Abstract
Background
Although previous reports suggest sex-specific differences in the antidepressant actions of (R,S)-ketamine, these differences in the antidepressant actions of (R)-ketamine, which is more potent than (S)-ketamine, are unknown.
Methods
Saline or (R)-ketamine was administered 23 hours post lipopolysaccharide administration to adult male or female mice. Subsequently, antidepressant effects were assessed using a forced swimming test. Furthermore, the concentration of (R)-ketamine and its 2 major metabolites, (R)-norketamine and (2R,6R)-hydroxynorketamine, was measured in the plasma and brain after the administration of (R)-ketamine in the mice.
Results
(R)-ketamine (10 mg/kg) significantly attenuated the increased immobility time of forced swimming test in the lipopolysaccharide-treated mice. There were no sex-specific differences in the concentrations of (R)-ketamine and its 2 metabolites in the plasma and brain.
Conclusions
These findings showed no sex-specific differences in terms of the acute antidepressant effects and pharmacokinetic profile of (R)-ketamine.
Keywords: (2R,6R)-hydroxynorketamine; (R)-ketamine; sex-specific difference
Introduction
Meta-analysis demonstrated that (R,S)-ketamine, a N-methyl-D-aspartate receptor antagonist, exhibits rapid and long-lasting antidepressant effects and antisuicidal ideation in treatment-resistant patients with major depressive or bipolar disorder (Newport et al., 2015; Wilkinson et al., 2018). At present, (R,S)-ketamine has gained attention as a rapid-acting antidepressant for treatment-resistant depression. Although the concerns about the safety of repeated (R,S)-ketamine infusions persist, it has been used as an off-label treatment for mood disorders (Sanacora et al., 2017). However, the precise mechanisms underlying the antidepressant actions of (R,S)-ketamine are unknown.
(R,S)-Ketamine, a racemic mixture, comprises equal parts of (R)-ketamine (or arketamine) and (S)-ketamine (or esketamine). (S)-ketamine shows approximately 3- to 4-fold greater anesthetic potency and undesirable psychotomimetic adverse effects than (R)-ketamine (Hashimoto, 2016a, 2016b). In several models of depression (e.g., neonatal dexamethasone exposure, chronic social defeat stress [CSDS], learned helplessness [LH], repeated corticosterone treatment), (R)-ketamine displays greater potency and long-lasting antidepressant effects than (S)-ketamine in mice (or rats) with a depression-like phenotype (Zhang et al., 2014a; Yang et al., 2015, 2018; Fukumoto et al., 2017). Unlike (S)-ketamine, (R)-ketamine does not induce psychotomimetic adverse effects or exhibit abuse potential in mice (Yang et al., 2015). Furthermore, a positron emission tomography study demonstrated a marked reduction in dopamine D2/3 receptor binding potential in the monkey striatum after a single infusion of (S)-ketamine but not after that of (R)-ketamine, suggesting that (S)-ketamine-induced dopamine release is associated with acute psychotomimetic and dissociative adverse effects in humans (Hashimoto et al., 2017). Collectively, (R)-ketamine could be a safer antidepressant alternative to (R,S)-ketamine and (S)-ketamine in humans (Hashimoto, 2016a, 2016b).
(R,S)-Ketamine is rapidly metabolized in the liver by microsomal cytochrome P450 enzymes into (R,S)-norketamine (via N-demethylation) and subsequently into (2S,6S; 2R,6R)-hydroxynorketamine (HNK). Zanos et al. (2016) reported that the metabolism of (R,S)-ketamine to (2R,6R)-HNK is essential for its antidepressant effects in rodents; they reported superior antidepressant-like actions of (R,S)-ketamine in female mice compared with those in male mice in the forced swimming test (FST) (Zanos et al., 2016). Although equivalent levels of (R,S)-ketamine and (R,S)-norketamine were observed, the concentration of (2S,6S; 2R,6R)-HNK was approximately 3-fold higher in the brain of female mice compared with that in the brain of male mice (Zanos et al., 2016). From these data, Zanos et al. (2016) claimed that (2R,6R)-HNK, the final metabolite from (R)-ketamine, plays a vital role in the antidepressant actions of (R,S)-ketamine. However, our recent preclinical studies in male mice did not replicate the antidepressant effects of (2R,6R)-HNK in an inflammation, a CSDS model (Yang et al., 2017), and a LH model of depression in male rats (Shirayama and Hashimoto, 2018). Currently, an increasing debate about the antidepressant actions of (2R,6R)-HNK in rodents exists (Abdallah, 2017; Chaki, 2018; Hashimoto and Shirayama, 2018). Furthermore, the sex-specific differences in the antidepressant actions of (R)-ketamine in rodents with a depression-like phenotype remain unclear.
This study aimed to examine whether sex-specific differences could influence the antidepressant effects of (R)-ketamine in an inflammation model of depression (Zhang et al., 2014b). Furthermore, we determined the levels of (R)-ketamine and its 2 major metabolites, (R)-norketamine and (2R,6R)-HNK, in the plasma and brain after the administration of (R)-ketamine in male and female mice with inflammation-induced depression-like phenotype.
Materials and Methods
Animals
Eight-week-old adult male and female C57BL/6 mice (weight, 20–25 g; Japan SLC, Inc) were used as model animals. They were housed under controlled temperatures and 12-hour-light/-dark cycles (lights were turned on between 7:00 am and 7:00 pm) with ad libitum access to food (CE-2; CLEA Japan, Inc.) and water. The Chiba University Institutional Animal Care and Use Committee approved the protocol (permission no. 29-317). The pharmacokinetic study of (R)-ketamine was conducted in accordance with the criteria of the Taisho Pharmaceutical Co., Ltd. The Animal Care Committee met the Japanese Experimental Animal Research Association standards as defined in the Guidelines for Animal Experiments. Prior to killing via cervical dislocation, mice were deeply anesthetized with isoflurane and all efforts were made to minimize pain and distress.
Chemical Reagents
Lipopolysaccharide (LPS; 0.5 mg/kg; L-4130, serotype 0111:B4; Sigma–Aldrich) was dissolved in physiological saline. (R)-Ketamine hydrochloride was prepared by the recrystallization of (R,S)-ketamine and D-(-)-tartaric acid, as per previous studies (Zhang et al., 2014b; Fukumoto et al., 2017). The dose (3 or 10 mg/kg) of (R)-ketamine to be dissolved in physiological saline was determined as per previous reports (Yang et al., 2015, 2018; Zanos et al., 2016). As analytical standard substances, (R,S)-ketamine hydrochloride solution (50 mg/mL as a free base; Fujita Pharmaceutical Co., Ltd), (R,S)-norketamine hydrochloride (Funakoshi Co., Ltd.), (2R,6R)-HNK, and (2S,6S)-HNK (Nacalai Tesque, Inc) were used. The analytical internal standard (IS), 3,4,5,6-tetradeuterophenyl-norketamine hydrochloride (2H4-norketamine), in methanol (100 µg/mL as a free base) was purchased from Sigma–Aldrich Co. Other reagents were commercially purchased.
Inflammation Model
The inflammation model using LPS (0.5 mg/kg) was performed as previously reported (Zhang et al., 2014b; Yang et al., 2017). Saline (10 mL/kg) or LPS (0.5 mg/kg) was i.p. injected into the male or female mice. Twenty-three hours later, the mice were i.p. treated with saline (10 mL/kg), (R)-ketamine (3 mg/kg), or (R)-ketamine (10 mg/kg). The locomotion test (LMT) and FST were performed 1 and 3 h after the administration of each compound, respectively.
LMT
LMT was performed using an animal movement analysis system (SCANET MV-40; MELQUEST Co., Ltd.). The mice were placed in experimental cages (560×560×330 mm), and cumulative exercise was recorded for 60 minutes. The cages were cleaned between the testing sessions.
FST
FST was conducted using an automated forced-swim apparatus (SCANET MV-40; MELQUEST Co., Ltd), wherein the mice were individually placed in a cylinder (diameter, 23 cm; height, 31 cm) containing 15 cm water maintained at a temperature of 23°C±1°C. The immobility time was calculated using the activity time as (total) − (active) time by the apparatus analysis software and was recorded for each mouse for 6 minutes.
Determination of (R)-Ketamine and Its Two Metabolites in the Plasma and Brain
LPS (0.5 mg/kg) was i.p. injected into the mice. Twenty-three hours later, the mice were i.p. treated with (R)-ketamine (3 mg/kg) or (R)-ketamine (10 mg/kg). To determine the plasma and brain concentration–time profiles, blood and brain samples were collected 10, 30, 60, 120, and 180 minutes after the administration of (R)-ketamine. Approximately 300 μL blood was collected from the jugular vein cava at designated times under isoflurane anesthesia and placed into a tube containing ethylenediamine-N,N,N’,N’-tetraacetic acid potassium salt dehydrate as an anticoagulant. To collect the brain, the animal was immediately killed by exsanguination. The analytical biological specimens were prepared and subjected to protein precipitation as previously reported (Toki et al., 2018). To simultaneously determine (R)-ketamine, (R)-norketamine, and (2R,6R)-HNK levels, 5 µL of the resulting supernatant was subjected to an enantioselective liquid chromatography–tandem mass spectrometry (LC–MS/MS) assay with some modifications to a procedure used in our previous study (Yamaguchi et al., 2018) regarding the separating conditions. The LC–MS/MS system comprised a Triple Quad 5500 mass spectrometer (AB SCIEX), 4 LC-30AD pumps, a SIL-30ACMP autosampler, a CTO-20AC column oven (Shimadzu), and 2 column-switching valves. Chromatographic separation was performed at 40°C on a Zorbax Eclipse C18 (4.6 mm i.d.×50 mm; 3.5-µm particles; Agilent Technologies), CHIRALPAK AY-RH (4.6 mm i.d.×150 mm; 5-µm particles; Daicel), and CHIRALPAK AS-RH (4.6 mm i.d.×150 mm; 5-µm particles; Daicel) columns using the mixtures of 1 mM ammonium bicarbonate and acetonitrile as mobile phases at a flow rate of 1.0 or 1.3 mL/min. For all analytes, the lower limits of quantification in the plasma and brain were 3 ng/mL and 1.5 ng/g, respectively.
Statistical Analysis
The data are represented as mean±SEM. The analysis was performed using PASW Statistics 20 (formerly SPSS Statistics; SPSS). Behavioral data were analyzed using 2-way ANOVA followed by the posthoc Tukey test. The Student’s t test was used to analyze the pharmacokinetic data at each time point. P <.05 were considered statistically significant. The pharmacokinetic parameters (Cmax and AUC0–3h) were calculated using a noncompartmental model with Phoenix WinNonlin 7.0 software.
Results
Sex-Specific Effects of (R)-Ketamine
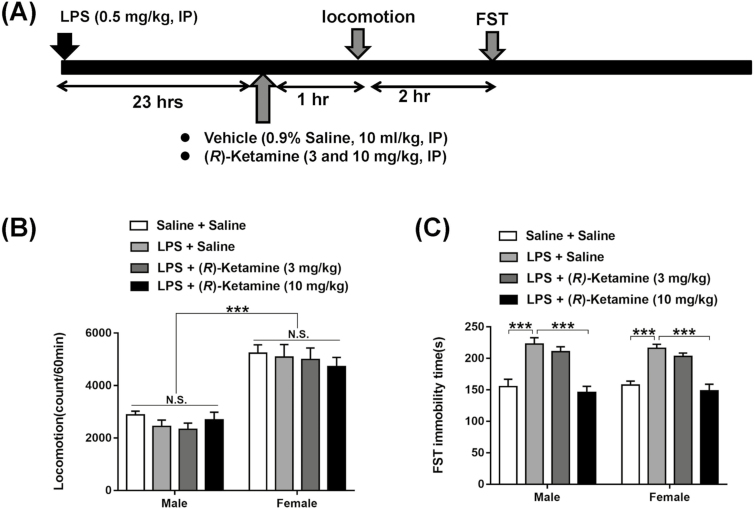
Saline or (R)-ketamine (3 or 10 mg/kg) was administered to male or female mice 23 hours after LPS administration (Figure 1A). Two-way ANOVA showed the following statistical results of the locomotion test: group, F3,56=0.594, P=.622; sex, F1,56=108.5, P<.001; and interaction, F3,56=0.416, P=.742 (Figure 1B). Notably, there were sex-specific differences in terms of the locomotion of LPS-treated mice.
Figure 1.
Effects of sex difference in the antidepressant effects of (R)-ketamine in an inflammation model. (A) Saline (10 mL/kg) or lipopolysaccharide (LPS) (0.5 mg/kg) was administered i.p. into the male and female mice. Twenty-three hours after injection, saline (10 mL/kg) or (R)-ketamine (3 and 10 mg/kg) was administered i.p. into mice. Locomotion and forced swimming test (FST) were performed 1 and 3 hours after a single injection of (R)-ketamine or saline, respectively. (B) Locomotion. (C) FST. The values represent the mean±SEM (n=8). ***P<.001. NS, not significant.
Furthermore, 2-way ANOVA showed the following statistical results for FST: group, F3,56=33.5, P<.001; sex, F1,56=0.148, P=.702; and interaction, F3,56=0.206, P=.892. The posthoc test revealed that a 10 mg/kg dose of (R)-ketamine significantly attenuated the increased immobility time of FST in the mice. However, the lower (R)-ketamine dose (3 mg/kg) did not show antidepressant effects in the male or female mice. These data suggest a lack of sex-specific differences in the antidepressant effects of (R)-ketamine in an inflammation model, yet there was a sex-specific difference in terms of the locomotion of the saline-treated mice.
Pharmacokinetic Profiles of (R)-Ketamine and Its Metabolites in Male and Female Mice
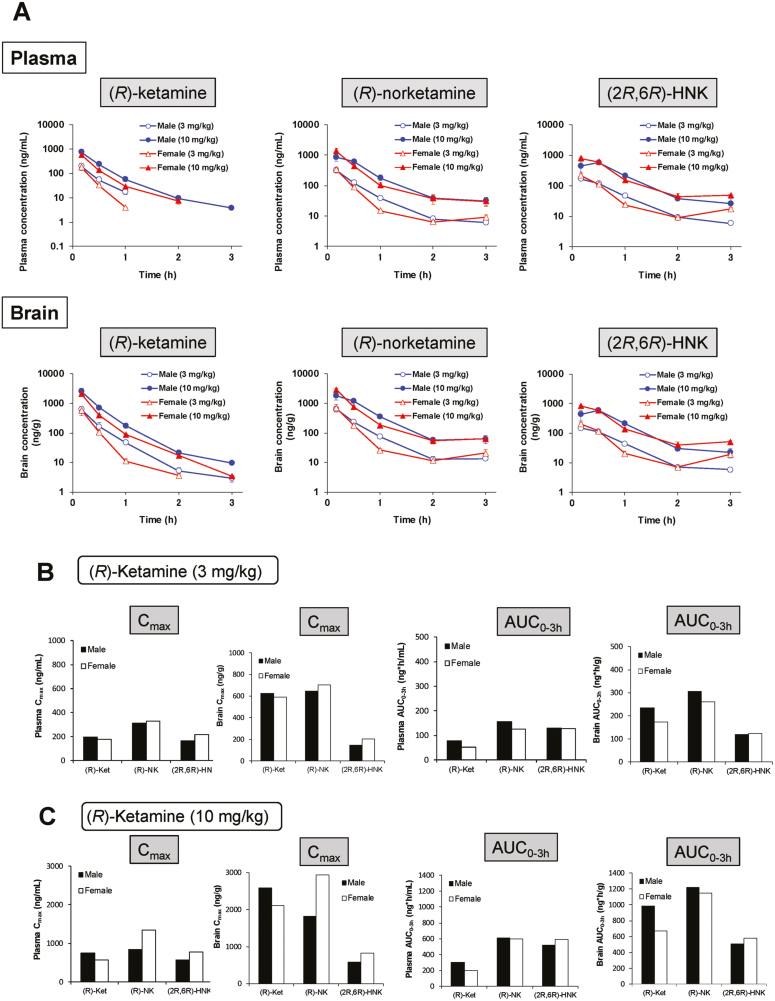
(R)-Ketamine (3 or 10 mg/kg) was administered to male and female mice 23 hours after the LPS administration. No sex-specific differences were observed in the plasma and brain concentrations of (R)-ketamine, (R)-norketamine, and (2R,6R)-HNK (Figure 2A). Furthermore, the pharmacokinetic parameters (Cmax and AUC0–3h) of (R)-ketamine, (R)-norketamine, and (2R,6R)-HNK in the brain and plasma after the administration of (R)-ketamine (3 or 10 mg/kg) were similar in both sexes (Figure 2B–C). These findings suggest no sex-specific differences in the concentrations of (R)-ketamine, (R)-norketamine, and (2R,6R)-HNK in the brain and plasma after the administration of (R)-ketamine.
Figure 2.
Pharmacokinetic profiles of (R)-ketamine and its metabolites after administration of (R)-ketamine. (A) Lipopolysaccharide (LPS) (0.5 mg/kg) was administered i.p. into the male and female mice. Twenty-three hours after injection, (R)-ketamine (3 and 10 mg/kg) was administered i.p. into mice. Sampling of brain and plasma was performed 10, 30, 60, 120, and 180 minutes after administration of (R)-ketamine. Concentration of (R)-ketamine, (R)-norketamine, and (2R,6R)-HNK in the plasma and brain was measured. The values at each time point represent the mean±SEM (n=3). The missing data were under the limit of detection (3 ng/mL). (B) Pharmacokinetic parameters of (R)-ketamine, (R)-norketamine, and (2R,6R)-HNK in the plasma and brain after administration of (R)-ketamine (3 mg/kg) were determined. There were no gender differences in the Cmax and AUC0-3h in the plasma and brain. (C) Pharmacokinetic parameters of (R)-ketamine, (R)-norketamine, and (2R,6R)-HNK in the plasma and brain after administration of (R)-ketamine (10 mg/kg) were determined. There were no gender differences in the Cmax and AUC0-3h in the plasma and brain.
Discussion
In this study, we demonstrated that (R)-ketamine showed antidepressant effects in the LPS-treated male and female mice when administered at 10 mg/kg dose but not at 3 mg/kg dose. Further, we found no sex-specific differences in the levels of (R)-ketamine and its 2 metabolites, (R)-norketamine and (2R,6R)-HNK, in the plasma and brain after a single administration of (R)-ketamine (3 or 10 mg/kg) in the LPS-treated mice. These findings show no sex-specific differences for the antidepressant actions and pharmacokinetic profiles of (R)-ketamine in the LPS-treated mice.
Previous reports demonstrated the sex-specific differences of (R,S)-ketamine’s antidepressant-like effects in rodents. In the FST, Carrier and Kabbaj (2013) reported antidepressant-like effects after a single administration of low-dose (R,S)-ketamine (2.5 mg/kg) in control female rats, but not control male rats. Furthermore, the antidepressant-like effects of low-dose (R,S)-ketamine (2.5 mg/kg) were completely abolished when the female rats were ovariectomized, suggesting a role of gonadal hormones in low-dose (R,S)-ketamine’s antidepressant-like actions in female rats (Carrier and Kabbaj, 2013). When the stage of estrous cycle is controlled, female mice with diestrus cycles and male mice exhibit similar antidepressant-like effects of (R,S)-ketamine in FST (Dossat et al., 2018). However, female mice with proestrus cycles exhibit superior antidepressant-like effects of (R,S)-ketamine (1.5 mg/kg) than female mice with other cycles and male mice (Dossat et al., 2018). Thus, menstrual cycle possibly affects the antidepressant-like effects of (R,S)-ketamine in control female mice, although the pharmacokinetic profiles of (R,S)-ketamine were not examined in these mice (Carrier and Kabbaj, 2013; Dossat et al., 2018).
It has been also reported that the immune response of female mice differs from male mice (Zellweger et al., 1997) and that sex hormones in female mice play a role in maintaining immune responses (Knöferl et al., 2002). Interestingly, Engler et al. (2016) reported the effects of a low dose of LPS (0.4 ng/kg) on inflammatory and neuroendocrine response in the blood of healthy volunteers (14 men, 14 women). Women exhibited a more profound proinflammatory response with significantly higher increases in tumor necrosis factor-α and interleukin (IL)-6. In contrast, the LPS-induced increase in antiinflammatory IL-10 was significantly higher in men. The LPS-induced increase in cortisol was significantly higher in woman. Despite these profound sex-specific differences in inflammatory and neuroendocrine responses, men and women did not differ in LPS-induced alterations in mood and state anxiety or nonspecific sickness symptoms (Engler et al., 2016). In this study, we did not find sex-specific differences in the acute antidepressant effects of (R)-ketamine, supporting the data in humans (Engler et al., 2016). The weakness of this paper is a lack of saline+drug control group (without LPS). A further study using the drug control group without LPS is needed.
Zanos et al. (2016) claim that (2R,6R)-HNK is essential for (R,S)-ketamine’s antidepressant actions in rodents since (2S,6S)-HNK’s antidepressant effects were weak. This hypothesis is also supported by the 3-fold higher levels of (2S,6S;2R,6R)-HNK in female brain compared with those in male brain after the administration of (R,S)-ketamine. Unfortunately, the authors did not measure the plasma levels of (2S,6S;2R,6R)-HNK after administering (R,S)-ketamine. In contrast, we found no sex-specific differences in the levels of (R)-ketamine, (R)-norketamine, or (2R,6R)-HNK in the plasma and brains of the LPS-treated male and female mice. Although the precise reasons for these discrepancies are currently unknown, several contributing factors might exist. First, the use of (R,S)-ketamine vs (R)-ketamine may have contributed to this discrepancy. In this study, we also measured the levels of (R)-norketamine and (2R,6R)-HNK in the plasma and brain, whereas Zanos et al. measured the racemic mixtures of (R,S)-norketamine and (2S,6S;2R,6R)-HNK in the brain (Zanos et al., 2016). Second, the use of rodents without (Zanos et al., 2016) vs those with depression-like phenotypes (in this study) may have also contributed to this discrepancy. Last, Zanos et al. (2016) reported that a 10 mg/kg dose of (R,S)-ketamine showed sustained antidepressant-like effects in control male and female mice, whereas a lower dose (3 mg/kg) showed antidepressant-like effects in female, but not male, mice. In contrast, we found that a 3 mg/kg dose of (R)-ketamine did not show antidepressant effects in LPS-treated male or female mice. Therefore, further detailed studies are warranted to study the role of sex-specific differences in the acute and sustained antidepressant effects of (R,S)-ketamine and its enantiomers in rodents with a depression-like phenotype.
In the LH paradigm, we demonstrated that a single bilateral infusion of (R)-ketamine into the infralimbic portion of the medial prefrontal cortex of rats with a depression-like phenotype improved depressive-like symptoms (Shirayama and Hashimoto, 2017), indicating that (R)-ketamine itself has antidepressant effects. Furthermore, we reported that an intraventricular administration of (R)-ketamine, but not (2R,6R)-HNK, showed rapid and long-lasting antidepressant effects in a CSDS model (Zhang et al., 2018). Moreover, a recent study demonstrated that pretreatment with cytochrome P450 inhibitors did not detect (2R,6R)-HNK in the blood after a single administration of (R)-ketamine, although its antidepressant effect was shown (Yamaguchi et al., 2018). Collectively, it is unlikely that (2R,6R)-HNK is essential for (R)-ketamine’s antidepressant actions in rodents, although further detailed studies are needed.
In conclusion, this study suggests no sex-specific differences in the acute antidepressant effects and pharmacokinetic profiles of (R)-ketamine in an inflammation model. Therefore, it is unlikely that sex plays a major role in the acute antidepressant actions of (R)-ketamine in rodents.
Acknowledgments
This study was supported by the Strategic Research Program for Brain Sciences from Japan Agency for Medical Research and Development, AMED (to K.H., JP17dm0107119).
Statement of Interest
Dr Hashimoto is an inventor on a filed patent application on “The use of (R)-ketamine in the treatment of psychiatric diseases” by Chiba University. Dr Hashimoto has received research support from Dainippon-Sumitomo, Otsuka, and Taisho. Drs Toki, Mizuno-Yasuhira, Yamaguchi, and Chaki are employees of Taisho Pharmaceutical Co., Ltd., Japan. Other authors declare no conflict of interest.
References
- Abdallah CG.(2017)What’s the buzz about hydroxynorketamine? Is it the history, the story, the debate, or the promise?Biol Psychiatry 81:e61–e63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrier N, Kabbaj M(2013)Sex differences in the antidepressant-like effects of ketamine. Neuropharmacology 70:27–34. [DOI] [PubMed] [Google Scholar]
- Chaki S.(2018)Is metabolism of (R)-ketamine essential for the antidepressant effects?Int J Neuropsychopharmacol 21:154–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dossat AM, Wright KN, Strong CE, Kabbaj M(2018)Behavioral and biochemical sensitivity to low doses of ketamine: influence of estrous cycle in C57BL/6 mice. Neuropharmacology 130:30–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engler H, Benson S, Wegner A, Spreitzer I, Schedlowski M, Elsenbruch S(2016)Men and women differ in inflammatory and neuroendocrine responses to endotoxin but not in the severity of sickness symptoms. Brain Behav Immun 52:18–26. [DOI] [PubMed] [Google Scholar]
- Fukumoto K, Toki H, Iijima M, Hashihayata T, Yamaguchi JI, Hashimoto K, Chaki S(2017)Antidepressant potential of (R)-ketamine in rodent models: comparison with (S)-ketamine. J Pharmacol Exp Ther 361:9–16. [DOI] [PubMed] [Google Scholar]
- Hashimoto K. (2016a) R-ketamine: a rapid-onset and sustained antidepressant without risk of brain toxicity. Psychol Med 46:2449–2451. [DOI] [PubMed] [Google Scholar]
- Hashimoto K. (2016b) Ketamine’s antidepressant action: beyond NMDA receptor inhibition. Expert Opin Ther Targets 20:1389–1392. [DOI] [PubMed] [Google Scholar]
- Hashimoto K, Kakiuchi T, Ohba H, Nishiyama S, Tsukada H(2017)Reduction of dopamine D2/3 receptor binding in the striatum after a single administration of esketamine, but not R-ketamine: a PET study in conscious monkeys. Eur Arch Psychiatry Clin Neurosci 267:173–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto K, Shirayama Y(2017)What are the causes for discrepancies of antidepressant actions of (2R,6R)-hydroxynorketamine?Biol Psychiatry 84:e7–e8. doi: 10.1016/j.biopsych.2017.12.007. [DOI] [PubMed] [Google Scholar]
- Knöferl MW, Angele MK, Diodato MD, Schwacha MG, Ayala A, Cioffi WG, Bland KI, Chaudry IH(2002)Female sex hormones regulate macrophage function after trauma-hemorrhage and prevent increased death rate from subsequent sepsis. Ann Surg 235:105–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newport DJ, Carpenter LL, McDonald WM, Potash JB, Tohen M, Nemeroff CB; APA Council of Research Task Force on Novel Biomarkers and Treatments (2015)Ketamine and other NMDA antagonists: early clinical trials and possible mechanisms in depression. Am J Psychiatry 172:950–966. [DOI] [PubMed] [Google Scholar]
- Sanacora G, Frye MA, McDonald W, Mathew SJ, Turner MS, Schatzberg AF, Summergrad P, Nemeroff CB; American Psychiatric Association (APA) Council of Research Task Force on Novel Biomarkers and Treatments (2017)A consensus statement on the use of ketamine in the treatment of mood disorders. JAMA Psychiatry 74:399–405. [DOI] [PubMed] [Google Scholar]
- Shirayama Y, Hashimoto K(2017)Effects of a single bilateral infusion of R-ketamine in the rat brain regions of a learned helplessness model of depression. Eur Arch Psychiatry Clin Neurosci 267:177–182. [DOI] [PubMed] [Google Scholar]
- Shirayama Y, Hashimoto K(2018)Lack of antidepressant effects of (2R,6R)-hydroxynorketamine in a rat learned helplessness model: comparison with (R)-ketamine. Int J Neuropsychopharmacol 21:84–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toki H, Ichikawa T, Mizuno-Yasuhira A, Yamaguchi J(2018)A rapid and sensitive chiral LC-MS/MS method for the determination of ketamine and norketamine in mouse plasma, brain and cerebrospinal fluid applicable to the stereoselective pharmacokinetic study of ketamine. J Pharm Biomed Anal 148:288–297. [DOI] [PubMed] [Google Scholar]
- Wilkinson ST, Ballard ED, Bloch MH, Mathew SJ, Murrough JW, Feder A, Sos P, Wang G, Zarate CA Jr, Sanacora G(2018)The effect of a single dose of intravenous ketamine on suicidal ideation: a systematic review and individual participant data meta-analysis. Am J Psychiatry 175:150–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi J, Toki H, Qu Y, Yang C, Koike H, Hashimoto K, Mizuno-Yasuhira A, Chaki S(2018)(2R,6R)-Hydroxynorketamine is not essential for the antidepressant actions of (R)-ketamine in mice. Neuropsychopharmacology doi: 10.1038/s41386-018-0084-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang C, Qu Y, Abe M, Nozawa D, Chaki S, Hashimoto K(2017)(R)-ketamine shows greater potency and longer lasting antidepressant effects than its metabolite (2R,6R)-hydroxynorketamine. Biol Psychiatry 82:e43–e44. [DOI] [PubMed] [Google Scholar]
- Yang C, Ren Q, Qu Y, Zhang JC, Ma M, Dong C, Hashimoto K(2018)Mechanistic target of rapamycin-independent antidepressant effects of (R)-ketamine in a social defeat stress model. Biol Psychiatry 83:18–28. [DOI] [PubMed] [Google Scholar]
- Yang C, Shirayama Y, Zhang JC, Ren Q, Yao W, Ma M, Dong C, Hashimoto K(2015)R-ketamine: a rapid-onset and sustained antidepressant without psychotomimetic side effects. Transl Psychiatry 5:e632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanos P, Moaddel R, Morris PJ, Georgiou P, Fischell J, Elmer GI, Alkondon M, Yuan P, Pribut HJ, Singh NS, Dossou KS, Fang Y, Huang XP, Mayo CL, Wainer IW, Albuquerque EX, Thompson SM, Thomas CJ, Zarate CA Jr, Gould TD(2016)NMDAR inhibition-independent antidepressant actions of ketamine metabolites. Nature 533:481–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zellweger R, Wichmann MW, Ayala A, Stein S, DeMaso CM, Chaudry IH(1997)Females in proestrus state maintain splenic immune functions and tolerate sepsis better than males. Crit Care Med 25:106–110. [DOI] [PubMed] [Google Scholar]
- Zhang K, Fujita Y, Hashimoto K(2018)Lack of metabolism in (R)-ketamine’s antidepressant actions in a chronic social defeat stress model. Sci Rep 8:4007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang JC, Li SX, Hashimoto K (2014a) R(-)-ketamine shows greater potency and longer lasting antidepressant effects than S (+)-ketamine. Pharmacol Biochem Behav 116:137–141. [DOI] [PubMed] [Google Scholar]
- Zhang JC, Wu J, Fujita Y, Yao W, Ren Q, Yang C, Li SX, Shirayama Y, Hashimoto K (2014b) Antidepressant effects of TrkB ligands on depression-like behavior and dendritic changes in mice after inflammation. Int J Neuropsychopharmacol 18:pyu077. [DOI] [PMC free article] [PubMed] [Google Scholar]