Abstract
Background
The purpose of the study was to investigate the association between radiomic features based on contrast‐enhanced multidetector computed tomography (CT) and the Ki‐67 proliferation index (PI) in patients with lung cancer.
Methods
One hundred and ten patients with lung cancer confirmed by surgical histology were retrospectively included. Radiomic features were extracted from preoperative contrast‐enhanced chest multidetector CT images for each tumor using open‐source three‐dimensional Slicer software. Statistical analysis was performed to determine significant radiomic features serving as image predictors of Ki‐67 status in lung cancer and to investigate the relationship between these features and Ki‐67 PI.
Results
Higher Ki‐67 expression was more common in men (P = 0.02) and patients with a smoking history (P = 0.01). Twelve radiomic features were significantly associated with Ki‐67 status. Multivariate logistic regression analysis identified inverse variance, minor axis, and elongation as independent predictors of Ki‐67 PI. There was a positive correlation between inverse variance, minor axis, elongation (P = 0.00, P = 0.02, and P = 0.14, respectively) and Ki‐67 PI. The area under the curve to identify high Ki‐67 status for inverse variance was 0.77 with a cutoff value of 0.47, which was significantly higher than for minor axis and elongation (P = 0.02 and P = 0.03, respectively).
Conclusion
Radiomic features based on contrast CT images, including inverse variance, minor axis, and elongation, can serve as noninvasive predictors of Ki‐67 status in patients with lung cancer. Inverse variance could be superior to the other radiomic features to identify high Ki‐67 status.
Keywords: Ki‐67, lung cancer, radiomics
Introduction
Lung cancer is the leading cause of cancer death worldwide despite advances in diagnosis and treatment, and incidence is steadily increasing in industrialized countries.1 The prognosis for patients with lung cancer is generally poor, even after complete surgical resection. There is an urgent need to determine reliable prognostic factors to predict clinical outcome and to more precisely stratify the group of patients susceptible to poorer outcomes.
Ki‐67, a nuclear protein, is expressed during the active phases of the cell cycle, except the G0 stage,2 and the Ki‐67 proliferation index (PI) has widely been used as a marker of cell proliferation. Previous studies have shown that a high Ki‐67 PI has a negative impact on disease‐free survival, relapse‐free survival, and overall survival in non‐small cell lung cancer.3, 4 Moreover, the most recent World Health Organization (WHO) classification has adopted the Ki‐67 PI for the diagnosis of small cell lung cancer (SCLC) and a higher Ki‐67 PI may represent a predictive factor for increased tumor radiosensitivity in SCLC.5 Hence, Ki‐67 may be a valuable biomarker for predicting the prognosis of lung cancer patients. The conventional method for detecting Ki‐67 expression is immunohistochemistry, which is a test involving invasive biopsy or surgical samples. In addition, biopsy samples may ignore intratumoral heterogeneity expression, which can induce sampling errors. Therefore, an accurate, noninvasive predictor of Ki‐67 status in patients with lung cancer is clinically desirable.
Radiomics, which differs from the traditional practice of using medical images solely for visual interpretation, is the transition of digital medical images into mineable data by high‐throughput extraction of abundant quantitative features based on shape, intensity, size, or volume, among others.6, 7 Quantitative image features, called also “radiomic features,” could provide richer information about intensity, shape, size, or volume, and texture of the tumor phenotype that is distinct or complementary to that provided by clinical reports, laboratory test results, and genomic or proteomic assays.8 Radiomic features have shown promise for the prediction of treatment response, differentiating benign and malignant tumors, and assessing prognosis and cancer genetics in many cancer types.9
However, to the best of our knowledge, no studies have examined the correlation between computed tomography (CT)‐based radiomic features and Ki‐67 status in lung cancer. Therefore, the aim of this study was to investigative whether radiomic features based on contrast CT images can serve as noninvasive predictors of Ki‐67 status in patients with lung cancer.
Methods
Patients
Our institutional review board approved this retrospective study, and the requirement for informed consent was waived. We collected data from patients who were pathologically diagnosed with lung cancer at our institute between January and December 2017. The inclusion criteria were: (i) visible lung cancer on preoperative CT images; (ii) surgical resection with histopathologically verified lung cancer; and (iii) immunohistochemical (IHC) examination of Ki‐67 expression level. The exclusion criteria were: (i) patients who underwent a biopsy before the CT examination; (ii) patients who underwent neoadjuvant chemotherapy before surgery; (iii) lesions displayed as ground‐glass or part‐solid nodules; and (iv) patients without Ki‐67 IHC examination data. In total, 110 patients met the requirements for the study. Clinical and pathologic information (age, gender, smoking history, histologic subtype, and tumor stage) were collected from the hospital's electronic medical record system.
Computed tomography examinations
Chest CT examinations were performed using a multidetector computed tomography (CT) system (Discovery CT750 HD scanner, GE Medical Systems, Milwaukee, WI, USA). The scanning parameters were as follows: tube voltage, 120 kVp; tube current, 150–200 mA; beam pitch, 0.969; reconstruction thickness, 1.25 mm; and reconstruction interval, 1.25 mm. All patients underwent contrast‐enhanced CT scans. Images were obtained after intravenous administration of 80–100 mL of non‐ionic iodine contrast material (Ultravist, Bayer Pharma, Berlin, Germany; Omnipaque, GE Company, Shanghai, China) at a rate of 2.5 mL/s using an automated injector. CT scanning was performed with a 70 second delay. All CT scans were obtained with the patient in the supine position holding their breath at the end of full inspiration. Image resolution was determined by pixel spacing, and each case had the same image resolution.
Tumor segmentation and radiomic feature extraction
Two reviewers in consensus retrospectively reviewed, analyzed, and segmented all scans. In cases of disagreement, the third reviewer, with 10 years of clinical experience in thoracic imaging, made the final decision. Each region of interest (ROI) was manually drawn around the boundary of the tumor, slice by slice, on contrasted CT images (Fig 1). After a tumor was segmented, radiomics analysis was performed using a designated multi‐platform, free and open source software package for visualization and medical image computing (3D slicer, version 4.8.1, available at: https://www.slicer.org/). In total, 105 radiomic features were extracted and were divided into seven categories: (i) shape (n = 13), (ii) Gray Level Dependence Matrix (GLDM) (n = 14), (iii) Gray Level Co‐occurrence Matrix (GLCM) (n = 23), (iv) first order (n = 18), (v) Gray Level Run Length Matrix (GLRLM) (n = 16), (vi) Gray Level Size Zone Matrix (GLSZM) (n = 16), and (vii) Neighboring Gray Tone Difference Matrix (NGTDM) (n = 5).
Figure 1.
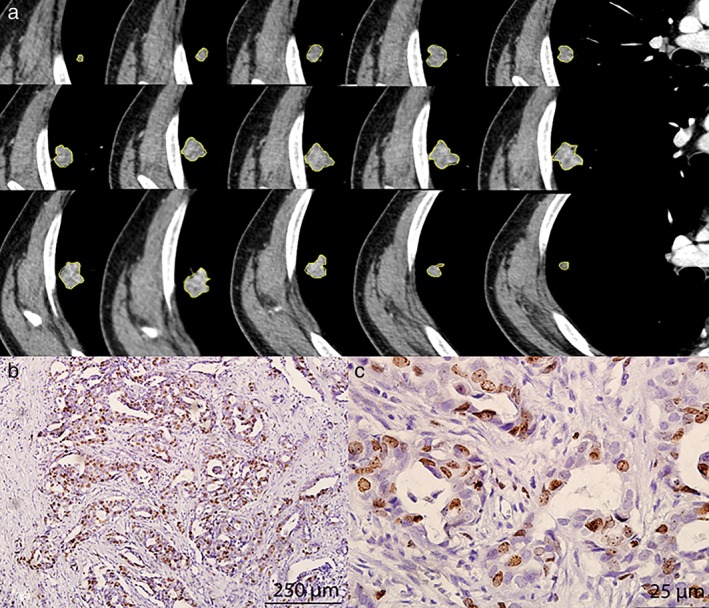
Examples of segmentation of lung cancer based on contrast‐enhanced computed tomography images and Ki‐67 status. (a) Semiautomatic tumor segmentation was performed on each slice of the tumor using a three‐dimensional slicer, which showed a high Ki‐67 expression level (b, magnification ×100; c, ×400).
Ki‐67 assessment
Experienced pathologists routinely evaluated the Ki‐67 expression levels using typical tumor samples collected from the patients. IHC staining was performed in accordance with the manufacturer's protocol. Briefly, formalin‐fixed, paraffin‐embedded tissue sections were cut into 4 μm sections, which were then dried, dewaxed in xylene, rinsed in graded ethanol, and rehydrated in double‐distilled water. IHC staining was performed with the Ki‐67 protein antibody (Santa Cruz Biotechnology, Santa Cruz, CA, USA) at a dilution of 1:100. Cells with brown nuclei were considered positive. After browsing the whole specimen, three regions with the highest density of positive cells were selected; 100 cells in each region were randomly counted under high magnification, and the Ki‐67 index was calculated as the percentage of positive cells. The Ki‐67 indices were calculated in the three regions and averaged. Low Ki‐67 expression was defined as ≤ 40% positive staining and high Ki‐67 expression as > 40% positive staining according to a previous study.3
Statistical analysis
Statistical analysis was carried out using two commercially available statistical software packages (SPSS v. 17.0, Chicago, IL, USA and MedCalc v. 16.2.1, Mariakerke, Belgium). Continuous variables were compared using an independent‐sample t or Mann‐Whitney U test; while categorical variables were presented as a frequency and were compared using chi‐square or Fisher's exact tests. Multivariate logistic regression analysis was subsequently developed to select statistically significant features that could predict the Ki‐67 expression level. In the multivariate logistic regression analysis, features with P < 0.05 in univariable analysis were entered into the initial model. A backward elimination method was used to select the final predictive model; at each step, a feature with P > 0.05 was eliminated.
Receiver operating characteristic (ROC) curves for the significant radiomic features were constructed and the areas under the curve (AUCs) were calculated with a cutoff value, sensitivity, specificity, and positive and negative likelihood ratios (+LR, −LR). The differences between the AUCs were then compared. Spearman's correlation coefficient was used to analyze the correlation between significant radiomic features and Ki‐67 PI. P < 0.05 was considered to indicate a statistically significant difference.
Results
One hundred and ten patients with 110 lung cancers were included in this study. The clinicopathological characteristics are summarized in Table 1. The median age was 62 years (range: 36–77). Of the 110 patients, 36 (32.7%) were never smokers, and 74 (67.3%) were current or former smokers. The majority of patients were male (64.5%). Ninety (81.8%) patients were at early stage (I or II) and 20 (18.2%) at advanced stage (III or IV). Tumor types included: 25 squamous cell carcinomas (22.7%), 62 adenocarcinomas (56.4%), and 23 neuroendocrine tumors (20.9%). Among the neuroendocrine tumors, there were seven typical carcinoids, six atypical carcinoids, six large cell neuroendocrine carcinomas, and four small cell neuroendocrine carcinomas. Among all of the tumors, 56 lesions had high Ki‐67 expression and 54 lesions had low Ki‐67 expression. Higher Ki‐67 expression was more common in men (P = 0.02) and patients with a smoking history (P = 0.01). Representative images of Ki‐67 immunostaining results are shown in Figure 1.
Table 1.
Association between clinicopathological characteristics and Ki‐67 status
| Clinicopathological characteristics | Total | Ki‐67 status | P | |
|---|---|---|---|---|
| Low (n = 54) | High (n = 56) | |||
| Age (mean, range) | 62 (36–77) | 62 (36–77) | 62 (42–76) | 0.73 |
| Gender (n, %) | ||||
| Male | 71 (64.5) | 29 (41.8) | 42 (59.1) | 0.02 |
| Female | 39 (35.5) | 25 (64.1) | 14 (35.9) | |
| Smoking history (n, %) | ||||
| Never smokers | 36 (32.7) | 24 (66.7) | 12 (33.3) | 0.01 |
| Smokers | 74 (67.3) | 30 (40.5) | 44 (59.5) | |
| Pathological type (n, %) | ||||
| Squamous cell carcinoma | 25 (22.7) | 12 (48.0) | 13 (52.0) | 0.26 |
| Adenocarcinoma | 62 (56.4) | 34 (54.8) | 28 (45.2) | |
| Neuroendocrine tumors | 23 (20.9) | 8 (34.8) | 15 (65.2) | |
| Stage (n, %) | ||||
| I or II | 90 (81.8) | 46 (64.4) | 44 (35.6) | 0.37 |
| III or IV | 20 (18.2) | 8 (55.0) | 12 (45.0) | |
A total of 105 available radiomic features were extracted from each tumor using the 3D Slicer software. There were significant differences between low and high Ki‐67 PI groups for 12 of these features (Table 2). Multivariate logistic regression analysis revealed that inverse variance (GLCM; P = 0.01), minor axis (shape; P = 0.03), and elongation (shape; P = 0.01) were independent predictors of Ki‐67 PI (Table 3). There was a positive correlation between inverse variance (GLCM), minor axis (shape), elongation (shape), and Ki‐67 PI (r = 0.46, r = 0.22, and r = 0.19; P = 0.00, P = 0.02, and P = 0.14, respectively). AUCs to identify high Ki‐67 status were 0.77, 0.64, and 0.61 for inverse variance (GLCM), minor axis (shape), and elongation (shape), respectively (Table 4). The cutoff value to identify high Ki‐67 status for inverse variance (GLCM) was 0.47 with a sensitivity of 0.66, specificity of 0.78, + LR of 2.97, and ‐LR of 0.44. The cutoff value of the minor axis (shape) was 26.56 with a sensitivity of 0.50, specificity of 0.81, + LR of 2.70, and ‐LR of 0.61, while the cutoff value of elongation (shape) was 0.80 with a sensitivity of 0.52, specificity of 0.69, + LR of 1.64, and ‐LR of 0.70 (Table 4). There were significant AUC differences between inverse variance (GLCM) and minor axis (shape) (P = 0.02), and inverse variance (GLCM) and elongation (shape) (P = 0.03), but no significant AUC difference existed between minor axis (shape) and elongation (shape) (P = 0.75) (Fig 2).
Table 2.
Significant radiomic features between low and high Ki‐67 expression level groups
| (a) | ||||
|---|---|---|---|---|
| Radiomic features | Ki‐67 status | t | P | |
| Low (n = 54) | High (n = 56) | |||
| Elongation (shape) | 0.74 ± 0.13 | 0.79 ± 0.11 | 2.26 | 0.03 |
| (b) | |||
|---|---|---|---|
| Radiomic features | Ki‐67 status | P | |
| Low (n = 54) | High (n = 56) | ||
| Inverse variance (GLCM) | 40.48 | 69.98 | 0.000 |
| Minor axis (shape) | 47.93 | 62.80 | 0.014 |
| Surface volume ratio (shape) | 63.65 | 47.64 | 0.009 |
| Volume (shape) | 47.28 | 63.43 | 0.008 |
| Surface area (shape) | 47.56 | 63.16 | 0.010 |
| Least axis (shape) | 46.26 | 64.41 | 0.003 |
| Maximum 2D diameter column (shape) | 47.94 | 62.79 | 0.015 |
| Maximum 2D diameter row (shape) | 47.20 | 63.50 | 0.007 |
| Gray Level Non‐uniformity (GLCM) | 45.76 | 64.89 | 0.002 |
| Zone variance (GLSZM) | 44.87 | 65.75 | 0.001 |
| Large area emphasis (GLSZM) | 44.85 | 65.77 | 0.001 |
Mean ± standard deviation of features, independent‐sample t test for comparison.
Mean rank of features, Mann–Whitney U‐test for comparison. GLCM, Gray Level Non‐uniformity; GLSZM, Gray Level Size Zone Matrix.
Table 3.
Multivariate logistic regression analysis of the significant radiomic features to predict Ki‐67 status
| Radiomic features | B | SE | Wald | df | Sig. | Exp (B) | 95% CI for EXP (B) | |
|---|---|---|---|---|---|---|---|---|
| Lower | Upper | |||||||
| Inverse variance (GLCM) | 18.30 | 6.68 | 7.50 | 1.00 | 0.01 | 88 591 554.41 | 181.63 | 4.3211E + 13 |
| Minor axis (shape) | −0.44 | 0.20 | 5.00 | 1.00 | 0.03 | 0.65 | 0.44 | 0.95 |
| Elongation (shape) | 9.91 | 4.00 | 6.13 | 1.00 | 0.01 | 20 160.92 | 7.88 | 51 564 672.82 |
| Surface volume ratio (shape) | 2.05 | 2.89 | 0.50 | 1.00 | 0.48 | 7.78 | 0.03 | 2250.67 |
| Volume (shape) | 0.00 | 0.00 | 1.90 | 1.00 | 0.17 | 1.00 | 1.00 | 1.00 |
| Surface area (shape) | 0.00 | 0.00 | 0.57 | 1.00 | 0.45 | 1.00 | 1.00 | 1.00 |
| Least axis (shape) | 0.18 | 0.12 | 2.29 | 1.00 | 0.13 | 1.20 | 0.95 | 1.52 |
| Maximum 2D diameter column (shape) | 0.10 | 0.08 | 1.68 | 1.00 | 0.19 | 1.11 | 0.95 | 1.29 |
| Maximum 2D diameter row (shape) | 0.11 | 0.06 | 3.08 | 1.00 | 0.08 | 1.12 | 0.99 | 1.26 |
| Gray Level Non‐uniformity (GLCM) | 0.00 | 0.00 | 1.18 | 1.00 | 0.28 | 1.00 | 1.00 | 1.00 |
| Zone variance (GLSZM) | 0.00 | 0.00 | 0.01 | 1.00 | 0.91 | 1.00 | 0.99 | 1.01 |
| Large area emphasis (GLSZM) | 0.00 | 0.00 | 0.01 | 1.00 | 0.91 | 1.00 | 0.99 | 1.01 |
2D, two‐dimensional; CI, confidence interval; GLCM, Gray Level Non‐uniformity; GLSZM, Gray Level Size Zone Matrix; SE, standard error.
Table 4.
ROC analyses of the significant radiomic features to identify high Ki‐67 status
| Radiomic features | Cutoff value | AUC | 95% CI | Sensitivity | Specificity | +LR | ‐LR | |
|---|---|---|---|---|---|---|---|---|
| Lower | Upper | |||||||
| Inverse variance (GLCM) | 0.47 | 0.77 | 0.68 | 0.85 | 0.66 | 0.78 | 2.97 | 0.44 |
| Minor axis (shape) | 26.56 | 0.64 | 0.54 | 0.73 | 0.50 | 0.81 | 2.70 | 0.61 |
| Elongation (shape) | 0.80 | 0.61 | 0.51 | 0.70 | 0.52 | 0.69 | 1.64 | 0.70 |
+LR, positive likelihood ratio; ‐LR, negative likelihood ratio; AUC, area under the curve; CI, confidence interval; GLCM, Gray Level Non‐uniformity; ROC, receiver operating characteristic.
Figure 2.
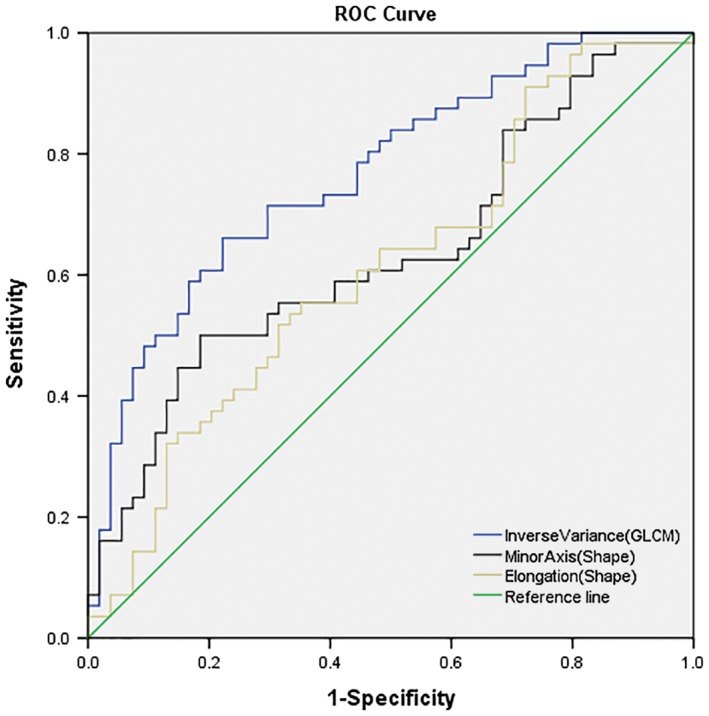
Receiver operating characteristic (ROC) analysis and comparison of the significant radiomic features to predict high Ki‐67 status in lung cancer. Inverse variance (Gray Level Non‐uniformity [GLCM]), minor axis (shape), elongation (shape), and reference line.
Discussion
The Ki‐67 PI is routinely used to evaluate various tumors and is a valuable prognostic factor for lung cancer.10, 11, 12 It can noninvasively and accurately predict Ki‐67 status in patients with lung cancer during routine clinical practice. The results of our preliminary study show that radiomic features based on contrast CT images could be helpful to predict Ki‐67 expression levels in lung cancer.
Radiomics is a developing field in which large amounts of advanced quantitative imaging features are extracted from medical images that can noninvasively predict nodule and tumor behavior. Radiomics has shown promising value in lung cancer research in terms of the determination of tumor viability or aggressiveness, response to chemotherapy and/or radiation, and genomic information.13 Radiomics also has the potential to revolutionize the diagnosis, surveillance, and treatment planning of lung cancer patients, allowing for personalized, noninvasive, and cost‐effective management.14 To the best of our knowledge, no studies have examined the correlation between CT‐based radiomic features and Ki‐67 status in lung cancer. Our present study is the first attempt to investigate this relationship. In our study, 105 available radiomic features were extracted and were divided into seven categories: (i) shape (n = 13), (ii) GLDM (n = 14), (iii) GLCM (n = 23), (iv) first order (n = 18), (v) GLRL (n = 16), (vi) GLSZM (n = 16), and (vii) NGTDM (n = 5). After univariable and multivariable regression analyses, the radiomic features of inverse variance (GLCM), minor axis (shape), and elongation (shape) were independent predictors of Ki‐67 PI. There was a positive correlation between radiomic features and Ki‐67 PI. Furthermore, the AUC to identify high Ki‐67 status for inverse variance (GLCM) was significantly higher than for minor axis (shape) and elongation (shape), which suggests that inverse variance (GLCM) could be superior to the other radiomic features to identify high Ki‐67 status. Our preliminary finding indicates that radiomic analysis as a noninvasive method may be of clinical benefit to predict Ki‐67 expression levels in lung cancer.
There is presently no consensus regarding the Ki‐67 cutoff value. The most widely used Ki‐67 cutoff value for prognostication in non‐small cell lung cancer ranges from 25% to 30%. However, even with the same cutoff value, the proportion of patients with high Ki‐67 expression varies across studies.15 Determining a cutoff value is often based on the median value. In accordance with a previous study and based on our data, we used a cutoff value of 40% for Ki‐67 expression.3 The difference in Ki‐67 cutoff value between our study and others could cause statistical bias. In our study, high Ki‐67 expression showed an association with poor prognostic clinical factors, including male gender and smoking history, consistent with the results of previous research.3 Despite this concordance, our results require verification by further study.
There are several limitations to our study. First, as this was a retrospective study with a single institution design, there may have been unavoidable selection bias. Second, the sample size was relatively small. Third, the follow‐up duration after surgery was not sufficient to evaluate the relationship between radiomic features and patient survival. Finally, the differences in radiomic features of different pathological types of lung cancer between low and high Ki‐67 expression groups were not assessed. Further research using a large sample size should be performed to probe this issue.
In summary, the preliminary results obtained from our study indicate that radiomic features derived from contrast‐enhanced CT images may serve as noninvasive predictors of Ki‐67 status in patients with lung cancer. Inverse variance (GLCM) could be the most promising radiomic feature to identify high Ki‐67 status in lung cancer.
Disclosure
No authors report any conflict of interest.
[Correction added on 5 October 2018, after first online publication: The affiliation no. 1 has been added to the second author, Jie Xu.]
References
- 1. Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. (Published erratum appears in CA Cancer J Clin 2014; 64: 364). CA Cancer J Clin 2014; 64: 9–29.24399786 [Google Scholar]
- 2. Gerdes J, Lemke H, Baisch H, Wacker HH, Schwab U, Stein H. Cell cycle analysis of a cell proliferation‐associated human nuclear antigen defined by the monoclonal antibody Ki‐67. J Immunol 1984; 133: 1710–5. [PubMed] [Google Scholar]
- 3. Ahn HK, Jung M, Ha SY et al Clinical significance of Ki‐67 and p53 expression in curatively resected non‐small cell lung cancer. Tumour Biol 2014; 35: 5735–40. [DOI] [PubMed] [Google Scholar]
- 4. Wen S, Zhou W, Li CM et al Ki‐67 as a prognostic marker in early‐stage non‐small cell lung cancer in Asian patients: A meta‐analysis of published studies involving 32 studies. BMC Cancer 2015; 15: 520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ishibashi N, Maebayashi T, Aizawa T, Sakaguchi M, Nishimaki H, Masuda S. Correlation between the Ki‐67 proliferation index and response to radiation therapy in small cell lung cancer. Radiat Oncol 2017; 12: 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lambin P, Rios‐Velazquez E, Leijenaar R et al Radiomics: Extracting more information from medical images using advanced feature analysis. Eur J Cancer 2012; 48: 441–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gillies RJ, Kinahan PE, Hricak H. Radiomics: Images are more than pictures, they are data. Radiology 2016; 278: 563–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Avanzo M, Stancanello J, El Naqa I. Beyond imaging: The promise of radiomics. Phys Med 2017; 38: 122–39. [DOI] [PubMed] [Google Scholar]
- 9. Yip SS, Aerts HJ. Applications and limitations of radiomics. Phys Med Biol 2016; 61: R150–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Martin B, Paesmans M, Mascaux C et al Ki‐67 expression and patients survival in lung cancer: Systematic review of the literature with meta‐analysis. Br J Cancer 2004; 91: 2018–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Warth A, Cortis J, Soltermann A et al Tumour cell proliferation (Ki‐67) in non‐small cell lung cancer: A critical reappraisal of its prognostic role. Br J Cancer 2014; 111: 1222–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Tabata K, Tanaka T, Hayashi T et al Ki‐67 is a strong prognostic marker of non‐small cell lung cancer when tissue heterogeneity is considered. BMC Clin Pathol 2014; 14: 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lee G, Lee HY, Park H et al Radiomics and its emerging role in lung cancer research, imaging biomarkers and clinical management: State of the art. Eur J Radiol 2017; 86: 297–307. [DOI] [PubMed] [Google Scholar]
- 14. Wilson R, Devaraj A. Radiomics of pulmonary nodules and lung cancer. Transl Lung Cancer Res 2017; 6: 86–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Jakobsen JN, Sørensen JB. Clinical impact of ki‐67 labeling index in non‐small cell lung cancer. Lung Cancer 2013; 79: 1–7. [DOI] [PubMed] [Google Scholar]


