Abstract
In patients with chronic heart failure, iron deficiency, even in the absence of anaemia, can aggravate the underlying disease and have a negative impact on clinical outcomes and quality of life. The 2016 European Society of Cardiology guidelines for the diagnosis and treatment of acute and chronic heart failure recognize iron deficiency as a co‐morbidity in chronic heart failure and recommend iron status screening in all newly diagnosed patients with chronic heart failure. Furthermore, the guidelines specifically recommend considerations of intravenous iron therapy, ferric carboxymaltose, for the treatment of iron deficiency. However, in spite of these recommendations, iron deficiency remains often overlooked and undertreated. This may be due, in part, to the lack of clinical context and practical guidance accompanying the guidelines for the treating physician. Here, we provide practical guidance complemented by a case study to assist and improve the timely diagnosis, treatment, and routine management of iron deficiency in patients with chronic heart failure.
Keywords: Iron deficiency, Chronic heart failure, Practical guidance, Ferric carboxymaltose, Case study
Introduction
Iron deficiency is a ‘health‐related condition in which iron availability is insufficient to meet the body's needs and which can be present with or without anaemia’.1 In chronic heart failure (CHF), iron deficiency is a recognized co‐morbidity affecting 37–61% of patients.2, 3, 4, 5 The causes of iron deficiency in the CHF setting can be multifactorial, arising from chronic inflammation, decreased iron intake (poor nutrition and loss of appetite), decreased gastrointestinal (GI) iron absorption due to oedema, and increased GI blood loss (partially resulting from antiplatelet and anticoagulant drugs).6, 7
Exercise intolerance and fatigue are defining features of patients with CHF. Studies have shown that iron deficiency, even before the onset of anaemia, can be particularly severe in patients with CHF, aggravating the underlying disease and negatively impacting symptoms, quality of life (QoL), exercise capacity, and clinical outcomes, and has been associated with an increased risk of mortality of 40–60%.2, 3, 4, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17 In addition, iron deficiency has been shown to increase the risk of hospitalization in patients with CHF by two‐fold (relative risk 2.23, 95% confidence interval 1.59–3.42; P < 0.001).8 Appropriate treatment of iron deficiency in CHF patients has been shown to alleviate symptoms and decrease hospitalizations for heart failure.10, 11, 12, 13, 14, 15
The 2016 European Society of Cardiology (ESC) guidelines for the diagnosis and treatment of acute and chronic heart failure recommend that all newly diagnosed patients with heart failure are tested for iron deficiency.18 Moreover, intravenous (i.v.) iron, ferric carboxymaltose, is specifically recommended in the ESC guidelines to be considered for the treatment of iron deficiency in order to alleviate heart failure symptoms and improve exercise capacity and QoL.18 Yet, in spite of these recommendations, iron deficiency remains underdiagnosed and undertreated.19 This may partially be due to the lack of clinical context accompanying available guidelines for the treating physician. This review thus aims to provide practical guidance, illustrated with a commonly encountered clinical case study, to help clinicians in the timely diagnosis and management of iron deficiency in patients with CHF.
The clinical case
A 73‐year‐old female patient was first admitted with acutely decompensated heart failure requiring inotropic support [New York Heart Association (NYHA) IV]. Echocardiogram showed left ventricular ejection fraction (LVEF) 22%, dilated left ventricle (68 mm), and moderate functional mitral regurgitation. No significant stenosis was observed on coronary angiography. Medical history included hyperlipidaemia and idiopathic dilated cardiomyopathy. Once the patient's condition had stabilized, the physician initiated heart failure treatment following ESC guideline recommendations, including carvedilol 12.5 mg/12 h, enalapril 10 mg/12 h, spironolactone 25 mg/24 h, and furosemide 20 mg/24 h. Ambulatory up‐titration of angiotensin‐converting enzyme inhibitors and beta‐blockers was limited because of symptomatic hypotension. The patient also received education and support from heart failure nurses prior to discharge.
An initial improvement in functional class to NYHA II was observed; however, the patient later presented with symptomatic deterioration to NYHA III. The patient required two consultations and day‐case hospital visits to receive diuretic therapy and an unplanned hospital admission for inotropic therapy. One month after discharge, the patient's status remained as NYHA III. She therefore continued with the medication previously prescribed at the initial diagnosis. However, she suffered several episodes of decompensation of heart failure, which were managed with furosemide but did not require admission. Up‐titration of renin–angiotensin–aldosterone blockade and beta‐blocker treatment was limited by symptomatic hypotension.
At a routine follow‐up examination, cardiac and respiratory auscultation was normal. There were no signs of organomegaly, and LVEF was 30%.
What would be the appropriate next steps in management?
The earlier illustrates a typical case of non‐ischaemic heart failure with reduced ejection fraction (HFrEF), with recurrent episodes of heart failure decompensation despite optimal first‐line guideline‐directed medical therapy. Consideration of additional heart failure therapy is warranted, including up‐titration of medications to guideline‐targeted doses, replacement of enalapril with angiotensin receptor/neprilysin inhibitors, addition of ivabradine, and evaluation for device therapy. Current ESC heart failure guidelines emphasize the importance of addressing potential precipitating causes of decompensation and managing co‐morbidities.
Moreover, physicians are recommended to measure iron parameters in all newly diagnosed heart failure patients (Class of Recommendation I, Level of Evidence C; Table 1), regardless of haemoglobin (Hb) status.18 A recent practical guidance consensus by a panel of recognized experts in the field of heart failure, based on the 2016 ESC guidelines, also recommends evaluation of iron status in patients with existing CHF, particularly if they are symptomatic, and monitoring once a year (Table 1).18
Table 1.
Diagnosis of iron deficiency in CHF: summary of ESC and practical guideline recommendations18
| In whom and when? | |
|---|---|
|
1. Diagnostic workup is recommended/should be considered for all newly diagnosed heart failure patients (Class of Recommendation I, Level of Evidence C). 2. Iron status evaluation is also recommended in patients with existing CHF, particularly if they are symptomatic. 3. Iron parameters should be evaluated at least once a year. |
| Which parameters should be assessed? | |
|---|---|
| Parameter | Threshold |
| Hb | Anaemia: 12 g/dL (women) and 13 g/dL (men) |
| Serum ferritin | Iron deficiency: <100 μg/L; if between 100 and <300 μg/L, TSAT results are required to confirm iron deficiency |
| TSAT | Iron deficiency: < 20% |
CHF, chronic heart failure; ESC, European Society of Cardiology; Hb, haemoglobin; TSAT, transferrin saturation.
Parameters should be assessed simultaneously.
Diagnosis of iron deficiency in chronic heart failure
The clinical case
As there was no resolution of the patient's NYHA III status, her serum ferritin, transferrin saturation (TSAT), and Hb levels were examined during a routine follow‐up assessment. Test results revealed serum ferritin to be 10 μg/L, with TSAT of 11% and Hb levels of 11.2 g/dL.
It is worth noting that the current patient was first diagnosed in 2010, when guidelines for the timely diagnosis of iron deficiency were not available. If the patient was diagnosed today, she would meet the guideline recommendations for checking iron status as part of the initial work up in patients with heart failure (Table 1).
How do we interpret these results?
The serum ferritin (<100 μg/L) and TSAT levels (< 20%) in this patient were diagnostic of iron deficiency (Figure 1 A, Step 1), while the Hb level (<12 g/dL) indicated mild anaemia (Figure 1 A, Step 2). Serum ferritin reflects total body iron stores, and low levels (<100 μg/L in heart failure patients) are highly specific for absolute iron deficiency (Table 2).6 Therefore, this patient unequivocally had absolute iron deficiency whereby total body iron stores are depleted and may occur with iron homeostasis mechanisms and erythropoiesis still intact.6, 21
Figure 1.
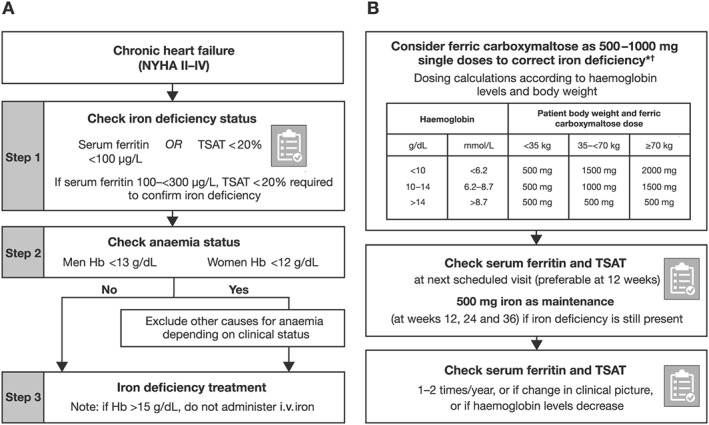
(A) Diagnostic and (B) treatment algorithms for iron deficiency in patients with chronic heart failure.1 *The use of ferric carboxymaltose has not been studied in children and is therefore not recommended in children under 14 years old. For full prescribing information, please refer to the summary of product characteristics.20 †The maximum dose per week of ferric carboxymaltose is 1000 mg. Two dosing sessions are required in case the patient needs >1000 mg ferric carboxymaltose.20 Algorithms adapted from Cappellini et al.1 and McDonagh and Macdougall.7 Hb, haemoglobin; i.v., intravenous; NYHA, New York Heart Association; TSAT, transferrin saturation.
Table 2.
| Absolute iron deficiency | Functional iron deficiency | |
|---|---|---|
| Aetiology | Decreased iron intake and GI absorption; increased blood loss | Pro‐inflammatory mediators, e.g. interleukin‐6 in chronic inflammation, trigger up‐regulation of the hepatic hormone hepcidin and subsequent internalization and degradation of the iron transporter ferroportin. This in turn causes iron sequestration in enterocytes and reticuloendothelial cellsa |
| Iron stores | Depleted | Normal or abundant but iron unavailable |
| Iron availability | Decreased | Decreased |
| Serum ferritin levels | <100 μg/Lb | >100 to <300 μg/L |
| TSAT levels | < 20% | < 20% |
It is critical, however, to recognize that functional iron deficiency can be present even if serum ferritin levels are seemingly normal or even elevated. This is because serum ferritin is an acute‐phase reactant that reflects the inflammatory milieu of heart failure.25 Furthermore, chronic kidney disease, another chronic inflammatory condition, is highly prevalent in patients with CHF.26 The patient in this case study had an estimated glomerular filtration rate of 51 mL/min/1.73 m2 suggesting mild to moderate loss of renal function (chronic kidney disease Stage 3).27 Notably, laboratory results commonly also report reference values for serum ferritin and TSAT as measured in patients without inflammation. It is therefore important to understand that these reference values do not apply to patients with heart failure, who are characterized by latent inflammation. Functional iron deficiency is due to inadequate transport of iron to iron‐utilizing tissues, despite seemingly sufficient body iron stores [indicated by low TSAT values (< 20%)].6, 21, 28 This is a result of chronic inflammation, which triggers regulatory mechanisms leading to iron sequestration in enterocytes and reticuloendothelial cells (Table 2).29, 30, 31 Importantly, for both forms of iron deficiency (absolute and functional), the treatment is the same (iron replenishment therapy).1, 6, 18, 21 Both types of iron deficiency may occur without concomitant presence of anaemia; however, with advancing severity of iron deficiency, overt anaemia may develop because of a lack of iron in utilizing tissues. In the current manuscript, ‘iron deficiency’ refers to both types unless otherwise stated.
In order to encompass iron deficiency in chronic inflammatory conditions such as CHF, the following single consensus diagnostic threshold criterion has been proposed: serum ferritin <100 μg/L or TSAT < 20% (if serum ferritin is between 100 and <300 μg/L, TSAT < 20% will be required to confirm iron deficiency).1
Treatment and management of iron deficiency in chronic heart failure
The clinical case
Iron deficiency anaemia was diagnosed (other causes of anaemia and occult blood loss accounting for absolute iron deficiency were excluded; Figure 1 A). The patient was subsequently treated with i.v. iron as shown in the diagnostic algorithm (Figure 1 A).1 The repletion dose for this patient, 1000 mg ferric carboxymaltose, was calculated according to her weight (66 kg) and Hb levels (11.9 g/dL; Figure 1 B). There was no change in her heart failure treatment.
Ferric carboxymaltose was administered with an infusion of 100 mL normal saline (a maximum of 250 mL diluting volume is allowed for this dose; Tables 3 and 4) in one session over 15 min, as outlined in Tables 3 and 4. A slow non‐diluted bolus injection of i.v. ferric carboxymaltose could also have been feasible in this patient following the treatment scheme suggested in the CONFIRM‐HF study.15
Table 3.
| In whom and when? |
Indications
|
Contraindications
|
Cautions/seek specialist advice
|
| Where? |
|
| How to use? |
|
| Monitoring |
|
CHF, chronic heart failure; ESC, European Society of Cardiology; Hb, haemoglobin; HFpEF, heart failure with preserved ejection fraction; HFrEF, heart failure with reduced ejection fraction; LVEF, left ventricular ejection fraction; TSAT, transferrin saturation.
Table 4.
Dilution plan and administration time for i.v. ferric carboxymaltose infusion20
| Volume of ferric carboxymaltose required (mL) | Equivalent iron dose (mg) | Maximum amount of sterile 0.9% m/V sodium chloride solution (mL) | Minimum administration time (min) |
|---|---|---|---|
| 2–4a | 100–200 | 50 | — |
| >4–10b | >200–500 | 100 | 6 |
| >10–20 | >500–1000 | 250 | 15 |
i.v., intravenous.
Notably, there are two possible modes of administration: (i) infusion using the dilution guidelines indicated in this table and (ii) injection (up to 1000 mg iron) without dilution and over a shorter administration time.20
Injection administration rates:
No minimal prescribed time.
100 mg iron/min.
At the 12 week follow‐up assessment, the patient's status had improved to functional class NYHA II. In accordance with the guidelines, blood parameter measurements were reassessed, and the patient was no longer anaemic (Hb: 12.3 g/dL), but she was still iron deficient (serum ferritin: 87 μg/L; TSAT: 19%; Figure 2 ). In line with the diagnostic and treatment algorithms (Figure 1 ), a further dose of i.v. ferric carboxymaltose (500 mg) was administered via infusion.
Figure 2.
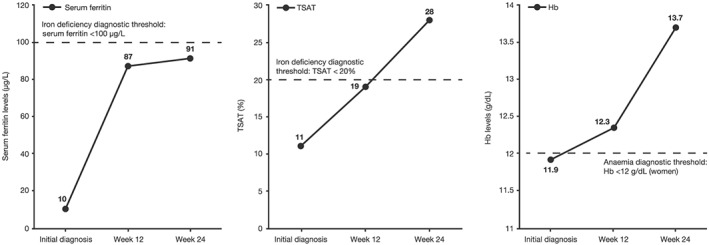
Patient's changes in serum ferritin, transferrin saturation (TSAT), and haemoglobin (Hb) levels over time.
At the 24 week follow‐up assessment, the patient's symptoms had improved to NYHA I, with no decompensation or hospitalization. However, she was still iron deficient (serum ferritin: 91 μg/L; TSAT: 28%; Figure 2 ) and required a further 500 mg dose of ferric carboxymaltose. This time, treatment was administered undiluted over 6 min in an i.v. bolus (Tables 3 and 4).
After this repeated iron replenishment, the patient was no longer iron deficient, and her CHF symptoms remained stable (NYHA I at Week 36), with no further hospitalizations or decompensation episodes. Ferric carboxymaltose was well tolerated, and no adverse events were reported throughout her treatment period.
The patient's iron parameters should be reassessed within 3 to 6 months to allow for the prompt detection and appropriate management of iron deficiency should it reoccur.
Treatment options and guideline recommendations
Treatment with i.v. ferric carboxymaltose is the correct evidence‐based treatment for this patient, based on data from randomized controlled trials. The efficacy of i.v. ferric carboxymaltose has been extensively examined in symptomatic iron‐deficient patients with CHF (HFrEF; LVEF ≤ 45%).2, 3, 4, 8, 9, 10, 11, 12, 13, 14, 15 In the FAIR‐HF study, iron‐deficient CHF patients treated with i.v. ferric carboxymaltose experienced significant improvements in symptoms compared with those receiving placebo at Week 24 [measured using the patient global assessment (P < 0.001), along with improvements in NYHA class (P < 0.001)] and in exercise capacity as measured by the 6 min walk test (6MWT; P < 0.001 at Weeks 4, 12, and 24).13 These findings are further supported by the 52 week CONFIRM‐HF study examining i.v. ferric carboxymaltose vs. placebo (6MWT at Week 24: P = 0.002; 6MWT at Week 52: P < 0.001; patient global assessment: P = 0.001; and NYHA improvement: P < 0.001).15 Furthermore, significant benefits were also observed in QoL questionnaires in the same patients in the FAIR‐HF (at Weeks 4, 12, and 24: P < 0.001) and CONFIRM‐HF (at Week 52: P < 0.05) studies.15, 32 In the most recent EFFECT‐HF study, treatment with i.v. ferric carboxymaltose in patients with HFrEF and iron deficiency increased iron stores. A favourable effect on peak volume of oxygen (VO2) was observed in the primary analysis in patients treated with i.v. ferric carboxymaltose compared with standard of care (including oral iron), yet the authors concluded that this effect was sensitive to the imputation strategy utilized in this unblinded trial.14 A recent individual patient data meta‐analysis revealed a significant decrease in the composite of recurrent heart failure hospitalizations and cardiovascular mortality (rate ratio 0.53, 95% confidence interval 0.33–0.86; P = 0.011) when patients were treated with i.v. ferric carboxymaltose compared with placebo.10 Although several i.v. iron preparations are available, only ferric carboxymaltose has been studied in patients with CHF.33 Accordingly, the 2016 ESC guidelines specifically recommend ferric carboxymaltose for HFrEF (LVEF ≤ 45%; Class of Recommendation IIa, Level of Evidence A).18
Oral iron is repeatedly discussed as a treatment option in the CHF context; however, the impaired enteral absorption in this setting and other conditions characterized by immune activation is considered to render oral iron administration ineffective. In the 16 week, randomized, prospective, IRONOUT HF study conducted in a similar population of HFrEF and iron‐deficient patients, high‐dose oral iron treatment was ineffective at correcting iron deficiency and did not improve peak VO2 (oral iron vs. placebo: P = 0.46) or other functional measures such as the 6MWT (P = 0.19), N‐terminal pro‐B‐type natriuretic peptide (P = 0.48), and Kansas City Cardiomyopathy Questionnaire clinical summary scores (P = 0.57).34 Owing to the lack of efficacy of oral iron in CHF, unfavourable GI adverse event profile, and low absorption in inflammatory conditions such as CHF,35, 36 current guidelines do not recommend oral iron for the treatment of iron deficiency in CHF.18
Practical considerations
Intravenous ferric carboxymaltose is generally easy to deliver and well tolerated. It can be administered intravenously as an infusion or injection, and to ensure that the full dose is given, the line should be flushed with normal saline (Table 3). Dilutions are not required, and administration time is shorter when i.v. ferric carboxymaltose is administered via injection vs. infusion (Table 4). For infusions, ferric carboxymaltose (500–1000 mg doses) can be diluted using a small bag of normal saline (50–100 mL, maximum 250 mL; Table 4). In contrast to older i.v. iron complexes, hypersensitivity reactions related to ferric carboxymaltose are rare (≥1/1000 to <1/100).20 However, equipment for the evaluation and treatment of potential hypersensitivity reactions should be available within the facility (Table 3).
Intravenous ferric carboxymaltose administration can be provided on an ambulatory basis (e.g. in a day hospital). In the case study example, only one dose of 1000 mg i.v. ferric carboxymaltose was required for the initial correction of iron deficiency. However, in some cases, depending on Hb levels and body weight, two doses of i.v. ferric carboxymaltose may be required over two sessions (Table 3); in this situation, the second dose would need to be administered 1 week after the initial dose.20
Follow‐up management of iron deficiency in chronic heart failure
Following initial ferric carboxymaltose administration, physicians are recommended to follow up with their patients at 12 weeks to evaluate the clinical and haematological response (serum ferritin, TSAT, and Hb; Figure 1 B and Table 3), even if patients are receiving optimal heart failure treatment.
Conclusions
Iron deficiency alone, even in the absence of anaemia, is a common co‐morbidity in patients with CHF. If left untreated, iron deficiency can account for poor clinical outcomes, as illustrated in the case study patient who was diagnosed when guidelines were unavailable. The case study provides a practical example of the diagnosis and management of iron deficiency in a patient with HFrEF, demonstrating the application of the current ESC guidelines in a clinical context. In support of clinical evidence and guidelines, the case study shows that treatment with i.v. ferric carboxymaltose improves clinical symptoms (from NYHA III to NYHA I; i.e. exercise capacity and QoL) with no adverse events. Furthermore, i.v. ferric carboxymaltose could provide cost‐effective savings as there was less intervention, that is, no hospitalizations or decompensations. Despite a solid evidence base and guideline recommendation, iron deficiency currently remains underdiagnosed and undertreated. The 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure recommend evaluating the iron status of all newly diagnosed CHF patients and consideration of i.v. ferric carboxymaltose for those patients with iron deficiency to alleviate CHF symptoms and improve exercise capacity and QoL.
Conflict of interest
C.S.P.L. has received research support/consultation fees from Abbott Diagnostics, AstraZeneca, Bayer, Boehringer Ingelheim, Boston Scientific, Janssen Research & Development, LLC, Medtronic, Menarini, Merck, Novartis, Takeda, Thermo Fisher, and Vifor Pharma. W.D. has received a grant and honoraria as advisor from Vifor Pharma. J.C.‐C. has received honoraria as advisor from Vifor Pharma.
Funding
This manuscript was sponsored by Vifor Pharma. Medical writing support was provided by Mai Kurihara, PhD, from Mudskipper Business Ltd, funded by Vifor Pharma.
Lam, C. S. P. , Doehner, W. , Comin‐Colet, J. , and on behalf of the IRON CORE Group (2018) Iron deficiency in chronic heart failure: case‐based practical guidance. ESC Heart Failure, 5: 764–771. 10.1002/ehf2.12333.
The IRON CORE Group includes Carolyn S.P. Lam, Wolfram Doehner, Josep Comin‐Colet, Maria Domenica Cappellini, Clara Camaschella, Angel de Francisco, Axel Dignass, Rezan Kadir, Iain C. Macdougall, Donat R. Spahn, Ali T. Taher, and Khaled M. Musallam.
Contributor Information
Josep Comin‐Colet, Email: josepcomin@gmail.com.
on behalf of the IRON CORE Group:
Carolyn S.P. Lam, Wolfram Doehner, Josep Comin‐Colet, Maria Domenica Cappellini, Clara Camaschella, Angel de Francisco, Axel Dignass, Rezan Kadir, Iain C. Macdougall, Donat R. Spahn, Ali T. Taher, and Khaled M. Musallam
References
- 1. Cappellini MD, Comin‐Colet J, De Francisco A, Dignass A, Doehner W, Lam SP, Macdougall IC, Rogler G, Camaschella C, Kadir R, Kassebaum NJ, Spahn DR, Taher AT, Musallam KM. Iron deficiency across chronic inflammatory conditions: international expert opinion on definition, diagnosis, and management. Am J Hematol 2017; 92: 1068–1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Jankowska EA, Rozentryt P, Witkowska A, Nowak J, Hartmann O, Ponikowska B, Borodulin‐Nadzieja L, Banasiak W, Polonski L, Filippatos G, McMurray JJ, Anker SD, Ponikowski P. Iron deficiency: an ominous sign in patients with systolic chronic heart failure. Eur Heart J 2010; 31: 1872–1880. [DOI] [PubMed] [Google Scholar]
- 3. Klip IT, Comin‐Colet J, Voors AA, Ponikowski P, Enjuanes C, Banasiak W, Lok DJ, Rosentryt P, Torrens A, Polonski L, van Veldhuisen DJ, van der Meer P, Jankowska EA. Iron deficiency in chronic heart failure: an international pooled analysis. Am Heart J 2013; 165: 575–582. [DOI] [PubMed] [Google Scholar]
- 4. Okonko DO, Mandal AK, Missouris CG, Poole‐Wilson PA. Disordered iron homeostasis in chronic heart failure: prevalence, predictors, and relation to anemia, exercise capacity, and survival. J Am Coll Cardiol 2011; 58: 1241–1251. [DOI] [PubMed] [Google Scholar]
- 5. Peyrin‐Biroulet L, Williet N, Cacoub P. Guidelines on the diagnosis and treatment of iron deficiency across indications: a systematic review. Am J Clin Nutr 2015; 102: 1585–1594. [DOI] [PubMed] [Google Scholar]
- 6. Jankowska EA, von Haehling S, Anker SD, Macdougall IC, Ponikowski P. Iron deficiency and heart failure: diagnostic dilemmas and therapeutic perspectives. Eur Heart J 2013; 34: 816–829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. McDonagh T, Macdougall IC. Iron therapy for the treatment of iron deficiency in chronic heart failure: intravenous or oral? Eur J Heart Fail 2015; 17: 248–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Toblli JE, Lombrana A, Duarte P, Di Gennaro F. Intravenous iron reduces NT‐pro‐brain natriuretic peptide in anemic patients with chronic heart failure and renal insufficiency. J Am Coll Cardiol 2007; 50: 1657–1665. [DOI] [PubMed] [Google Scholar]
- 9. Avni T, Leibovici L, Gafter‐Gvili A. Iron supplementation for the treatment of chronic heart failure and iron deficiency: systematic review and meta‐analysis. Eur J Heart Fail 2012; 14: 423–429. [DOI] [PubMed] [Google Scholar]
- 10. Anker SD, Kirwan BA, van Veldhuisen DJ, Filippatos G, Comin‐Colet J, Ruschitzka F, Luscher TF, Arutyunov GP, Motro M, Mori C, Roubert B, Pocock SJ, Ponikowski P. Effects of ferric carboxymaltose on hospitalisations and mortality rates in iron‐deficient heart failure patients: an individual patient data meta‐analysis. Eur J Heart Fail 2018; 20: 125–133. [DOI] [PubMed] [Google Scholar]
- 11. Jankowska EA, Tkaczyszyn M, Suchocki T, Drozd M, von Haehling S, Doehner W, Banasiak W, Filippatos G, Anker SD, Ponikowski P. Effects of intravenous iron therapy in iron‐deficient patients with systolic heart failure: a meta‐analysis of randomized controlled trials. Eur J Heart Fail 2016; 18: 786–795. [DOI] [PubMed] [Google Scholar]
- 12. Enjuanes C, Bruguera J, Grau M, Cladellas M, Gonzalez G, Merono O, Moliner‐Borja P, Verdu JM, Farre N, Comin‐Colet J. Iron status in chronic heart failure: impact on symptoms, functional class and submaximal exercise capacity. Rev Esp Cardiol (Engl Ed) 2016; 69: 247–255. [DOI] [PubMed] [Google Scholar]
- 13. Anker SD, Comin CJ, Filippatos G, Willenheimer R, Dickstein K, Drexler H, Luscher TF, Bart B, Banasiak W, Niegowska J, Kirwan BA, Mori C, von Eisenhart RB, Pocock SJ, Poole‐Wilson PA, Ponikowski P. Ferric carboxymaltose in patients with heart failure and iron deficiency. N Engl J Med 2009; 361: 2436–2448. [DOI] [PubMed] [Google Scholar]
- 14. van Veldhuisen DJ, Ponikowski P, van der Meer P, Metra M, Bohm M, Doletsky A, Voors AA, Macdougall IC, Anker SD, Roubert B, Zakin L, Cohen‐Solal A. Effect of ferric carboxymaltose on exercise capacity in patients with chronic heart failure and iron deficiency. Circulation 2017; 136: 1374–1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ponikowski P, van Veldhuisen DJ, Comin‐Colet J, Ertl G, Komajda M, Mareev V, McDonagh T, Parkhomenko A, Tavazzi L, Levesque V, Mori C, Roubert B, Filippatos G, Ruschitzka F, Anker SD. Beneficial effects of long‐term intravenous iron therapy with ferric carboxymaltose in patients with symptomatic heart failure and iron deficiency. Eur Heart J 2015; 36: 657–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Comin‐Colet J, Enjuanes C, Gonzalez G, Torrens A, Cladellas M, Merono O, Ribas N, Ruiz S, Gomez M, Verdu JM, Bruguera J. Iron deficiency is a key determinant of health‐related quality of life in patients with chronic heart failure regardless of anaemia status. Eur J Heart Fail 2013; 15: 1164–1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Enjuanes C, Klip IT, Bruguera J, Cladellas M, Ponikowski P, Banasiak W, van Veldhuisen DJ, van der MP, Jankowska EA, Comin‐Colet J. Iron deficiency and health‐related quality of life in chronic heart failure: results from a multicenter European study. Int J Cardiol 2014; 174: 268–275. [DOI] [PubMed] [Google Scholar]
- 18. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JG, Coats AJ, Falk V, Gonzalez‐Juanatey JR, Harjola VP, Jankowska EA, Jessup M, Linde C, Nihoyannopoulos P, Parissis JT, Pieske B, Riley JP, Rosano GM, Ruilope LM, Ruschitzka F, Rutten FH, van der Meer P. ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: the Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J 2016; 37: 2129–2200. [DOI] [PubMed] [Google Scholar]
- 19. Belmar Vega L, De Francisco A, Albines Fiestas Z, Serrano Soto M, Kislikova M, Seras Mozas M, Unzueta MG, Arias Rodriguez M. Investigation of iron deficiency in patients with congestive heart failure: a medical practice that requires greater attention. Nefrologia 2016; 36: 249–254. [DOI] [PubMed] [Google Scholar]
- 20. Vifor Pharma Ltd . Ferinject 50 mg iron/mL solution for injection/infusion SmPC. https://www.medicines.org.uk/emc/medicine/24167 2013.
- 21. Wish JB. Assessing iron status: beyond serum ferritin and transferrin saturation. Clin J Am Soc Nephrol 2006; 1: S4–S8. [DOI] [PubMed] [Google Scholar]
- 22. Polin V, Coriat R, Perkins G, Dhooge M, Abitbol V, Leblanc S, Prat F, Chaussade S. Iron deficiency: from diagnosis to treatment. Dig Liver Dis 2013; 45: 803–809. [DOI] [PubMed] [Google Scholar]
- 23. Camaschella C. Iron‐deficiency anemia. N Engl J Med 2015; 372: 1832–1843. [DOI] [PubMed] [Google Scholar]
- 24. Lopez A, Cacoub P, Macdougall IC, Peyrin‐Biroulet L. Iron deficiency anaemia. Lancet 2016; 387: 907–916. [DOI] [PubMed] [Google Scholar]
- 25. Jankowska EA, Malyszko J, Ardehali H, Koc‐Zorawska E, Banasiak W, von Haehling S, Macdougall IC, Weiss G, McMurray JJ, Anker SD, Gheorghiade M, Ponikowski P. Iron status in patients with chronic heart failure. Eur Heart J 2013; 34: 827–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ahmed A, Campbell RC. Epidemiology of chronic kidney disease in heart failure. Heart Fail Clin 2008; 4: 387–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. KDIGO . Clinical practice guideline for anemia in chronic kidney disease. Kidney Int Suppl 2012; 2: 279–335. [Google Scholar]
- 28. Ganz T. Systemic iron homeostasis. Physiol Rev 2013; 93: 1721–1741. [DOI] [PubMed] [Google Scholar]
- 29. Cherayil BJ. The role of iron in the immune response to bacterial infection. Immunol Res 2011; 50: 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ganz T. Iron in innate immunity: starve the invaders. Curr Opin Immunol 2009; 21: 63–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ganz T, Nemeth E. The hepcidin–ferroportin system as a therapeutic target in anemias and iron overload disorders. Hematology Am Soc Hematol Educ Program 2011; 2011: 538–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Comin‐Colet J, Lainscak M, Dickstein K, Filippatos GS, Johnson P, Luscher TF, Mori C, Willenheimer R, Ponikowski P, Anker SD. The effect of intravenous ferric carboxymaltose on health‐related quality of life in patients with chronic heart failure and iron deficiency: a subanalysis of the FAIR‐HF study. Eur Heart J 2013; 34: 30–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Drozd M, Jankowska EA, Banasiak W, Ponikowski P. Iron therapy in patients with heart failure and iron deficiency: review of iron preparations for practitioners. Am J Cardiovasc Drugs 2017; 17: 183–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lewis GD, Malhotra R, Hernandez AF, McNulty SE, Smith A, Felker GM, Tang WHW, LaRue SJ, Redfield MM, Semigran MJ, Givertz MM, Van Buren P, Whellan D, Anstrom KJ, Shah MR, Desvigne‐Nickens P, Butler J, Braunwald E. Effect of oral iron repletion on exercise capacity in patients with heart failure with reduced ejection fraction and iron deficiency: the IRONOUT HF randomized clinical trial. JAMA 2017; 317: 1958–1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Geisser P, Burckhardt S. The pharmacokinetics and pharmacodynamics of iron preparations. Pharmaceutics 2011; 3: 12–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Morris CC. Pediatric iron poisonings in the United States. South Med J 2000; 93: 352–358. [PubMed] [Google Scholar]


