In addition to cerebral metabolism and autonomic control, cerebral blood flow (CBF) is principally regulated by the partial pressure of arterial carbon dioxide () and mean arterial pressure (MAP). The early understanding of the cerebrovascular pressure–flow relationship was ‘pressure‐passive’; this prevailing view indicates that a rise in MAP increases CBF and vice versa. Conversely, Lassen (1959) reviewed the CBF response across groups and clinical populations and suggested that CBF is stable across a relatively wide range of MAP (e.g. 60–150 mmHg), termed static cerebral autoregulation (CA); however, when considered within the same participants, the CBF vs. MAP relationship likely occurs over a much narrower range. Additionally, it has been shown that there is an interaction between CA and the sensitivity of CBF to changes in . For example, a reduction in MAP (i.e. hypotension) effectively attenuates the cerebrovascular responsiveness to changes in (Harper & Glass, 1965). The understanding of cerebrovascular regulation has been extended over the last 20 years with sophisticated models of MAP regulation in both animal and human studies. Together with various models of hypotension (e.g. pharmacological interventions, head‐up tilt, lower‐body negative pressure) and non‐invasive measures of volumetric CBF (e.g. extra‐cranial duplex ultrasound), recent research has provided new insights into the competing influences of MAP and on the regulation of CBF.
In a recent issue of The Journal of Physiology, Olesen et al. (2018) evaluated the influence of hypotension via intravenous infusion of sodium nitroprusside (SNP) on regional CBF regulation in humans with an aimed 20% and 40% reduction in MAP, while keeping MAP ≥50 mmHg. In 19 healthy males (24 ± 4 years), regional CBF of the right internal carotid artery (ICA) and vertebral artery (VA) was assessed via duplex ultrasound together with transcranial Doppler (TCD) measures of right middle cerebral artery blood velocity (MCAv). Arterial catheterization was performed to measure arterial blood gases, metabolic substrates and cardiovascular variables. To account for the possible influence of hypocapnic cerebral vasoconstriction, Olesen et al. evaluated CO2 reactivity for ICA and VA via hyperventilation at rest and have reported CBF with ‘correction’ for changes in with hypotension (i.e. 2.8% reduction in CBF per mmHg reduction in ). The primary finding was that during SNP‐induced reduction in MAP (and ), global CBF and VA blood flow were maintained via an increase in cerebrovascular conductance. In contrast, ICA blood flow was elevated at the moderate reduction in MAP but returned to the baseline value at the more severe level of hypotension. Following ‘correction’ for hypocapnia with progressive hypotension, global CBF and ICA blood flow were elevated; however, VA blood flow was unchanged. Overall, these results indicate a differential regional CBF regulation in the anterior (ICA) and posterior (VA) cerebral circulations during progressive hypotension; together these changes mediated the maintenance of global CBF. These novel findings merit further discussion with respect to: (1) the effectiveness of CA; (2) global and regional changes in CBF regulation during experimental models of hypotension; and (3) possible clinical implications.
Cerebral autoregulation and hypotension
Contrary to previous reports of a narrow CA range, Olesen et al. reported a relatively wide CA range during progressive hypotension (see Fig. 1). To extend these findings, we calculated the CA slope between the percentage change in cerebrovascular resistance (i.e. CVR = MAP/CBF) and relative reduction in MAP using the regression coefficient from three key studies to date. Notably, whereas Olesen et al. have shown stable CA across progressive hypotension using a pharmacological intervention, Sato et al. (2012) and Lewis et al. (2015) have reported a compromise in CA when hypotension was induced via head‐up tilt with thigh cuff release and lower‐body negative pressure (LBNP), respectively. Whereas the latter experimental models of hypotension evoke marked elevations in sympathetic nervous activity, the use of SNP seems to help facilitate CA via direct cerebral vasodilatation irrespective of modest increases in sympathetic activity. In other words, while all models of hypotension should theoretically result in some cerebral vasodilatation (i.e. CA), the use of SNP seems to cause additional dilatation (i.e. further facilitation of CA).
Figure 1. Summary of the key studies that have investigated cerebral blood flow changes during systemic hypotension.
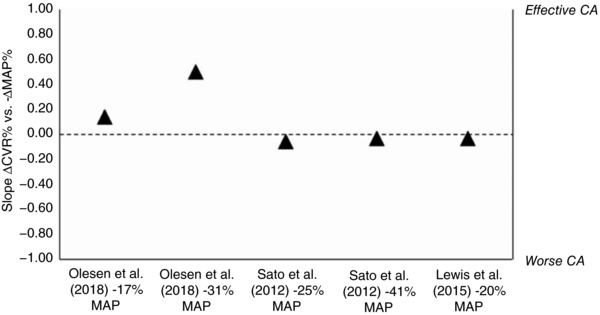
The slope of cerebral autoregulation (CA) is percentage change in cerebrovascular resistance (i.e. CVR = MAP/CBF; △CVR%) and mean arterial pressure relative to baseline values (−△MAP%) and is calculated via the regression coefficient from each of the estimated equations. These values are ‘uncorrected’ for changes in arterial CO2 tension () in poikilocapnic conditions. A CA slope of 1 would correspond to complete CA (i.e. △CVR% = △MAP% for a constant CBF), and a value of 0 corresponds to no CA (i.e. △CBF% = △MAP% without any changes in CVR). The unexpected 7% increase in global CBF with modest hypotension (i.e. −17% MAP) is notable. As MAP was further reduced by −31%, the CA slope increased; these results indicate lower CVR for a given decrease in MAP. Lastly, the results by Sato et al. (2012) and Lewis et al. (2015) indicate less effective CA during the respective reductions in MAP.
Global and regional changes in CBF regulation during experimental hypotension
Regulation of ICA and VA blood flow with alterations in is also somewhat equivocal and may be related to the different physiological stresses elicited by different models of hypotension. Lewis and colleagues (2015) reported that independent of , the reduction in global CBF with hypotension (i.e. 20% reduction in MAP) is influenced by vasoconstriction of both the ICA and VA. In contrast, in the study by Olesen et al. during poikilocapnic conditions (i.e. reduction in from 41 to 39 mmHg), the 17% SNP‐induced reduction in MAP evoked vasodilatation of both ICA and VA. Additionally, even at the highest rate of SNP infusion – provoking a 31% reduction in MAP – was only lowered to 37 mmHg in the study by Olesen et al. compared to a marked reduction in of 26 mmHg with a modest 20% decrease in MAP via LBNP by Lewis and colleagues (2015). The inconsistency between constriction vs. dilatation of the cerebral arteries is likely explained by differences in sympathetic activity and, therefore, differences in hyperventilatorily induced reductions in evoked by LBNP and SNP.
Consistent with the Olesen et al. study, Sato and colleagues (2012) reported a preserved VA blood flow response to orthostatic stress (e.g. induced via 60° head‐up tilt), indicating CBF regulation favouring the vertebro‐basilar cerebral circulation supplying important cardiac, vasomotor and respiratory control centres. In contrast, Lewis et al. (2015) reported that VA and not ICA blood flow was sensitive to changes in hypotension and related hypocapnia. The relative reduction in regional VA blood flow by Lewis et al. (2015) was significantly correlated with the respective level of hypocapnia (i.e. 2.3% reduction in VA blood flow per mmHg reduction in ); these data indicate that VA reactivity to CO2 is present during LBNP‐induced hypotension. As recognized by Olesen et al. a future perspective will be to investigate the effect of SNP on CBF when is maintained. Alternatively, the influence of systemic hypotension with and without SNP on CO2 reactivity can also be explored.
Clinical relevance
Notably, SNP is an endothelium‐independent relaxation agent that lowers MAP via systemic vasodilatation; however, SNP may also reduce CBF. This SNP‐induced decrease in CBF may be related to arterial hypotension as well as an elevation in intra‐cranial pressure (ICP), thereby reducing both MAP and cerebral perfusion pressure (CPP), respectively (i.e. CPP = MAP – ICP). Additionally, in some locations, SNP is used clinically to induce hypotensive anaesthesia to reduce surgical blood loss, and thus it is important whether SNP reduces the lower limit of CA or not. Notably, hypotension‐induced hyperventilation and subsequent reduction in may influence regional CBF regulation. As such, the contradictory reports of SNP on CBF may be related to reductions in and thereby CBF. Additionally, irrespective of systemic hypotension, SNP may also directly influence both the extra‐ and intracranial cerebral arteries, thereby possibly contributing to and facilitating CA via additional cerebral vasodilatation. A future consideration for the current results from Olesen et al. would be to explore the effects of SNP on CBF regulation when MAP is maintained, perhaps with direct ICA administration of SNP utilizing carotid duplex ultrasound or magnetic resonance angiography assessment of CBF. This method would allow for cerebral vasodilatation via direct increased cerebral NO availability while controlling for the influence of systemic hypotension. Alternatively, the influence of SNP on CBF may be investigated in patients with neurological disease or traumatic brain injury who have been instrumented with a ventricular catheter for continuous ICP measurement. This assessment would further explain the effects of SNP on CBF regulation with the competing influence of a change in MAP.
Experimental considerations
Olesen et al. estimated changes in MCA diameter by assuming similar changes in blood flow for the ICA and MCA. With the observed decrease in MCAv with progressive infusion of SNP (by 14 ± 7% and 25 ± 10%, respectively), Olesen et al. estimated an increase in MCA diameter at both infusion rates (by 12 ± 7% and 18 ± 8%, respectively). Consistent with these results, Lewis et al. (2015) reported that independent of hypocapnia, the reduction in ICA and VA blood flow due to hypotension was consistently larger than the decrease in MCAv and PCAv, respectively; these data indicate that TCD measures of blood velocity underestimate changes in CBF during hypotension. Additionally, although the attempt to post hoc correct for reductions holds merit, an improvement in the current study design would be to utilize an approach to ‘clamp’ directly independent of ventilation. Lastly, while pharmacologically induced hypotension elicits ‘static CA’ reported by Olesen et al., the studies by Lewis et al. (2015) and Sato et al. (2012) reflect ‘dynamic CA’, thereby further adding to the experimental difficulties and interpretation of MAP control. Overall, Olesen et al. have offered valuable insights on cerebrovascular regulation during pharmacologically induced hypotension and have provided direction for many future follow‐up studies.
Additional information
Competing interests
None declared.
Author contributions
Sole author
Funding
Hannah G. Caldwell was funded by a NSERC CGS‐Master’s Scholarship.
Acknowledgements
Dr Philip N. Ainslie, University of British Columbia Okanagan, is acknowledged for helpful discussion and insightful feedback on this article.
Edited by: Ole Petersen & Laura Bennet
Linked articles This Journal Club article highlights an article by Olesen et al. To read this article, visit http://doi.org/10.1113/JP275887
References
- Harper AM & Glass HI (1965). Effect of alterations in the arterial carbon dioxide tension on the blood flow through the cerebral cortex at normal and low arterial blood pressures. J Neurol Neurosurg Psychiatry 28, 449–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lassen NA (1959). Cerebral blood flow and oxygen consumption in man. Physiol Rev 39, 183–238. [DOI] [PubMed] [Google Scholar]
- Lewis NCS, Smith KJ, Bain AR, Wildfong KW, Numan T & Ainslie PN (2015). Impact of transient hypotension on regional cerebral blood flow in humans. Clin Sci (Lond) 129, 169–178. [DOI] [PubMed] [Google Scholar]
- Olesen ND, Fischer M & Secher NH (2018). Sodium nitroprusside dilates cerebral vessels and enhances internal carotid artery flow in young men. J Physiol 596, 3967–3976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato K, Fisher JP, Seifert T, Overgaard M, Secher NH & Ogoh S (2012). Blood flow in internal carotid and vertebral arteries during orthostatic stress. Exp Physiol 97, 1272–1280. [DOI] [PubMed] [Google Scholar]


