Abstract
Background
The neuropeptide oxytocin can extensively modulate human social behavior and affective processing, and its effects can be interpreted in terms of mediating approach-avoidance motivational processes. However, little is known about how oxytocin mediates approach-avoidance behavior and particularly the underlying neural mechanisms.
Methods
In a randomized, double-blind, between-subject design, the present pharmaco-fMRI study used an approach-avoidance paradigm to investigate oxytocin’s effects on approach-avoidance behavior and associated neural mechanisms.
Results
Results revealed that oxytocin generally decreased activity in the right striatum irrespective of response (approach/avoidance) and social context, suggesting an inhibitory effect on motivational representation during both appetitive approach and aversive avoidance. Importantly, while on the behavioral level oxytocin selectively enhanced accuracy when approaching social positive stimuli, on the neural level it decreased left ventral and right dorsal anterior insula activity in response to social vs nonsocial positive stimuli compared with the placebo treatment. The left ventral anterior insula activity was negatively correlated with the corresponding accuracy difference scores in the oxytocin but not in the placebo group.
Conclusion
Given the role of the ventral anterior insula in emotional processing and the dorsal anterior insula in salience processing, the oxytocin-induced suppression of activity in these regions may indicate that oxytocin is acting to reduce interference from hyper-activity in core regions of the emotional and salience networks when approaching salient positive social stimuli and thereby to promote social interaction. Thus, oxytocin may be of potential therapeutic benefit for psychiatric disorders exhibiting avoidance of social stimuli.
Keywords: oxytocin, approach/avoidance behavior, anterior insula, positive social stimuli
Significance Statement
The hypothalamic neuropeptide oxytocin (OT) plays an important role in modulating human social behavior. These effects can be interpreted in terms of OT’s actions on basal approach-avoidance (AA) motivational processes, as proposed by the general AA hypothesis of OT (GAAO). However, few studies have evaluated the proposed OT effects on AA behavior and particularly the underlying neural mechanisms. Using neuroimaging combined with intranasal OT administration, the present study revealed that while OT selectively enhanced behavioral accuracy when approaching social positive stimuli, it decreased left ventral and right dorsal anterior insula (AI) activity in response to social vs nonsocial positive stimuli compared to the PLC treatment, with the left ventral AI activity being negatively correlated with the corresponding behavioral accuracy only in the OT group. These findings provide the first confirmatory evidence for the GAAO by demonstrating that OT facilitates human approach behavior to social positive stimuli via inhibiting AI activity.
Introduction
Across species, the hypothalamic neuropeptide oxytocin (OT) regulates social behavior, particularly bonding and maternal care (Striepens et al., 2011; Kendrick et al., 2018). During the last 2 decades, the number of studies examining oxytocinergic regulation of human behavior via intranasal administration of OT has steadily increased. While OT can facilitate appetitive approach behaviors, such as interpersonal trust and generosity (Kosfeld et al., 2005; Zak et al., 2007), pair bonding and maternal behavior (Strathearn et al., 2009; Scheele et al., 2012) and emotional empathy and face recognition (Hurlemann et al., 2010; Marsh et al., 2010), it can also promote aversive avoidance behavior by increasing envy and schadenfreude (Shamay-Tsoory et al., 2009), ethnocentrism (e.g., trust and empathy; De Dreu et al., 2010; Sheng et al., 2013), group-serving dishonesty (Shalvi and De Dreu, 2014), and noncooperation (Bartz et al., 2011; De Dreu et al., 2012).
To account for these complex and somewhat contradictory findings, Harari-Dahan and Bernstein (2014) proposed in their general approach-avoidance (AA) hypothesis of OT (GAAO) that the broad effects of OT on human behavior are mediated by its actions on basal AA motivational processes. More specifically, within this extended overarching framework, OT’s complex behavioral effects are considered to be rooted in its modulation of the salience of personally relevant and emotionally evocative stimuli not necessarily restricted to social contexts (Harari-Dahan and Bernstein, 2014; cf. Kemp and Guastella, 2011 for the social-approach/withdrawal hypothesis and Shamay-Tsoory and Abu-Akel, 2016 for the social salience hypothesis). However, surprisingly few studies have experimentally evaluated the proposed effects of OT on AA behavior and particularly the underlying neural mechanisms.
In a previous study that examined the effects of intranasal OT on human AA behavior, OT was found to accelerate both approach and avoidance behavior towards emotionally negative stimuli such as disgusted faces (Theodoridou et al., 2013; Harari-Dahan and Bernstein, 2017). Using similar paradigms, OT also facilitated approach towards angry faces with a direct gaze (Radke et al., 2013). Furthermore, in the context of pair bonding, OT was found to modulate interpersonal space by decreasing the preferred distance men in a romantic relationship kept between themselves and an unknown attractive woman (Scheele et al., 2012). However, these studies predominantly used emotional faces to investigate OT’s actions on AA behavior and were thus unable to determine whether observed effects were driven by its well-established actions on increased attention and attraction to faces per se (Theodoridou et al., 2009; Domes et al., 2013; Shahrestani et al., 2013; Striepens et al., 2014; Xu et al., 2015). Moreover, the neural substrates mediating OT’s effects on AA behavior also remain unclear, with initial evidence showing decreased amygdala activity only when approaching angry faces (Radke et al., 2017). Since this latter study also used facial stimuli, this again precludes any definitive conclusion as to whether findings simply reflect the well-documented anxiolytic effect of OT in decreasing amygdala responses to threatening facial expressions (Heinrichs et al., 2003; Kirsch et al., 2005) rather than specific effects on AA behavior.
The present study has therefore employed a face-independent AA task combined with fMRI to investigate OT’s specific effects on AA behavior and the underlying neural mechanisms involved. Thus, social and nonsocial scenes rather than facial experimental stimuli were used to determine specific effects of OT on AA behavior per se to avoid its potential confounding effects on face processing. Participants were instructed to approach positive (appetitive approach) and avoid negative (aversive avoidance) stimuli during the AA task. In accordance with the GAAO that OT modulates salience of cues that are personally relevant and emotionally evocative but not necessarily specific to social contexts (Harari-Dahan and Bernstein, 2014), we hypothesized that OT would (1) facilitate approach behavior to positive stimuli, particularly more emotionally evocative social ones, and associated activity in the motivational and emotional salience core regions such as the striatum and anterior insula, and (2) decrease avoidance behavior via attenuating amygdala reactivity to negative stimuli, particularly more emotionally evocative negative social ones.
Methods And Materials
Participants and Treatment
A total of 83 healthy male students (mean age=21.35 years, SD=2.48) participated in a randomized, double-blind, between-subject experiment and were randomly assigned to receive either intranasal OT (40 IU; Oxytocin Spray, Sichuan Meike Pharmacy Co. Ltd, China) or placebo (PLC; same ingredients other than OT, i.e., sodium chloride and glycerin). To control for potential confounding effects from personality traits or mood states, subjects completed Chinese versions of validated psychometric questionnaires before treatment, including the Positive and Negative Affect Schedule (Watson et al., 1988), Autism Spectrum Quotient (Baron-Cohen et al., 2001), Empathy Quotient (Baron-Cohen and Wheelwright, 2004), and NEO 5-factor inventory (Costa and MacCrae, 1992). To further control for a potentially confounding influence of altered mood states, subjects were asked to complete the Positive and Negative Affect Schedule 3 times: after they first arrived (pretreatment), before MRI scanning (posttreatment), and finally after scanning (post-scan). Subjects received OT/PLC treatment in accordance with a standardized protocol (Guastella et al., 2013), and fMRI acquisition started 45 minutes after treatment. A total of 7 subjects were excluded due to technical issues during data acquisition (4 subjects) or excessive head movement (3 subjects). Thus, 39 subjects in the OT group and 37 subjects in the PLC group were included in the final analysis. In postscan interviews, subjects were unable to identify better than chance whether they had received OT or PLC (χ2=0.21, P=.646). Written informed consent was obtained from all subjects before study inclusion. All procedures were in accordance with the latest version of the Declaration of Helsinki and approved by the ethical committee of University of Electronic Science and Technology of China.
The AA Task
In a revised AA task (Chen and Bargh, 1999), participants were instructed to make approach responses to positive social or nonsocial stimuli (e.g., happy friends meeting or beautiful landscapes) and avoidance responses to social or nonsocial negative stimuli (e.g., victims or environmental pollution). In a prestudy, we selected 620 pictures mostly from the International Affective Picture System (Lang et al., 2008) and additionally from the Internet that were rated in terms of valence and arousal (9-point Likert scale) by an independent sample of 34 healthy volunteers (18 males). Based on this data, a total of 112 stimuli (28 pictures per category, positive vs negative and social vs nonsocial) were selected: social positive (valence: mean±SD=2.36±0.27; arousal: 6.27±0.35), social negative (valence: 2.47±0.26; arousal: 6.65±0.46), nonsocial positive (valence: 1.68±0.39; arousal: 6.25±0.42), and nonsocial negative (valence: 1.87±0.55; arousal: 6.10±0.70). Note that the valence rating scores were transformed to the distance from the neutral midpoint of the 9-point scale. Each picture was presented for 3 seconds at a 624- × 468-pixel resolution followed by a jittered inter-stimulus interval of 2 to 6 seconds. Subjects were instructed to pull the positive stimuli towards their body by pressing the “down” key and push the negative stimuli away from their body by pressing the “up” key successively via a response pad during the 3-second presentation. To realistically convey approach and avoidance of the stimuli for the subjects, each pulling-associated button press would enlarge the picture by 100×75 pixels while each pushing response would decrease the size of the picture by 100×75 pixels. All subjects preformed 10 practice trials before entering the scanner and were instructed to respond as fast and accurately as possible during the experiment.
Image Acquisition and Data Analysis
Images were collected using a 3 Tesla, GE Discovery MR750 system (General Electric Medical System, Milwaukee, WI). During each fMRI scan, a time series of volumes was acquired using a T2*-weighted echo-planar pulse sequence (repetition time: 2000 ms; echo time: 30 ms; number of slices: 39; slice thickness: 4 mm; gap: 1 mm; field of view: 240×240 mm; resolution: 64×64; flip angle: 90°). To control for any anatomic abnormalities and increase normalization accuracy during preprocessing, additional T1-weighted images were acquired obliquely with a 3-dimensional spoiled gradient echo pulse sequence (repetition time: 6 milliseconds; echo time: 2 milliseconds; flip angle: 9°; field of view: 256×256 mm; acquisition matrix: 256×256; number of slices: 156; slice thickness: 1 mm).
Images were processed using SPM8 (Wellcome Department of Cognitive Neurology, London; https://www.fil.ion.ucl.ac.uk/spm/software/spm8/) (Friston et al., 1994). The first 5 functional images were deleted to achieve magnet-steady images, and the remaining images were realigned to correct for head movement based on a 6-parameter rigid body algorithm. After co-registering the mean functional image and the T1 image, the T1 image was segmented to determine the parameters for normalizing the functional images to Montreal Neurological Institute (MNI) space. These normalized images were finally spatially smoothed using a Gaussian kernel (8 mm full-width at half maximum).
The first-level design matrix included 4 condition-specific regressors (social positive/negative, nonsocial positive/negative) convolved with the canonical hemodynamic response function and the 6 head-motion parameters as nuisance regressors. Contrast images for each stimulus condition, all positive and all negative were created separately. On the second level, group differences were analyzed using 2-sample t tests. Interactions were tested using an ANOVA model implemented in a flexible factorial design. Based on our region-specific hypotheses, the analysis focused on core regions involved in salience processing (Menon, 2015; Uddin, 2015) and appetitive/approach (social/nonsocial reward) and withdrawal/avoidance (punishment/threat) motivational processes (Izuma et al., 2008; Delgado et al., 2008, 2009; Rademacher et al., 2010; Schlund and Cataldo, 2010; Palminteri et al., 2012), that is, the amygdala, the AI, and the striatum. Importantly, these regions strongly overlap with the network mediating the social cognitive and affective effects of intranasal OT (Kirsch et al., 2005; Wigton et al., 2015; Shamay-Tsoory and Abu-Akel, 2016; Kendrick et al., 2018; Yao et al., 2018). Regions-of-interest (ROIs) were anatomically defined using the Automated Anatomic Labeling atlas (Tzourio-Mazoyer et al., 2002). Within the unilateral a priori ROIs, a threshold of P<.05 family-wise error (FWE) peak-level correction was set for multiple comparisons using small volume correction (SVC). Parameter estimates used for plotting and brain behavior associations analysis were extracted for each subject from a 6-mm sphere centered on the peak voxel within corresponding ROIs. For additional exploratory whole-brain analyses, a threshold of PFWE<.05 corrected at peak level was used and only clusters >10 voxels are reported.
Results
Questionnaires
Two-sample t tests on questionnaires measuring mood, autistic traits, empathy, and personality traits revealed no significant differences between the treatment groups (Ps>.136; supplementary Table 1).
Behavioral Results
For response times, a repeated-measures ANOVA was performed with social context (social vs nonsocial) and response type (approach vs avoidance) as within-subject factors and treatment (OT vs PLC) as between-subject factor. This revealed a significant main effect of response type (F(1, 74)=250.46, P<.001), with subjects being significantly faster to approach positive than to avoid negative stimuli (1617.33±176.84 vs 1776.95±184.28). The interaction between social context and response was also significant (F(1, 74)=19.04, P<.001), with posthoc tests reavealing that subjects were faster to approach social compared with nonsocial positive stimuli (1600.86±172.73 vs 1633.79±180.49) but slower to avoid social than nonsocial negative stimuli (1784.72±181.76 vs 1769.18±187.65). There were no other significant effects (P>.106).
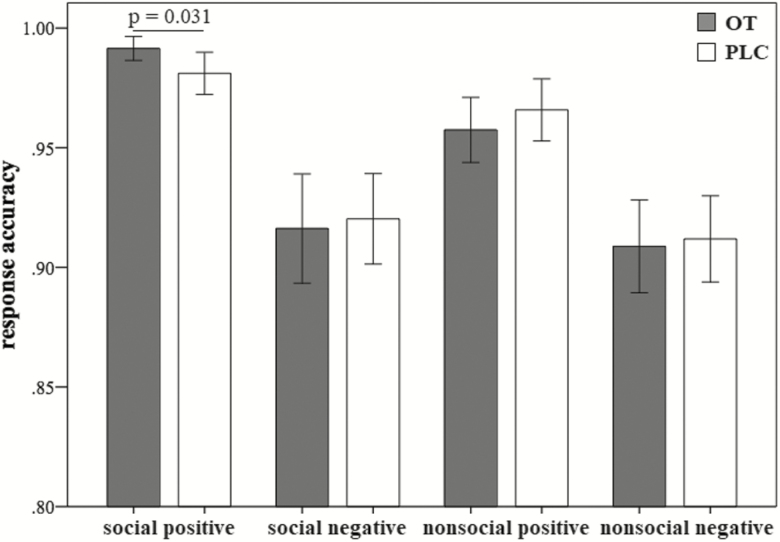
In terms of response accuracy (RA), there was a significant main effect of social context (F(1, 74)=15.75, P<.001), with a higher accuracy for social compared with nonsocial stimuli (95.4%±5.7% vs 93.7%±5.4%). The main effect of response type was also significant (F(1, 74)=109.59, P<.001), with a higher accuracy for approaching positive than avoiding negative stimuli (97.5%±3.4% vs 91.7%±5.8%). While the interaction between social context, response type, and treatment was not significant (F(1, 74)=0.76, P=.385), an exploratory pairwise comparison revealed a significantly higher RA only for social (P=.031) but not nonsocial positive stimuli (P=.568) in the OT relative to the PLC group (Figure 1). The interaction between social context and response type was marginally significant (F(1, 74)=3.18, P=.079), suggesting a trend of higher accuracy to social vs nonsocial stimuli for positive (P<.001; 98.8%±2.1% vs 96.2%±4.0%) but not negative stimuli (P=.280; 92.1%±6.1% vs 91.3%±5.6%). There were no other significant effects (P>.361). Given the slightly higher valence scores for social compared with nonsocial positive stimuli, to clarify whether the valence difference would confound the significant OT’s effects, we further conducted a correlation analysis between valence scores for individual pictures by independent raters and RA for social and nonsocial positive stimuli in the experimental subjects and found no significant associations either for social (Pearson r=0.061, df=28, P=.759) or nonsocial positive stimuli (Pearson r=- 0.044, df=28, P=.823). Thus, the magnitude of the positive valence score for the individual pictures did not influence response accuracy.
Figure 1.
Response accuracy in response to each condition in the oxytocin and placebo groups. Error bars show standard errors.
fMRI Results
We first examined unspecific effects of treatment (OT vs PLC) independent of response type and social context using a 2-sample t test. This revealed decreased right striatum activity (MNI=22, 2, 8, t=4.05, PFWE=.025 SVC, voxels=48; Figure 2A) in the OT compared with the PLC group (OT all conditions<PLC all conditions).
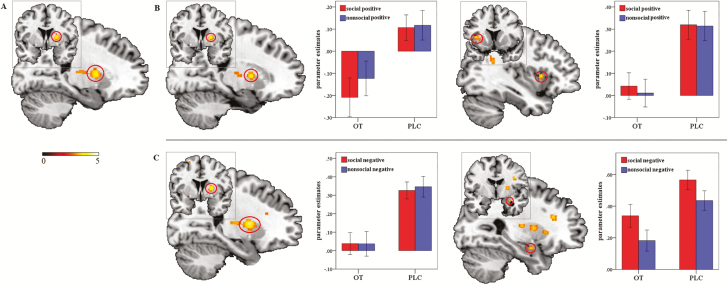
Figure 2.
(A) Decreased right striatum activity in the oxytocin (OT) compared with the placebo (PLC) group. (B) Decreased activity in the right striatum and the left dorsal anterior insula (AI) in the OT compared with the PLC group during appetitive approach. (C) Decreased activity in the right striatum and the right amygdala in the OT compared with the PLC group during aversive avoidance. Statistic maps were displayed with a P<.005 uncorrected threshold. Error bars show standard errors.
Next, we examined treatment effects and interactions between treatment and social context on appetitive approach and aversive avoidance separately. For appetitive approach, decreased activity in the right striatum (MNI=20, 2, 6, t=3.96, PFWE=.031 SVC, voxels=29) and the left dorsal AI (MNI=-38, 12, 6, t=3.52, PFWE=.040 SVC, voxels=11; Figure 2B) was found in the OT compared with the PLC group (OT all positive<PLC all positive). Examining the interaction between treatment and social context (OT social positive>nonsocial positive<PLC social positive>nonsocial positive) during approach behavior revealed significant interaction effects in the left ventral AI (MNI=-42, 6, -6, t=3.57, PFWE=.022 SVC, voxels=14; Figure 3A) and the right dorsal AI (MNI=48, 12, 2, t=3.40, PFWE=.036 SVC, voxels=7; Figure 3B), suggesting that OT decreased activity in these regions during approach of social relative to nonsocial positive stimuli.
Figure 3.
Oxytocin (OT) decreased the left ventral anterior insula (AI) (A) and the right dorsal AI (B) activity in response to social relative to nonsocial positive stimuli (OT social positive>nonsocial positive<placebo [PLC] social positive>nonsocial positive). Statistic maps were displayed with a P<.005 uncorrected threshold. Error bars show standard errors.
With respect to aversive avoidance, OT decreased activity in the right striatum (MNI=24, 2, 10, t=4.31, PFWE=.011 SVC, voxels=58) and the right amygdala (MNI=28, -4, -18, t=3.34, PFWE=.025 SVC, voxels=2; Figure 2C) irrespective of social context (OT all negative<PLC all negative). Examination of interaction effects between treatment and social context during avoidance behavior (OT social negative>nonsocial negative<PLC social negative>nonsocial negative) revealed no significant effects (PFWE<.05 SVC). There were also no other significant effects in the a priori ROIs (PFWE<.05 SVC). For completeness, additional effects beyond the a priori ROIs on the whole-brain level are reported in supplementary Table 2 (PFWE<.05).
Brain Behavior Associations
Correlation analyses between extracted parameter estimates from the left ventral and the right dorsal AI (social positive>nonsocial positive) and RA difference scores (social positive − nonsocial positive) were conducted separately to explore associations between OT-induced modulation on neural responses and corresponding behavioral indices. Results showed a significant negative correlation between activity in the left ventral AI (MNI=-42, 6, -6) and RA difference scores in the OT (Pearson r=- 0.346, df=39, P=.031; Figure 3A) but not in the PLC group (Pearson r=0.039, df=37; P=.818). The correlation difference between groups was tested using the Fisher z-transformation test and revealed a marginally significant difference between the OT and PLC groups (Fishers z-score=-1.672, P=.094).
Discussion
The present study investigated OT’s effects on AA behavior and corresponding neural mechanisms using emotional scenes and specifically examined whether the effects generalize across social and nonsocial contexts. On the neural level, a significant main effect of treatment was observed in the right striatum, with OT generally decreasing activity in this region irrespective of response type and social context. Furthermore, separate examination of appetitive approach and aversive avoidance revealed that while OT specifically decreased the left dorsal AI activity during approaching positive stimuli, it decreased right amygdala activity during avoidance of negative stimuli. Additionally, exploring the role of social context revealed evidence for a selective enhancement effect of OT on RA when approaching social relative to nonsocial positive stimuli. This behavioral effect was accompanied by decreased left ventral and right dorsal AI activity in response to social vs nonsocial positive stimuli in the OT compared with the PLC group, with the relative difference in left ventral AI activity and RA for social vs nonsocial positive stimuli exhibiting a negative association following OT. By contrast, during aversive avoidance, no evidence for the social specificity of OT was observed. These findings provide support for the GAAO by showing that OT modulates activation of motivational and emotional salience core regions during both approach to positive and avoidance to negative stimuli across social and nonsocial contexts and that OT specifically facilitates approach behavior to more personally relevant and emotionally evocative stimuli, namely positive social stimuli, via inhibiting AI activity.
Examination of unspecific OT effects on AA behavior revealed significantly decreased activity in the right striatum following OT across both response type and social contexts. The striatum has been strongly involved in both approach (social/nonsocial reward) and avoidance (punishment/threat)-motivated behavior (Izuma et al., 2008; Delgado et al., 2008, 2009; Rademacher et al., 2010; Palminteri et al., 2012). Thus, OT may inhibit motivational representation both during appetitive approach and aversive avoidance. Furthermore, OT additionally decreased left dorsal AI activity during approach to positive stimuli across social and nonsocial contexts. As a core hub of the salience network (Menon, 2015; Uddin, 2015), the reduced dorsal AI activity may thus reflect an OT-evoked decrease in the salience of positive stimuli when subjects approached them.
The observed inhibitory effects of OT on striatum and AI activity seem to conflict with both the proposal that it enhances the salience of social cues (Shamay-Tsoory and Abu-Akel, 2016) and some previous findings that OT-induced alterations on human social behavior are associated with increased activity in the striatum and AI (Baumgartner et al., 2008; Strathearn et al., 2009; Striepens et al., 2011; Yao et al., 2018). Given that OT effects on social behavior are often highly context and person dependent (Bartz et al., 2011), this inconsistency could be due to different paradigms and contexts used in these previous studies. In previous studies, subjects were asked to passively process certain stimuli, whereas the present study using an AA task required subjects to actively approach or avoid them.
It is notable that OT also decreased activation of the left ventral and right dorsal AI during approaching social relative to nonsocial positive stimuli. Given the role of the ventral AI in emotional processing (Nieuwenhuys, 2012; Uddin, 2015; Wager and Barrett, 2017) and the dorsal AI in salience processing (Menon, 2015; Uddin, 2015), these attenuated AI activities may indicate a more robust inhibitory effect of OT on decreasing both the emotional and salience processing of social positive stimuli that are more personally relevant and emotionally evocative (Harari-Dahan and Bernstein, 2014). Consistent with previous observations that OT can enhance processing of positive facial emotion (Guastella et al., 2008; Marsh et al., 2010; Domes et al., 2013; Xu et al., 2015), the present study found evidence that OT may specifically facilitate RA during approaching social but not nonsocial positive stimuli. Thus, the oxytocinergic downregulation of AI activation may act to attenuate interference caused by hyperactivation of the AI when subjects approach external social positive stimuli, resulting in enhanced accuracy of social information processing and facilitation of social interaction. This assumption is further supported by the presence of a significant negative correlation between the left ventral AI activity and the RA difference between social and nonsocial positive stimuli following OT but not PLC administration. However, it should be noted that the difference in brain-behavior correlation between groups was only marginally significant, and thus inferences regarding this effect of OT need to be drawn with caution.
Additionally, OT was found to decrease amygdala activity during avoidance responses to negative stimuli independent of social context. This finding is in line with the anxiolytic action of OT via inhibiting amygdala responses to threatening stimuli (Kirsch et al., 2005; Petrovic et al., 2008). Based on the GAAO (Harari-Dahan and Bernstein, 2014), the absence of a social-specific effect of OT on avoiding negative stimuli suggests that threatening social and nonsocial stimuli may be comparable in terms of personal relevance and emotional evocation, perhaps due to the high survival relevance of threating events during evolution (Woody and Szechtman, 2011).
Given that we found no significant personality and mood difference between OT and PLC groups, this argues against confounding effects of pretreatment between-group differences on the observed effects of OT. However, individual differences in personality traits have been shown to mediate approach and avoidance behavior, as proposed by the (revised) Reinforcement Sensitivity Theory (Gray and McNaughton, 2000; Corr, 2004). More specifically, previous studies have demonstrated associations between the functional organization of the salience network, particularly the AI, and its interactions with anxiety-related traits such as harm avoidance (Baur et al., 2013; Markett et al., 2013, 2016). Thus, future studies should consider examining the role of individual differences in personality traits on AA behavior and their potential modulatory effects on the effects of OT in this domain. Moreover, within this context, the present findings may lend support for potential therapeutic benefits of OT for psychiatric disorders such as social anxiety and autism exhibiting altered approach/avoidance towards social stimuli (Heuer et al., 2007; Davis et al., 2010; Roelofs et al., 2010; Tanaka and Sung, 2016).
There are several limitations in the present study. Firstly, considering that organisms have primarily evolved mechanisms to approach stimuli associated with positive outcomes and to avoid those associated with aversive ones, we only asked subjects to approach positive and avoid negative stimuli. Effects of OT on approaching negative and avoiding positive stimuli thus remain to be determined. Secondly, we used only male subjects to avoid possible confounding effects from menstrual cycle; thus, the present conclusions are limited to males and sex differences remain to be explored. Thirdly, another potential limitation is that we cannot completely exclude the possibility of a complex interaction involving differences in social and nonsocial stimuli valence and OT effects on response accuracy during approach behavior. However, for positive valence stimuli, we found no association between individual picture valence and response accuracy for either social or nonsocial stimuli, suggesting that valence differences are unlikely to have had a major impact on our results. Finally, we do not know if the effects we observed with a 40-IU dose of OT may also be observed with lower doses. A previous study has revealed a dose-dependent effect of OT (12, 24, and 48 IU), with the 24-IU dose being most effective in inhibiting amygdala activity during negative emotional processing (Spengler et al., 2017). However, in 2 previous studies from our group, we reported evidence for comparable effects of 24- and 40-IU doses on both behavioral and neural changes in the context of empathy and self-processing in humans (Zhao et al., 2017; Geng et al., 2018).
In conclusion, the present study provides first evidence for the GAAO by demonstrating that while OT inhibits motivational representations both during appetitive approach and aversive avoidance, it facilitates human approach behavior to more general positive social stimuli via inhibiting AI activity. This inhibitory effect of OT may reduce interference from emotion-facilitated hyperactivation of core regions of the emotional and salience networks when approaching external social salient positive stimuli and consequently be of benefit in promoting social interaction.
Supplementary Materials
Supplementary data are available at International Journal of Neuropsychopharmacology (IJNPPY) online.
Acknowledgments
This work was supported by National Natural Science Foundation of China grants (31700998 to S.Y. and 31530032 to K.M.K.), Fundamental Research Funds for the Central Universities (ZYGX2015Z002 to B.B.), and the Science, Innovation and Technology Department of the Sichuan Province (2018JY0001 to B.B.).
Statement of Interest
None.
References
- Baron-Cohen S, Wheelwright S, Skinner R, Martin J, Clubley E(2001)The autism-spectrum quotient (AQ): evidence from asperger syndrome/high-functioning autism, males and females, scientists and mathematicians. J Autism Dev Disord 31:5–17. [DOI] [PubMed] [Google Scholar]
- Baron-Cohen S, Wheelwright S(2004)The empathy quotient: an investigation of adults with asperger syndrome or high functioning autism, and normal sex differences. J Autism Dev Disord 34:163–175. [DOI] [PubMed] [Google Scholar]
- Bartz J, Simeon D, Hamilton H, Kim S, Crystal S, Braun A, Vicens V, Hollander E(2011)Oxytocin can hinder trust and cooperation in borderline personality disorder. Soc Cogn Affect Neurosci 6:556–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumgartner T, Heinrichs M, Vonlanthen A, Fischbacher U, Fehr E(2008)Oxytocin shapes the neural circuitry of trust and trust adaptation in humans. Neuron 58:639–650. [DOI] [PubMed] [Google Scholar]
- Baur V, Hänggi J, Langer N, Jäncke L(2013)Resting-state functional and structural connectivity within an insula-amygdala route specifically index state and trait anxiety. Biol Psychiatry 73:85–92. [DOI] [PubMed] [Google Scholar]
- Corr PJ.(2004)Reinforcement sensitivity theory and personality. Neurosci Biobehav Rev 28:317–332. [DOI] [PubMed] [Google Scholar]
- Costa PT, MacCrae RR(1992)Revised NEO personality inventory (NEO PI-R) and NEO five-factor inventory (NEO FFI): Professional manual. Odessa, FL: Psychological Assessment Resources. [Google Scholar]
- Chen M, Bargh JA(1999)Consequences of automatic evaluation: immediate behavioral predispositions to approach or avoid the stimulus. Pers Soc Psychol Bull 25:215–224. [Google Scholar]
- Davis TE III, Fodstad JC, Jenkins WS, Hess JA, Moree BN, Dempsey T, Matson JL(2010)Anxiety and avoidance in infants and toddlers with autism spectrum disorders: evidence for differing symptom severity and presentation. Res Autism Spectr Disord 4:305–313. [Google Scholar]
- De Dreu CK, Greer LL, Handgraaf MJ, Shalvi S, Van Kleef GA, Baas M, Ten Velden FS, Van Dijk E, Feith SW(2010)The neuropeptide oxytocin regulates parochial altruism in intergroup conflict among humans. Science 328:1408–1411. [DOI] [PubMed] [Google Scholar]
- De Dreu CK, Shalvi S, Greer LL, Van Kleef GA, Handgraaf MJ(2012)Oxytocin motivates non-cooperation in intergroup conflict to protect vulnerable in-group members. Plos One 7:e46751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delgado MR, Li J, Schiller D, Phelps EA(2008)The role of the striatum in aversive learning and aversive prediction errors. Philos Trans R Soc Lond B Biol Sci 363:3787–3800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delgado MR, Jou RL, Ledoux JE, Phelps EA(2009)Avoiding negative outcomes: tracking the mechanisms of avoidance learning in humans during fear conditioning. Front Behav Neurosci 3:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domes G, Sibold M, Schulze L, Lischke A, Herpertz SC, Heinrichs M(2013)Intranasal oxytocin increases covert attention to positive social cues. Psychol Med 43:1747–1753. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Holmes AP, Worsley KJ, Poline JP, Frith CD, Frackowiak RS(1994)Statistical parametric maps in functional imaging: a general linear approach. Hum Brain Mapp 2:189–210. [Google Scholar]
- Geng Y, Zhao W, Zhou F, Ma X, Yao S, Hurlemann R, Becker B, Kendrick K(2018). Oxytocin enhancement of emotional empathy: generalization across cultures and effects on amygdala activity. bioRxiv 307256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray JA, McNaughton N(2000)The Neuropsychology of anxiety: an enquiry into the functions of the septo-hippocampal system. Oxford: Oxford University Press. [Google Scholar]
- Guastella AJ, Mitchell PB, Mathews F(2008)Oxytocin enhances the encoding of positive social memories in humans. Biol Psychiatry 64:256–258. [DOI] [PubMed] [Google Scholar]
- Guastella AJ, Hickie IB, McGuinness MM, Otis M, Woods EA, Disinger HM, Chan HK, Chen TF, Banati RB(2013)Recommendations for the standardisation of oxytocin nasal administration and guidelines for its reporting in human research. Psychoneuroendocrinology 38:612–625. [DOI] [PubMed] [Google Scholar]
- Harari-Dahan O, Bernstein A(2014)A general approach-avoidance hypothesis of oxytocin: accounting for social and non-social effects of oxytocin. Neurosci Biobehav Rev 47:506–519. [DOI] [PubMed] [Google Scholar]
- Harari-Dahan O, Bernstein A(2017)Oxytocin attenuates social and non-social avoidance: re-thinking the social specificity of oxytocin. Psychoneuroendocrinology 81:105–112. [DOI] [PubMed] [Google Scholar]
- Heinrichs M, Baumgartner T, Kirschbaum C, Ehlert U(2003)Social support and oxytocin interact to suppress cortisol and subjective responses to psychosocial stress. Biol Psychiatry 54:1389–1398. [DOI] [PubMed] [Google Scholar]
- Heuer K, Rinck M, Becker ES(2007)Avoidance of emotional facial expressions in social anxiety: the approach-avoidance task. Behav Res Ther 45:2990–3001. [DOI] [PubMed] [Google Scholar]
- Hurlemann R, Patin A, Onur OA, Cohen MX, Baumgartner T, Metzler S, Dziobek I, Gallinat J, Wagner M, Maier W, Kendrick KM(2010)Oxytocin enhances amygdala-dependent, socially reinforced learning and emotional empathy in humans. J Neurosci 30:4999–5007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izuma K, Saito DN, Sadato N(2008)Processing of social and monetary rewards in the human striatum. Neuron 58:284–294. [DOI] [PubMed] [Google Scholar]
- Kemp AH, Guastella AJ(2011)The role ofoxytocin in human affect a novel hypothesis. Curr Dir Psychol Sci 20:222–231. [Google Scholar]
- Kendrick KM, Guastella AJ, Becker B(2018)Overview of human oxytocin research. Curr Top Behav Neurosci 35:321–348. [DOI] [PubMed] [Google Scholar]
- Kirsch P, Esslinger C, Chen Q, Mier D, Lis S, Siddhanti S, Gruppe H, Mattay VS, Gallhofer B, Meyer-Lindenberg A(2005)Oxytocin modulates neural circuitry for social cognition and fear in humans. J Neurosci 25:11489–11493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosfeld M, Heinrichs M, Zak PJ, Fischbacher U, Fehr E(2005)Oxytocin increases trust in humans. Nature 435:673–676. [DOI] [PubMed] [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN(2008)International affective picture system (IAPS): affective ratings of pictures and instruction manual. Technical Report A-8. Gainesville, FL: University of Florida. [Google Scholar]
- Markett S, Weber B, Voigt G, Montag C, Felten A, Elger C, Reuter M(2013)Intrinsic connectivity networks and personality: the temperament dimension harm avoidance moderates functional connectivity in the resting brain. Neuroscience 240:98–105. [DOI] [PubMed] [Google Scholar]
- Markett S, Montag C, Melchers M, Weber B, Reuter M(2016)Anxious personality and functional efficiency of the insular-opercular network: a graph-analytic approach to resting-state fMRI. Cogn Affect Behav Neurosci 16:1039–1049. [DOI] [PubMed] [Google Scholar]
- Marsh AA, Yu HH, Pine DS, Blair RJ(2010)Oxytocin improves specific recognition of positive facial expressions. Psychopharmacology (Berl) 209:225–232. [DOI] [PubMed] [Google Scholar]
- Menon V.(2015)Salience network. In: Brain mapping: an encyclopedic reference (Toga AW, ed), pp597–611. London: Elsevier. [Google Scholar]
- Nieuwenhuys R.(2012)The insular cortex: a review. Prog Brain Res 195:123–163. [DOI] [PubMed] [Google Scholar]
- Palminteri S, Justo D, Jauffret C, Pavlicek B, Dauta A, Delmaire C, Czernecki V, Karachi C, Capelle L, Durr A, Pessiglione M(2012)Critical roles for anterior insula and dorsal striatum in punishment-based avoidance learning. Neuron 76:998–1009. [DOI] [PubMed] [Google Scholar]
- Petrovic P, Kalisch R, Singer T, Dolan RJ(2008)Oxytocin attenuates affective evaluations of conditioned faces and amygdala activity. J Neurosci 28:6607–6615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rademacher L, Krach S, Kohls G, Irmak A, Gründer G, Spreckelmeyer KN(2010)Dissociation of neural networks for anticipation and consumption of monetary and social rewards. Neuroimage 49:3276–3285. [DOI] [PubMed] [Google Scholar]
- Radke S, Roelofs K, de Bruijn ER(2013)Acting on anger: social anxiety modulates approach-avoidance tendencies after oxytocin administration. Psychol Sci 24:1573–1578. [DOI] [PubMed] [Google Scholar]
- Radke S, Volman I, Kokal I, Roelofs K, de Bruijn ERA, Toni I(2017)Oxytocin reduces amygdala responses during threat approach. Psychoneuroendocrinology 79:160–166. [DOI] [PubMed] [Google Scholar]
- Roelofs K, Putman P, Schouten S, Lange WG, Volman I, Rinck M(2010)Gaze direction differentially affects avoidance tendencies to happy and angry faces in socially anxious individuals. Behav Res Ther 48:290–294. [DOI] [PubMed] [Google Scholar]
- Scheele D, Striepens N, Güntürkün O, Deutschländer S, Maier W, Kendrick KM, Hurlemann R(2012)Oxytocin modulates social distance between males and females. J Neurosci 32:16074–16079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlund MW, Cataldo MF(2010)Amygdala involvement in human avoidance, escape and approach behavior. Neuroimage 53:769–776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahrestani S, Kemp AH, Guastella AJ(2013)The impact of a single administration of intranasal oxytocin on the recognition of basic emotions in humans: a meta-analysis. Neuropsychopharmacology 38:1929–1936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shalvi S, De Dreu CK(2014)Oxytocin promotes group-serving dishonesty. Proc Natl Acad Sci U S A 111:5503–5507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shamay-Tsoory SG, Abu-Akel A(2016)The social salience hypothesis of oxytocin. Biol Psychiatry 79:194–202. [DOI] [PubMed] [Google Scholar]
- Shamay-Tsoory SG, Fischer M, Dvash J, Harari H, Perach-Bloom N, Levkovitz Y(2009)Intranasal administration of oxytocin increases envy and schadenfreude (gloating). Biol Psychiatry 66:864–870. [DOI] [PubMed] [Google Scholar]
- Sheng F, Liu Y, Zhou B, Zhou W, Han S(2013)Oxytocin modulates the racial bias in neural responses to others’ suffering. Biol Psychol 92:380–386. [DOI] [PubMed] [Google Scholar]
- Spengler FB, Schultz J, Scheele D, Essel M, Maier W, Heinrichs M, Hurlemann R(2017)Kinetics and dose dependency of intranasal oxytocin effects on amygdala reactivity. Biol Psychiatry 82:885–894. [DOI] [PubMed] [Google Scholar]
- Strathearn L, Fonagy P, Amico J, Montague PR(2009)Adult attachment predicts maternal brain and oxytocin response to infant cues. Neuropsychopharmacology 34:2655–2666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Striepens N, Kendrick KM, Maier W, Hurlemann R(2011)Prosocial effects of oxytocin and clinical evidence for its therapeutic potential. Front Neuroendocrinol 32:426–450. [DOI] [PubMed] [Google Scholar]
- Striepens N, Matusch A, Kendrick KM, Mihov Y, Elmenhorst D, Becker B, Lang M, Coenen HH, Maier W, Hurlemann R, Bauer A(2014)Oxytocin enhances attractiveness of unfamiliar female faces independent of the dopamine reward system. Psychoneuroendocrinology 39:74–87. [DOI] [PubMed] [Google Scholar]
- Theodoridou A, Rowe AC, Penton-Voak IS, Rogers PJ(2009)Oxytocin and social perception: oxytocin increases perceived facial trustworthiness and attractiveness. Horm Behav 56:128–132. [DOI] [PubMed] [Google Scholar]
- Theodoridou A, Penton-Voak IS, Rowe AC(2013)A direct examination of the effect of intranasal administration of oxytocin on approach-avoidance motor responses to emotional stimuli. Plos One 8:e58113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka JW, Sung A(2016)The “eye avoidance” hypothesis of autism face processing. J Autism Dev Disord 46:1538–1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, Mazoyer B, Joliot M(2002)Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage 15:273–289. [DOI] [PubMed] [Google Scholar]
- Uddin LQ.(2015)Salience processing and insular cortical function and dysfunction. Nat Rev Neurosci 16:55–61. [DOI] [PubMed] [Google Scholar]
- Wager TD, Barrett LF(2017)From affect to control: Functional specialization of the insula in motivation and regulation. bioRxiv 102368. [Google Scholar]
- Watson D, Clark LA, Tellegen A(1988)Development and validation of brief measures of positive and negative affect: the PANAS scales. J Pers Soc Psychol 54:1063–1070. [DOI] [PubMed] [Google Scholar]
- Wigton R, Radua J, Allen P, Averbeck B, Meyer-Lindenberg A, McGuire P, Shergill SS, Fusar-Poli P(2015)Neurophysiological effects of acute oxytocin administration: systematic review and meta-analysis of placebo-controlled imaging studies. J Psychiatry Neurosci 40:E1–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woody EZ, Szechtman H(2011)Adaptation to potential threat: the evolution, neurobiology, and psychopathology of the security motivation system. Neurosci Biobehav Rev 35:1019–1033. [DOI] [PubMed] [Google Scholar]
- Xu L, Ma X, Zhao W, Luo L, Yao S, Kendrick KM(2015)Oxytocin enhances attentional bias for neutral and positive expression faces in individuals with higher autistic traits. Psychoneuroendocrinology 62:352–358. [DOI] [PubMed] [Google Scholar]
- Yao S, Becker B, Zhao W, Zhao Z, Kou J, Ma X, Geng Y, Ren P, Kendrick KM(2018)Oxytocin modulates attention switching between interoceptive signals and external social cues. Neuropsychopharmacology 43:294–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zak PJ, Stanton AA, Ahmadi S(2007)Oxytocin increases generosity in humans. Plos One 2:e1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao W, Geng Y, Luo L, Zhao Z, Ma X, Xu L, Yao S, Kendrick KM(2017)Oxytocin increases the perceived value of both self- and other-owned items and alters medial prefrontal cortex activity in an endowment task. Front Hum Neurosci 11:272. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.