Abstract
Background
Immune checkpoint inhibitors (ICIs) have revolutionized the clinical treatment of multiple cancers. Recent studies revealed the potential prognostic value of the neutrophil to lymphocyte ratio (NLR) and platelet to lymphocyte ratio (PLR) in patients receiving ICIs; however, the results were inconsistent. We conducted a meta‐analysis to identify the prognostic significance of baseline NLR and PLR in cancer patients treated with ICIs.
Methods
We conducted a thorough literature search of PubMed, Embase, and Cochrane databases for studies dealing with the prognostic impact of pretreatment NLR and/or PLR levels in cancer patients treated with ICIs. The clinical outcomes were progression‐free survival (PFS) and overall survival (OS). Hazard ratios (HRs) and 95% confidence intervals (CIs) were calculated, and sensitivity and subgroup analyses were performed to investigate heterogeneity.
Results
Seventeen articles involving 2092 patients were included in the final analysis. The pooled HRs of PFS and OS for NLR were 1.81(95% CI 1.36–2.41) and 2.26 (95% CI 1.68–3.03), respectively, suggesting that patients with higher baseline NLRs had significantly poorer PFS and OS. Similar results were detected in sensitivity and subgroup analyses. However, no significant relevance was found between PLR and clinical endpoints in patients treated with ICIs (HR = 1.14, 95% CI 0.88–1.48 for PFS; HR = 1.35, 95% CI 0.86–2.12 for OS).
Conclusion
These results indicate that high pretreatment NLR but not PLR level, as a routinely obtained hematological parameter, is a potential prognostic predictor for poor PFS and OS in cancer patients receiving ICIs.
Keywords: Cancer, immune checkpoint inhibitor, marker, neutrophil to lymphocyte ratio, platelet to lymphocyte ratio
Introduction
Cancer is the leading cause of death in many countries and the incidence rate is assumed to consistently increase across the world over the next decades.1 Although forecasted demographic estimations indicate that the number of cancer patients will increase, targeted intervention and increasing curative treatment options may help to control incidence.2
Immune checkpoint inhibitors (ICIs), mainly targeting CTLA‐4, PD‐1, and its associated ligand PD‐L1, have led to a new era of treatment for a wide range of cancers.3 To date, six drugs (anti‐CTLA‐4 antibodies ipilimumab; anti‐PD‐1 antibodies nivolumab and pembrolizumab; and anti‐PD‐L1 antibodies atezolizumab, avelumab, and durvalumab) have been approved for the treatment of multiple malignancies, including melanoma, non‐small cell lung cancer (NSCLC), metastatic renal cell carcinoma, and urothelial cancer.4 ICIs have improved clinical outcomes compared to standard chemotherapy in various cancers; however, only a proportion of patients can benefit and a small number of patients that achieve a clinical benefit suffer a period of progression (pseudoprogression).5 Thus, the identification of potential biomarkers to discern patients best suited to immune therapy and to predict clinical response is urgently required.
A great deal of research has focused on determining the potential predictive biomarkers of ICI treatment, such as PD‐L1 expression and tumor mutational load; however, these are invasive or expensive methods.6 Several studies have explored the predictive value of existing peripheral blood markers, such as peripheral blood cell count, which may be promising predictors of response to ICI therapy.7 Hematological markers are convenient to obtain and readily accessible in clinical practice, and thus would assist to make clinical decisions.
Among these hematological markers, the neutrophil to lymphocyte ratio (NLR) and platelet to lymphocyte ratio (PLR) can reflect inflammation and host immune reaction. Numerous studies have elucidated the mutual influence of host immune activity and cancer progression, especially in patients who have undergone ICI treatment.8, 9 A higher NLR level indicates elevated protumor inflammation and weaker anti‐tumor immune ability.10 Several meta‐analyses have reported the prognostic value of NLR and PLR in various cancers, but have not taken the therapy regimen into consideration. Recently, several studies have demonstrated the prognostic significance of NLR and/or PLR in patients treated with ICI;11, 12, 13 however, the results of these studies were inconsistent, thus the prognostic significance of NLR and PLR in ICI treatment remains uncertain. Therefore, we searched relevant studies and performed this meta‐analysis to obtain a more reliable result assessing the value of NLR and PLR in predicting response to ICI treatment in multiple cancer patients.
Methods
Literature search
This meta‐analysis was carried out according to the instructions of the Preferred Reporting Items for Systematic Review and Meta‐Analyses (PRISMA). A systematic electronic search of PubMed, Embase, and Cochrane databases was conducted in November 2017, and re‐run in June 2018. The search terms were: “immune checkpoint blockade,” or “immune checkpoint inhibitor,” or “anti‐PD‐1 antibody,” or “anti‐PD‐L1 antibody,” or “anti‐CTLA‐4 antibody,” or “pembrolizumab,” or “nivolumab,” or “atezolizumab,” or “durvalumab,” or “avelumab,” or “ipilimumab,” or “tremelimumab,” or “cancer,” or “tumor,” or “melanoma,” or “malignancy,” or “sarcoma,” or “neoplasms,” or “neutrophil‐to‐lymphocyte ratio,” or “NLR,” or “platelet‐to‐lymphocyte ratio,” or “PLR.” The titles and abstracts were scanned carefully to exclude irrelevant articles. The remaining studies were comprehensively reviewed by reading the full text.
Selection criteria
This meta‐analysis was limited to studies dealing with the prognostic implications of pretreatment NLR and PLR levels. The NLR was defined as the absolute neutrophil count divided by the absolute lymphocyte count, and the PLR as the ratio of absolute platelet count to absolute lymphocyte count. Studies were eligible if they met the following conditions: (i) all patients were histologically diagnosed with cancer and treated with ICIs; (ii) the predictive or prognostic value of NLR or PLR was evaluated; (iii) NLR or PLR was assessed before initiating ICI treatment; (iv) hazard ratios (HRs) and corresponding 95% confidence intervals (CIs) comparing patients with high and low level NLR or PLR were described or statistically extractable from the data; and (v) when several studies reported the same patient population, the newest or most informative study was included. The exclusion criteria were: (i) review articles, editorial comments, letters, expert opinions, conference abstracts, or case reports; (ii) the full text was unavailable; (iii) non‐human studies; and (iv) non‐English publications, with translation unavailable.
Data extraction
Two investigators independently performed data extraction and disagreements were resolved by discussion. The following information was extracted from each study: first author's name, publication year, country of origin, number of patients, therapeutic regimen, cancer type, and clinical factors. HRs and 95% CIs were used to combine these data, they were obtained directly from 13 articles when described in text or tables,14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27 or calculated from available numerical data using methods reported by Tierney et al.,28, 29, 30or by emailing the authors.31 Multivariate data were preferable to univariate data if both were provided; however, univariate data were acceptable if multivariate results were not presented.
Statistical analysis
The prognostic values of NLR and PLR were evaluated using HR and 95% CIs. The pooled HRs were presented in the form of a forest plot. HR > 1 implied poor survival, with a higher pretreatment NLR or PLR. Prognostic outcomes were primarily progression‐free survival (PFS) or overall survival (OS); median PFS and OS time were presented using descriptive statistics. Heterogeneity across studies was assessed by Cochran Q and I 2 tests. Statistically significant heterogeneity was considered when the Cochran Q test P value was ≤ 0.10 and/or the I 2 value was ≥ 50%; random effects models were adopted regardless of heterogeneity.
Sensitivity analysis was performed by removing one study at a time in order and examining the pooled results of the rest. In addition, subgroup analyses stratified by potential confounding factors were conducted to explore the sources of heterogeneity. Begg's and Egger's tests were performed to detect publication bias. Stata statistical software, version 12.0 (Stata Corporation, College Station, TX, USA) was used for the analyses.
Two investigators independently assessed the quality of each study using the Newcastle‐Ottawa Quality Assessment Scale (NOS): a study with a score ≥ 7 was considered high‐quality.32
Results
Study characteristics
In total, 40 studies were selected for full‐text review and 17 were eligible for the final meta‐analysis. The reasons for exclusion are shown in Figure 1. Detailed information about the studies is shown in Table 1. A total of 2092 patients were included in the final analysis, ranging from 27 to 209 cases per study. Treatment strategies included: 826 patients treated with ipilimumab, 1190 with anti‐PD‐1 antibodies, and 76 with anti‐PD‐L1 antibodies. Among the 17 studies, the histological types were: 7 NSCLC, 7 melanoma, and 3 metastatic renal cell carcinoma or other cancers. The study by Ferrucci et al. included a training group and three validation groups (henceforth referred to as Ferrucci tr and Ferrucci va1–3).19
Figure 1.
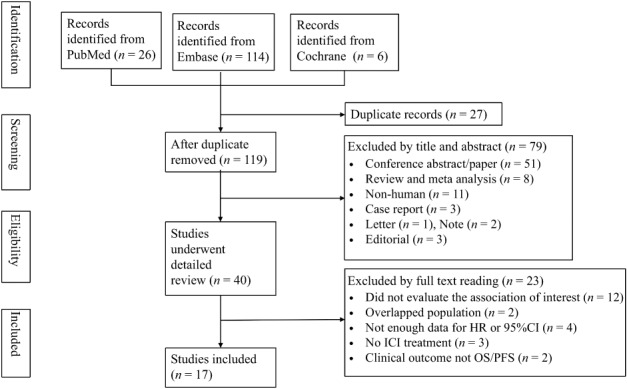
Flow diagram of study selection. CI, confidence interval; HR, hazard ratio; ICI, immune checkpoint inhibitor; OS, overall survival; PFS, progression‐free survival.
Table 1.
Characteristics of the included studies
| Author | Year | Country | Duration | Sample Size | Cancer type | Median age | Clinical parameters | Therapy administered | Cutoff value (NLR/PLR) |
Median follow‐up (months) |
Clinical factor |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Suh et al.31 | 2017 | Korea | 2013–2016 | 54 | NSCLC | 68 (43–80) | 1,3,4,5,6,7,8,9,10 | Nivo, Pemb | 5/169 | 26.2 (6.8–36.2) | OS, PFS |
| Bagley et al.14 | 2017 | USA | 2015–2016 | 175 | NSCLC | 68 (33–88) | 1,2,3,5,6,7,9,10 | Nivo | 5 | NR | OS, PFS |
| Shiroyama et al.15 | 2017 | Japan | 2015–2016 | 201 | NSCLC | 68 (27–87) | 1,3,5,10 | Nivo | 4 | 12.4 | PFS |
| Diem et al.16 | 2017 | Switzerland | 2015–2016 | 52 | NSCLC | 66 (46–88) | 1,3,5,7,8,9,10 | Nivo | 5/262 | 0–14 | OS, PFS |
| Sun et al.17 | 2017 | France | 2011–2014 | 167 | Multiple | 55 (20–82) | 1,3,8,10 | PD‐1, PD‐L1 | 4 | 12.9 (1.2–47.2) | OS |
| Cassidy et al.18 | 2017 | USA | 2006–2011 | 197 | Melanoma | 63 (10–91) | 1,3,4,5 | Ipi | 5 | 54.3 (5109) | OS, PFS |
| Ferrucci et al.19 | 2015 | Italy | 2010–2013 | 69(tr) | Melanoma | 62 (33–87) | 1,3,4,5,6,7,10 | Ipi | 5 | 10.6 | OS, PFS |
| 115(va1) | Melanoma | 63 | 1,3,5,10 | Ipi | 5 | 16.4 | OS | ||||
| 72(va2) | Melanoma | 62 | 1,3,5,10 | Ipi | 5 | 10.6 | OS | ||||
| 27(va3) | Melanoma | 55 (23–77) | 1,3,4,5 | Ipi | 5 | 9.6 | OS | ||||
| Jung et al.28 | 2017 | Korea | 2014–2015 | 104 | Melanoma | 58 (50–66) | 1,3,4,5,6,7,9 | Ipi | 5 | 7.1 (5.9–8.3) | OS, PFS |
| Khoja et al.20 | 2016 | Canada | 2008–2014 | 183 | Melanoma | 58 (24–89) | 1,3,4,6,9 | Ipi | 4/− | 7.5 (0.3,49.5) | OS, PFS |
| Zaragoza et al.21 | 2016 | France | 2015–2016 | 58 | Melanoma | 54.7 | 1,3,4,7 | Ipi | 4 | 31 | OS |
| Jeyakumar et al.22 | 2017 | USA | NR | 42 | mRCC | 42 (24–85) | 2,5 | Multiple | 3 | NR | OS, PFS |
| Fukui et al.23 | 2018 | Japan | 2016–2017 | 52 | NSCLC | 69 (46–83) | 1.3.4.5.6.7.9.10 | Nivo | 5 | 10.9 (5.6–16.4) | OS, PFS |
| Russo et al.24 | 2018 | Italy | NR | 28 | NSCLC | 68 (45–82) | 1.5.9.10 | Nivo | −/160 | NR | OS, PFS |
| Rosner et al.25 | 2018 | USA | NR | 209 | Melanoma | 60.5 (22–86) | 1.4.10 | Nivo, Ipi | 4.73 | 13.1 | OS, ORR |
| Fujisawa et al.30 | 2018 | Japan | NR | 90 | Melanoma | NR | NR | Nivo | 2.2 | NR | OS |
| Bilen et al.26 | 2018 | USA | 2015–2016 | 38 | mRCC | 69 (28–80) | 1.2.3. | Nivo | 5.5 | NR | OS, PFS |
| Park et al.27 | 2018 | USA | 2015–2017 | 159 | NSCLC | 68 (41–91) | 1.2.3.4.9.10 | Nivo | 5 | NR | OS, PFS |
Ipi, ipilimumab; mRCC, metastatic renal cell carcinoma; Nivo, nivolumab; NR, not reported; NSCLC, non‐small cell lung cancer; OS, overall survival; PD‐1, anti‐PD‐1 antibody; PD‐L1, anti‐PD‐L1 antibody; Pemb, pembrolizumab; PFS, progression‐free survival; tr, training group; va, validation group; 1, gender; 2, race; 3, performance status; 4, stage; 5, histological subtype; 6, adverse effect; 7, metastatic disease; 8, PD‐L1expression; 9, driver mutation; 10, previous lines of treatment.
Association between pretreatment neutrophil to lymphocyte ratio (NLR) and progression‐free survival (PFS)
The association between pretreatment NLR and PFS was analyzed in 11 studies including 1274 patients. As shown in Figure 2, patients with high pretreatment NLR levels experienced earlier progression than those with low NLR levels. The pooled HR for PFS was 1.81 (95% CI 1.36–2.41) with existing heterogeneity (I 2 = 85.1%; P < 0.001), suggesting elevated NLR was associated with an increased risk of progression.
Figure 2.
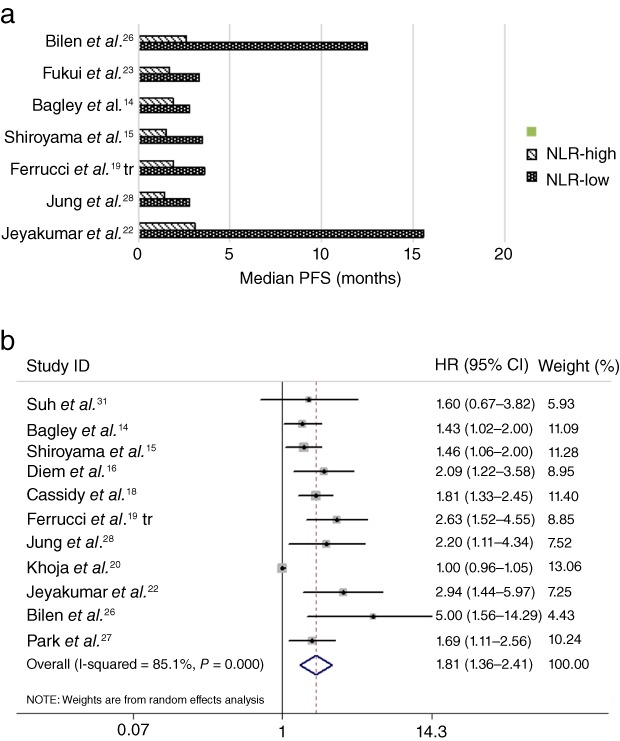
(a) Median progression‐free survival (PFS) according to pretreatment neutrophil to lymphocyte ratio (NLR). (b) Forest plot of the hazard ratio (HR) of the impact of NLR on PFS. CI, confidence interval.
Association between pretreatment NLR and overall survival (OS)
Fifteen studies reported OS in patients treated with ICIs according to high or low NLR levels. As shown in Figure 3a, patients with high pretreatment NLR levels generally experienced shorter OS than those with low NLR levels. Pooled analysis demonstrated that patients with high NLR levels had a significantly increased risk of death (HR = 2.26, 95% CI 1.68–3.03) (Fig 3b), suggesting that a high pretreatment NLR was a predictive marker of poor OS. However, high heterogeneities were present in these analyses (I2 = 86.4%; P < 0.001).
Figure 3.
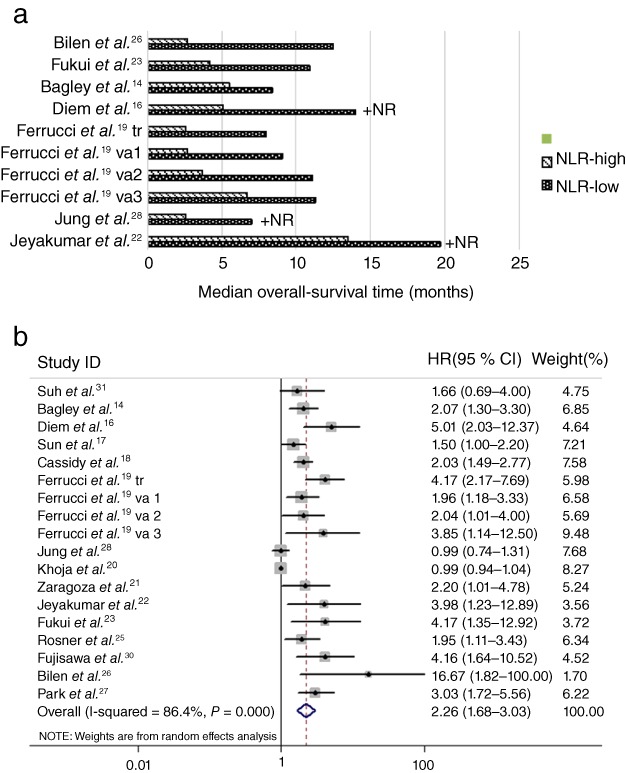
(a) Median overall survival (OS) according to neutrophil to lymphocyte ratio (NLR). (b) Forest plot of the hazard ratio (HR) of the impact of NLR on OS. +NR: not reached (OS is shown based on median follow‐up duration). CI, confidence interval.
Association of pretreatment platelet to lymphocyte ratio (PLR) with PFS and OS
Four studies analyzed an association between pretreatment PLR and PFS and OS. As shown in Figure 4, the combined HR for PFS was 1.14 (95% CI 0.88–1.48), suggesting that there were no significant PFS differences between patients grouped according to pretreatment PLR level. Moreover, there was no significant difference in OS between patients with different PLR levels (HR = 1.35, 95% CI 0.86–2.12). Thus, pretreatment PLR may not be a prognostic factor for clinical outcome in patients treated with ICIs.
Figure 4.
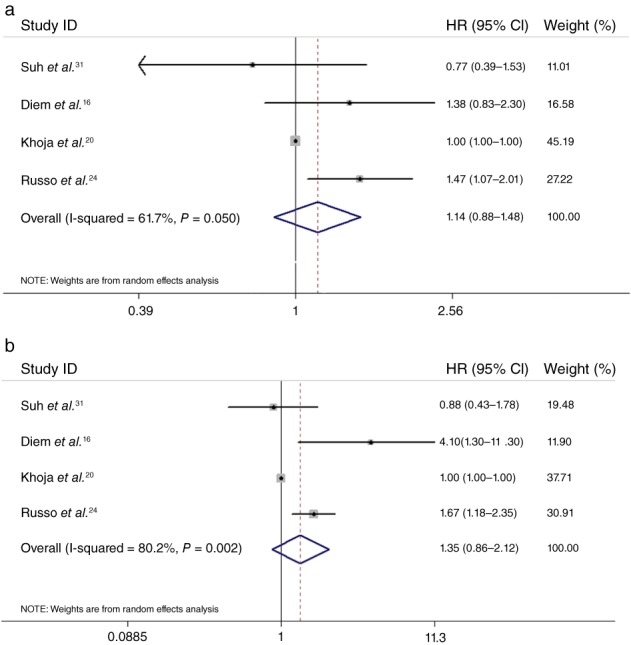
Forest plot of the hazard ratio (HR) of the impact of pretreatment platelet to lymphocyte ratio (PLR) on progression‐free survival (PFS) and overall survival (OS). The impact of PLR on (a) PFS and (b) OS. CI, confidence interval.
Sensitivity analysis
To assess the stability of the studies included, we performed sensitivity analysis by sequential omission of individual studies. As summarized in Table 2, the pooled OS and PFS results were not significantly affected by removing any of the studies, but heterogeneity reduced significantly when the study by Khoja et al. was excluded.20 This may be the source of heterogeneity. When this outlier study was removed, as expected there was no evidence of heterogeneity in the remaining studies referring to NLR and PFS (I 2 = 19.4%; P = 0.27).
Table 2.
Sensitivity analysis of NLR results of progression‐free and overall survival by random effect model
| Study omitted | Progression‐free survival | Overall survival | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| HR | LCL | UCL | I 2 | P | HR | LCL | UCL | I 2 | P | |
| Suh et al.31 | 1.82 | 1.35 | 2.46 | 86.40 | < 0.01 | 2.30 | 1.70 | 3.11 | 87.10 | < 0.01 |
| Bagley et al.14 | 1.88 | 1.36 | 2.59 | 85.90 | < 0.01 | 2.28 | 1.68 | 3.09 | 86.30 | < 0.01 |
| Shiroyama et al.15 | 1.87 | 1.36 | 2.59 | 85.70 | < 0.01 | |||||
| Diem et al.16 | 1.78 | 1.32 | 2.39 | 85.20 | < 0.01 | 2.16 | 1.61 | 2.89 | 85.90 | < 0.01 |
| Sun et al.17 | 2.35 | 1.71 | 3.22 | 86.90 | < 0.01 | |||||
| Cassidy et al.18 | 1.81 | 1.33 | 2.46 | 83.60 | < 0.01 | 2.28 | 1.68 | 3.11 | 85.20 | < 0.01 |
| Ferrucci et al.19 tr | 1.73 | 1.30 | 2.31 | 84.10 | < 0.01 | 2.15 | 1.61 | 2.87 | 85.00 | < 0.01 |
| Ferrucci et al.19 va1 | 2.28 | 1.68 | 3.10 | 86.60 | < 0.01 | |||||
| Ferrucci et al.19 va2 | 2.27 | 1.68 | 3.08 | 86.80 | < 0.01 | |||||
| Ferrucci et al.19 va3 | 2.21 | 1.64 | 2.97 | 86.70 | < 0.01 | |||||
| Jung et al.28 | 1.78 | 1.32 | 2.39 | 85.60 | < 0.01 | 2.46 | 1.76 | 3.43 | 87.10 | < 0.01 |
| Khoja et al.20 | 1.82 | 1.54 | 2.16 | 19.40 | 0.27 | 2.35 | 1.81 | 3.04 | 65.80 | < 0.01 |
| Zaragoza et al.21 | 2.26 | 1.67 | 3.06 | 86.80 | < 0.01 | |||||
| Jeyakumar et al.22 | 1.73 | 1.30 | 2.31 | 84.80 | < 0.01 | 2.21 | 1.64 | 2.97 | 86.70 | < 0.01 |
| Fukui et al.23 | 2.20 | 1.64 | 2.96 | 86.60 | < 0.01 | |||||
| Rosner et al.25 | 2.28 | 1.68 | 3.10 | 86.70 | < 0.01 | |||||
| Fujisawa et al.30 | 2.18 | 1.63 | 2.93 | 86.30 | < 0.01 | |||||
| Bilen et al.26 | 1.72 | 1.30 | 2.28 | 84.90 | < 0.01 | 2.17 | 1.63 | 2.90 | 86.40 | < 0.01 |
| Park et al.27 | 1.83 | 1.34 | 2.48 | 85.60 | < 0.01 | 2.20 | 1.64 | 2.96 | 85.80 | < 0.01 |
| Combined | 1.81 | 1.36 | 2.41 | 85.10 | < 0.01 | 1.72 | 1.27 | 2.33 | 86.40 | < 0.01 |
HR, hazard ratio; LCL, lower confidence limit; NLR, neutrophil to lymphocyte ratio; UCL, upper confidence limit.
Subgroup analysis
To explore the possible sources of heterogeneity, we conducted further subgroup analysis taking cutoff value, sample size, therapy, cancer type, analysis method, follow‐up duration, and median age into consideration. The results indicated that the predictive value for OS or PFS remained unchanged by most confounders; the results for the subgroups based on cancer type and treatment regimen suggest the prognostic potential of NLR for all kinds of cancer patients receiving ICI treatment (Table 3). Interestingly, in studies with median follow‐up durations of > 12 months or median age > 60 years, NLR was no longer associated with PFS. Heterogeneity no longer existed in small sample size, PD‐1/PD‐L1, and NSCLC groups in regard to PFS.
Table 3.
Results of subgroup analysis
| NLR & OS | NLR & PFS | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Factor | No. | HR | P | Heterogeneity | No. | HR | P | Heterogeneity | |||
| I 2 | P | I 2 | P | ||||||||
| Cut‐off | < 5 | 6 | 2.26 (1.68,3.03) | 0.008 | 81.90% | < 0.001 | 7 | 1.84 (1.50–2.26) | 0.042 | 18.80% | 0.286 |
| ≥ 5 | 12 | 2.44 (1.74,3.43) | < 0.001 | 73.10% | < 0.001 | 4 | 1.58 (1.02–2.46) | < 0.001 | 85.70% | 0.001 | |
| Sample size | < 100 | 12 | 2.94 (1.79,4.83) | < 0.001 | 76.30% | < 0.001 | 6 | 2.42 (1.83–3.19) | < 0.001 | 0% | 0.661 |
| ≥ 100 | 7 | 1.79 (1.22,2.63) | 0.003 | 89.40% | < 0.001 | 5 | 1.42 (1.05–1.91) | 0.023 | 85.90% | < 0.001 | |
| Therapy | PD‐1/PD‐L1 | 9 | 2.76 (1.91–3.98) | < 0.001 | 50.50% | 0.004 | 7 | 1.75 (1.40–2.19) | < 0.001 | 28.40% | 0.212 |
| CTLA‐4 | 8 | 1.82 (1.25,2.64) | 0.002 | 87.90% | < 0.001 | 4 | 1.71 (1.02–2.86) | 0.041 | 91.20% | < 0.001 | |
| Cancer type | NSCLC | 4 | 2.62 (1.62–4.25) | < 0.001 | 33.60% | 0.211 | 4 | 1.53 (1.25–1.89) | < 0.001 | 0% | 0.674 |
| Melanoma | 11 | 2.04 (1.45,2.89) | < 0.001 | 88.10% | < 0.001 | 5 | 1.70 (1.10–2.62) | 0.017 | 88.90% | < 0.001 | |
| Others | 3 | 3.43 (1.02–11.54) | 0.047 | 72.70% | 0.026 | 2 | 3.43 (1.89–6.25) | < 0.001 | 85.10% | < 0.001 | |
| Analysis | Uni | 5 | 2.36 (1.51,3.69) | < 0.001 | 48.60% | 0.100 | 5 | 1.71 (1.39–2.12) | < 0.001 | 0.00% | 0.442 |
| Multi | 13 | 2.19 (1.56,3.07) | < 0.001 | 87.40% | < 0.001 | 6 | 1.80 (1.20–2.71) | 0.005 | 88.20% | < 0.001 | |
| Follow‐up (month) | < 12 | 7 | 2.5 (1.34,3.44) | 0.002 | 87.20% | < 0.001 | 3 | 1.71 (1.27–2.33) | 0.149 | 88.20% | < 0.001 |
| ≥ 12 | 6 | 1.86 (1.53,2.59) | < 0.001 | 0.00% | 0.881 | 4 | 1.69 (1.38–2.06) | < 0.001 | 0% | 0.654 | |
| NA | 5 | 3.07 (1.99,4.73) | < 0.001 | 30.70% | 0.217 | 4 | 2.00 (1.32–3.04) | 0.001 | 56.50% | 0.075 | |
| Median age (year) | < 60 | 6 | 1.38 (1.01,1.90) | 0.045 | 72.70% | 0.003 | 4 | 1.81 (0.99–3.33) | 0.055 | 85.60% | < 0.001 |
| ≥ 60 | 10 | 2.59 (1.99,3.37) | < 0.001 | 29.80% | 0.171 | 6 | 1.74 (1.35–2.23) | < 0.001 | 36.80% | 0.161 | |
NA, not available; NLR, neutrophil to lymphocyte ratio; NSCLC, non‐small cell lung cancer; OS, overall survival; PD‐1, anti‐PD‐1 antibody; PD‐L1, anti‐PD‐L1 antibody; PFS, progression‐free survival.
Quality assessment and publication bias analysis
The mean quality score was 7.1 (range: 6–8) (Table 4). A funnel plot suggested asymmetry, indicating the existence of publication bias (Fig S1). No evidence of publication bias was demonstrated by Begg's regression tests for PFS (P = 0.533) or OS (P = 0.449), while the Egger's tests show significant bias for PFS (P = 0.015 slope, P < 0.001 bias) and OS (P = 0.018 slope, P < 0.001 bias).
Table 4.
Assessment of study quality by Newcastle‐Ottawa scale
| Study | Selection | Comparability | OUTCOME assessment | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | Score | |
| Suh et al.31 | x | x | x | x | x | — | x | x | x | 8 |
| Bagley et al.14 | x | x | x | x | — | — | x | x | x | 7 |
| Shiroyama et al.15 | x | x | x | x | — | — | x | x | x | 7 |
| Diem et al.16 | x | x | x | x | — | — | x | — | x | 6 |
| Sun et al.17 | x | x | x | x | x | — | x | x | x | 8 |
| Cassidy et al.18 | x | x | x | x | — | — | x | x | x | 7 |
| Ferrucci et al.19 | x | x | x | x | — | — | x | x | x | 7 |
| Jung et al.28 | x | x | x | x | — | — | x | — | x | 6 |
| Khoja et al.20 | x | x | x | x | — | — | x | x | x | 7 |
| Zaragoza et al.21 | x | x | x | x | — | — | x | x | x | 7 |
| Jeyakumar et al.22 | x | x | x | x | x | x | x | x | 8 | |
| Fukui et al.23 | x | x | x | x | — | — | x | x | x | 7 |
| Russo et al.24 | x | x | x | x | — | — | x | x | x | 7 |
| Rosner et al.25 | x | x | x | x | — | — | x | x | x | 7 |
| Fujisawa et al.30 | x | x | x | x | — | — | x | x | x | 7 |
| Bilen et al.26 | x | x | x | x | — | x | x | x | x | 8 |
| Park et al.27 | x | x | x | x | — | — | x | x | x | 7 |
x: For cohort studies: 1 truly representative of the exposed cohort; 2, non‐exposed cohort drawn from the same community; 3, ascertainment of exposure; 4, outcome of interest not present at start; 5, cohorts comparable on basis of PD‐L1 expression; 6, cohorts comparable on other factor(s); 7, quality of outcome assessment; 8, follow‐up long enough for outcomes to occur; and 9, complete accounting for cohorts.
Discussion
There is sufficient evidence of the association between inflammation and immune response with prognosis in patients with diverse histological types of neoplasia. Outcomes after ICI treatment, which reactivates the immune system and eradicates tumors, are closely connected to immune status.33 Previous studies revealed the prognostic potential of several blood and clinical markers, including NLR and PLR, which are routinely available in daily practice. Our meta‐analysis of 17 studies comprising 2092 patients demonstrated that a high pretreatment NLR level is correlated with a 1.81‐fold increased risk of progression (HR = 1.81, 95% CI 1.36–2.41) and a 2.26‐fold increased risk of death (HR = 2.26, 95% CI 1.68–3.03). However, no significant differences in PFS (HR = 1.14, 95% CI 0.88–1.48) or OS (HR = 1.35, 95% CI 0.86–2.12) were identified when patients were dichotomized according to pretreatment PLR levels. No significant association between PLR and PFS or OS was found, which may have been a result of the small number of studies included. Our conclusions require confirmation by further studies.
In‐deep investigation targeting immune regulatory mechanisms within the tumor microenvironment has yielded a deeper understanding of ICI therapeutic activity. Because T cells undertake the role of immunosurveillance, tumor‐infiltrating lymphocytes (Tils) are reported to occupy an important position in the tumor microenvironment of patients treated with ICIs.34 Several factors mediating T cell proliferation, viability, and spatial distribution are also related to the response to ICI therapy: silencing genes encoding chemokines can lead T cell trafficking to the tumor35 while age‐related immunity decline (immunosenescence) also influences the response to ICI therapy.36 Immunity function plays a vital role in ICI treatment, thus any element affecting immunity can impact the response to ICI treatment. On the other hand, inflammatory factor interleukin‐6 (IL‐6) has been identified as promoting ICI resistance,37, 38 and tumor cell cyclooxygenase (COX) can produce prostaglandin (E2), which suppresses immunity and builds an inflammatory environment favoring tumor growth.39 Based on the results of previous studies, we propose that the prognostic value of NLR in cancer patients administered ICI treatment is associated with different functions of lymphocytes and neutrophils. Recruited neutrophils could stimulate the secretion of many inflammatory cytokines, such as IL‐1, IL‐6, and tumor necrosis factor (TNF), and fuels a favorable environment for tumor development and progression.40 In contrast, lymphocytes are considered immune cells and exert anti‐tumor effects. An increased NLR implies an elevated neutrophil count and/or a reduced lymphocyte count; therefore, a higher NLR level reflects an advantage of anti‐tumor over protumor activity, which implies an unfavorable prognostic factor for patients treated with ICIs. Because NLR is a systemic inflammation indicator that can predict the efficacy of ICI treatment, will the combination of immunomodulating drugs and ICIs deliver a better outcome? No prospective study has investigated the interaction efficacy of immunomodulating drugs with ICIs,41 nonetheless, retrospective results show that patients who received immunomodulators for adverse events did not achieve better efficacy than those who had not.42, 43 More details of the influence of immunomodulating drugs (types, timing, duration) on the clinical outcome of ICIs are needed.
Several meta‐analyses have demonstrated the predictive value of NLR or PLR in multiple cancers, including hepatocellular carcinoma, nasopharyngeal carcinoma and ovarian cancer;11, 12, 13 however, treatment has not been consolidated. In recent years, the development of ICIs has changed the landscape of cancer treatment; therefore it is critical to identify biomarkers that predict a response to ICIs. Although a meta‐analysis investigating the prognostic role of NLR in patients receiving ICI treatment has previously been conducted,44 there are several advantages of our study. First, we included 17 full articles (2092 patients) compared to the 7 studies (4 of which were abstracts) included in the previous meta‐analysis. We included a greater number of studies and data to ensure the validity and reliability of our research. Second, we evaluated not only HR as a parameter of the prognostic value of NLR, but also median PFS and OS, which are consistent with HR results. Third, we conducted sensitivity and subgroup analyses of cutoff values, sample size, therapy, cancer type, analysis methods, follow‐up duration, and median age. The significance of our results remained, increasing their reliability. Finally, to the best of our knowledge, this is the first meta‐analysis to evaluate the prognostic value of both pretreatment peripheral blood NLR and PLR in patients treated with ICIs.
Despite these advantages, there are several limitations to our study. First, almost all eligible studies were retrospective observational studies and only one prospective study was included, which may cause bias in the final analysis. Second, heterogeneity was observed in this study, which may partially be attributed to different study designs. For example, the study by Khoja et al. comprised only cutaneous melanoma patients, which is quite different from other studies. As various histological cancers show diverse responses to ICIs, consolidation of cancer histological types may result in heterogeneity.20 Heterogeneity may also derive from other variations, such as the treatment administered after ICI failure, complications, and because the peripheral blood cell count can easily be influenced by inflammatory diseases. It is noteworthy that heterogeneity in our study decreased in small sample size, PD‐1/PD‐L1 treatment, and NSCLC subgroups, demonstrating varying degrees of the prognostic value of NLR for different cancer patients treated with various ICI regimens. Third, publication bias was detected in this study and we cannot ignore the fact that positive results are preferentially published. Fourth, we only analyzed the relationship between pretreatment NLR or PLR level with clinical outcomes. Studies by Suh et al.31 and Di Giacomo et al.45 indicated no significant association between pretreatment NLR level but an association with post‐treatment NRL level, while Cassidy et al.18 evaluated the changes in the NLR after ICI treatment. The comprehensive meaning of different periods of these biomarkers and their serial changes requires further investigation.
In summary, our meta‐analysis demonstrates that high pretreatment peripheral blood NLR might be a predictor of poor PFS/OS in cancer patients treated with ICIs; however, no significant correlation between PLR and PFS/OS was observed. Baseline NLR could serve as a promising prognostic predictor for ICI treatment, as it is convenient to obtain from a routine blood test.
Disclosure
No authors report any conflict of interest.
Supporting information
Figure S1 Funnel plots presenting meta‐analyses of neutrophil to lymphocyte ratio (NLR) in progression‐free survival (PFS) and overall survival (OS). Funnel plot of (a) 11 studies reporting PFS and (b) 14 studies reporting OS.
Acknowledgments
This work was supported by grants from the Chinese National Major Project for New Drug Innovation (2017ZX09304015).
References
- 1. Torre LA, Bray F, Siegel RL, Ferlay J, Lortet‐Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin 2015; 65: 87–108. [DOI] [PubMed] [Google Scholar]
- 2. Bray F, Jemal A, Grey N, Ferlay J, Forman D. Global cancer transitions according to the Human Development Index (2008–2030): A population‐based study. Lancet Oncol 2012; 13: 790–801. [DOI] [PubMed] [Google Scholar]
- 3. Gong J, Chehrazi‐Raffle A, Reddi S, Salgia R. Development of PD‐1 and PD‐L1 inhibitors as a form of cancer immunotherapy: A comprehensive review of registration trials and future considerations. J Immunother Cancer 2018; 6: 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Yoest JM. Clinical features, predictive correlates, and pathophysiology of immune‐related adverse events in immune checkpoint inhibitor treatments in cancer: A short review. Immunotargets Ther 2017; 6: 73–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sharma P, Hu‐Lieskovan S, Wargo JA, Ribas A. Primary, adaptive, and acquired resistance to cancer immunotherapy. Cell 2017; 168: 707–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Thallinger C, Füreder T, Preusser M et al Review of cancer treatment with immune checkpoint inhibitors: Current concepts, expectations, limitations and pitfalls. Wien Klin Wochenschr 2018; 130: 85–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hopkins AM, Rowland A, Kichenadasse G et al Predicting response and toxicity to immune checkpoint inhibitors using routinely available blood and clinical markers. Br J Cancer 2017; 117: 913–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wang D, DuBois R. The role of prostaglandin E2 in tumor‐associated immunosuppression. Trends Mol Med 2016; 22: 1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Klevorn LE, Teague RM. Adapting cancer immunotherapy models for the real world. Trends Immunol 2016; 37: 354–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Moses K, Brandau S. Human neutrophils: Their role in cancer and relation to myeloid‐derived suppressor cells. Semin Immunol 2016; 28: 187–96. [DOI] [PubMed] [Google Scholar]
- 11. Huang QT, Zhou L, Zeng WJ et al Prognostic significance of neutrophil‐to‐lymphocyte ratio in ovarian cancer: A systematic review and meta‐analysis of observational studies. Cell Physiol Biochem 2017; 41: 2411–8. [DOI] [PubMed] [Google Scholar]
- 12. Yin J, Qin Y, Luo YK, Feng M, Lang JY. Prognostic value of neutrophil‐to‐lymphocyte ratio for nasopharyngeal carcinoma: A meta‐analysis. Medicine 2017; 96 (29): e7577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Liu X, Qu JK, Zhang J et al Prognostic role of pretreatment neutrophil to lymphocyte ratio in breast cancer patients: A meta‐analysis. Medicine 2017; 96: e8101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bagley SJ, Kothari S, Aggarwal C et al Pretreatment neutrophil‐to‐lymphocyte ratio as a marker of outcomes in nivolumab‐treated patients with advanced non‐small‐cell lung cancer. Lung Cancer 2017; 106: 1–7. [DOI] [PubMed] [Google Scholar]
- 15. Shiroyama T, Suzuki H, Tamiya M et al Pretreatment advanced lung cancer inflammation index (ALI) for predicting early progression in nivolumab‐treated patients with advanced non‐small cell lung cancer. Cancer Med 2018; 7: 13–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Diem S, Schmid S, Krapf M et al Neutrophil‐to‐lymphocyte ratio (NLR) and platelet‐to‐lymphocyte ratio (PLR) as prognostic markers in patients with non‐small cell lung cancer (NSCLC) treated with nivolumab. Lung Cancer 2017; 111: 176–81. [DOI] [PubMed] [Google Scholar]
- 17. Sun R, Champiat S, Dercle L et al Baseline lymphopenia should not be used as exclusion criteria in early clinical trials investigating immune checkpoint blockers (PD‐1/PD‐L1 inhibitors). Eur J Cancer 2017; 84: 202–11. [DOI] [PubMed] [Google Scholar]
- 18. Cassidy MR, Wolchok RE, Zheng J et al Neutrophil to lymphocyte ratio is associated with outcome during ipilimumab treatment. EBio Medicine 2017; 18: 56–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ferrucci PF, Gandini S, Battaglia A et al Baseline neutrophil‐to‐lymphocyte ratio is associated with outcome of ipilimumab‐treated metastatic melanoma patients. Br J Cancer 2015; 112: 1904–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Khoja L, Atenafu EG, Templeton A et al The full blood count as a biomarker of outcome and toxicity in ipilimumab‐treated cutaneous metastatic melanoma. Cancer Med 2016; 5: 2792–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zaragoza J, Caille A, Beneton N et al High neutrophil to lymphocyte ratio measured before starting ipilimumab treatment is associated with reduced overall survival in patients with melanoma. Br J Dermatol 2016; 174: 146–51. [DOI] [PubMed] [Google Scholar]
- 22. Jeyakumar G, Kim S, Bumma N et al Neutrophil lymphocyte ratio and duration of prior anti‐angiogenic therapy as biomarkers in metastatic RCC receiving immune checkpoint inhibitor therapy. J Immuno Ther Cancer 2017; 5: 82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Fukui T, Okuma Y, Nakahara Y et al Activity of nivolumab and utility of neutrophil‐to‐lymphocyte ratio as a predictive biomarker for advanced non‐small‐cell lung cancer: A prospective observational study. Clin Lung Cancer 2018; S1525‐7304 (18): 30103–7. [DOI] [PubMed] [Google Scholar]
- 24. Russo A, Franchina T, Ricciardi GRR et al Baseline neutrophilia, derived neutrophil‐to‐lymphocyte ratio (dNLR), platelet‐to‐lymphocyte ratio (PLR), and outcome in non small cell lung cancer (NSCLC) treated with nivolumab or docetaxel. J Cell Physiol 2018; 233: 6337–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Rosner S, Kwong E, Shoushtari AN et al Peripheral blood clinical laboratory variables associated with outcomes following combination nivolumab and ipilimumab immunotherapy in melanoma. Cancer Med 2018; 7: 690–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bilen MA, Dutcher GMA, Liu Y et al Association between pretreatment neutrophil‐to‐lymphocyte ratio and outcome of patients with metastatic renal‐cell carcinoma treated with nivolumab. Clin Genitourin Cancer 2018; 16: e563–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Park W, Kwon D, Saravia D et al Developing a predictive model for clinical outcomes of advanced non‐small cell lung cancer patients treated with nivolumab. Clin Lung Cancer 2018; 19: 280–8.e4. [DOI] [PubMed] [Google Scholar]
- 28. Jung M, Lee J, Kim TM et al Ipilimumab real‐world efficacy and safety in Korean melanoma patients from the Korean named‐patient program cohort. Cancer Res Treat 2017; 49: 44–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical methods for incorporating summary time‐to‐event data into meta‐analysis. Trials 2007; 8 (8): 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Fujisawa Y, Yoshino K, Otsuka A et al Baseline neutrophil to lymphocyte ratio combined with serum lactate dehydrogenase level associated with outcome of nivolumab immunotherapy in a Japanese advanced melanoma population. Br J Dermatol 2018; 179: 213–5. [DOI] [PubMed] [Google Scholar]
- 31. Suh KJ, Kim SH, Kim YJ et al Post‐treatment neutrophil‐to‐lymphocyte ratio at week 6 is prognostic in patients with advanced non‐small cell lung cancers treated with anti‐PD‐1 antibody. Cancer Immunol Immunother 2018; 67: 459–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wells GA, Shea B, O'Connell J et al The Newcastle‐Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta‐analyses 2014. [Cited 3 July 2018.] Available from URL: http://www.ohri.ca/programs/oxford.asp/oxford.asp
- 33. Pitt JM, Vétizou M, Daillère R et al Resistance mechanisms to immune‐checkpoint blockade in cancer: Tumor‐intrinsic and ‐extrinsic factors. Immunity 2016; 44: 1255–69. [DOI] [PubMed] [Google Scholar]
- 34. Wei SC, Levine JH, Cogdill AP et al Distinct cellular mechanisms underlie anti‐CTLA‐4 and anti‐PD‐1 checkpoint blockade. Cell 2017; 170: 1120–33.e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Schumacher TN, Schreiber RD. Neoantigens in cancer immunotherapy. Science 2015; 348: 69–74. [DOI] [PubMed] [Google Scholar]
- 36. Nishijima TF, Muss HB, Shachar SS, Moschos SJ. Comparison of efficacy of immune checkpoint inhibitors (ICIs) between younger and older patients: A systematic review and meta‐analysis. Cancer Treat Rev 2016; 45: 30–7. [DOI] [PubMed] [Google Scholar]
- 37. Liu H, Shen J, Lu K. IL‐6 and PD‐L1 blockade combination inhibits hepatocellular carcinoma cancer development in mouse model. Biochem Biophys Res Commun 2017; 486: 239–44. [DOI] [PubMed] [Google Scholar]
- 38. Mace TA, Shakya R, Pitarresi JR et al IL‐6 and PD‐L1 antibody blockade combination therapy reduces tumour progression in murine models of pancreatic cancer. Gut 2018; 67: 320–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Zelenay S, van der Veen AG, Böttcher JP et al Cyclooxygenase‐dependent tumor growth through evasion of immunity. Cell 2015; 162: 1257–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell 2010; 140: 883–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Postow MA, Sidlow R, Hellmann MD. Immune‐related adverse events associated with immune checkpoint blockade. N Engl J Med 2018; 378: 158–68. [DOI] [PubMed] [Google Scholar]
- 42. Horvat TZ, Adel NG, Dang TO et al Immune‐related adverse events, need for systemic immunosuppression, and effects on survival and time to treatment failure in patients with melanoma treated with Ipilimumab at Memorial Sloan Kettering Cancer Center. J Clin Oncol 2015; 33: 3193–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Weber JS, Hodi FS, Wolchok JD et al Safety profile of nivolumab monotherapy: A pooled analysis of patients with advanced melanoma. J Clin Oncol 2017; 35: 785–92. [DOI] [PubMed] [Google Scholar]
- 44. Sacdalan DB, Lucero JA, Sacdalan DL. Prognostic utility of baseline neutrophil‐to‐lymphocyte ratio in patients receiving immune checkpoint inhibitors: A review and meta‐analysis. Onco Targets Ther 2018; 11: 955–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Di Giacomo AM, Ascierto PA, Queirolo P et al Three‐year follow‐up of advanced melanoma patients who received ipilimumab plus fotemustine in the Italian Network for Tumor Biotherapy (NIBIT)‐M1 phase II study. Ann Oncol 2015; 26: 798–803. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 Funnel plots presenting meta‐analyses of neutrophil to lymphocyte ratio (NLR) in progression‐free survival (PFS) and overall survival (OS). Funnel plot of (a) 11 studies reporting PFS and (b) 14 studies reporting OS.


