Abstract
Background
The aim of this study was to compare the safety, efficacy, and prognostic value of radiofrequency ablation (RFA) and microwave ablation (MWA) for lung tumors.
Methods
Between March 2012 and January 2018, 238 patients with lung cancer were treated with MWA (139, 58.4%) or RFA (99, 41.6%) in our center. Patient and tumor characteristics, complications, complete ablation (CA) rate, and prognosis were compared between the groups. Meta‐analysis was used to systematically compare the outcomes of RFA and MWA for the treatment of lung tumors.
Results
Ablation was successfully completed in all patients and no procedure‐related death occurred. The total complication rates in the RFA and MWA groups were 24.2% (24/99) and 16.5% (23/139), respectively, and there was no statistically significant difference (P = 0.142). The initial and total CA rates were similar at P > 0.05 (RFA vs. MWA: initial CA, 97.0% vs. 96.4%; total CA, 99.0% vs. 98.6%, respectively). During follow‐up, there was no significant difference in median progression‐free (RFA vs. MWA: 12.5 months, 95% confidence interval [CI] 5.002–19.998 vs. 9.5 months, 95% CI 6.623–12.377; P = 0.673) or overall survival (RFA vs. MWA: 33 months, 95% CI 27.070–38.930 vs. 30 months, 95% CI, 18.482–41.518; P = 0.410) between the groups. Combined with the results of published comparison studies, meta‐analysis further confirmed that the outcomes of these two treatments were similar.
Conclusion
Both RFA and MWA are safe and effective treatments with a survival benefit for selected patients with primary and metastatic lung tumors.
Keywords: Lung tumor, microwave ablation, radiofrequency ablation
Introduction
Lung cancer is one of the most common cancers worldwide, and the leading cause of cancer death in both men and women. The lung is the most common site of metastases in liver and colorectal cancers.1, 2 Currently, surgery is the main treatment option; however, many high‐risk, advanced‐stage, or older patients refuse surgery because of the high risk of complications that can occur during surgery, such as atrial fibrillation, prolonged air leaks, myocardial infarction, recurrent nerve injury, bleeding, pneumonia, and bronchial stenosis.3, 4 Currently, minimally invasive percutaneous thermal ablation therapies, such as radiofrequency ablation (RFA) and microwave ablation (MWA) have emerged as safe and effective treatment alternatives for patients for whom surgery is not suitable.5 In recent years, many studies have shown that computed tomography (CT)‐guided RFA and MWA are feasible for patients with primary lung cancer or unresectable pulmonary metastases.6, 7, 8, 9, 10 RFA and MWA are effective and safe with negligible mortality, low morbidity, involve a short hospital stay, and result in a better quality of life.
Radiofrequency ablation has been widely used in the treatment of both primary and metastatic lung cancer. Its advantage lies in the ability to locally heat a tumor to a lethal temperature while incurring minimal damage to surrounding normal lung tissue. However, RFA has higher impedance in the lung than in the liver, which means poor energy is spread because of high impedance and charring, thus it is difficult to identify whether the lesions are completely ablated.11 MWA is another heat‐based ablation technique, with theoretical advantages over RFA, including enhanced thermocoagulation of tumor cells as a result of improved energy deposition in an aerated lung and increased heating near blood vessels. It could allow for increased intratumoral temperatures with the generation of a larger ablation zone (up to 2 cm from the probe tip) in a shorter period of time compared to RFA.12 Recently, a few studies have compared RFA and MWA outcomes for the treatment of lung tumors; however, these studies were small single‐center reports.13, 14, 15, 16, 17, 18 In this study, we retrospectively evaluated the safety, efficacy, and prognostic value of CT‐guided RFA and MWA for the treatment of 238 patients with lung tumors in our center and compared our results with the outcomes of RFA and MWA in published comparison studies.
Methods
Patients
Between March 2012 and January 2018, 238 patients diagnosed with primary or metastatic lung cancer by pulmonary biopsy were treated with MWA/RFA in our center. Patients were informed in detail about the risks and benefits associated with MWA/RFA treatment and provided written informed consent of the ablation procedure. The study was approved by the ethics committee of our hospital and conformed to the ethical guidelines of the World Medical Association Declaration of Helsinki. The tolerance of interventional surgery for all patients was evaluated based on cardiopulmonary reserve and/or medical comorbidities. The inclusion criteria were: (i) primary lung cancers or pulmonary metastases with controlled original disease; (ii) peripheral tumors; (iii) ≤ 5 tumors and largest tumor size ≤ 10cm; (iv) unable to undergo thoracic surgery because of advanced age or poor cardiopulmonary function; (v) not suitable for chemotherapy or radiotherapy; (vi) normal cognition and without serious mental disease, such as schizophrenia or suicidal tendencies; (vii) no pleural effusion; and (viii) platelet count ≥ 100 × 109/L, international normalized ratio ≤ 1.5. The exclusion criteria were: (i) serious organ failure; (ii) central tumors; (iii) metastasis to neck and thoracic vertebrae, with serious vertebral injury; (iv) diffuse pulmonary metastases; and (v) severe psychological diseases.
Pre‐ablation preparations
The size, location, and number of tumors were evaluated by conventional CT scan. Routine blood tests, evaluation of prothrombin time and activated partial thromboplastin time, liver and kidney function tests, and electrocardiography were all conducted prior to ablation.
Therapeutic methods
All procedures were performed with a curative intent under conscious sedation and local anesthesia. The procedures were performed using CT guidance (GE Discovery CT750 HD, GE Healthcare, Milwaukee, WI, USA). Patients’ cardiac status and vital signs were continuously monitored. MWA was performed with a 2450 MHZ MTC‐3C microwave generator (Vision Medical, Nanjing, China), which has a 25 cm cooled‐shaft electrode probe (15‐gauge) with a 1.5 cm expandable tip. Power output was set at 40–100 watts. RFA was performed using the RITA system (MEDSPHERE S‐1500, MEDSPHERE, Shanghai, China) powered by 40 W or 100 W generators with a 17‐gauge multi‐tined expandable electrode.
During the procedure, deployment of the needle was staged based on the size of the tumor. Under CT guidance, the probes were introduced into the tumor and the path was planned to avoid vessels, bronchi, blebs, and fissures. The nearest point between the chest skin and the center of the tumor was selected as the puncture point. The electrode needles were pushed forward and unfolded gradually until they reached or crossed the borders of the tumor. Once the CT scan confirmed proper electrode positioning, electrodes were attached to the generator and ablation commenced.
Follow‐up and outcome measure
Technical success was defined as correct placement of the ablation device into all target tumors with completion of the planned ablation protocol. Complications were observed and the grade was evaluated based on the Clavien–Dindo classification. Chest radiographs were performed two hours after ablation to exclude pneumothorax. Patients were observed overnight and generally discharged from the hospital the next day if there were no complications.
Follow‐up examination of contrast‐enhanced CT was performed one month after ablation, and then every three months. Initial complete ablation (CA) was defined as complete coverage of the tumor by the ablation zone and no irregular enhancement existed at the treatment margin one month after ablation. If the tumor had not been completely ablated, an additional session of ablation was considered and the patients were reevaluated. Progression‐free survival (PFS) was defined as the duration from the initiation of ablation treatment to the recurrence of tumors. Overall survival (OS) was defined as the duration from the initiation of ablation treatment to death from any cause.
Systematic literature review
A systematic literature review was performed in PubMed Entrez using combinations of the following keywords: (lung OR pulmonary) AND (cancer OR tumor) AND ([radiofrequency ablation] OR RFA) AND ([microwave ablation] OR MWA). Studies that met the following criteria were included: (i) reported clinical outcomes of RFA and MWA; (ii) comparison studies; and (iii) with adequate information reported.
Statistical analysis
The data was processed using spss version 19.0 (IBM Corp., Armonk, NY, USA). Comparative analysis was conducted using the chi‐square test for categorical variables and independent t‐test analysis for continuous variables. Survival curves were constructed using the Kaplan–Meier method and the log‐rank (Mantel–Cox) test was used for comparison. Review Manager 5.3 (Cochrane Collaboration, Oxford, UK) was used to conduct the meta‐analysis and calculate odds ratios (OR) with 95% confidence intervals (CI), while the Mantel‐Haenszel statistical method and random effects model were used to calculate the ORs. A P value of < 0.05 was considered statistically significant.
Results
Clinical features
This study included 238 patients with primary (96, 40.3%) or metastatic (142, 59.7%) lung tumors. The RFA group consisted of 99 (41.6%) patients, while the MWA group consisted of 139 (58.4%) patients. The baseline patient characteristics are shown in Table 1.
Table 1.
Clinical characteristics of patients
| Characteristics | MWA | RFA | P |
|---|---|---|---|
| No. of patients (%) | 139 (58.4%) | 99 (41.6%) | — |
| Age, years, mean ± SD | 61 ± 13 | 61 ±12 | 0.530 |
| Gender, M/F, n (%) | 102 (73.4%)/37 (26.6%) | 76 (76.8%)/23 (23.2%) | 0.553 |
| Tumor origin, n (%) | 0.001 | ||
| Primary/metastasis | 68 (48.9%)/71 (51.1%) | 28 (28.3%)/71 (71.7%) | |
| Smoking, n (%) | 0.147 | ||
| Yes/No | 38 (27.3%)/101 (72.7%) | 19 (19.2%)/80 (80.8%) | |
| Cough, n (%) | 0.937 | ||
| Yes/No | 23 (16.5%)/116 (83.5%) | 16 (16.2%)/83 (83.8%) | |
| Tumors, n (%) | < 0.001 | ||
| Single/multiple | 107(77.0%)/32(23.0%) | 52(52.5%)/47(47.5%) | |
| Tumor size, cm, mean ± SD | 2.87 ± 1.76 | 2.41 ± 1.18 | 0.057 |
| Tumor location, n (%) | 0.161 | ||
| Risk†/non‐risk | 36(25.9%)/103(74.1%) | 18(18.2%)/81(81.8%) | |
| UICC Stage, n (%) | 0.145 | ||
| I | 40(28.8%) | 18(18.2%) | |
| II | 13(9.4%) | 7(7.1%) | |
| III | 5(3.6%) | 2(2.0%) | |
| IV | 81(58.3%) | 72(72.7%) |
Tumors in risk areas refer to those located within 5 mm of pleura, diaphragm, big vessels, bronchi, or mediastinum. MWA, microwave ablation; RFA, radiofrequency ablation; SD, standard deviation; UICC, Union for International Cancer Control.
Safety and complications
Both RFA and MWA were successfully completed in all patients with a mean hospital stay of 3.45 postoperative days (standard deviation 3.57 days). No procedure‐related death occurred within 30 days after ablation. Complications occurred in 23 (16.5%) patients in the MWA group and 24 (24.2%) in the RFA group. There was no significant difference in the total complication rates between the groups (P = 0.142). Pneumothorax was the most common side effect to occur after RFA (19.2%) and MWA (13.7%), but there was no statistically significant difference between the groups (P = 0.252). Subcutaneous emphysema developed in 8.1% of patients after RFA and 2.2% after MWA, with significantly higher occurrence in the RFA group (P = 0.032). Although comparable analysis found that the total occurrence rate of complications was not associated with tumor factors, including tumor size, number, or location, or Union for International Cancer Control stage, multiple tumors were a risk factor for hemoptysis (P = 0.030) and subcutaneous emphysema (P = 0.028) (Table 2).
Table 2.
Complications and associated factors
| Characteristics | Total Complications | Hemoptysis | Pleural effusion | Pneumothorax | Subcutaneous emphysema | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Rate | P | Rate | P | Rate | P | Rate | P | Rate | P | |
| Treatment | 0.142 | 0.386 | 0.672 | 0.252 | 0.032 | |||||
| MWA | 23 (16.5%) | 4 (2.9%) | 3 (2.2%) | 19 (13.7%) | 3 (2.2%) | |||||
| RFA | 24(24.2%) | 5 (5.1%) | 3 (3.0%) | 19 (19.2%) | 8 (8.1%) | |||||
| Tumor size | 0.450 | 0.669 | 0.419 | 0.530 | 0.267 | |||||
| ≤ 3 cm | 32 (17.9%) | 6 (3.4%) | 4 (2.2%) | 26 (14.5%) | 6 (3.4%) | |||||
| 3–5 cm | 12 (25.5%) | 2 (4.3%) | 1 (2.1%) | 10 (21.3%) | 4 (8.5%) | |||||
| > 5 cm | 3 (25.0%) | 1 (8.3%) | 1 (8.3%) | 2 (16.7%) | 1 (8.3%) | |||||
| Tumor number | 0.062 | 0.030 | 0.376 | 0.370 | 0.028 | |||||
| Single | 26(16.4%) | 3 (1.9%) | 3 (1.9%) | 23 (14.5%) | 4 (2.5%) | |||||
| Multiple | 21 (26.6%) | 6 (7.6%) | 3 (3.8%) | 15 (19.0%) | 7 (8.9%) | |||||
| Location | 0.603 | 0.398 | 0.721 | 0.315 | 0.715 | |||||
| Risk | 12 (22.2%) | 1 (1.9%) | 1 (1.9%) | 11 (20.4%) | 2 (3.7%) | |||||
| Non‐risk | 35 (19.0%) | 8 (4.3%) | 5 (2.7%) | 27 (14.7%) | 9 (4.9%) | |||||
| UICC stage | 0.878 | 0.158 | 0.841 | 0.494 | 0.410 | |||||
| I | 13 (22.4%) | 0 (0%) | 1 (1.7%) | 13(22.4%) | 1 (1.7%) | |||||
| II | 3 (15.0%) | 0 (0%) | 1 (5.0%) | 3 (15.0%) | 2 (10.0%) | |||||
| III | 1 (14.3%) | 0 (0%) | 0 (0%) | 1 (14.3%) | 0 (0%) | |||||
| IV | 30 (19.6%) | 9 (5.9%) | 4 (2.6%) | 21 (13.7%) | 8 (5.2%) | |||||
MWA, microwave ablation; RFA, radiofrequency ablation; UICC, Union for International Cancer Control.
Effectiveness
Two hundred and thirty eight patients with 375 lesions from 0.6 to 10 cm were treated with MWA or RFA. Enhanced CT taken one month post‐ablation showed enhancement in eight (3.4%) patients, including five (3.6%) in the MWA group and three (3.0%) in the RFA group. However, a second ablation was only performed in five patients. Three (1.3%) patients did not receive a second ablation because of the small size and lack of growth of the residual tumor. A total of 235 (98.7%) patients underwent complete ablation. The initial and total complete response rate was not affected by the ablation method (P > 0.05) (Table 3).
Table 3.
Clinical responses to treatment
| Responses | MWA | RFA | P |
|---|---|---|---|
| Complete ablation | |||
| Initial session | 134 (96.4%) | 96 (97.0%) | 0.811 |
| Two sessions | 137 (98.6%) | 98 (99.0%) | 0.770 |
| Median progression‐free survival | 9.5 months (95% CI 6.623–12.377) |
12.5 months (95% CI 5.002–19.998) |
0.673 |
| Median overall survival | 30 months (95% CI 18.482–41.518) |
33 months (95% CI 27.070–38.930) |
0.410 |
CI, confidence interval; MWA, microwave ablation; RFA, radiofrequency ablation.
Long‐term survival
Survival data was available for 214 (89.9%) patients. At a median follow‐up duration of 13.6 months (range 1–47.5), 24 (10.1%) patients had been lost to follow‐up, including 11 in the MWA group and 13 in the RFA group. During the follow‐up period, the PFS rates at 6 and 12 months were 62.7% and 41.7% for patients treated with MWA and 66.0% and 51.3% for patients treated with RFA, respectively. The OS rates at 6, 12, and 24 months were 90.0%, 76.9%, and 55.7% for patients treated with MWA and 88.4%, 80.5%, and 67.0% for patients treated with RFA, respectively (Fig 1). There were no significant differences in the median PFS and OS durations between the groups (P > 0.05) (Table 3).
Figure 1.
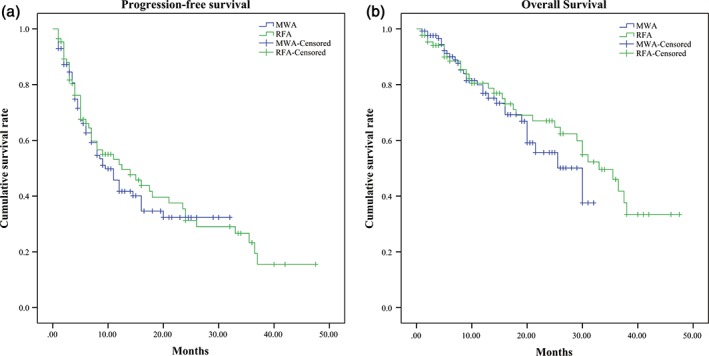
(a) The progression‐free survival curves at six months and one year were 62.7 and 41.7 for patients treated with microwave ablation (MWA) and 66.0 and 51.3% for patients treated with radiofrequency ablation (RFA), respectively. (b) The overall survival curves at six months, one and two‐years were 90, 76.9, and 55.7 for patients treated with MWA and 88.4, 80.5 and 67.0% for patients treated with RFA, respectively.
Meta‐analysis comparing RFA with MWA for lung tumors
A PubMed search identified 110 citations. After abstract and full‐text review, five studies were selected for meta‐analysis. The characteristics of the patients presented in these five reports are summarized in Table 4. The complication and CR rates were only available for three published trials and our study. Meta‐analysis showed that either the complication rate or the CR rate was comparable between the RFA and MWA groups (P > 0.05), and there was no significant statistical heterogeneity (complication rate: I2 = 45%, P = 0.14; CR rate: I2 = 44%, P = 0.15) among the results of the included studies.
Table 4.
Characteristics of published comparison studies of RFA and MWA
| Reference | Period | Tumor type | RFA | MWA | ||
|---|---|---|---|---|---|---|
| Tumor size (cm) | Number of patients | Tumor size (cm) | Number of patients | |||
| Carrafiello et al.16 | 2003.1–2009.1 | Primary or secondary lung tumors | 2.8 (range 1.5–5) | 29 | 3.75 (range 2.8–4.7) | 16 |
| Vogl et al.17 | 2000.5–2014.5 | Colorectal lung metastases | 0.8–4.2 | 41 | 0.5–5 | 47 |
| Macchi et al 15 | — | Primary or secondary lung tumors | 1.64 ± 0.80 | 28 | 2.21 ± 0.89 | 24 |
| Nour‐Eldin et al.18 | — | Non‐colorectal cancer lung metastases | 0.8–4.5 | 29 | 0.6–5 | 63 |
| Feng et al.13 | 2007.3–2014.11 | Primary or secondary lung tumors | 3.00 ± 1.75 | 43 | 3.46 ± 2.02 | 32 |
MWA, microwave ablation; RFA, radiofrequency ablation.
Meta‐analysis according to 12 month PFS, and 12 and 24 month OS was performed to compare the prognostic value of RFA and MWA. The 12‐month PFS rate was significantly higher in the RFA (101/164, 61.59%) than in the MWA group (114/242, 47.11%) (OR = 0.51, 95% CI 0.28–0.95; P = 0.003). However, there was no statistically significant difference in the 12‐month (RFA vs. MWA 187/236, 79.24% vs. 277/337, 82.20; P = 0.52) and 24‐month OS rates (RFA vs. MWA 138/236, 58.47% vs. 211/337, 62.61; P = 0.58) between the groups.
Discussion
Lung cancer is a significant cause of cancer‐related death worldwide. Lobectomy is the standard treatment for early‐stage lung cancer. However, many patients with lung cancers are not medically fit for lobectomy because of insufficient pulmonary reserve, significant comorbidities, or other risk factors. Recently, emerging studies have depicted thermal ablation, including RFA and MWA, for tumors. Kwan et al. reported no difference in OS following sublobar resection or thermal ablation for comparable elderly patients with stage I non‐small cell lung cancer.19 Several studies have also retrospectively evaluated the safety and efficacy of CT‐guided MWA in lung cancer patients.20
Compared to RFA, less research is available on MWA, but it is a promising option because it offers larger ablation zones, reduced procedure times, and decreased heat‐sink effects.21, 22 In our study, we focused on a comparison of RFA and MWA for the treatment of lung tumors. We revealed the safety, efficacy, and prognostic value of these two treatments through retrospective comparison of clinical cases in our center and meta‐analysis of the results of our study and published comparison studies.
In our study, no procedure‐related death occurred in either group. Although subcutaneous emphysema was more likely to occur in patients treated with RFA, this might be a result of the higher number of patients in the RFA group with multiple tumors (Table 2). There was no significant difference in the total complication rate between the groups.
With regard to the efficacy, our study revealed that both RFA and MWA could provide complete tumor ablation in the majority of cases (initial complete response: 97.0% for MWA and 96.4% for RFA). For patients with residual tumors, a second ablation was considered and total CA could be achieved. Only one patient in the RFA and two in the MWA group did not receive a second ablation for residual tumors one month later as the tumors were small and were not growing; a second ablation was performed when the tumor began to grow. Compared with published studies, the CR rate was higher in our study. No significant difference was found between RFA and MWA groups in our study or the studies analyzed.
Concerning follow‐up survival, PFS and OS rates were similar between the groups (Table 3, Fig 1). Meta‐analysis showed comparable 12 and 24‐month OS rates were between the groups (Fig 2).
Figure 2.
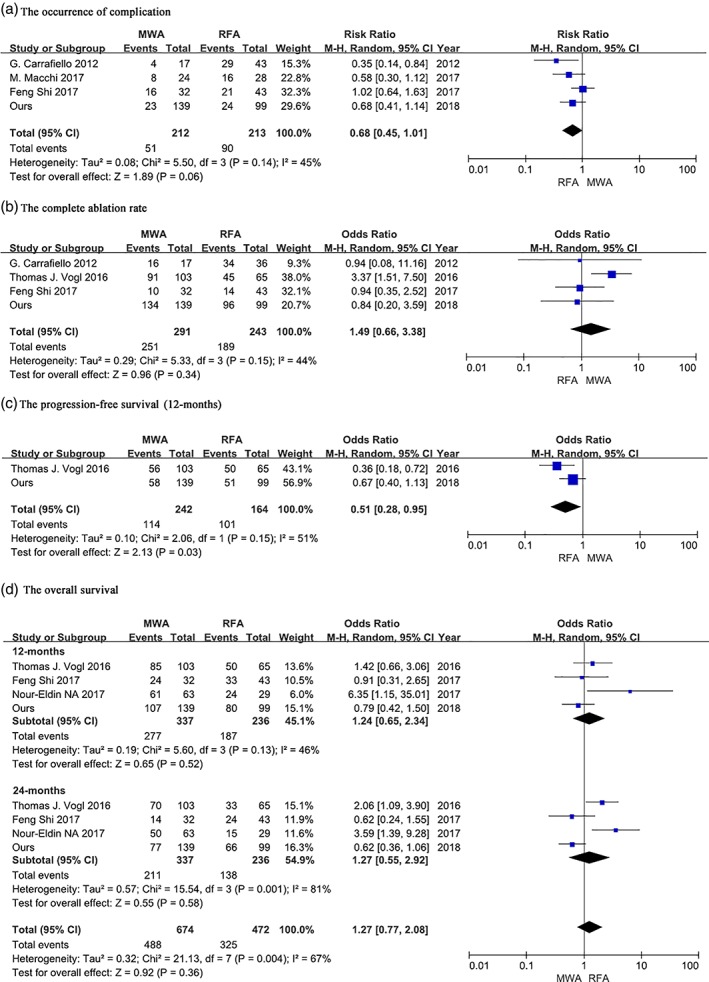
Meta‐analysis of published comparison studies of microwave ablation (MWA) and radiofrequency ablation (RFA) for pulmonary tumors. (a) Complication, (b) complete ablation, (c) progression‐free survival (12 months), and (d) overall survival rates. CI, confidence interval, M–H, Mantel–Haenszel.
Despite the promising results, our study does have some limitations. First, because of its retrospective nature, inevitable selection bias was present in the study population. Second, the treatments were not randomly selected. The two populations were not well balanced in terms of histology and tumor dimension. Third, our study included patients with primary or metastatic lung tumors, which may affect the survival results. Finally, the follow‐up period was inadequate.
In conclusion, our study showed that both RFA and MWA were safe and effective treatments with a survival benefit for selected patients with primary and metastatic lung tumors.
Disclosure
No authors report any conflict of interest.
References
- 1. Katyal S, Oliver JHIII, Peterson MS, Ferris JV, Carr BS, Baron RL. Extrahepatic metastases of hepatocellular carcinoma. Radiology 2000; 216: 698–703. [DOI] [PubMed] [Google Scholar]
- 2. Rama N, Monteiro A, Bernardo JE, Eugénio L, Antunes MJ. Lung metastases from colorectal cancer: Surgical resection and prognostic factors. Eur J Cardiothoracic Surg 2009; 35: 444–9. [DOI] [PubMed] [Google Scholar]
- 3. D'Andrilli A, Maurizi G, Andreetti C et al Sleeve lobectomy versus standard lobectomy for lung cancer: Functional and oncologic evaluation. Ann Thorac Surg 2016; 101: 1936–42. [DOI] [PubMed] [Google Scholar]
- 4. Pompili C, Miserocchi G. Air leak after lung resection: Pathophysiology and patients' implications. J Thorac Dis 2016; 8(Suppl 1): S46–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Dupuy DE. Image‐guided thermal ablation of lung malignancies. Radiology 2011; 260: 633–55. [DOI] [PubMed] [Google Scholar]
- 6. Iguchi T, Hiraki T, Ishii H et al Transosseous route for CT fluoroscopy‐guided radiofrequency ablation of lung tumors. J Vasc Interv Radiol 2015; 26: 1694–8. [DOI] [PubMed] [Google Scholar]
- 7. Ni X, Han JQ, Ye X, Wei ZG. Percutaneous CT‐guided microwave ablation as maintenance after first‐line treatment for patients with advanced NSCLC. OncoTargets Ther 2015; 8: 3227–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sun YH, Song PY, Guo Y, Sheng LJ. Computed tomography‐guided percutaneous microwave ablation therapy for lung cancer. Genet Mol Res 2015; 14: 4858–64. [DOI] [PubMed] [Google Scholar]
- 9. Splatt AM, Steinke K. Major complications of high‐energy microwave ablation for percutaneous CT‐guided treatment of lung malignancies: Single‐centre experience after 4 years. J Med Imaging Radiat Oncol 2015; 59: 609–16. [DOI] [PubMed] [Google Scholar]
- 10. Belfiore G, Ronza F, Belfiore MP et al Patients' survival in lung malignancies treated by microwave ablation: Our experience on 56 patients. Eur J Radiol 2013; 82: 177–81. [DOI] [PubMed] [Google Scholar]
- 11. Zhao Q, Tian G, Chen F, Zhong L, Jiang T. CT‐guided percutaneous laser ablation of metastatic lung cancer: Three cases report and literature review. Oncotarget 2017; 8: 2187–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Simon CJ, Dupuy DE, Mayo‐Smith WW. Microwave ablation: Principles and applications. Radiographics 2005; 25(Suppl 1): S69–83. [DOI] [PubMed] [Google Scholar]
- 13. Shi F, Li G, Zhou Z et al Microwave ablation versus radiofrequency ablation for the treatment of pulmonary tumors. Oncotarget 2017; 8: 109791–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Botsa EI, Thanou IL, Papatheodoropoulou AT, Thanos LI. Thermal ablation in the management of adrenal metastasis originating from non‐small cell lung cancer: A 5‐year single‐center experience. Chin Med J (Engl) 2017; 130: 2027–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Macchi M, Belfiore MP, Floridi C et al Radiofrequency versus microwave ablation for treatment of the lung tumours: LUMIRA (lung microwave radiofrequency) randomized trial. Med Oncol 2017; 34: 96. [DOI] [PubMed] [Google Scholar]
- 16. Carrafiello G, Mangini M, Fontana F et al Complications of microwave and radiofrequency lung ablation: Personal experience and review of the literature. Radiol Med 2012; 117: 201–13. [DOI] [PubMed] [Google Scholar]
- 17. Vogl TJ, Eckert R, Naguib NN, Beeres M, Gruber‐Rouh T, Nour‐Eldin NA. Thermal ablation of colorectal lung metastases: Retrospective comparison among laser‐induced thermotherapy, radiofrequency ablation, and microwave ablation. AJR Am J Roentgenol 2016; 207: 1340–9. [DOI] [PubMed] [Google Scholar]
- 18. Nour‐Eldin NA, Exner S, Al‐Subhi M et al Ablation therapy of non‐colorectal cancer lung metastases: Retrospective analysis of tumour response post‐laser‐induced interstitial thermotherapy (LITT), radiofrequency ablation (RFA) and microwave ablation (MWA). Int J Hyperthermia 2017; 33: 820–9. [DOI] [PubMed] [Google Scholar]
- 19. Kwan SW, Mortell KE, Talenfeld AD, Brunner MC. Thermal ablation matches sublobar resection outcomes in older patients with early‐stage non‐small cell lung cancer. J Vasc Interven Radiol 2014; 25 (1): 1–9 e1. [DOI] [PubMed] [Google Scholar]
- 20. Zhong L, Sun S, Shi J et al Clinical analysis on 113 patients with lung cancer treated by percutaneous CT‐guided microwave ablation. J Thorac Dis 2017; 9: 590–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lee H, Jin GY, Han YM et al Comparison of survival rate in primary non‐small‐cell lung cancer among elderly patients treated with radiofrequency ablation, surgery, or chemotherapy. Cardiovasc Intervent Radiol 2012; 35: 343–50. [DOI] [PubMed] [Google Scholar]
- 22. Planché O, Teriitehau C, Boudabous S et al In vivo evaluation of lung microwave ablation in a porcine tumor mimic model. Cardiovasc Intervent Radiol 2013; 36: 221–8. [DOI] [PubMed] [Google Scholar]


