopamine D2 receptors (e.g., pre- vs postsynaptic receptors), it was suggested that aripiprazole was functionally selective at D2 receptors (Mailman, 2007; Mailman and Murthy, 2010). Subsequent work demonstrated that the pharmacological profile of aripiprazole acting at other receptors and various signaling cascades, including gene transcription, was also complex (for review, see Shapiro et al., 2003; Mailman and Murthy, 2010; de Bartolomeis et al., 2015), which led to the suggestion that it is the functional selectivity properties of aripiprazole acting at a variety of receptors that are responsible for its therapeutic mechanism of action (de Bartolomeis et al., 2015; Tuplin and Holahan, 2017). It should be noted that as in the case of pimavanserin (vide supra), this conclusion is based on characterization of aripiprazole in cell systems in vitro and in physiological animal models. It has not been established that functional selectivity underlies its therapeutic mechanism of action.
As described above, from in vitro and in vivo studies there is reason to believe that a mu opioid receptor agonist that is biased toward G protein signaling and away from ß-arrestin signaling would be a better analgesic drug with a reduced adverse effect profile. Oliceridine (Olinvo, TRV130) has similar efficacy as morphine (80%) to activate G protein signaling, but much less activity (20%) toward recruitment of ß-arrestin in cell models (DeWire et al., 2013). In rats and mice, oliceridine exhibited similar analgesic activity as morphine, but produced less constipation and respiratory depression. In humans, oliceridine has passed phase II clinical trials for treatment of postoperative pain and has analgesic efficacy similar to that of morphine but with fewer adverse effects. Notably, in February 2016, the FDA has conferred breakthrough therapy status to oliceridine.
It is important to note that a majority of studies of ligand functional selectivity focus on 2 signaling pathways (most often G protein and ß-arrestin). Receptors, however, can regulate many signaling pathways, and it will be important when developing therapeutically useful, functionally selective ligands that ligand activity at all of the signaling pathways coupled to a receptor be taken into consideration. Important as well is ensuring that the cellular phenotype and physiological state of the in vitro model system used to characterize the potential drug matches that of the in vivo target cells. The success of oliceridine vs the failure of TRV120027 may reflect this latter issue.
Conclusions
It has been clear for some time that development of new or improved drugs has slowed dramatically over the past decade or two (Filmore et al., 2004; Pammolli et al., 2011; Scannell and Bosley, 2016). Clearly, new approaches to drug development must be implemented. The old concepts of affinity to define drug selectivity and intrinsic efficacy to define drug action that have been guiding principles for drug development for over 50 years are no longer tenable. It is important to consider that most, if not all, receptors have constitutive activity and most, if not all, antagonist drugs have inverse agonist properties. However, it can be very difficult to establish that an in vivo effect, or therapeutic effect, of a drug is in fact due to inverse agonism. Consequently, more research is needed to better understand the role of constitutive receptor activity in physiological functions and disease to determine if inverse agonism is an important pharmacotherapeutic property. In addition, we need new ways to assess ligand activity toward multiple signaling pathways in physiologically relevant systems to generate functional selectivity fingerprints that can be used as templates for continued drug development. Ideally, such fingerprints can be obtained in cells systems that faithfully reproduce in vivo target cell phenotypes or even directly in vivo. Development of genetically encoded biosensors for intracellular signaling molecules (Jones-Tabah et al., 2017) may facilitate identification and characterization of functionally selective ligands in physiologically relevant systems. It is hoped that exploitation of the new pharmacology will allow for improved treatment of neuropsychiatric diseases with more selectivity and fewer adverse effects.
Acknowledgments
We gratefully acknowledge Dr Elaine Jennings for helpful comments.
This work was supported by US Public Health Service grants from the National Institutes of Health: R01 GM 106035, R21 DA 037572, and R01 DA 038645.
Statement of Interest
None.
References
- Ahlquist RP.(1948)A study of the adrenotropic receptors. Am J Physiol 153:586–600. [DOI] [PubMed] [Google Scholar]
- Ahn KH, Scott CE, Abrol R, Goddard WA III, Kendall DA(2013)Computationally-predicted CB1 cannabinoid receptor mutants show distinct patterns of salt-bridges that correlate with their level of constitutive activity reflected in G protein coupling levels, thermal stability, and ligand binding. Proteins 81:1304–1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alewijnse AE, Timmerman H, Jacobs EH, Smit MJ, Roovers E, Cotecchia S, Leurs R(2000)The effect of mutations in the DRY motif on the constitutive activity and structural instability of the histamine H(2) receptor. Mol Pharmacol 57:890–898. [PubMed] [Google Scholar]
- Allen LF, Lefkowitz RJ, Caron MG, Cotecchia S(1991)G-protein-coupled receptor genes as protooncogenes: constitutively activating mutation of the alpha 1B-adrenergic receptor enhances mitogenesis and tumorigenicity. Proc Natl Acad Sci U S A 88:11354–11358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ango F, Prézeau L, Muller T, Tu JC, Xiao B, Worley PF, Pin JP, Bockaert J, Fagni L(2001)Agonist-independent activation of metabotropic glutamate receptors by the intracellular protein homer. Nature 411:962–965. [DOI] [PubMed] [Google Scholar]
- Arrowsmith J, Miller P(2013)Trial watch: phase II and phase III attrition rates 2011-2012. Nat Rev Drug Discov 12:569. [DOI] [PubMed] [Google Scholar]
- Audigier Y, Picault FX, Chaves-Almagro C, Masri B(2013)G protein-coupled receptors in cancer: biochemical interactions and drug design. Prog Mol Biol Transl Sci 115:143–173. [DOI] [PubMed] [Google Scholar]
- Aurelio L, Baltos JA, Ford L, Nguyen ATN, Jörg M, Devine SM, Valant C, White PJ, Christopoulos A, May LT, Scammells PJ(2018)A structure-activity relationship study of bitopic N6-substituted adenosine derivatives as biased adenosine A1 receptor agonists. J Med Chem 61:2087–2103. [DOI] [PubMed] [Google Scholar]
- Baltos JA, Paoletta S, Nguyen AT, Gregory KJ, Tosh DK, Christopoulos A, Jacobson KA, May LT(2016)Structure-activity analysis of biased agonism at the human adenosine A3 receptor. Mol Pharmacol 90:12–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barak LS, Wilbanks AM, Caron MG(2003)Constitutive desensitization: a new paradigm for g protein-coupled receptor regulation. Assay Drug Dev Technol 1:339–346. [DOI] [PubMed] [Google Scholar]
- Berg KA, Maayani S, Goldfarb J, Scaramellini C, Leff P, Clarke WP(1998)Effector pathway-dependent relative efficacy at serotonin type 2A and 2C receptors: evidence for agonist-directed trafficking of receptor stimulus. Mol Pharmacol 54:94–104. [PubMed] [Google Scholar]
- Berg KA, Stout BD, Cropper JD, Maayani S, Clarke WP(1999)Novel actions of inverse agonists on 5-HT2C receptor systems. Mol Pharmacol 55:863–872. [PubMed] [Google Scholar]
- Berg KA, Patwardhan AM, Sanchez TA, Silva YM, Hargreaves KM, Clarke WP(2007)Rapid modulation of μ-opioid receptor signaling in primary sensory neurons. J Pharmacol Exp Ther 321:839–847. [DOI] [PubMed] [Google Scholar]
- Berg KA, Dunlop J, Sanchez T, Silva M, Clarke WP(2008)A conservative, single-amino acid substitution in the second cytoplasmic domain of the human serotonin2c receptor alters both ligand-dependent and -independent receptor signaling. J Pharmacol Exp Ther 324:1084–1092. [DOI] [PubMed] [Google Scholar]
- Berg KA, Rowan MP, Sanchez TA, Silva M, Patwardhan AM, Milam SB, Hargreaves KM, Clarke WP(2011)Regulation of κ-opioid receptor signaling in peripheral sensory neurons in vitro and in vivo. J Pharmacol Exp Ther 338:92–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg KA, Rowan MP, Gupta A, Sanchez TA, Silva M, Gomes I, McGuire BA, Portoghese PS, Hargreaves KM, Devi LA, Clarke WP(2012)Allosteric interactions between δ and κ opioid receptors in peripheral sensory neurons. Mol Pharmacol 81:264–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianchi MT.(2010)Promiscuous modulation of ion channels by anti-psychotic and anti-dementia medications. Med Hypotheses 74:297–300. [DOI] [PubMed] [Google Scholar]
- Black J.(1989)Drugs from emasculated hormones: the principle of syntopic antagonism. Science 245:486–493. [DOI] [PubMed] [Google Scholar]
- Bockaert J, Fagni L, Dumuis A, Marin P(2004)GPCR interacting proteins (GIP). Pharmacol Ther 103:203–221. [DOI] [PubMed] [Google Scholar]
- Boerrigter G, Lark MW, Whalen EJ, Soergel DG, Violin JD, Burnett JC Jr(2011)Cardiorenal actions of TRV120027, a novel ß-arrestin-biased ligand at the angiotensin II type I receptor, in healthy and heart failure canines: a novel therapeutic strategy for acute heart failure. Circ Heart Fail 4:770–778. [DOI] [PubMed] [Google Scholar]
- Bohn LM, Lefkowitz RJ, Gainetdinov RR, Peppel K, Caron MG, Lin FT(1999)Enhanced morphine analgesia in mice lacking beta-arrestin 2. Science 286:2495–2498. [DOI] [PubMed] [Google Scholar]
- Bond RA, Ijzerman AP(2006)Recent developments in constitutive receptor activity and inverse agonism, and their potential for GPCR drug discovery. Trends Pharmacol Sci 27:92–96. [DOI] [PubMed] [Google Scholar]
- Bruchas MR, Yang T, Schreiber S, Defino M, Kwan SC, Li S, Chavkin C(2007)Long-acting kappa opioid antagonists disrupt receptor signaling and produce noncompetitive effects by activating c-Jun N-terminal kinase. J Biol Chem 282:29803–29811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burris KD, Molski TF, Xu C, Ryan E, Tottori K, Kikuchi T, Yocca FD, Molinoff PB(2002)Aripiprazole, a novel antipsychotic, is a high-affinity partial agonist at human dopamine D2 receptors. J Pharmacol Exp Ther 302:381–389. [DOI] [PubMed] [Google Scholar]
- Burstein ES, Spalding TA, Brann MR(1997)Pharmacology of muscarinic receptor subtypes constitutively activated by G proteins. Mol Pharmacol 51:312–319. [DOI] [PubMed] [Google Scholar]
- Cahill TJ III, et al. (2017)Distinct conformations of GPCR-β-arrestin complexes mediate desensitization, signaling, and endocytosis. Proc Natl Acad Sci USA 114:2562–2567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerione RA, Codina J, Benovic JL, Lefkowitz RJ, Birnbaumer L, Caron MG(1984)The mammalian beta 2-adrenergic receptor: reconstitution of functional interactions between pure receptor and pure stimulatory nucleotide binding protein of the adenylate cyclase system. Biochemistry 23:4519–4525. [DOI] [PubMed] [Google Scholar]
- Chang SD, Mascarella SW, Spangler SM, Gurevich VV, Navarro HA, Carroll FI, Bruchas MR(2015)Quantitative signaling and structure-activity analyses demonstrate functional selectivity at the nociceptin/orphanin FQ opioid receptor. Mol Pharmacol 88:502–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Sassano MF, Zheng L, Setola V, Chen M, Bai X, Frye SV, Wetsel WC, Roth BL, Jin J(2012)Structure-functional selectivity relationship studies of β-arrestin-biased dopamine D₂ receptor agonists. J Med Chem 55:7141–7153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chun LS, Vekariya RH, Free RB, Li Y, Lin DT, Su P, Liu F, Namkung Y, Laporte SA, Moritz AE, Aubé J, Frankowski KJ, Sibley DR(2018)Structure-activity investigation of a G protein-biased agonist reveals molecular determinants for biased signaling of the D2 dopamine receptor. Front Synaptic Neurosci 10:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke WP, Bond RA(1998)The elusive nature of intrinsic efficacy. Trends Pharmacol Sci 19:270–276. [DOI] [PubMed] [Google Scholar]
- Clarke WP, Chavera TA, Silva M, Sullivan LC, Berg KA(2013)Signalling profile differences: paliperidone versus risperidone. Br J Pharmacol 170:532–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connor M, Traynor J(2010)Constitutively active μ-opioid receptors. Methods Enzymol 484:445–469. [DOI] [PubMed] [Google Scholar]
- Cordeaux Y, Hill SJ(2002)Mechanisms of cross-talk between G-protein-coupled receptors. Neurosignals 11:45–57. [DOI] [PubMed] [Google Scholar]
- Cosi C, Carilla-Durand E, Assié MB, Ormiere AM, Maraval M, Leduc N, Newman-Tancredi A(2006)Partial agonist properties of the antipsychotics SSR181507, aripiprazole and bifeprunox at dopamine D2 receptors: G protein activation and prolactin release. Eur J Pharmacol 535:135–144. [DOI] [PubMed] [Google Scholar]
- Costa T, Herz A(1989)Antagonists with negative intrinsic activity at delta opioid receptors coupled to GTP-binding proteins. Proc Natl Acad Sci USA 86:7321–7325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa-Neto C, Parreiras-E-Silva L, Bouvier M(2016)A Pluridimensional view of biased agonism. Mol Pharmacol 90:587–595. [DOI] [PubMed] [Google Scholar]
- Cummings J, Isaacson S, Mills R, Williams H, Chi-Burris K, Corbett A, Dhall R, Ballard C(2014)Pimavanserin for patients with Parkinson’s disease psychosis: a randomised, placebo-controlled phase 3 trial. Lancet 383:533–540. [DOI] [PubMed] [Google Scholar]
- de Bartolomeis A, Tomasetti C, Iasevoli F(2015)Update on the mechanism of action of aripiprazole: translational insights into antipsychotic strategies beyond dopamine receptor antagonism. CNS Drugs 29:773–799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Deurwaerdère P, Navailles S, Berg KA, Clarke WP, Spampinato U(2004)Constitutive activity of the serotonin2c receptor inhibits in vivo dopamine release in the rat striatum and nucleus accumbens. J Neurosci 24:3235–3241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Ligt RA, Kourounakis AP, IJzerman AP(2000)Inverse agonism at G protein-coupled receptors: (patho) physiological relevance and implications for drug discovery. Br J Pharmacol 130:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Castillo J, Katz B(1957)Interaction at end-plate receptors between different choline derivatives. Proc R Soc Lond B Biol Sci 146:369–381. [DOI] [PubMed] [Google Scholar]
- DeWire SM, Yamashita DS, Rominger DH, Liu G, Cowan CL, Graczyk TM, Chen XT, Pitis PM, Gotchev D, Yuan C, Koblish M, Lark MW, Violin JD(2013)A G protein-biased ligand at the μ-opioid receptor is potently analgesic with reduced gastrointestinal and respiratory dysfunction compared with morphine. J Pharmacol Exp Ther 344:708–717. [DOI] [PubMed] [Google Scholar]
- Dorsam RT, Gutkind JS(2007)G-protein-coupled receptors and cancer. Nat Rev Cancer 7:79–94. [DOI] [PubMed] [Google Scholar]
- Fathy DB, Leeb T, Mathis SA, Leeb-Lundberg LM(1999)Spontaneous human B2 bradykinin receptor activity determines the action of partial agonists as agonists or inverse agonists. Effect of basal desensitization. J Biol Chem 274:29603–29606. [DOI] [PubMed] [Google Scholar]
- Filmore D, Thayer A, Willis R(2004)Pipeline challenges. Mod Drug Discovery 7:28–34. [Google Scholar]
- Furchgott RF.(1966)The use of ß-haloalkylamines in the differentiation of receptors and in the determination of dissociation constants of receptor-agonist complexes. In: Advances in Drug Research (Harper NJ, Simmonds AB, eds), pp21–55. London, New York: Academic Press. [Google Scholar]
- Galandrin S, Onfroy L, Poirot MC, Sénard JM, Galés C(2016)Delineating biased ligand efficacy at 7TM receptors from an experimental perspective. Int J Biochem Cell Biol 77:251–263. [DOI] [PubMed] [Google Scholar]
- Gether U, Ballesteros JA, Seifert R, Sanders-Bush E, Weinstein H, Kobilka BK(1997)Structural instability of a constitutively active G protein-coupled receptor. Agonist-independent activation due to conformational flexibility. J Biol Chem 272:2587–2590. [DOI] [PubMed] [Google Scholar]
- Grammatopoulos DK.(2017)Regulation of G-protein coupled receptor signalling underpinning neurobiology of mood disorders and depression. Mol Cell Endocrinol 449:82–89. [DOI] [PubMed] [Google Scholar]
- Greasley PJ, Clapham JC(2006)Inverse agonism or neutral antagonism at G-protein coupled receptors: a medicinal chemistry challenge worth pursuing?Eur J Pharmacol 553:1–9. [DOI] [PubMed] [Google Scholar]
- Grob F, Deladoëy J, Legault L, Spigelblatt L, Fournier A, Vassart G, Van Vliet G(2014)Autonomous adenomas caused by somatic mutations of the thyroid-stimulating hormone receptor in children. Horm Res Paediatr 81:73–79. [DOI] [PubMed] [Google Scholar]
- Gurevich VV, Gurevich EV(2014)Overview of different mechanisms of arrestin-mediated signaling. Curr Protoc Pharmacol 67:Unit 2.10–1–9.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall RA, Premont RT, Lefkowitz RJ(1999)Heptahelical receptor signaling: beyond the G protein paradigm. J Cell Biol 145:927–932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser AS, Attwood MM, Rask-Andersen M, Schiöth HB, Gloriam DE(2017)Trends in GPCR drug discovery: new agents, targets and indications. Nat Rev Drug Discov 16:829–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermans E.(2003)Biochemical and pharmacological control of the multiplicity of coupling at G-protein-coupled receptors. Pharmacol Ther 99:25–44. [DOI] [PubMed] [Google Scholar]
- Herrick-Davis K, Grinde E, Teitler M(2000)Inverse agonist activity of atypical antipsychotic drugs at human 5-hydroxytryptamine2C receptors. J Pharmacol Exp Ther 295:226–232. [PubMed] [Google Scholar]
- Hughes JP, Rees S, Kalindjian SB, Philpott KL(2011)Principles of early drug discovery. Br J Pharmacol 162:1239–1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hur EM, Kim KT(2002)G protein-coupled receptor signalling and cross-talk: achieving rapidity and specificity. Cell Signal 14:397–405. [DOI] [PubMed] [Google Scholar]
- Insel PA, Sriram K, Wiley SZ, Wilderman A, Katakia T, McCann T, Yokouchi H, Zhang L, Corriden R, Liu D, Feigin ME, French RP, Lowy AM, Murray F(2018)GPCRomics: GPCR expression in cancer cells and tumors identifies new, potential biomarkers and therapeutic targets. Front Pharmacol 9:431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamshidi RJ, Jacobs BA, Sullivan LC, Chavera TA, Saylor RM, Prisinzano TE, Clarke WP, Berg KA(2015)Functional selectivity of kappa opioid receptor agonists in peripheral sensory neurons. J Pharmacol Exp Ther 355:174–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamshidi RJ, Sullivan LC, Jacobs BA, Chavera TA, Berg KA, Clarke WP(2016)Long-term reduction of kappa opioid receptor function by the biased ligand, norbinaltorphimine, requires c-jun N-terminal kinase activity and new protein synthesis in peripheral sensory neurons. J Pharmacol Exp Ther 359:319–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones-Tabah J, Clarke PB, Hébert TE(2017)Measuring G protein-coupled receptor signalling in the brain with resonance energy transfer based biosensors. Curr Opin Pharmacol 32:44–48. [DOI] [PubMed] [Google Scholar]
- Kenakin T.(2004)Efficacy as a vector: the relative prevalence and paucity of inverse agonism. Mol Pharmacol 65:2–11. [DOI] [PubMed] [Google Scholar]
- Kenakin T.(2009)Quantifying biological activity in chemical terms: a pharmacology primer to describe drug effect. ACS Chem Biol 4:249–260. [DOI] [PubMed] [Google Scholar]
- Kenakin T.(2012)The potential for selective pharmacological therapies through biased receptor signaling. BMC Pharmacol Toxicol 13:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenakin T.(2013)New concepts in pharmacological efficacy at 7TM receptors: IUPHAR review 2. Br J Pharmacol 168:554–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenakin T.(2017)Signaling bias in drug discovery. Expert Opin Drug Discov 12:321–333. [DOI] [PubMed] [Google Scholar]
- Kenakin T.(2018)Is the quest for signaling bias worth the effort?Mol Pharmacol 93:266–269. [DOI] [PubMed] [Google Scholar]
- Kenakin T, Miller LJ(2010)Seven transmembrane receptors as shapeshifting proteins: the impact of allosteric modulation and functional selectivity on new drug discovery. Pharmacol Rev 62:265–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenakin TP, Beek D(1980)Is prenalterol (H133/80) really a selective beta 1 adrenoceptor agonist? Tissue selectivity resulting from differences in stimulus-response relationships. J Pharmacol Exp Ther 213:406–413. [PubMed] [Google Scholar]
- Khilnani G, Khilnani AK(2011)Inverse agonism and its therapeutic significance. Indian J Pharmacol 43:492–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komazawa Y, Adachi K, Mihara T, Ono M, Kawamura A, Fujishiro H, Kinoshita Y(2003)Tolerance to famotidine and ranitidine treatment after 14 days of administration in healthy subjects without Helicobacter pylori infection. J Gastroenterol Hepatol 18:678–682. [DOI] [PubMed] [Google Scholar]
- Kyrilli A, Paternot S, Miot F, Corvilain B, Vassart G, Roger PP, Dumont JE(2017)Commentary: thyrotropin stimulates differentiation not proliferation of normal human thyrocytes in culture. Front Endocrinol (Lausanne) 8:214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leake CD.(1975)An historical account of pharmacology. Springfield, IL: Thomas. [Google Scholar]
- Lee TW, Cotecchia S, Milligan G(1997)Up-regulation of the levels of expression and function of a constitutively active mutant of the hamster alpha1B-adrenoceptor by ligands that act as inverse agonists. Biochem J 325:733–739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leeb-Lundberg LM, Kang DS, Lamb ME, Fathy DB(2001)The human B1 bradykinin receptor exhibits high ligand-independent, constitutive activity. Roles of residues in the fourth intracellular and third transmembrane domains. J Biol Chem 276:8785–8792. [DOI] [PubMed] [Google Scholar]
- Leff P.(1995)The two-state model of receptor activation. Trends Pharmacol Sci 16:89–97. [DOI] [PubMed] [Google Scholar]
- Leff P, Scaramellini C, Law C, McKechnie K(1997)A three-state receptor model of agonist action. Trends Pharmacol Sci 18:355–362. [DOI] [PubMed] [Google Scholar]
- Leggio GM, Cathala A, Neny M, Rouge-Pont F, Drago F, Piazza PV, Spampinato U(2009)In vivo evidence that constitutive activity of serotonin2C receptors in the medial prefrontal cortex participates in the control of dopamine release in the rat nucleus accumbens: differential effects of inverse agonist versus antagonist. J Neurochem 111:614–623. [DOI] [PubMed] [Google Scholar]
- Li S, Huang S, Peng SB(2005)Overexpression of G protein-coupled receptors in cancer cells: involvement in tumor progression. Int J Oncol 27:1329–1339. [PubMed] [Google Scholar]
- Liu S, Zeng Y, Li Y, Guo W, Liu J, Ouyang N(2014)VPAC1 overexpression is associated with poor differentiation in colon cancer. Tumour Biol 35:6397–6404. [DOI] [PubMed] [Google Scholar]
- Lovell KM, Frankowski KJ, Stahl EL, Slauson SR, Yoo E, Prisinzano TE, Aubé J, Bohn LM(2015)Structure-activity relationship studies of functionally selective kappa opioid receptor agonists that modulate ERK ½ phosphorylation while preserving G protein over βarrestin2 signaling bias. ACS Chem Neurosci 6:1411–1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luttrell LM.(2014)Minireview: more than just a hammer: ligand “bias” and pharmaceutical discovery. Mol Endocrinol 28:281–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luttrell LM, Maudsley S, Bohn LM(2015)Fulfilling the promise of “biased” G protein-coupled receptor agonism. Mol Pharmacol 88:579–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macey TA, Bobeck EN, Suchland KL, Morgan MM, Ingram SL(2015)Change in functional selectivity of morphine with the development of antinociceptive tolerance. Br J Pharmacol 172:549–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mailman RB.(2007)GPCR functional selectivity has therapeutic impact. Trends Pharmacol Sci 28:390–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mailman RB, Murthy V(2010)Third generation antipsychotic drugs: partial agonism or receptor functional selectivity?Curr Pharm Des 16:488–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manglik A, Lin H, Aryal DK, McCorvy JD, Dengler D, Corder G, Levit A, Kling RC, Bernat V, Hübner H, Huang XP, Sassano MF, Giguère PM, Löber S, Da Duan, Scherrer G, Kobilka BK, Gmeiner P, Roth BL, Shoichet BK(2016)Structure-based discovery of opioid analgesics with reduced side effects. Nature 537:185–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Männel B, Jaiteh M, Zeifman A, Randakova A, Möller D, Hübner H, Gmeiner P, Carlsson J(2017)Structure-guided screening for functionally selective D2 dopamine receptor ligands from a virtual chemical library. ACS Chem Biol 12:2652–2661. [DOI] [PubMed] [Google Scholar]
- McLaughlin JN, Shen L, Holinstat M, Brooks JD, Dibenedetto E, Hamm HE(2005)Functional selectivity of G protein signaling by agonist peptides and thrombin for the protease-activated receptor-1. J Biol Chem 280:25048–25059. [DOI] [PubMed] [Google Scholar]
- Melief EJ, Miyatake M, Carroll FI, Béguin C, Carlezon WA Jr, Cohen BM, Grimwood S, Mitch CH, Rorick-Kehn L, Chavkin C(2011)Duration of action of a broad range of selective κ-opioid receptor antagonists is positively correlated with c-Jun N-terminal kinase-1 activation. Mol Pharmacol 80:920–929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meltzer HY.(2017)New trends in the treatment of schizophrenia. CNS Neurol Disord Drug Targets 16:900–906. [DOI] [PubMed] [Google Scholar]
- Meltzer HY, Roth BL(2013)Lorcaserin and pimavanserin: emerging selectivity of serotonin receptor subtype-targeted drugs. J Clin Invest 123:4986–4991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michel MC, Charlton SJ(2018)Biased agonism in drug discovery-is it too soon to choose a path?Mol Pharmacol 93:259–265. [DOI] [PubMed] [Google Scholar]
- Millan MJ, Goodwin GM, Meyer-Lindenberg A, Ove Ögren S(2015)Learning from the past and looking to the future: emerging perspectives for improving the treatment of psychiatric disorders. Eur Neuropsychopharmacol 25:599–656. [DOI] [PubMed] [Google Scholar]
- Milligan G.(2003)Constitutive activity and inverse agonists of G protein-coupled receptors: a current perspective. Mol Pharmacol 64:1271–1276. [DOI] [PubMed] [Google Scholar]
- Milligan G, Bond RA(1997)Inverse agonism and the regulation of receptor number. Trends Pharmacol Sci 18:468–474. [DOI] [PubMed] [Google Scholar]
- M’Kadmi C, Leyris JP, Onfroy L, Galés C, Saulière A, Gagne D, Damian M, Mary S, Maingot M, Denoyelle S, Verdié P, Fehrentz JA, Martinez J, Banères JL, Marie J(2015)Agonism, antagonism, and inverse agonism bias at the ghrelin receptor signaling. J Biol Chem 290:27021–27039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mon SY, Riedlinger G, Abbott CE, Seethala R, Ohori NP, Nikiforova MN, Nikiforov YE, Hodak SP(2018)Cancer risk and clinicopathological characteristics of thyroid nodules harboring thyroid-stimulating hormone receptor gene mutations. Diagn Cytopathol 46:369–377. [DOI] [PubMed] [Google Scholar]
- Moody TW, Nuche-Berenguer B, Jensen RT(2016)Vasoactive intestinal peptide/pituitary adenylate cyclase activating polypeptide, and their receptors and cancer. Curr Opin Endocrinol Diabetes Obes 23:38–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morisset S, Rouleau A, Ligneau X, Gbahou F, Tardivel-Lacombe J, Stark H, Schunack W, Ganellin CR, Schwartz JC, Arrang JM(2000)High constitutive activity of native H3 receptors regulates histamine neurons in brain. Nature 408:860–864. [DOI] [PubMed] [Google Scholar]
- Moya PR, Berg KA, Gutiérrez-Hernandez MA, Sáez-Briones P, Reyes-Parada M, Cassels BK, Clarke WP(2007)Functional selectivity of hallucinogenic phenethylamine and phenylisopropylamine derivatives at human 5-hydroxytryptamine (5-HT)2A and 5-HT2C receptors. J Pharmacol Exp Ther 321:1054–1061. [DOI] [PubMed] [Google Scholar]
- Mukhopadhyay S, Howlett AC(2005)Chemically distinct ligands promote differential CB1 cannabinoid receptor-Gi protein interactions. Mol Pharmacol 67:2016–2024. [DOI] [PubMed] [Google Scholar]
- Mullane K, Winquist RJ, Williams M(2014)Translational paradigms in pharmacology and drug discovery. Biochem Pharmacol 87:189–210. [DOI] [PubMed] [Google Scholar]
- Musk P.(2004)Magic shotgun methods for developing drugs for CNS disorders. Discov Med 4:299–302. [PubMed] [Google Scholar]
- Navailles S, Moison D, Ryczko D, Spampinato U(2006)Region-dependent regulation of mesoaccumbens dopamine neurons in vivo by the constitutive activity of central serotonin2C receptors. J Neurochem 99:1311–1319. [DOI] [PubMed] [Google Scholar]
- Nutt D, Stahl S, Blier P, Drago F, Zohar J, Wilson S(2017)Inverse agonists - what do they mean for psychiatry?Eur Neuropsychopharmacol 27:87–90. [DOI] [PubMed] [Google Scholar]
- Nwokolo CU, Smith JT, Gavey C, Sawyerr A, Pounder RE(1990)Tolerance during 29 days of conventional dosing with cimetidine, nizatidine, famotidine or ranitidine. Aliment Pharmacol Ther 4:29–45. [PubMed] [Google Scholar]
- Pammolli F, Magazzini L, Riccaboni M(2011)The productivity crisis in pharmaceutical R&D. Nat Rev Drug Discov 10:428–438. [DOI] [PubMed] [Google Scholar]
- Parnot C, Miserey-Lenkei S, Bardin S, Corvol P, Clauser E(2002)Lessons from constitutively active mutants of G protein-coupled receptors. Trends Endocrinol Metab 13:336–343. [DOI] [PubMed] [Google Scholar]
- Parra S, Bond RA(2007)Inverse agonism: from curiosity to accepted dogma, but is it clinically relevant?Curr Opin Pharmacol 7:146–150. [DOI] [PubMed] [Google Scholar]
- Patwardhan AM, Berg KA, Akopain AN, Jeske NA, Gamper N, Clarke WP, Hargreaves KM(2005)Bradykinin-induced functional competence and trafficking of the delta-opioid receptor in trigeminal nociceptors. J Neurosci 25:8825–8832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pei G, Samama P, Lohse M, Wang M, Codina J, Lefkowitz RJ(1994)A constitutively active mutant beta 2-adrenergic receptor is constitutively desensitized and phosphorylated. Proc Natl Acad Sci USA 91:2699–2702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson YK, Luttrell LM(2017)The diverse roles of arrestin scaffolds in G protein-coupled receptor signaling. Pharmacol Rev 69:256–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postiglione MP, Parlato R, Rodriguez-Mallon A, Rosica A, Mithbaokar P, Maresca M, Marians RC, Davies TF, Zannini MS, De Felice M, Di Lauro R(2002)Role of the thyroid-stimulating hormone receptor signaling in development and differentiation of the thyroid gland. Proc Natl Acad Sci USA 99:15462–15467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Premont RT, Hall RA(2002)Identification of novel G protein-coupled receptor-interacting proteins. Methods Enzymol 343:611–621. [DOI] [PubMed] [Google Scholar]
- Raehal KM, Walker JK, Bohn LM (2005a) Morphine side effects in beta-arrestin 2 knockout mice. J Pharmacol Exp Ther 314:1195–1201. [DOI] [PubMed] [Google Scholar]
- Raehal KM, Lowery JJ, Bhamidipati CM, Paolino RM, Blair JR, Wang D, Sadée W, Bilsky EJ (2005b) In vivo characterization of 6beta-naltrexol, an opioid ligand with less inverse agonist activity compared with naltrexone and naloxone in opioid-dependent mice. J Pharmacol Exp Ther 313:1150–1162. [DOI] [PubMed] [Google Scholar]
- Rajagopal K, Lefkowitz RJ, Rockman HA(2005)When 7 transmembrane receptors are not G protein-coupled receptors. J Clin Invest 115:2971–2974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajagopal K, Whalen EJ, Violin JD, Stiber JA, Rosenberg PB, Premont RT, Coffman TM, Rockman HA, Lefkowitz RJ(2006)Beta-arrestin2-mediated inotropic effects of the angiotensin II type 1A receptor in isolated cardiac myocytes. Proc Natl Acad Sci USA 103:16284–16289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rankovic Z, Brust TF, Bohn LM(2016)Biased agonism: an emerging paradigm in GPCR drug discovery. Bioorg Med Chem Lett 26:241–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinartz MT, Kälble S, Littmann T, Ozawa T, Dove S, Kaever V, Wainer IW, Seifert R(2015)Structure-bias relationships for fenoterol stereoisomers in six molecular and cellular assays at the β2-adrenoceptor. Naunyn Schmiedebergs Arch Pharmacol 388:51–65. [DOI] [PubMed] [Google Scholar]
- Roth BL, Sheffler DJ, Kroeze WK(2004)Magic shotguns versus magic bullets: selectively non-selective drugs for mood disorders and schizophrenia. Nat Rev Drug Discov 3:353–359. [DOI] [PubMed] [Google Scholar]
- Rowan MP, Ruparel NB, Patwardhan AM, Berg KA, Clarke WP, Hargreaves KM(2009)Peripheral delta opioid receptors require priming for functional competence in vivo. Eur J Pharmacol 602:283–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samama P, Bond RA, Rockman HA, Milano CA, Lefkowitz RJ(1997)Ligand-induced overexpression of a constitutively active beta2-adrenergic receptor: pharmacological creation of a phenotype in transgenic mice. Proc Natl Acad Sci USA 94:137–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato M, Horinouchi T, Hutchinson DS, Evans BA, Summers RJ(2007)Ligand-directed signaling at the beta3-adrenoceptor produced by 3-(2-ethylphenoxy)-1-[(1,S)-1,2,3,4-tetrahydronapth-1-ylamino]-2S-2-propanol oxalate (SR59230A) relative to receptor agonists. Mol Pharmacol 72:1359–1368. [DOI] [PubMed] [Google Scholar]
- Scannell JW, Bosley J(2016)When quality beats quantity: decision theory, drug discovery, and the reproducibility crisis. Plos One 11:e0147215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro DA, Renock S, Arrington E, Chiodo LA, Liu LX, Sibley DR, Roth BL, Mailman R(2003)Aripiprazole, a novel atypical antipsychotic drug with a unique and robust pharmacology. Neuropsychopharmacology 28:1400–1411. [DOI] [PubMed] [Google Scholar]
- Shenoy S, Lefkowitz R(2005)Seven-transmembrane receptor signaling through beta-arrestin. Sci STKE 308:cm10. [DOI] [PubMed] [Google Scholar]
- Shonberg J, Herenbrink CK, López L, Christopoulos A, Scammells PJ, Capuano B, Lane JR(2013)A structure-activity analysis of biased agonism at the dopamine D2 receptor. J Med Chem 56:9199–9221. [DOI] [PubMed] [Google Scholar]
- Shonberg J, Lopez L, Scammells PJ, Christopoulos A, Capuano B, Lane JR(2014)Biased agonism at G protein-coupled receptors: the promise and the challenges–a medicinal chemistry perspective. Med Res Rev 34:1286–1330. [DOI] [PubMed] [Google Scholar]
- Smit MJ, Leurs R, Alewijnse AE, Blauw J, Van Nieuw Amerongen GP, Van De Vrede Y, Roovers E, Timmerman H(1996)Inverse agonism of histamine H2 antagonist accounts for upregulation of spontaneously active histamine H2 receptors. Proc Natl Acad Sci USA 93:6802–6807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smit MJ, Vischer HF, Bakker RA, Jongejan A, Timmerman H, Pardo L, Leurs R(2007)Pharmacogenomic and structural analysis of constitutive g protein-coupled receptor activity. Annu Rev Pharmacol Toxicol 47:53–87. [DOI] [PubMed] [Google Scholar]
- Smith JS, Rajagopal S(2016)The β-arrestins: multifunctional regulators of G protein-coupled receptors. J Biol Chem 291:8969–8977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spengler D, Waeber C, Pantaloni C, Holsboer F, Bockaert J, Seeburg PH, Journot L(1993)Differential signal transduction by five splice variants of the PACAP receptor. Nature 365:170–175. [DOI] [PubMed] [Google Scholar]
- Stein C, Zollner C(2009)Opioids and sensory nerves. Handb Exp Pharmacol 194:495–518. [DOI] [PubMed] [Google Scholar]
- Stout BD, Clarke WP, Berg KA(2002)Rapid desensitization of the serotonin(2C) receptor system: effector pathway and agonist dependence. J Pharmacol Exp Ther 302:957–962. [DOI] [PubMed] [Google Scholar]
- Sullivan LC, Clarke WP, Berg KA (2015a) Atypical antipsychotics and inverse agonism at 5-HT2 receptors. Curr Pharm Des 21:3732–3738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan LC, Berg KA, Clarke WP (2015b) Dual regulation of δ-opioid receptor function by arachidonic acid metabolites in rat peripheral sensory neurons. J Pharmacol Exp Ther 353:44–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan LC, Chavera TS, Jamshidi RJ, Berg KA, Clarke WP(2016)Constitutive desensitization of opioid receptors in peripheral sensory neurons. J Pharmacol Exp Ther 359:411–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan LC, Chavera TA, Gao X, Pando MM, Berg KA(2017)Regulation of δ opioid receptor-mediated signaling and antinociception in peripheral sensory neurons by arachidonic acid-dependent 12/15-lipoxygenase metabolites. J Pharmacol Exp Ther 362:200–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabo M, Klein Herenbrink C, Christopoulos A, Lane JR, Capuano B(2014)Structure-activity relationships of privileged structures lead to the discovery of novel biased ligands at the dopamine D₂ receptor. J Med Chem 57:4924–4939. [DOI] [PubMed] [Google Scholar]
- Tuplin EW, Holahan MR(2017)Aripiprazole, a drug that displays partial agonism and functional selectivity. Curr Neuropharmacol 15:1192–1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urban JD, Clarke WP, von Zastrow M, Nichols DE, Kobilka B, Weinstein H, Javitch JA, Roth BL, Christopoulos A, Sexton PM, Miller KJ, Spedding M, Mailman RB(2007)Functional selectivity and classical concepts of quantitative pharmacology. J Pharmacol Exp Ther 320:1–13. [DOI] [PubMed] [Google Scholar]
- Vassart G, Dumont JE(1992)The thyrotropin receptor and the regulation of thyrocyte function and growth. Endocr Rev 13:596–611. [DOI] [PubMed] [Google Scholar]
- Violin JD, Crombie AL, Soergel DG, Lark MW(2014)Biased ligands at G-protein-coupled receptors: promise and progress. Trends Pharmacol Sci 35:308–316. [DOI] [PubMed] [Google Scholar]
- Violin JD, DeWire SM, Yamashita D, Rominger DH, Nguyen L, Schiller K, Whalen EJ, Gowen M, Lark MW(2010)Selectively engaging β-arrestins at the angiotensin II type 1 receptor reduces blood pressure and increases cardiac performance. J Pharmacol Exp Ther 335:572–579. [DOI] [PubMed] [Google Scholar]
- Wacker D, Stevens RC, Roth BL(2017)How ligands illuminate GPCR molecular pharmacology. Cell 170:414–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wadud A, Prasad PV, Rao MM, Narayana A(2007)Evolution of drug: a historical perspective. Bull Indian Inst Hist Med Hyderabad 37:69–80. [PubMed] [Google Scholar]
- Walther C, Ferguson SS(2015)Minireview: role of intracellular scaffolding proteins in the regulation of endocrine G protein-coupled receptor signaling. Mol Endocrinol 29:814–830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D, Sun X, Sadee W(2007)Different effects of opioid antagonists on mu-, delta-, and kappa-opioid receptors with and without agonist pretreatment. J Pharmacol Exp Ther 321:544–552. [DOI] [PubMed] [Google Scholar]
- Watson C, Chen G, Irving P, Way J, Chen WJ, Kenakin T(2000)The use of stimulus-biased assay systems to detect agonist-specific receptor active states: implications for the trafficking of receptor stimulus by agonists. Mol Pharmacol 58:1230–1238. [DOI] [PubMed] [Google Scholar]
- Weiner DM, Burstein ES, Nash N, Croston GE, Currier EA, Vanover KE, Harvey SC, Donohue E, Hansen HC, Andersson CM, Spalding TA, Gibson DF, Krebs-Thomson K, Powell SB, Geyer MA, Hacksell U, Brann MR(2001)5-hydroxytryptamine2a receptor inverse agonists as antipsychotics. J Pharmacol Exp Ther 299:268–276. [PubMed] [Google Scholar]
- Welsby PJ, Kellett E, Wilkinson G, Milligan G(2002)Enhanced detection of receptor constitutive activity in the presence of regulators of G protein signaling: applications to the detection and analysis of inverse agonists and low-efficacy partial agonists. Mol Pharmacol 61:1211–1221. [DOI] [PubMed] [Google Scholar]
- Werry TD, Wilkinson GF, Willars GB(2003)Mechanisms of cross-talk between G-protein-coupled receptors resulting in enhanced release of intracellular Ca2+. Biochem J 374:281–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White KL, Scopton AP, Rives ML, Bikbulatov RV, Polepally PR, Brown PJ, Kenakin T, Javitch JA, Zjawiony JK, Roth BL(2014)Identification of novel functionally selective κ-opioid receptor scaffolds. Mol Pharmacol 85:83–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu C, Zheng L, Li D, Chen G, Gu J, Chen J, Yao Q(2018)CXCR4 overexpression is correlated with poor prognosis in colorectal cancer. Life Sci. (in press). [DOI] [PubMed] [Google Scholar]
- Zhao H, Guo L, Zhao H, Zhao J, Weng H, Zhao B(2015)CXCR4 over-expression and survival in cancer: a system review and meta-analysis. Oncotarget 6:5022–5040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou L, Bohn LM(2014)Functional selectivity of GPCR signaling in animals. Curr Opin Cell Biol 27:102–108. [DOI] [PMC free article] [PubMed] [Google Scholar]