Abstract
Background
Several currently available animal models reproduce select behavioral facets of human mania as well as the abnormal glutamatergic neurotransmission and dysregulation of glycogen synthase kinase 3β that accompanies this disease.
Methods
In this study, we addressed the therapeutic potential of ligands of sigma receptor type 1 (σ1R) in 2 putative models of mania: the “manic” Black Swiss outbred mice from Taconic farms (BStac) and mice with the 129 genetic background and histidine triad nucleotide-binding protein 1 (HINT1) deletion (HINT1-/- mice) that exhibit bipolar-like behaviors.
Results
The activity of control mice, which do not exhibit manic-like behaviors in the forced swim test, was significantly enhanced by MK801, an inhibitor of glutamate N-methyl-D-aspartate receptor activity, an effect that was not or barely observed in manic-like mice. Typical mood stabilizers, such as glycogen synthase kinase 3β inhibitors, but not σ1R ligands, reduced the N-methyl-D-aspartate receptor-mediated behaviors in control mice. Notably, σ1R antagonists S1RA, PD144418, BD1047, and BD1063, but not σ1R agonists PRE084 and PPCC, attenuated the manic-like behaviors of BStac and HINT1-/- mice by increasing antiactivity behaviors. The antimanic effects of a single administration of σ1R antagonists persisted for at least 24 hours, and these drugs did not alter the behavior of the “bipolar” HINT1−/− mice during pro-depressive episodes.
Conclusions
σ1R antagonists exhibit a selective normalizing effect on specific behavioral domains of mania without altering control (normal) or depressive-like behaviors.
Keywords: mania, sigma receptor type 1, mood stabilizers, NMDA receptor, HINT1 protein
Significance Statement
Mania had long been recognized as an aberrant behavior indicative of mental illness. The present study explored the potential of sigma 1 receptor ligands as antimanic/mood stabilizers in 2 validated animal models of mania, a specific strain of Black Swiss mice from Taconic and mice with genetic deletion of the HINT1 protein. The antagonists of the sigma 1 receptor diminished the incidence of specific behavioral domains of mania without modifying the behavior of control mice. Thus, our data evidence the potential of these drugs to improve the quality of life of subjects affected by such manic disease.
Introduction
Mania has long been recognized as aberrant behavior indicative of mental illness. Manic states include a variety of complex and multifaceted symptoms that create a challenge for the establishment of clear clinical distinctions (Cassidy et al., 1998a, 1998b; Grunze et al., 2009). Recent factor analyses of manic signs and symptoms (Gupta et al., 2011; Young et al., 2011) have identified between 4 and 7 independent factors, including dysphoria (characterized by anxiety and depressed mood associated with mixed episodes), psychomotor acceleration (increased motor activity, pressured speech), psychosis (grandiosity, psychotic symptoms), and irritability/aggression. Despite the diversity of manic subgroups (irritable, euphoric, psychotic, depressed), psychomotor agitation appears to be a common denominator, leading some to hypothesize that increased activation or “overactivity” represents the core underlying feature of mania (Savitz et al., 2005; Perry et al., 2010). In the context of clinical therapeutics, the etiology and pathophysiology of the disease remains unclearly defined. Notwithstanding, abnormal glutamatergic neurotransmission has been implicated in the pathophysiology of acute mania (Ongür et al., 2008; Gao et al., 2013). Glial dysfunction has also been proposed to be important to this pathology (Mitterauer, 2011). In fact, a multitude of studies have found reduced glial cell number to be associated with acute mania (Rajkowska, 2000; Ongür et al., 2008). At the molecular level, dysregulation of kinases such as glycogen synthase kinase-3β (GSK3β) (Jope and Roh, 2006) and PKC (Manji and Lenox, 1999) may occur. In fact, neurotransmitter systems that are implicated in the pathophysiology of mood disorders regulate GSK3β/PKC in the brain.
Several currently available animal models reproduce select behavioral facets of human mania and may be used to reliable detect novel drugs that may be administered for palliative treatment of this disease. Black Swiss (BS) mice exhibit an increased motor activity compared with C57bl/6, CBA/J, and A/J strains (Flaisher-Grinberg and Einat, 2010; Hannah-Poquette et al., 2011). BS mice from Taconic (BStac) show a higher preference for sweet solutions and higher motor activity than other BS mouse colonies, that is, from Charles River (BScr). A series of studies from different groups has provided the etiological validity supporting the utilization of BStac mice to model behavioral domains of human mania, including reward-seeking, risk-taking, increased saccharin preference, reduced immobility in the forced swim test (FST), and sensitivity to psychostimulants (Flaisher-Grinberg and Einat, 2010;Hannah-Poquette et al., 2011;Kalinichev and Dawson, 2011). Interestingly, mood stabilizers such as lithium salts and valproate diminished these activities displayed by BStac mice without altering those of BScr mice (Juetten and Einat, 2012). Thus, the activity of BStac mice is highly sensitive to low doses of amphetamine, and its enhancement is prevented by mood stabilizers (Flaisher-Grinberg and Einat, 2010) and GSK3β inhibitors (Kalinichev and Dawson, 2011; Garzón-Niño et al., 2017). On the other hand, mice with a genetic deletion of histidine triad nucleotide-binding protein 1 (HINT1) also display manic-like behaviors. HINT1-/- mice rapidly switch into depressive-like behaviors in response to stressful situations (Garzón-Niño et al., 2017). In contrast, the elevated manic activity of BStac mice predominates and prevents stress from triggering the mechanisms necessary to reduce mobility in the FST. Because human mania is associated with altered glutamatergic neurotransmission (Ongür et al., 2008; Gao et al., 2013), a valid animal model of mania is expected to exhibit such a relevant molecular feature. In this respect, alterations in the glutamate N-methyl-D-aspartate receptor (NMDAR) apparently facilitate the differential responses exhibited by BStac (acute mania/bipolar type I) and HINT1-/- mice (bipolar mania/bipolar type II) in response to stressful paradigms and pharmacological interventions (Garzón-Niño et al., 2017). Thus, the characteristics of BStac and HINT1-/- mice may assist in the detection/development of suitable drugs for treating human mania.
Evidence suggests that sigma receptors type 1 (σ1Rs) are involved in several neurological and psychiatric conditions (Kourrich et al., 2012), and hence sigma receptor ligands may serve as the next generation of psychotherapeutic drugs (Hayashi and Su, 2004). Because NMDARs are implicated in mental illnesses such as schizophrenia, depression, and bipolar disorder (Mathews et al., 2012; Naughton et al., 2014; Poels et al., 2014; Deutschenbaur et al., 2016), the capacity of σ1Rs to modulate NMDAR transmission (Martina et al., 2007) and particularly that of σ1R antagonists to prevent NMDAR hypofunction (Sánchez-Blázquez et al., 2014) could be useful for designing pharmacological interventions to alleviate the aforementioned mood dysfunctions. The HINT1 protein collaborates with σ1Rs to bring glutamate NMDARs under control of certain G protein-coupled receptors (GPCRs) (Rodríguez-Muñoz et al., 2015b). In this context, σ1R antagonism prevents HINT1 proteins from coupling GPCR function to that of NMDARs, thereby controlling the cellular impact of this glutamatergic activity (Rodríguez-Muñoz et al., 2015b). The antagonists of σ1Rs take advantage of this mechanism, and in inflammatory pain models involving NMDAR activation these drugs effectively diminish pain-related behaviors (Vela et al., 2015). Thus, the present study explores the potential of σ1R ligands as antimanic/mood stabilizers in BStac and HINT1-/- “manic” mice.
Materials and Methods
Animals and Drugs
Two strains of male BScr and BStac mice were used in this study. Knock-out mice with a 129 genetic background that exhibit targeted disruption of the HINT1 gene (HINT1-/-, a gift from I. B. Weinstein/J. B. Wang) and wild-type littermate mice (HINT1+/+) were also utilized. Male mice were housed at a constant temperature (22±1°C) under a 12-h-light/-dark cycle and allowed unlimited access to food and water. Experiments were regularly performed in different cohorts of mice to avoid any variations caused by handling stress or exposure to stressful paradigms. Each mouse was used only once. Experiments were performed according to the European regulations for experimental work with animals (directive 2010/63/EU) and were approved by the Ethical Committee for Research at CSIC (SAF2012-34991 and CAM PROEX 225/14).
The σ1R antagonist S1RA: 4-[2-[[5-methyl-1-(2-naphthalenyl)-1H-pyrazol-3-yl]oxy]ethyl] morpholine) was obtained from Cayman Chemical USA (# 16279); TDZD8 (#T8325) and valproic acid sodium salt (#P4543) were purchased from Sigma Aldrich. BD1047 (#0956), BD1063 (#0883), PD144418 (#2606), (±)-PPCC oxalate (#3870), PRE084 (#0589), amphetamine (# 2813), and MK801 (#0924) were obtained from Tocris Bioscience. Test drugs were dissolved in saline except PPCC, PD144418, and TDZD8, which were prepared in 1:1:18 (v/v/v) mixture of ethanol:Kolliphor EL (Sigma, C5135):physiological saline. To facilitate a selective and direct access to their targets, the compounds were each injected into the lateral ventricles of mice at 4-μL volume as previously described (Rodríguez-Muñoz et al., 2012; Garzón et al., 2015). Animals were lightly anesthetized and intracerebroventricular (icv) injections were performed with a 10-μL Hamilton syringe at a depth of 3 mm at a point of 2 mm lateral and 2 mm caudal from the bregma. The 4 μL were infused at a rate of 1 μL every 5 seconds. After that, the needle was maintained for an additional 10 seconds. The administration of amphetamine and valproate was by the i.p. route. Groups of 6 to 10 mice were treated with the selected compounds.
Behavioral Testing
Before beginning behavioral testing, the mice were allowed to acclimate to the testing room for 2 consecutive days (60 min/d). On the third day (testing), these animals were transferred to the testing room 30 minutes prior to each test session. To exclude potential changes in behavior, each test was performed with a different cohort of animals. Drug doses were selected based on previous works (Vela et al., 2015; Garzón-Niño et al., 2017). Vehicle control animals received equivalent volumes of saline. The icv administration did not alter the behavioral assessment of the mice, since the injection of saline did not modify their performance in the tests.
Spontaneous Activity
Mice were tested individually for spontaneous activity using 20×20×28-cm transparent plastic automated activity monitors (Accuscan activity analyzer, Versamax 260 v2.4; Omnitech Electronics, Inc.). Infrared beam crossings were recorded for 90 minutes at 10-minute intervals. At the end of the session, mice were returned to their home cages, and the boxes were wiped clean with a 10% alcohol solution.
Response to Psychostimulants
We tested separate cohorts of BStac and BScr mice to evaluate the hyperlocomotor-inducing effects of psychostimulants. Mice were allowed a 30-minute habituation period, injected with amphetamine (2 mg/kg, i.p.), and returned to their activity cages. Additionally, the horizontal activities of mice pretreated with either the selective GSK3β inhibitor TDZD-8 (20 nmol per mouse, icv) or sigma ligands (3 nmol, icv) were also recorded. The effects of genotype on drug-induced increases in the total distance travelled (calculated by the pre-drug distance travelled subtracted from the post-drug distance travelled) were analyzed by comparing them with those of the control group.
Sweet Solution Preference Test
Over a period of 48 hours, mice were given the option of consuming a 1% sucrose solution (Sigma Aldrich) in addition to their regular supply of water and food. The bottle containing the sweet solution was made available to the mice throughout the entire preference test period. Sucrose solution and water bottle weights were recorded at the beginning of the experiment and every 24 hours thereafter. To avoid the possible effect of a side preference on drinking behavior, the positions of the bottles were changed every 12 hours. Mice were not subjected to food or water deprivation prior to the test. Drugs were administered twice a day; drug administration started 1 day before the sweet solution consumption testing began and continued throughout the 2 days of the experiment. Vehicle control groups received an equivalent volume of saline. Preference for sucrose was calculated as the percentage of sucrose solution consumed relative to the total amount of liquid consumed.
Forced Swim Test
This test was based on the original version of the Porsolt forced swim test for mice (Porsolt et al., 1977). Mice were placed in a 5-L cylinder (40 cm high, 25 cm diameter) filled with 3.5 L of water and swam without the ability to touch the bottom. Mice were placed in water for 6 minutes, and the last 4 minutes of the session were manually scored for total time of activity (swimming, defined as correlated movements of all 4 limbs, and struggling, defined as intensive movements of all paws, mostly against the container wall) vs immobility (floating with only minimal movements needed to keep their heads above water).
Membrane Preparation and Protein Detection
Briefly, frontal cortex synaptosomal membranes were obtained from groups of 6 to 10 mice that were killed via decapitation after receiving the drugs being investigated. Cerebral cortices were homogenized in 10 volumes of 25 mM Tris-HCl, pH 7.4, and 0.32 M sucrose supplemented with a phosphatase inhibitor mixture (P2850; Sigma Aldrich), H89 (B1427; Sigma Aldrich), and a protease inhibitor cocktail (P8340; Sigma Aldrich). The homogenate was centrifuged at 1000 g for 10 minutes to remove the nuclear fraction. The supernatant (S1) was centrifuged twice at 20000 g for 20 minutes to obtain the crude synaptosomal pellet (P2). The final pellet was diluted in Tris buffer supplemented with a mixture of protease inhibitors (0.2 mM phenylmethylsulphonyl fluoride, 2 μg/mL leupeptin, and 0.5 μg/mL aprotinin) then divided into aliquots and processed for protein determinations. Proteins were resolved by SDS/PAGE, transferred onto 0.2 μm polyvinylidene difluoride membranes (162–0176; Bio-Rad), and probed overnight at 6ºC with the selected primary antibodies diluted in Tris-buffered saline pH 7.7 +0.05% Tween 20. Those were detected using secondary antibodies conjugated to horseradish peroxidase. The western-blot images, antibody binding, were visualized by chemiluminescence (#170–5061; Bio-Rad) and recorded using a ChemiImager IS-5500 (Alpha Innotech). Protein immunosignals, and those of α-tubulin, were measured using the area of the strongest signal of each studied group of samples (average optical density of the pixels within the object area/mm2; AlphaEase FC software), and the grey values of the means were then normalized within the 8 bit/256 grey levels [(256-computed value)/computed value]. Equal loading was verified and adjusted, if necessary, vs α-tubulin. Immunosignals were computed relative to those of controls that were attributed an arbitrary value of 1.
Antibodies
The following primary antibodies were used in this study: anti-NR1 (#MAB1586 Millipore), anti-NR1C1 (#AB5046P Millipore), anti-NR2A (#ab14596 Abcam), anti-NR2B (#ab14400 Abcam), anti-NR1 C1 P-S897 (#ABN99 Millipore), anti-NR2B P-Y1472 (#AB5403 Millipore), anti-NR2B P-Y1303 (#ab81271 Abcam), anti-NR2B P-Y1480 (#PA1-4733 ThermoFisher Scientific), anti NR2B P-Y1252 (#ab18532 Abcam), anti-GSK3β (#9315 Cell Signaling Technology), anti-P-S9 GSK3β (#9336 Cell Signaling Technology), anti-P-Y216 GSK3β (#ab75745 Abcam), anti-Akt (#4691 Cell Signaling Technology), anti-P-S473 Akt (#4060 Cell Signaling Technology), anti-P-T308 Akt (#2965 Cell Signaling Technology), anti-PKC sampler kit (#611421 BD Biosciences), anti-PKCγ (#ab71558 Abcam), and anti-α tubulin (#T9026 Sigma Aldrich).
Statistical Analyses
Graphs were generated using Sigmaplot v.13 (SPSS Science Software). Statistical analyses were performed using IBM Statistics SPSS 24. Data for the amphetamine-induced hyperactivity test and sweet solution preference (preference ratio) were analyzed using 2-way ANOVA with genotype and treatment as main factors. Data for FST (immobility time) were analyzed using 3-way ANOVA with genotype, MK801/saline, and treatment as main factors. Experiments performed across multiple days were analyzed using a repeated-measures ANOVA. All experiments produced a significant interaction; thus, the follow-up analysis involved 1-way ANOVAs for each genotype and MK801/saline treatment followed by all pairwise Holm-Sidak multiple comparison test as indicated in the legends to the figures. Data for protein expression were analyzed using 1-way ANOVA followed by Holm-Sidak test. Statistical significance was defined as P<.05.
Results
Mood-Stabilizing Property of Sigma 1 Receptor Antagonists
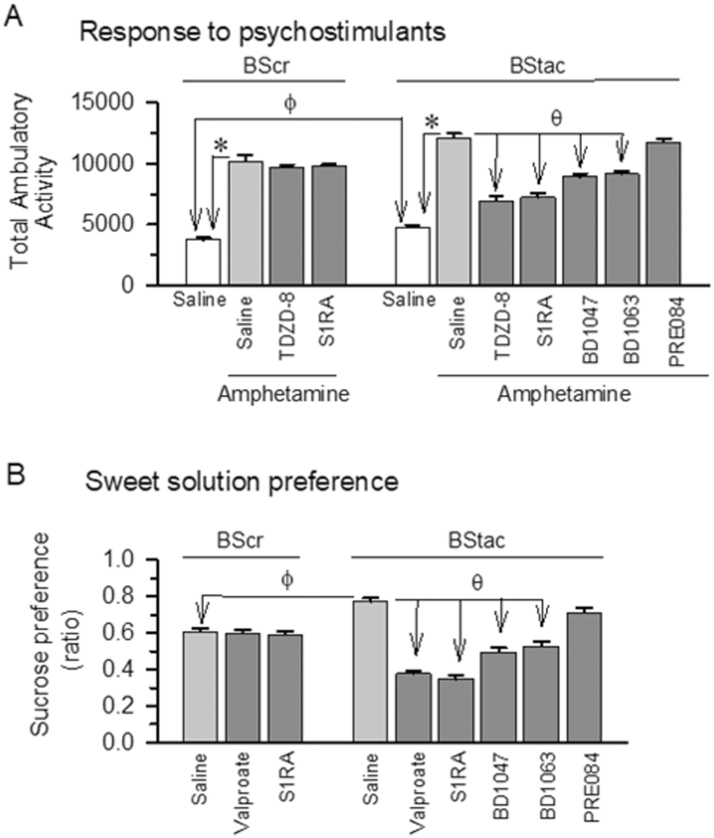
As a control in studies with BStac mice, we compared their behavioral phenotype with that of BScr mice. Bstac mice exhibited a higher locomotor activity [F(1,10)=15.45, t=3.93 P=.003] and sweet solution preference [F(8,45)=49.24 P<.001; t=5.88 P<.001] than BScr mice (Figure 1A, B). As aforementioned, human antimania drugs did not alter amphetamine-induced hyperactivity [F(8,45)=26.23 P<.001; TDZD-8 t=0.99 P=.94] or sucrose preference in BScr mice [F(8,45)=49.24 P<.001; valproate t=0.23 P=.96]; however, these behaviors were significantly diminished by these mood stabilizers in BStac mice (TDZD-8 t=10.19, P<.001 and valproate t=14.01, P<.001). As observed for mood stabilizers, administration of the σ1R antagonist S1RA did not alter these behaviors in BScr mice; however, S1RA and other σ1R antagonists such as BD1047 and BD1063 but not the agonist PRE084 attenuated the manic-like behaviors displayed by BStac mice in these paradigms [amphetamine-induced hyperactivity: F(8,45)=26.23, P<.001; S1RA t=10.57, P<.001; BD1047 t=5.49, P<.001; BD1063 t=6.24, P<.001; sucrose preference: F(8,45)=49.24, P<.001; S1RA t=15.07, P<.001; BD1047 t=9.89, P<.001; BD1063 t=8.65, P<.001]. These results indicate that BStac mice exhibit manic-like behaviors and that BScr mice are suitable for use as their controls.
Figure 1.
Black Swiss (BS) mice exhibit manic-like behaviors: effect of σ1R ligands and mood stabilizers. (A) Hyperactivity evoked by acute systemic amphetamine administration (hypersensitivity to psychostimulants). Horizontal activity of BScr (BS Charles River) and BStac (BS Taconic Farms) mice pretreated (i.p., at time 0) with vehicle (saline), TDZD-8 (20 nmol, icv), or σ1R ligands (3 nmol, icv). After 30 minutes of activity, the mice received saline or amphetamine (2 mg/kg, i.p.) and were observed during the subsequent 70-minute period. (B) Sweet solution preference ratios (hedonistic drive) on days 1 and 2 of the test. Each assay was performed on different cohorts of mice. Preference ratio was calculated as the percentage of sucrose solution consumed relative to the total amount of liquid consumed. Compounds were administered twice a day either via systemic i.p. injections (valproate, 200 mg/kg) or icv injections (σ1R ligands, 3 nmol). Each bar is the computed mean±SEM of the groups, n=6. ϕ In the absence of drugs (saline), the behavior of the BS strains was significantly different; *significantly different compared to the BS control group, which received saline instead of amphetamine. Within amphetamine-treated BS mice, θ indicates a significant difference from the group that received saline instead of the drug under study. ANOVA, Holm-Sidak multiple comparisons, P<.05. See further details in “ Methods.”
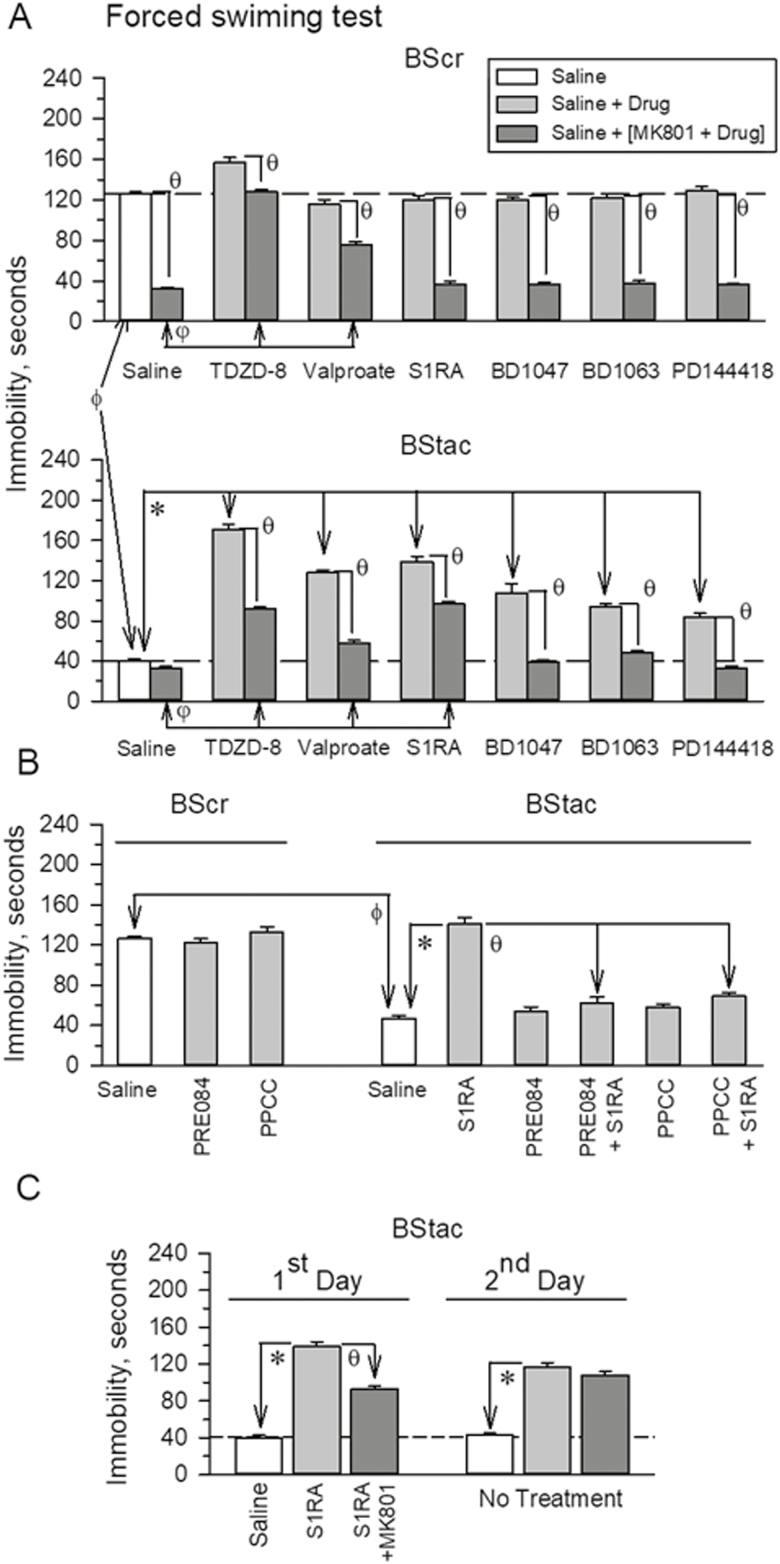
BStac mice exhibited much higher swim activity than BScr mice in the FST [F (1,17)=536.44, t=23.16 P<.001]. Then, we addressed the influence of the mood stabilizers valproate and TDZD-8 and of σ1R antagonists on the activity of these BS mice in this paradigm (Figure 2A). With the exception of TDZD-8, which moderately increased BScr swim activity [F (13,126)=196.50, P<.001; TDZD-8 t=6.81, P<.001], these drugs did not significantly alter this behavior in BScr mice; however, they clearly diminished swim activity in BStac mice [F(13,112)=127.40, P<.001]. The NMDAR mediates pro-depressive (antiactivity) behaviors in the FST, and as reported (Garzón-Niño et al., 2017) the NMDAR antagonist MK801 increased swim activity in BScr mice that received only saline [F(13,126)=196.50, P<.001; MK801 t=20.45, P<.001] but failed to do so in saline-treated “manic” BStac mice [F(13,112)=127.40, P<.001; MK801 t=2.54, P=0.20]. In MK801-injected BScr mice, TDZD-8 and valproate produced a significant reduction in the NMDAR-mediated component of their swim behavior [F(13,126)=196.50, P<.001, TDZD-8 t=20.97, P<.001; valproate t=9.68, P<.001] (Figure 2A). In contrast, σ1R antagonists did not produce such an alteration, and in their presence the NMDAR antagonist MK801 retained its full capacity to increased BScr swim activity. Notably, in BStac mice, these drugs facilitated the expression of the NMDAR-mediated component during the FST (Figure 2A). After the mood stabilizers and the σ1R antagonists diminished the swim activity of BStac mice, MK801 was able to significantly increase their activity in the FST [F(13,112)=127.40, P<.001]. In the presence of MK801, the activity of BStac mice treated with BD1047, BD1063, or PD144418 did not differ from that of saline-treated BStac mice. The activity of MK801-treated mice when TDZD-8, S1RA, and valproate were the drugs co-administered was lesser than that of saline-treated BStac mice [F(13,112)=127.40, P<.001; TDZD-8 t=11.96, P<.001; S1RA t=12.84, P<.001; valproate t=5.88, P<.001]. The agonists of σ1Rs, PRE084, and PPCC did not alter swim activity in control BScr [F(8,53)=56.19, P<.001; PRE084 t=1.00, P=.91; PPCC t=1.73, P=.56] or “manic” BStac mice [F(8,53)=56.19, P<.001; PRE084 t=0.76, P=.90; PPCC t=0.43, P=.88] in the FST; however, these σ1R agonists diminished the antimanic activity exhibited with S1RA treatment in BStac mice [PRE084 t=11.73, P<.001; PPCC t=10.43, P<.001] (Figure 2B). The antimanic effect of a single administration of S1RA to BStac mice persisted at least for 24 hours [F (5,48)=124.11, P<.001; 1st day t=19.4, P<.001; 2nd day t=14.24, P<.001] (Figure 2C). At this time point, the MK801-sensitive antiactivity component of S1RA-treated mice on day 1 was reduced, suggesting that the σ1R antagonist primarily diminishes hyperactivity in these mice by reducing proactivity behaviors.
Figure 2.
Effect of σ1R ligands on the activity of (Black Swiss [BS] Charles River) and BS Taconic Farms (BStac) mice in the forced swim test (FST). (A) Compounds were acutely administered either systemic i.p. injections (valproate, 200 mg/kg, 60 minutes prior the FST) or icv injections (TDZD-8, 20 nmol; σ1R ligands, 3 nmol, 30 minutes prior the test). These doses did not significantly affect spontaneous mouse activity. ϕ Indicates that in the absence of drugs (saline), the BS strains significantly differed in their swim activity in the FST; *within BStac mice, significant difference from the group receiving saline instead of the drug under study. The NMDAR antagonist, MK801 (light grey bars), was given in an icv injection at 1 nmol 30 minutes before the FST. θ Significant difference compared to the control group that received saline or the drugs. Within MK801-treated mice, φ indicates a significant difference compared to the control group that received saline instead of the drug. (B) The σ1R agonists, PRE084 or PPCC, reduced the normalizing effect of S1RA in “manic” BStac mice. ϕ As above; *significantly different from the group that received saline instead of S1RA; θ significantly different from the group that received only S1RA. (C) The antimanic effect of a single icv injection of S1RA persisted for at least 24 hours. BStac mice received S1RA±MK801 and were tested in the FST. After 24 hours, the same mice were subjected to a second FST without additional pharmacological treatment. *Significantly different from the control group that received saline instead of S1RA on day 1. Within BStac mice treated on day 1 with S1RA, θ indicates a significant difference from the group that also received MK801 on day 1. (A–C) Each bar represents the mean±SEM of the data obtained from 7 to 10 mice. ANOVA, Holm-Sidak multiple comparisons, P<.05.
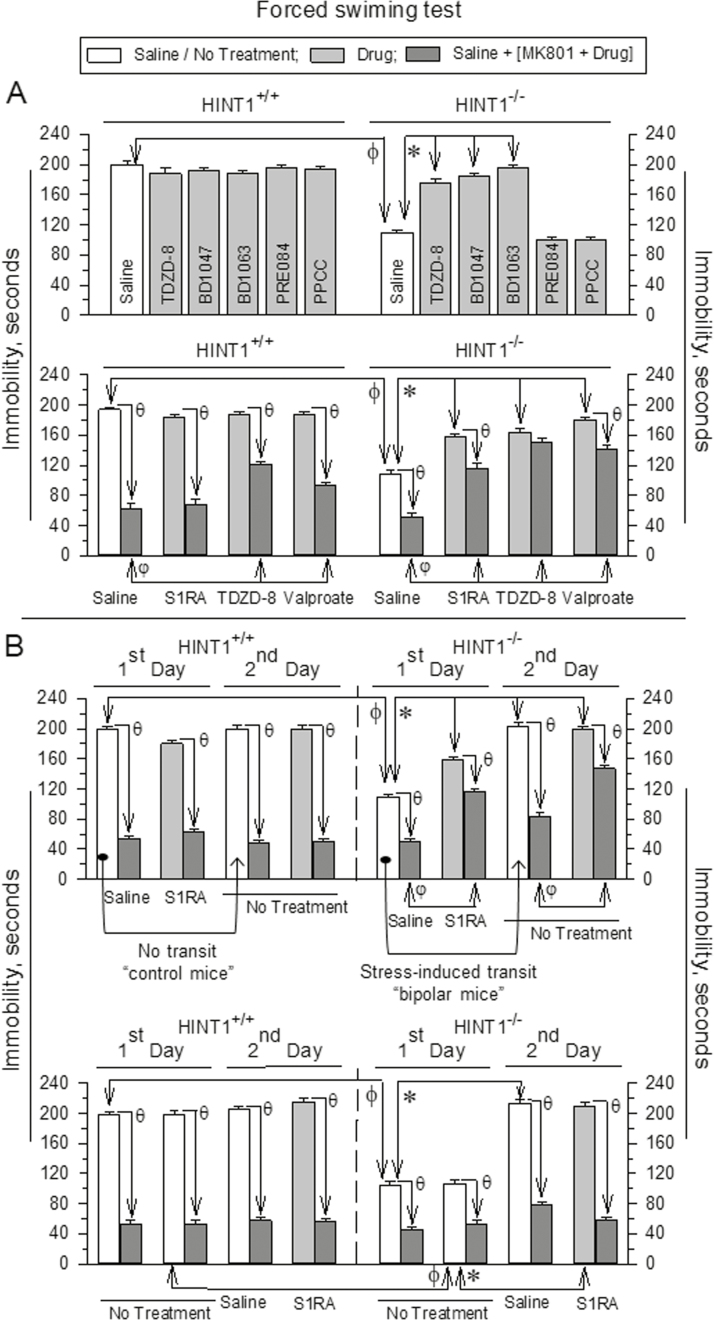
To further substantiate the profile of σ1R antagonists as mood stabilizers, we tested their effects in another animal model of mania, the “bipolar” HINT1-/- mice (Garzón-Niño et al., 2017), which compared to HINT1+/+ wild-type littermates exhibit increased activity in the FST [F (11,78)=122.99; t=17.53 P<.001] (Figure 3A). As observed in BStac mice, the GSK3β inhibitor TDZD-8 and σ1R antagonists but not agonists significantly decreased swimming activity in HINT1-/- mice [F (11,78)=122.99, P<.001; TDZD-8 t=10.92, P<.001; BD1047 t=14.92, P<.001; BD1063 t=17.33, P<.001] without altering that in HINT1+/+ controls (Figure 3A). HINT1+/+ mice and, to a minor extent, HINT1-/- mice exhibit MK801-sensitive, prodepressive behaviors in the FST. This observation suggests that HINT1-/- mice exhibit manic-like behaviors but of lower intensity than those in BStac mice (Garzón-Niño et al., 2017). In the present study, administration of S1RA did not alter the enhancing effect of MK801 on the swim behavior exhibited by HINT1+/+ mice [F(15,100)=111,82, P<.001; S1RA t=2.6, P=.20]. However, MK801 produced a lower level of swim stimulation when HINT1-/- mice had received S1RA [F(15,100)=111,82 P<.001; S1RA=10.14, P<.001]. In both groups of HINT1 mice, TDZD-8 and valproate weakened the stimulatory swim effects of MK801 [HINT1+/+ mice: TDZD-8 t=4.73, P<.001; valproate t=4.77, P<.001; HINT1-/- mice: TDZD-8 t=15.50, P<.001; valproate t=14.06, P<.001] (Figure 3A). The “bipolar” HINT1-/- mice exhibit manic-like behaviors prior to exposure to stressful paradigms, and after being challenged, they progressively develop depression-like behaviors, that is, decreased activity time in the FST (Garzón-Niño et al., 2017). This particular feature of these bipolar mice was confirmed in the present study [F(7,64)=136.86, P<.001; HINT1-/- mice, stress-induced transit t=16.82, P<.001] (Figure 3B). As aforementioned, S1RA diminished the activating effect of MK801 on the swim behavior of HINT1-/- mice, and this reduction was also observed 24 hours later during the second FST, which was performed in the absence of further treatment (Figure 3B). This normalizing effect of S1RA was not observed if the HINT1-/- mice had already switched into the depressive-like state. Interestingly, the stimulatory effect of MK801 on the swim behavior of the mice is also produced by drugs with antidepressant activity in humans (Garzón-Niño et al., 2017). Thus, S1RA and probably other σ1R antagonists selectively act as antimaniac drugs but not as antidepressants.
Figure 3.
Effect of σ1R ligands on the activity of HINT1+/+ and “bipolar” HINT1-/- mice in the FST. (A) Details are the same as in Figure 2. (B) Upper panel: saline or S1RA (3 nmol) was administered via icv injection before the first FST challenge. After 24 hours, the same animals were tested in the FST a second time. The arrows indicate that HINT1-/- “manic” mice but not their controls littermates exposed to the stressful FST on day 1 exhibited a depression-like and MK801-sensitive behavior during the second FST (Garzón-Niño et al., 2017). ϕ Significantly different from the activity of wild type mice in the FST; *significantly different from the control group that received saline on the 1st day. θ Significantly different from the corresponding group that did not receive MK801; φ significantly different from the group that received MK801 on the 1st day. Lower panel: on the first day, the mice received no treatment before the FST; 24 hours later, they were given an icv injection of saline or S1RA and reexposed to the FST. ϕ Significantly different from the activity of wild type mice in the FST; *significantly different from the control group that received no treatment on the 1st day; θ significantly different from the corresponding group that did not receive MK801. Each bar represents the mean±SEM of data obtained from 7 to 10 mice. ANOVA, Holm-Sidak multiple comparisons, P<.05.
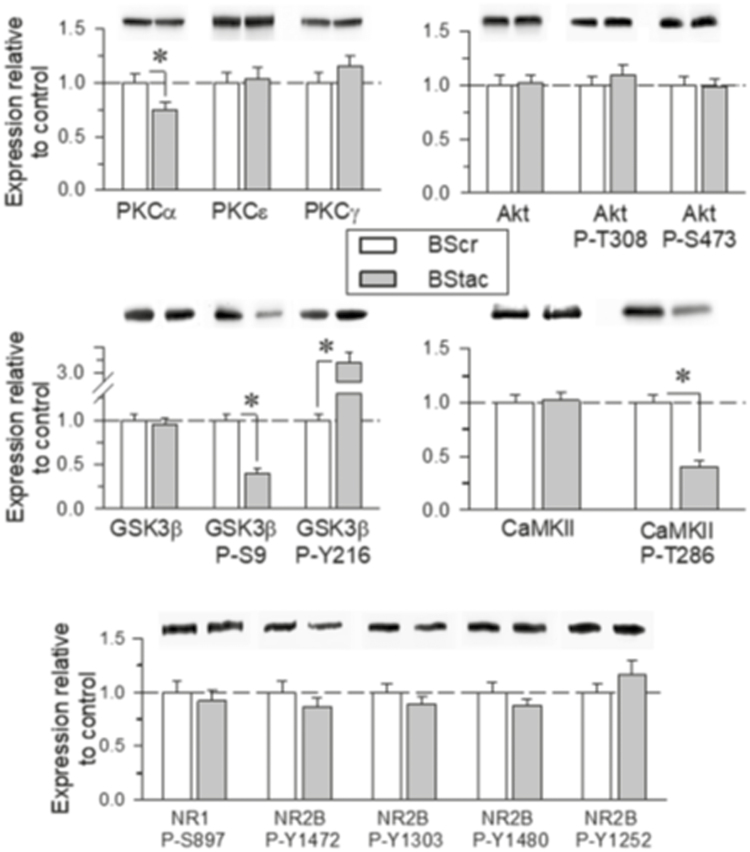
BStac Mice Display Altered Akt/GSK3β Signaling
Our previous work revealed that “manic-depressive bipolar” HINT1-/- mice present a series of molecular changes, such as increased PKC, PKA, and GSK3β activity and altered glutamate NMDAR composition and function (Garzón-Niño et al., 2017). Thus, we evaluated possible alterations in the Akt/GSK3β pathway and their regulatory phosphorylation in BStac and BScr mice (Figure 4). In the frontal cortex, the total levels of Akt and GSK3β did not significantly differ between the 2 groups of BS mice. However, phosphorylation analysis revealed marked differences between these mice. Compared to the phosphorylation status of GSK3β in BScr mice, Akt-mediated inhibitory phosphorylation of GSK3β at serine 9 (P-S9) was higher in BStac mice. Analysis of the activating phosphorylation of GSK3β at tyrosine 216 (Y216) revealed that BStac mice exhibited much higher levels of P-Y216 GSK3β than BScr mice. Manic-like behaviors are usually associated with high GSK3β activity. In BStac mice, activating P-Y216 was likely more predominant than the inhibitory P-S9, and consequently, the resulting GSK3β activity may have been higher than that in BScr mice. This result could explain the rapid cessation of manic-like behavior after GSK3β pharmacological inhibition by TDZD-8 (Figures 1 and 2).
Figure 4.
PKC, Akt/GSK3β, and N-methyl-D-aspartate receptor (NMDAR)/ calmodulin-dependent kinase II (CaMKII) signaling pathways in Black Swiss (BS) mice. Total levels and phosphorylated forms of the proteins were determined by SDS-PAGE/western-blot analysis in frontal cortical tissue obtained from BS Taconic Farms (BStac) and BS Charles River (BScr) mice. The immunosignal corresponding to the BScr control was assigned an arbitrary value of 1.The assay was repeated thrice on samples from different mice that were not previously subjected to behavioral testing. *Significantly different from the BScr control group with ANOVA, Holm-Sidak multiple comparisons, P<.05. Representative blots are shown.
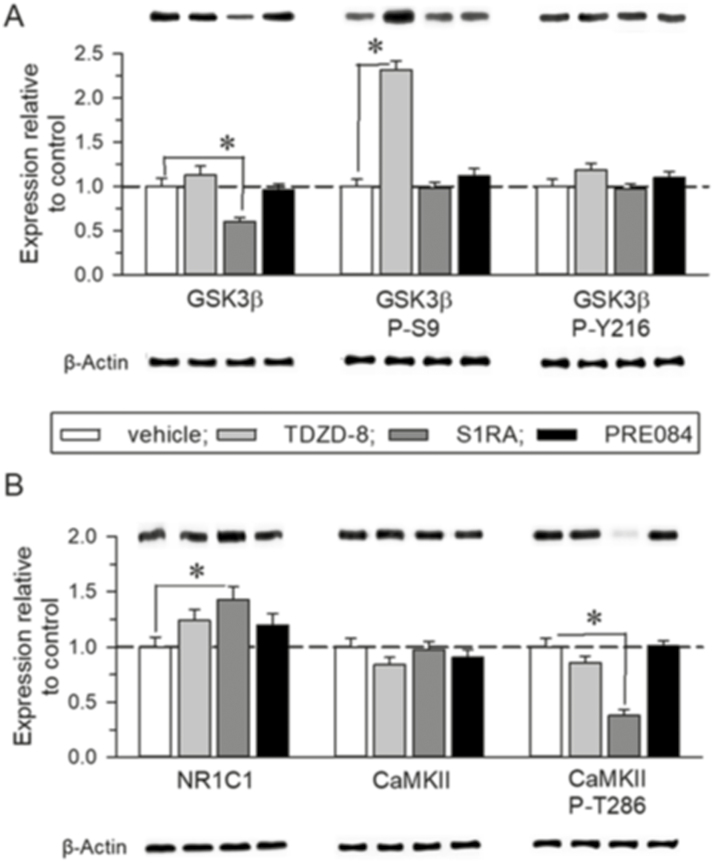
The administration of TDZD-8 to BStac mice reduced GSK3β activity by increasing levels of P-S9 GSK3β without altering those of P-Y216 GSK3β (Figure 5A). This effect was not reproduced by sigma ligands. However, in these “manic” mice TDZD-8 did not alter NMDAR-related parameters such as the presence of NR1 subunits carrying the C1 cytosolic segment, which enables GPCR-NMDAR cross-regulation (Rojas and Dingledine, 2013), or the levels of calcium and calmodulin-dependent kinase II (CaMKII) and its activating auto-phosphorylation on T286. In contrast, the selective σ1R antagonist S1RA increased NR1 C1 levels, and although it did not modify CaMKII levels, S1RA decreased the extent of P-T286 CaMKII, effects not observed with the administration of the σ1R agonist PRE084 (Figure 5B). Thus, although administration of S1RA and TDZD-8 to “manic” BStac mice led to a similar antimanic profile, their effects were likely achieved through a different molecular mechanism. Our data suggest that σ1R antagonists, such as S1RA and BD1063, produce mood stabilization by also acting at the NMDAR level.
Figure 5.
Effect of TDZD-8, S1RA, and PRE084 on the N-methyl-D-aspartate receptor (NMDAR)/ calmodulin-dependent kinase II (CaMKII) pathway of Black Swiss Taconic Farms (BStac) mice. (A–B) Effect of TDZD-8 (20 nmol, icv), S1RA (3 nmol, icv), and PRE084 (3 nmol, icv) on BStac frontal cortical levels of GSK3β, NMDAR NR1 C1, and CaMKII. The mice were killed 30 minutes after the last drug/saline administration. The immunosignal corresponding to the BStac control was assigned an arbitrary value of 1. The assay was repeated thrice on samples from different mice, which were not previously subjected to behavioral testing. *Significantly different from the control group. ANOVA, Holm-Sidak multiple comparisons, P<.05. Representative blots are shown.
Discussion
BStac and HINT1-/- mice when compared to their corresponding controls, BScr and HINT1+/+ mice, exhibit higher swim activity in the FST, an increased sensitivity to psychostimulants, and augmented aggressive and reward-seeking behaviors. These differential features are proposed to reflect the increased vigor and goal-directed behaviors that characterize the human mania (Flaisher-Grinberg and Einat, 2010; Garzón-Niño et al., 2017). To avoid possible differences in bioavailability after crossing the BBB, we administered the σ1R ligands by icv route at equimolar concentrations, similar to those found effective in mu-opioid-induced analgesia regulation (Sánchez-Blázquez et al., 2014; Rodríguez-Muñoz et al., 2015b). Interestingly, the present study showed that σ1R antagonists such as S1RA, BD1047, BD1063, and PD144418 normalized the conduct of “manic” BStac and HINT1-/- mice without altering that of BScr and HINT1+/+ mice. The σ1R antagonist S1RA was more efficacious at counteracting the manic-like behaviors than the other antagonists evaluated. This finding corroborates previous data showing that S1RA is the most effective antagonist in an in vivo experimental model evaluating the agonist/antagonist performance of σ1R ligands (Sánchez-Blázquez et al., 2014). The antimanic effects promoted by σ1R antagonists were reduced by σ1R agonists, thus indicating the participation of σ1Rs in the control of the mood exhibited by HINT1-/- and BStac mice.
The glutamatergic system, particularly the NMDAR, plays a critical role in the setting of normal behavior, probably via control of antiactivity processes (Garzón-Niño et al., 2017). Thus, the downregulation of this glutamatergic function facilitates the expression of mania-like behaviors (Szabo et al., 2009), which are apparently related to abnormal increases in GSK3β activity (Polter et al., 2010), and accordingly, inhibitors of this enzyme diminish the expression of these conducts (O’Brien et al., 2004). NMDARs together with GSK3β determine the synaptic tone and, thus, the quality of neuronal communication (Zhu et al., 2007). On the other hand, NMDAR activity is regulated by different GPCRs, some of which enhance this glutamatergic signaling via PKC and nonreceptor tyrosine kinases such as Src (Rojas and Dingledine, 2013). The increased activity of PKC in the prefrontal cortex may also account for the symptoms of mania, and in fact, lithium, a major treatment for mania, diminishes PKC signaling cascade in which GSK3β is also implicated (Sourial-Bassillious et al., 2009; Szabo et al., 2009; Garzón-Niño et al., 2017). Thus, mania-like behaviors can be induced by increasing proactivity moods, which may occur after increasing PKC/GSK3β activity in cortical areas. However, mania-like behaviors can also appear after a reduction in NMDAR-mediated antiactivity processes (Garzón-Niño et al., 2017). In that sense, NMDAR antagonism causes transient mood elevation or euphoria and psychotomimetic effects similar to a manic-like syndrome (Lu et al., 2016).
Our study further substantiates the efficacy of GSK3β inhibitors in the reduction of manic-like behaviors in HINT1-/- and BStac mice. Mood stabilizers, such as valproate and lithium salts, are effective in treating bipolar mania, probably because they indirectly inhibit PKC signaling and consequently diminish GSK3β function (Zarate and Manji, 2009). Our results suggest that valproate and GSK3β inhibitors exert antimanic effects by reducing proactivity behaviors without facilitating expression of antiactivity (prodepressive) episodes, which are more related to the function of NMDARs. We have reported that administration of the NMDAR antagonist MK801 to BStac mice do not significantly increase their “manic-like” behavior, which suggests that their intrinsic impulsive (proactivity) behavior restrains the development or expression of NMDAR-mediated anti-activity behavior during the FST (Garzón-Niño et al., 2017). In “bipolar” HINT1-/- mice, administration of MK801 still increased their activity to levels that approached those exhibited by “manic” BStac mice.
GSK3β inhibitors and σ1R antagonists reduced the hyperactivity of both strains of “manic” mice, normalizing their swim behavior in the FST. Notably, MK801 revealed certain differences in the ways in which these drugs both achieved the control of manic-like behaviors. Whereas MK801 dampened the antimanic effects of valproate, BD1047, BD1063, PD144418, S1RA, and TDZD-8 in BStac mice, the NMDAR antagonist was less effective at counteracting the effects of S1RA, TDZD-8, and valproate in HINT1-/- mice. Thus, BStac and HINT1-/- mice may differ in the types of human mania modeled, such as bipolar type I and II, respectively. The inhibition of GSK3β mostly reduced the expression of proactivity (promanic) behaviors without increasing that of NMDAR-mediated antiactivity (prodepressive) behaviors. In BStac mice, GSK3β inhibition decreased the expression of proactivity behaviors but also promoted NMDAR-mediated antiactivity behaviors, probably as an indirect effect of GSK3β inhibition in these “manic” mice. Antiactivity processes may have been restrained by GSK3β-mediated promaniac mechanisms that are free to develop upon GSK3β inhibition. The HINT1-/- mice exhibit manic-like behaviors of lower intensity than these of BStac mice, and then, when proactivity behaviors were reduced, prodepressive behaviors barely develop (Garzón-Niño et al., 2017). In both strains of “manic” mice, S1RA reduced proactivity behaviors and apparently promoted NMDAR-mediated antiactivity behaviors; however, after 24 hours the “normalized” behaviors of these mice were mostly accompanied by a discrete MK801-sensitive component. Thus, in “manic” mice, inhibition of proactivity behaviors releases the restrained NMDAR-mediated antiactivity behaviors; however, under the beneficial effects of the antimanic drugs these prodepressive behaviors diminish to adapt their influence to that of the actual proactivity behaviors.
As mentioned previously, the NMDAR receptor participates in the expression of behaviors that oppose activity, and thus these behaviors are less evident in the manic-like mice (Garzón-Niño et al., 2017). We have reported that expression of NMDAR NR1 subunits is similar in cortical synaptosomes of HINT1-/- and HINT1+/+ wild type mice, while expression of the NR1 subtype that carries the C1 cytosolic segment able to couple to GPCRs is 2-fold higher in HINT1-/- mice. Additionally, the levels of NR2A subunits are comparable in these 2 groups of mice, but the NR2B subunit levels are higher in HINT1-/- than HINT1+/+ in mice. Differences in NMDAR subunit levels are not observed in BS mouse strains (Garzón-Niño et al., 2017), suggesting that subunit changes are not directly related to mania but mostly facilitate the transition from mania to depression that stressful situations promote in the “bipolar” HINT1-/- mice. Even though BStac and HINT1-/- mice exhibit manic-like behaviors, the 2 strains differ in the cortical content of phosphorylated and nonphosphorylated forms of GSK3β. These differences may be reconciled in both strains of mice if the activating phosphorylation at Y216 is more predominant than the inhibitory P-S9, thus likely resulting in higher GSK3β activity in BStac and HINT1-/- mice than in their respective control mice (Garzón-Niño et al., 2017). Accordingly, the antimanic effects of the GSK3β inhibitor TDZD-8 in BStac mice correlated with increased expression of inhibitory P-S9 GSK3β without alterations in P-Y216 GSK3β expression.
Interestingly, S1RA promoted antimanic effects in these mice without significantly modifying GSK3β total levels or its phosphorylated forms. Instead, the selective σ1R antagonist increased the presence of glutamate NMDAR NR1 C1 subunits at the plasma membrane. The antagonism of σ1Rs promotes the physical and functional uncoupling of certain GPCRs from NMDAR function (Rodríguez-Muñoz et al., 2015a). Thus, the rapid increase of NR1 C1 levels observed in BStac mice after S1RA treatment may be a consequence of a reduced co-internalization of NR1 C1 subunits with GPCRs. This possibility has been documented for the cannabinoid CB1Rs, which exert an inhibitory influence on NMDAR function (Sánchez-Blázquez et al., 2013); their co-internalization with NR1 C1 subunits can be disrupted by σ1R antagonists (Sánchez-Blázquez et al., 2014). Notably, by reducing the influence of activating GPCRs on NMDAR function, σ1R antagonists reduce allodynia and neural damage in animal models of neuropathy and stroke, respectively (Rodríguez-Muñoz et al., 2015b; Sánchez-Blázquez et al., 2018). At the molecular level, the σ1R forms a functional complex with HINT1 to coordinate the activity of certain GPCRs with those of NMDARs (Rodríguez-Muñoz et al., 2015b). In this scenario, GPCR signaling leads to the PKC/Src-mediated phosphorylation of NMDARs and their subsequent activation (Rodríguez-Muñoz et al., 2015a, 2016). The antagonists of σ1Rs provoke the transfer of HINT1 proteins from activated GPCRs to NMDARs, thereby disrupting the GPCR control of NMDAR function, and increasing inactivation of those already primed by GPCRs.
Certainly, several studies have suggested the potential of σ1R ligands in the treatment of mental illnesses such as schizophrenia (Hayashi and Su, 2004) and depression (Wang et al., 2007;Fishback et al., 2010). However, to the best of our knowledge, the present study is the first to report that σ1R antagonists can ameliorate manic-like symptoms in animal models of disease. Most relevantly, while these drugs normalized the mood exhibited by “manic” mice, they did not alter the behavior of the control mice. This selective profile is not exhibited by antimanic drugs such as inhibitors of GSK3β activity, which also affect the behavior of control mice. Thus, drugs targeting the σ1R may offer therapeutic protection against manic-like behavior.
Acknowledgments
We thank Gabriela de Alba and Veronica Merino for their excellent technical assistance. The authors are entirely responsible for the scientific content of the paper. The authors acknowledge the assistance of Springer Nature Editing Service in the preparation of this manuscript.
Funding
The work was supported by Ministerio de Sanidad, Servicios Sociales e Igualdad “Plan Nacional de Drogas” [grant no. 2014-012] and MINECO Plan Nacional I+D+i [grant no. SAF-2015-65420R].
Statement of Interest
None.
References
- Cassidy F, Forest K, Murry E, Carroll BJ (1998a) A factor analysis of the signs and symptoms of mania. Arch Gen Psychiatry 55:27–32. [DOI] [PubMed] [Google Scholar]
- Cassidy F, Murry E, Forest K, Carroll BJ (1998b) Signs and symptoms of mania in pure and mixed episodes. J Affect Disord 50:187–201. [DOI] [PubMed] [Google Scholar]
- Deutschenbaur L, Beck J, Kiyhankhadiv A, Mühlhauser M, Borgwardt S, Walter M, Hasler G, Sollberger D, Lang UE(2016)Role of calcium, glutamate and NMDA in major depression and therapeutic application. Prog Neuropsychopharmacol Biol Psychiatry 64:325–333. [DOI] [PubMed] [Google Scholar]
- Fishback JA, Robson MJ, Xu YT, Matsumoto RR(2010)Sigma receptors: potential targets for a new class of antidepressant drug. Pharmacol Ther 127:271–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flaisher-Grinberg S, Einat H(2010)Strain-specific battery of tests for domains of mania: effects of valproate, lithium and imipramine. Front Psychiatry 1:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Y, Jhaveri M, Lei Z, Chaneb BL, Lingrel J, El-Mallakh RS(2013)Glial-specific gene alterations associated with manic behaviors. Int J Bipolar Disord 1:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garzón J, Herrero-Labrador R, Rodríguez-Muñoz M, Shah R, Vicente-Sánchez A, Wagner CR, Sánchez-Blázquez P(2015)HINT1 protein: a new therapeutic target to enhance opioid antinociception and block mechanical allodynia. Neuropharmacology 89:412–423. [DOI] [PubMed] [Google Scholar]
- Garzón-Niño J, Rodríguez-Muñoz M, Cortés-Montero E, Sánchez-Blázquez P(2017)Increased PKC activity and altered GSK3Β/NMDAR function drive behavior cycling in HINT1-deficient mice: bipolarity or opposing forces. Sci Rep 7:43468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grunze H, Vieta E, Goodwin GM, Bowden C, Licht RW, Moller HJ, Kasper S(2009)The World Federation of Societies of Biological Psychiatry (WFSBP) guidelines for the biological treatment of bipolar disorders: update 2009 on the treatment of acute mania. World J Biol Psychiatry 10:85–116. [DOI] [PubMed] [Google Scholar]
- Gupta SC, Sinha VK, Praharaj SK, Gandotra S(2011)Factor structure of manic symptoms in adolescents. Ann Clin Psychiatry 23:243–249. [PubMed] [Google Scholar]
- Hannah-Poquette C, Anderson GW, Flaisher-Grinberg S, Wang J, Meinerding TM, Einat H(2011)Modeling mania: further validation for Black Swiss mice as model animals. Behav Brain Res 223:222–226. [DOI] [PubMed] [Google Scholar]
- Hayashi T, Su TP(2004)Sigma-1 receptor ligands: potential in the treatment of neuropsychiatric disorders. CNS Drugs 18:269–284. [DOI] [PubMed] [Google Scholar]
- Jope RS, Roh MS(2006)Glycogen synthase kinase-3 (GSK3) in psychiatric diseases and therapeutic interventions. Curr Drug Targets 7:1421–1434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juetten J, Einat H(2012)Behavioral differences in black Swiss mice from separate colonies: implications for modeling domains of mania. Behav Pharmacol 23:211–214. [DOI] [PubMed] [Google Scholar]
- Kalinichev M, Dawson LA(2011)Evidence for antimanic efficacy of glycogen synthase kinase-3 (GSK3) inhibitors in a strain-specific model of acute mania. Int J Neuropsychopharmacol 14:1051–1067. [DOI] [PubMed] [Google Scholar]
- Kourrich S, Su TP, Fujimoto M, Bonci A(2012)The sigma-1 receptor: roles in neuronal plasticity and disease. Trends Neurosci 35:762–771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu YY, Lin CH, Lane HY(2016)Mania following ketamine abuse. Neuropsychiatr Dis Treat 12:237–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manji HK, Lenox RH(1999)Ziskind-somerfeld research award. Protein kinase C signaling in the brain: molecular transduction of mood stabilization in the treatment of manic-depressive illness. Biol Psychiatry 46:1328–1351. [DOI] [PubMed] [Google Scholar]
- Martina M, Turcotte ME, Halman S, Bergeron R(2007)The sigma-1 receptor modulates NMDA receptor synaptic transmission and plasticity via SK channels in rat hippocampus. J Physiol 578:143–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathews DC, Henter ID, Zarate CA(2012)Targeting the glutamatergic system to treat major depressive disorder: rationale and progress to date. Drugs 72:1313–1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitterauer BJ.(2011)Downregulation and upregulation of glial connexins may cause synaptic imbalances responsible for the pathophysiology of bipolar disorder. CNS Neurosci Ther 17:281–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naughton M, Clarke G, O’Leary OF, Cryan JF, Dinan TG(2014)A review of ketamine in affective disorders: current evidence of clinical efficacy, limitations of use and pre-clinical evidence on proposed mechanisms of action. J Affect Disord 156:24–35. [DOI] [PubMed] [Google Scholar]
- O’Brien WT, Harper AD, Jové F, Woodgett JR, Maretto S, Piccolo S, Klein PS(2004)Glycogen synthase kinase-3beta haploinsufficiency mimics the behavioral and molecular effects of lithium. J Neurosci 24:6791–6798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ongür D, Jensen JE, Prescot AP, Stork C, Lundy M, Cohen BM, Renshaw PF(2008)Abnormal glutamatergic neurotransmission and neuronal-glial interactions in acute mania. Biol Psychiatry 64:718–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry W, Minassian A, Henry B, Kincaid M, Young JW, Geyer MA(2010)Quantifying over-activity in bipolar and schizophrenia patients in a human open field paradigm. Psychiatry Res 178:84–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poels EM, Kegeles LS, Kantrowitz JT, Slifstein M, Javitt DC, Lieberman JA, Abi-Dargham A, Girgis RR(2014)Imaging glutamate in schizophrenia: review of findings and implications for drug discovery. Mol Psychiatry 19:20–29. [DOI] [PubMed] [Google Scholar]
- Polter A, Beurel E, Yang S, Garner R, Song L, Miller CA, Sweatt JD, McMahon L, Bartolucci AA, Li X, Jope RS(2010)Deficiency in the inhibitory serine-phosphorylation of glycogen synthase kinase-3 increases sensitivity to mood disturbances. Neuropsychopharmacology 35:1761–1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porsolt RD, Bertin A, Jalfre M(1977)Behavioral despair in mice: a primary screening test for antidepressants. Arch Int Pharmacodyn Ther 229:327–336. [PubMed] [Google Scholar]
- Rajkowska G.(2000)Postmortem studies in mood disorders indicate altered numbers of neurons and glial cells. Biol Psychiatry 48:766–777. [DOI] [PubMed] [Google Scholar]
- Rodríguez-Muñoz M, Sánchez-Blázquez P, Vicente-Sánchez A, Berrocoso E, Garzón J(2012)The mu-opioid receptor and the NMDA receptor associate in PAG neurons: implications in pain control. Neuropsychopharmacology 37:338–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodríguez-Muñoz M, Cortés-Montero E, Pozo-Rodrigálvarez A, Sánchez-Blázquez P, Garzón-Niño J (2015a) The ON:OFF switch, σ1r-HINT1 protein, controls GPCR-NMDA receptor cross-regulation: implications in neurological disorders. Oncotarget 6:35458–35477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodríguez-Muñoz M, Sánchez-Blázquez P, Herrero-Labrador R, Martínez-Murillo R, Merlos M, Vela JM, Garzón J (2015b) The σ1 receptor engages the redox-regulated HINT1 protein to bring opioid analgesia under NMDA receptor negative control. Antioxid Redox Signal 22:799–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodríguez-Muñoz M, Sánchez-Blázquez P, Merlos M, Garzón-Niño J(2016)Endocannabinoid control of glutamate NMDA receptors: the therapeutic potential and consequences of dysfunction. Oncotarget 7:55840–55862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojas A, Dingledine R(2013)Ionotropic glutamate receptors: regulation by G-protein-coupled receptors. Mol Pharmacol 83:746–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sánchez-Blázquez P, Pozo-Rodrigálvarez A, Merlos M, Garzón J(2018)The sigma-1 receptor antagonist, S1RA, reduces stroke damage, ameliorates post-stroke neurological deficits and suppresses the overexpression of MMP-9. Mol Neurobiol 55:4940–4951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sánchez-Blázquez P, Rodríguez-Muñoz M, Vicente-Sánchez A, Garzón J(2013)Cannabinoid receptors couple to NMDA receptors to reduce the production of NO and the mobilization of zinc induced by glutamate. Antioxid Redox Signal 19:1766–1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sánchez-Blázquez P, Rodríguez-Muñoz M, Herrero-Labrador R, Burgueño J, Zamanillo D, Garzón J(2014)The calcium-sensitive sigma-1 receptor prevents cannabinoids from provoking glutamate NMDA receptor hypofunction: implications in antinociception and psychotic diseases. Int J Neuropsychopharmacol 17:1943–1955. [DOI] [PubMed] [Google Scholar]
- Savitz J, Solms M, Ramesar R(2005)Neuropsychological dysfunction in bipolar affective disorder: a critical opinion. Bipolar Disord 7:216–235. [DOI] [PubMed] [Google Scholar]
- Sourial-Bassillious N, Rydelius PA, Aperia A, Aizman O(2009)Glutamate-mediated calcium signaling: a potential target for lithium action. Neuroscience 161:1126–1134. [DOI] [PubMed] [Google Scholar]
- Szabo ST, Machado-Vieira R, Yuan P, Wang Y, Wei Y, Falke C, Cirelli C, Tononi G, Manji HK, Du J(2009)Glutamate receptors as targets of protein kinase C in the pathophysiology and treatment of animal models of mania. Neuropharmacology 56:47–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vela JM, Merlos M, Almansa C(2015)Investigational sigma-1 receptor antagonists for the treatment of pain. Expert Opin Investig Drugs 24:883–896. [DOI] [PubMed] [Google Scholar]
- Wang J, Mack AL, Coop A, Matsumoto RR(2007)Novel sigma (sigma) receptor agonists produce antidepressant-like effects in mice. Eur Neuropsychopharmacol 17:708–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young JW, Henry BL, Geyer MA(2011)Predictive animal models of mania: hits, misses and future directions. Br J Pharmacol 164:1263–1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarate CA, Manji HK(2009)Protein kinase C inhibitors: rationale for use and potential in the treatment of bipolar disorder. CNS Drugs 23:569–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu LQ, Wang SH, Liu D, Yin YY, Tian Q, Wang XC, Wang Q, Chen JG, Wang JZ(2007)Activation of glycogen synthase kinase-3 inhibits long-term potentiation with synapse-associated impairments. J Neurosci 27:12211–12220. [DOI] [PMC free article] [PubMed] [Google Scholar]