Abstract
Key points
The female hormone oestrogen may protect muscle from injury by reducing inflammation but this is debatable.
In this study, the inflammatory response of injured muscle from oestrogen‐replete mice was comprehensively compared to that from oestrogen‐deficient mice.
We show that oestrogen markedly promotes movement of neutrophils, an inflammatory white blood cell type, into muscle over the first few days after injury but has only a minor effect on the movement of macrophages, another inflammatory cell type.
Despite the enhancement of inflammation by oestrogen in injured muscle, we found strength in oestrogen‐replete mice to recover faster and to a greater extent than it does in oestrogen‐deficient mice.
Our study and others indicate that lower doses of oestrogen, such as that used in our study, may affect muscle inflammation and injury differently from higher doses.
Abstract
Oestrogen has been shown to protect against skeletal muscle injury and a reduced inflammatory response has been suggested as a possible protective mechanism. There are, however, dissenting reports. Our objective was to conduct an unbiased, comprehensive study of the effect of oestradiol on the inflammatory response following muscle injury. Female C57BL6/J mice were ovariectomized and supplemented with and without oestradiol. Tibialis anterior muscles were freeze injured and studied primarily at 1–4 days post‐injury. Oestradiol supplementation increased injured muscle gene expression of neutrophil chemoattractants (Cxcl1 and Cxcl5) and to a lesser extent that of monocyte/macrophage chemoattractants (Ccl2 and Spp1). Oestradiol markedly increased gene expression of the neutrophil cell surface marker (Ly6g) but had less consistent effects on the monocyte/macrophage cell surface markers (Cd68, Cd163 and Cd206). These results were confirmed at the protein level by immunoblot with oestradiol increasing LY6G/C content and having no significant effect on CD163 content. These findings were confirmed with fluorescence‐activated cell sorting counts of neutrophils and macrophages in injured muscles; oestradiol increased the proportion of CD45+ cells that were neutrophils (LY6G+) but not the proportion that were macrophages (CD68+ or CD206+). Physiological impact of the oestradiol‐enhanced neutrophil response was assessed by strength measurements. There was no significant difference in strength between oestradiol‐supplemented and ‐unsupplemented mice until 2 weeks post‐injury; strength was 13–24% greater in supplemented mice at 2–6 weeks post‐injury. In conclusion, a moderate level of oestradiol supplementation enhances neutrophil infiltration in injured muscle and this is associated with a beneficial effect on strength recovery.
Keywords: Estrogen, Inflammation, Skeletal muscle
Key points
The female hormone oestrogen may protect muscle from injury by reducing inflammation but this is debatable.
In this study, the inflammatory response of injured muscle from oestrogen‐replete mice was comprehensively compared to that from oestrogen‐deficient mice.
We show that oestrogen markedly promotes movement of neutrophils, an inflammatory white blood cell type, into muscle over the first few days after injury but has only a minor effect on the movement of macrophages, another inflammatory cell type.
Despite the enhancement of inflammation by oestrogen in injured muscle, we found strength in oestrogen‐replete mice to recover faster and to a greater extent than it does in oestrogen‐deficient mice.
Our study and others indicate that lower doses of oestrogen, such as that used in our study, may affect muscle inflammation and injury differently from higher doses.
As compared to males, females have been associated with decreased muscle injury susceptibility and/or a faster recovery therefrom (Kendall & Eston, 2002; Tiidus, 2003; Enns & Tiidus, 2010); however, there are dissenting reports (Clarkson & Hubal, 2001). This sex difference has been attributed primarily to the hormone oestrogen. Mechanisms hypothesized to explain oestrogen's protective effect on muscle include: (a) increased stability of cell membranes, including the plasmalemma, (b) antioxidant activity of oestradiol decreasing injury‐induced oxidative stress, (c) decreased inflammatory response, and/or (d) increased satellite cell and myoblast number during recovery (Enns & Tiidus, 2010). The first two proposed mechanisms, although popular two to three decades ago, are now thought to play a lesser, if any, role in manifesting oestrogen's protective effect.
Of the two latter proposed mechanisms, the effect of oestrogen or sex on the inflammatory response has been more studied. However, the results of these studies appear discrepant even after a comprehensive review of the literature up to the current year. Of five human studies probing the effect of sex or oestrogen on the inflammatory response following injury, two studies found oestrogen to attenuate the inflammatory response (Dieli‐Conwright et al. 2009; MacNeil et al. 2011), whereas two studies found the female sex to enhance the inflammatory response (MacIntyre et al. 2000; Stupka et al. 2001), and one study found no significant effect of sex (Stupka et al. 2000). Of 14 studies using rodents, seven found the female sex or oestrogen to reduce the inflammatory response (St Pierre Schneider et al. 1999; Tiidus & Bombardier, 1999; Stupka & Tiidus, 2001; Tiidus et al. 2001, 2005b; Enns et al. 2008; Iqbal et al. 2008), but four found the contrary (Tiidus et al. 2005a; Schneider et al. 2012; Fulkerson et al. 2015; Fearing et al. 2016), and three studies found no effect or contradictory effects (Schneider et al. 2002; McHale et al. 2012; Velders et al. 2012). Though all four human studies observing oestrogen‐ or sex‐modulated changes in the inflammatory response attempted to associate those changes with ones observed in the markers of muscle injury (MacIntyre et al. 2000; Stupka et al. 2001; Dieli‐Conwright et al. 2009; MacNeil et al. 2011), only four of the animal studies did so (Stupka & Tiidus, 2001; Tiidus et al. 2005a; Enns et al. 2008; Velders et al. 2012). Of these eight studies that did look at injury markers, four found oestrogen or the female sex to decrease the inflammatory response following injury (Stupka & Tiidus, 2001; Enns et al. 2008; Dieli‐Conwright et al. 2009; MacNeil et al. 2011), but in only two of these studies were the injury markers decreased (Enns et al. 2008; Dieli‐Conwright et al. 2009), while the markers were increased in one study (Stupka & Tiidus, 2001). Likewise, three of the eight studies found oestrogen or the female sex to increase the inflammatory response (MacIntyre et al. 2000; Stupka et al. 2001; Tiidus et al. 2005a), but injury markers were increased in one study (Tiidus et al. 2005a), and unchanged in two (MacIntyre et al. 2000; Stupka et al. 2001).
The explanation for the above discrepant findings is not clear but may be related to many studies being less than comprehensive. Of the five human and 14 animal studies mentioned above, only three made assessments of the inflammatory response at more than two times post‐injury (St Pierre Schneider et al. 1999; Tiidus et al. 2005a; Fearing et al. 2016). Furthermore, only three studies used more than one methodology to assess the inflammatory response (Stupka & Tiidus, 2001; Tiidus et al. 2001; McHale et al. 2012) and most studies (n = 10) relied solely on histological techniques (St Pierre Schneider et al. 1999; Stupka et al. 2000, 2001; Schneider et al. 2002, 2012; Tiidus et al. 2005b; Enns et al. 2008; Iqbal et al. 2008; MacNeil et al. 2011; Fulkerson et al. 2015). Of the 19 studies, only three assessed inflammation beyond determining phagocyte (neutrophil and/or macrophage) count or content in the injured muscle (Dieli‐Conwright et al. 2009; McHale et al. 2012; Velders et al. 2012); these three studies quantified cytokines with or without phagocyte counts.
The means by which the muscle injury was induced might also explain the disparate findings. Exercise or a bout of eccentric contractions was used to induce the injury in all but five of the 19 studies; in those five studies, injury was induced via a toxin injection or hindlimb ischaemia–reperfusion (Stupka & Tiidus, 2001; Tiidus et al. 2005a; McHale et al. 2012; Velders et al. 2012; Fearing et al. 2016). This is important because we have previously shown that the inflammatory response following muscle injury induced by trauma to be greater and qualitatively different from that induced by eccentric contractions (Warren et al. 2007). However, the effect of oestrogen on inflammation in these five studies using traumatic injuries was equally variable. One study showed oestrogen to decrease the inflammatory response (Stupka & Tiidus, 2001) while two showed it to increase the response (Tiidus et al. 2005a; Fearing et al. 2016); one study showed no effect (McHale et al. 2012) and the last showed contradictory effects (Velders et al. 2012).
The objective of the present work was to conduct an unbiased, comprehensive study of the effect of oestradiol, i.e. the principal oestrogen, on the inflammatory response following traumatic muscle injury. We conducted a series of experiments assessing multiple aspects of the inflammatory response using several quantitative methodologies at four different times post‐injury. Upon observing a strong effect of oestradiol on the inflammatory response, we sought to determine if the effect was associated with an oestradiol effect on the preferred marker of injury, i.e. strength loss (Warren et al. 1999b).
Methods
Ethical approval
All protocols were approved by the Institutional Animal Care and Use Committee at the University of Minnesota, which operates under the national guidelines set by the Association for Assessment and Accreditation of Laboratory Animal Care. Investigators understand the ethical principles and ensure that the work complies with the animal ethics checklist of The Journal.
Animals and procedures common across experiments
Female C57BL/6J mice aged 3–4 months were obtained from The Jackson Laboratory (Bar Harbor, ME, USA). Mean (SD) body mass at the beginning of experiments was 22.4 (2.5) g. Mice were housed in groups of four to five and had access to phytooestrogen‐free rodent chow (Harlan‐Teklad no. 2019; Indianapolis, IN, USA) and water ad libitum. The housing room was maintained on a 12:12 h light:dark cycle with controlled temperature and humidity.
After at least 1 week of the mice acclimating to their new housing, ovariectomy and hormone pellet implantation procedures were conducted. Under aseptic conditions, bilateral ovariectomy was performed through two small dorsal incisions between the iliac crest and the lower ribs (Moran et al. 2007). Immediately after ovariectomy, mice were randomly assigned to receive either a slow‐release oestradiol‐containing pellet (OVX+E2) or a placebo pellet (OVX+placebo) (or no pellet (OVX) in two experiments). Pellets were placed intraperitoneal through one of the dorsal incisions. Oestradiol‐containing pellets had 0.18 mg of 17β‐oestradiol released over a 60‐day period (Innovative Research of America, Sarasota, FL, USA) except where noted otherwise. In subsets of mice, blood was collected by facial vein bleed 2–4 weeks following pellet implantation and serum or plasma was stored at −80°C until assayed for oestradiol by liquid chromatography‐tandem mass spectrometry (LC‐MS/MS).
After at least 3 weeks of recovery from the surgical procedures and thus when the mice were at 4–5 months of age (i.e. mature adults), freeze injury to tibialis anterior (TA) muscles was performed as described previously (Warren et al. 2002). In brief, mice were anaesthetized via isoflurane and injury was induced by applying a dry ice‐cooled steel probe to the TA muscle for 10 s. Skin incisions were closed using 6‐0 silk suture and n‐butyl cyanoacrylate tissue adhesive (Vetbond®; 3M, St Paul, MN, USA). With this injury, ∼60% of the muscle is affected histologically; the greatest damage occurs beneath where the probe is applied with ∼75% of the muscle cross‐section being affected (Warren et al. 2002, 2007).
Each mouse received extended‐release buprenorphine subcutaneously (1.0 mg kg−1 body mass) as an analgesic immediately prior to injury induction or any surgical procedure. Muscle injury induction and surgical procedures were performed under anaesthesia using an induction chamber containing isoflurane and then maintained using inhalation of ∼2% isoflurane in oxygen at a flow rate of 200–250 ml min−1. Depth of anaesthesia was assessed and maintained by monitoring respiratory rate and toe pinch withdrawal reflex.
At the time of euthanasia, mice were first anaesthetized by an intraperitoneal injection of pentobarbital sodium (100 mg kg−1 body mass; Diamondback Drugs, Scottsdale, AZ, USA). TA muscles were dissected and prepared for experiments. Uteri were dissected and weighed (mean (SD) uterine mass for OVX+placebo, OVX and OVX+E2 mice: 18.0 (2.9), 18.3 (5.2) and 112.7 (19.6) mg, respectively). Based on our experience, uterine mass of <30 mg in OVX+placebo or OVX mice reflects successful ovariectomy surgery. Mice were killed by an overdose of pentobarbital sodium (200 mg kg−1).
Experimental design
To begin to determine how oestradiol modulates skeletal muscle inflammation following injury, a gene expression array with 84 chemokines, cytokines and their receptors was used on injured and uninjured skeletal muscles. Ovariectomized mice were implanted with placebo (OVX+placebo; n = 22) or oestradiol (OVX+E2; n = 23) pellets and 21 days later, freeze injury was induced to left TA muscles. Mice were sacrificed at 1 or 3 days post‐injury along with uninjured control mice (6–8 mice per oestradiol condition (placebo/oestradiol) and time point). The gene arrays were run using SYBR Green quantitative polymerase chain reaction (qPCR) methodology.
Based on the gene array results, four genes (Ccl2, Cxcl1, Cxcl5, and Spp1) that were significantly upregulated by oestradiol in injured muscle at 1 or 3 days post‐injury were selected for confirmatory gene expression. Selection of these genes was also based on their known roles for modulating phagocyte function (Warren et al. 2007). Mice were ovariectomized and implanted with oestradiol (OVX+E2; n = 25) or placebo (OVX+placebo; n = 25) pellets. Three weeks later, freeze injury was induced to left and right TA muscles. Mice were sacrificed at 1, 2, 3 and 4 days post‐injury along with uninjured controls (n = 5 mice per oestradiol condition per time point). Expression of the four genes was assessed using TaqMan qPCR methodology.
The gene array and confirmatory qPCR data indicated that chemokines and cytokines known to modulate phagocytes were affected by oestradiol following injury. Thus, gene expression of cell surface markers for neutrophils (Ly6g), M1 macrophages (Cd68), M2 macrophages (Cd163 and Cd206), and leukocytes (Cd11b) were assessed using RNA from the same muscle samples that were used to confirm the gene array data.
Gene expression of the cell surface markers Ly6g and Cd163 were most affected by oestradiol treatment. To determine if protein levels mirrored gene expression, immunoblotting of LY6G/C and CD163 was conducted on TA muscles from OVX and OVX+E2 mice (n = 25 each). The mice were sacrificed 1, 2, 3 and 4 days post‐injury along with uninjured control mice. From these mice, we also measured myeloperoxidase enzyme activity of the TA muscles as a biochemical marker of inflammation related predominantly to neutrophils (n = 5 mice per oestradiol condition per time point).
Results of the immunoblotting and myeloperoxidase assays suggested that neutrophil number and activity in injured muscle were sensitive to oestradiol treatment. To objectively measure the number of LY6G+ cells infiltrating the injured muscle, flow cytometry was used to compare TA muscles of OVX (n = 24) and OVX+E2 mice (n = 21) that were freeze injured 21 days after ovariectomy surgeries. Mice were sacrificed 1, 2, 3 and 4 days post‐injury, and TA muscles were processed for cell sorting (n = 4–6 mice per oestradiol condition per time point).
To determine the extent that oestradiol‐mediated changes in our indices of muscle inflammation translated to the recovery of muscle strength after injury, the following experiment was conducted. Twenty‐seven mice were chronically implanted with a stimulating nerve cuff on the common peroneal nerve of the left leg; two mice were lost to technical failure. Mice were ovariectomized 28 days later and given either a placebo (OVX+placebo; n = 12) or oestradiol pellet (OVX+E2; n = 13). In this experiment, 90‐day release pellets containing 0.25 mg of 17β‐oestradiol were used due to the extended duration of the experiment; this pellet yielded an oestradiol release rate 7% lower than that in the other studies. Twenty‐eight days after the ovariectomy surgery, strength of left anterior crural muscles was measured (Pre) and then the TA muscle was subjected to freeze injury. Strength was reassessed from immediately post‐injury out to 6 weeks after injury.
RNA isolation and quantitative PCR
Eighty‐four genes encoding chemokines, cytokines and their receptors (PAMM‐011A; RT2 Profiler Array; SABiosciences, Frederick, MA, USA; Table 1) were screened to determine the extent to which gene expression was altered by oestradiol at 1 and 3 days post‐injury. The arrays were run as described previously (Baltgalvis et al. 2010). In short, RNA was isolated by mechanical homogenization of the TA muscles in TRI Reagent followed by a Qiagen RNeasy clean‐up kit (Qiagen, Valencia, CA, USA). For each muscle, cDNA was synthesized from 1 μg of RNA using a SABiosciences RT2 First Strand kit and the cDNA was subsequently loaded on the SYBR Green PCR array and run using an iCycler real‐time PCR system (Bio‐Rad, Hercules, CA, USA). Hprt was used as the reference gene.
Table 1.
Gene expression list of inflammatory chemokines/cytokines and related receptors
| Injury effect, relative to OVX+placebo, uninjured (fold change) | Oestradiol effect, relative to OVX+placebo, injured (fold change) | ||||
|---|---|---|---|---|---|
| Gene name | Symbol | 1 day | 3 days | 1 day | 3 days |
| ATP‐binding cassette, subfamily F member 1 | Abcf1 | 1.32 | 1.57* | 0.99 | 1.00 |
| B‐cell leukemia/lymphoma 6 | Bcl6 | 1.41* | 1.59* | 0.92 | 0.86 |
| Caspase 1 | Casp1 | 11.85* | 31.72* | 1.04 | 0.92 |
| CD40 ligand | Cd40lg | 0.98 | 1.97* | 0.60 | 0.94 |
| Chemokine (C motif) receptor 1 | Xcr1 | 1.87* | 6.72* | 0.65 | 0.72 |
| Chemokine (C‐C motif) ligand 1 | Ccl1 | 0.76 | 0.92 | – | – |
| Chemokine (C‐C motif) ligand 2 | Ccl2 | 1105.74* | 389.82* | 1.27 † | 1.03 |
| Chemokine (C‐C motif) ligand 3 | Ccl3 | 348.95* | 163.62* | 0.85 | 1.19 |
| Chemokine (C‐C motif) ligand 4 | Ccl4 | 563.42* | 253.62* | 0.81 | 0.86 |
| Chemokine (C‐C motif) ligand 5 | Ccl5 | 7.87* | 26.56* | 0.83 | 0.50 † |
| Chemokine (C‐C motif) ligand 6 | Ccl6 | 112.05* | 51.76* | 1.65 † | 1.37 † |
| Chemokine (C‐C motif) ligand 7 | Ccl7 | 1633.13* | 654.49* | 1.26 | 1.06 |
| Chemokine (C‐C motif) ligand 8 | Ccl8 | 135.16* | 447.64* | 0.87 | 0.87 |
| Chemokine (C‐C motif) ligand 9 | Ccl9 | 81.35* | 48.05* | 1.40 | 1.17 † |
| Chemokine (C‐C motif) ligand 11 | Ccl11 | 1.10 | 1.23 | – | – |
| Chemokine (C‐C motif) ligand 12 | Ccl12 | 458.71* | 327.39* | 1.34 | 1.03 |
| Chemokine (C‐C motif) ligand 17 | Ccl17 | 1.97 | 0.67 | – | – |
| Chemokine (C‐C motif) ligand 19 | Ccl19 | 5.56* | 3.99* | 0.97 | 0.75 |
| Chemokine (C‐C motif) ligand 20 | Ccl20 | 0.77 | 3.32* | 0.94 | 1.02 |
| Chemokine (C‐C motif) ligand 22 | Ccl22 | 8.66* | 6.08* | 0.97 | 0.56 † |
| Chemokine (C‐C motif) ligand 24 | Ccl24 | 2.88* | 2.05* | 1.73 † | 1.23 |
| Chemokine (C‐C motif) ligand 25 | Ccl25 | 0.70 | 0.97 | – | – |
| Chemokine (C‐C motif) receptor 1 | Ccr1 | 527.32* | 297.01* | 1.20 † | 1.03 |
| Chemokine (C‐C motif) receptor 2 | Ccr2 | 59.86* | 50.69* | 0.86 | 1.41 |
| Chemokine (C‐C motif) receptor 3 | Ccr3 | 83.97* | 123.01* | 0.78 | 0.85 † |
| Chemokine (C‐C motif) receptor 4 | Ccr4 | 1.67* | 4.58* | 1.42 | 0.74 |
| Chemokine (C‐C motif) receptor 5 | Ccr5 | 149.33* | 356.51* | 2.22 | 1.02 |
| Chemokine (C‐C motif) receptor 6 | Ccr6 | 1.26 | 3.55* | 0.77 | 1.19 |
| Chemokine (C‐C motif) receptor 7 | Ccr7 | 3.48* | 6.47* | 0.70 | 0.40 † |
| Chemokine (C‐C motif) receptor 8 | Ccr8 | 0.95 | 1.00 | – | – |
| Chemokine (C‐C motif) receptor 9 | Ccr9 | 2.30* | 3.58* | 1.06 | 1.22 |
| Chemokine (C‐C motif) receptor 10 | Ccr10 | 1.13 | 1.06 | – | – |
| Chemokine (C‐X3‐C motif) ligand 1 | Cx3cl1 | 2.94* | 6.93* | 0.95 | 0.97 |
| Chemokine (C‐X‐C motif) ligand 1 | Cxcl1 | 99.76* | 44.37* | 3.01 † | 1.12 |
| Chemokine (C‐X‐C motif) ligand 5 | Cxcl5 | 3272.97* | 2544.69* | 1.96 † | 1.07 |
| Chemokine (C‐X‐C motif) ligand 9 | Cxcl9 | 1.02 | 5.47* | 0.76 | 0.28 † |
| Chemokine (C‐X‐C motif) ligand 10 | Cxcl10 | 84.26* | 315.23* | 0.48 | 0.53 † |
| Chemokine (C‐X‐C motif) ligand 11 | Cxcl11 | 0.94 | 1.71* | 0.59 | 0.72 |
| Chemokine (C‐X‐C motif) ligand 12 | Cxcl12 | 2.14* | 5.63* | 0.80 † | 1.17 |
| Chemokine (C‐X‐C motif) ligand 13 | Cxcl13 | 2.28 | 10.28* | 0.73 | 1.03 |
| Chemokine (C‐X‐C motif) ligand 15 | Cxcl15 | 0.76 | 0.92 | – | – |
| Chemokine (C‐X‐C motif) receptor 3 | Cxcr3 | 9.21* | 44.88* | 0.81 | 0.97 |
| Chemokine (C‐X‐C motif) receptor 5 | Cxcr5 | 6.55* | 3.07* | 1.39 | 1.21 |
| Complement component 3 | C3 | 3.17* | 5.99* | 1.00 | 0.96 |
| C‐reactive protein, pentraxin‐related | Crp | 0.89 | 0.92 | – | – |
| Integrin alpha M | Itgam | 95.06* | 88.51* | 1.14 | 1.09 |
| Integrin beta 2 | Itgb2 | 66.62* | 77.64* | 0.99 | 1.07 |
| Interferon gamma | Ifng | 1.02 | 3.89* | 1.01 | 0.52 † |
| Interleukin 1 alpha | Il1a | 16.84* | 8.85* | 0.56 | 0.89 |
| Interleukin 1 beta | Il1b | 1178.28* | 634.82* | 1.36 | 0.65 |
| Interleukin 1 family, member 6 | Il1f6 | 0.78 | 0.92 | – | – |
| Interleukin 1 family, member 8 | Il1f8 | 0.76 | 0.92 | – | – |
| Interleukin 1 receptor, type I | Il1r1 | 8.38* | 22.64* | 1.16 | 1.24 |
| Interleukin 1 receptor, type II | Il1r2 | 360.55* | 83.26* | 1.61 | 2.66 |
| Interleukin 2 receptor, beta chain | Il2rb | 3.03* | 10.00* | 0.82 | 0.56 |
| Interleukin 2 receptor, gamma chain | Il2rg | 13.96* | 35.44* | 0.91 | 0.99 |
| Interleukin 3 | Il3 | 0.76 | 0.92 | – | – |
| Interleukin 4 | Il4 | 0.87 | 0.93 | – | – |
| Interleukin 5 receptor, alpha | Il5rα | 2.10* | 2.22* | 0.37 † | 0.33 † |
| Interleukin 6 receptor, alpha | Il6ra | 11.29* | 18.21* | 0.99 | 0.91 |
| Interleukin 6 signal transducer | Il6st | 1.76* | 1.22* | 0.97 | 3.10 |
| Interleukin 8 receptor, beta | Il8rb | 975.86* | 389.53* | 1.24 | 0.86 |
| Interleukin 10 | Il10 | 56.68* | 150.23* | 1.01 | 0.83 |
| Interleukin 10 receptor, alpha | Il‐10ra | 23.20* | 101.04* | 0.85 | 0.69 † |
| Interleukin 10 receptor, beta | Il‐10rb | 10.45* | 21.61* | 0.84 | 0.90 |
| Interleukin 11 | Il11 | 7.52* | 3.25* | 1.19 | 0.74 |
| Interleukin 13 | Il13 | 0.87 | 0.98 | – | – |
| Interleukin 13 receptor, alpha 1 | Il13ra1 | 15.15* | 24.12* | 1.15 | 1.02 |
| Interleukin 15 | Il‐15 | 0.81* | 1.67* | 0.98 | 0.79 † |
| Interleukin 16 | Il16 | 4.61* | 11.36* | 0.87 | 0.85 |
| Interleukin 17B | Il‐17b | 0.89 | 23.48* | 0.90 | 0.50 † |
| Interleukin 18 | Il18 | 15.90* | 65.62* | 1.19 | 0.92 |
| Interleukin 20 | Il20 | 0.76 | 0.92 | – | – |
| Lymphotoxin A | Lta | 0.76 | 0.92 | – | – |
| Lymphotoxin B | Ltb | 30.89* | 14.44* | 0.90 | 0.46 |
| Macrophage migration inhibitory factor | Mif | 1.80* | 4.02* | 1.17 | 1.00 |
| Platelet factor 4 | Pf4 | 83.68* | 117.65* | 1.12 | 0.96 |
| Secreted phosphoprotein 1 | Spp1 | 377.24* | 1323.28* | 0.82 | 2.00 † |
| Small inducible cytokine subfamily E, member 1 | Scye1 | 0.90 | 1.02 | – | – |
| Toll interacting protein | Tollip | 2.33* | 1.69* | 0.94 | 1.05 |
| Transforming growth factor, beta 1 | Tgfb1 | 9.70* | 25.44* | 0.95 | 0.92 |
| Tumor necrosis factor | Tnf | 80.17* | 39.96* | 0.76 | 1.03 |
| Tumor necrosis factor receptor superfamily, member 1a | Tnfrsf1a | 9.89* | 14.84* | 1.01 | 1.01 |
| Tumor necrosis factor receptor superfamily, member 1b | Tnfrsf1b | 18.46* | 38.60* | 0.87 | 0.96 |
Complete gene list from the SYBR Green‐based real‐time PCR array. Data are presented as the fold change relative to uninjured, OVX+placebo (left two columns) or to injured, OVX+placebo (right two columns). *Significantly different from uninjured, OVX+placebo (P<0.05); †significantly different from OVX+placebo, injured (P < 0.05); the effect of oestradiol was not determined for genes whose expression was not altered by injury.
Quantitative PCR using TaqMan methodology was also performed to confirm gene expression of the following chemokines and cytokines (Cxcl1 [Mm04207460_m1], Cxcl5 [Mm00436451_g1], Ccl2 [Mm00441242_m1] and Spp1 [Mm00436767_m1]) as well as leukocyte cell surface markers (Ly6g [Mm04934123_m1], Cd68 [Mm03047340_m1], Cd206 [Mm00485148_m1], Cd163 [Mm00474091_m1] and Cd11b [Mm00434455_m1]). TA muscles were homogenized in TRI Reagent and total RNA was extracted using a RNeasy Plus Universal Mini kit (Qiagen). cDNA synthesis was performed using 2 μg RNA for each sample and a High Capacity RNA‐to‐cDNA kit (Thermo Fisher Scientific, Waltham, MA, USA). For each assay, qPCR was performed using 50 ng of cDNA in TaqMan Fast‐Advanced master mix on an ABI 7900HT (Thermo Fisher Scientific) real‐time PCR system. The combination of Hprt (Mm01545399_m1) and Hsp90ab1 (Mm00833431_g1) was used as the reference gene.
Immunoblotting
Frozen TA muscles were homogenized in 500–700 μl ice‐cold NP‐40 lysis buffer (20 mm Tris–HCl, pH 7.4, 150 mm NaCl, 1 mm EDTA, NP‐40). Homogenates were centrifuged at 10,000 g and 4°C for 10 min and the supernatants stored at −80°C. Proteins were measured by SDS‐PAGE and immunoblotting using a rat biotinylated antibody for LY6G/C (GR‐1, 1:1000 dilution; Acris Antibodies, Inc., San Diego, CA, USA) (Jutila et al. 1988), a rabbit anti‐mouse CD163 antibody (Clone M‐96, 1:800 dilution, sc‐33560; Santa Cruz Biotechnology Inc., Dallas, TX, USA) (Apolloni et al. 2016) and a rabbit anti‐glyceraldehyde 3‐phosphate dehydrogenase (GAPDH) antibody (ab9485, 1:10,000 dilution; Abcam, Cambridge, MA, USA) for loading control. After incubation with a secondary antibody, membranes were scanned and bands quantified using the Odyssey® Infrared Imaging System (LI‐COR Biosciences; Lincoln, NE, USA).
Myeloperoxidase enzyme activity
A highly specific and sensitive enzyme‐linked immunosorbent assay (ELISA) as described by Pulli et al. (2013) was used to measure myeloperoxidase activity in TA muscles. All samples were assayed in duplicate.
Fluorescence‐activated cell sorting
For fluorescence‐activated cell sorting (FACS), freshly harvested TA muscles were minced and digested with 0.2% collagenase II containing 0.4% dispase (Thermo Fisher Scientific). Cell suspensions were filtered through 40‐μm cell strainers and counted using a haemocytometer. Cell suspensions were incubated with antibodies for 1 h on ice. Propidium iodide (PI) (1 g ml−1, Sigma‐Aldrich, St Louis, MO, USA) was added to differentiate between live and dead cells and only live cells (PI negative) were counted. Cells were analysed with a BD FACSAria™ III flow cytometer (BD Biosciences, San Jose, CA, USA) and FlowJo software. The following antibodies (all from BioLegend, San Diego, CA, USA) were used: anti‐CD45 (clone 30‐F11), anti‐CD11b (clone M1/70), anti‐LY6G (clone 1A8), anti‐CD68 (clone FA‐11) and anti‐CD206 (clone C068C2). CD45+ cells were gated and within this cell population, neutrophils were identified as CD11b+ and LY6G+, M1 macrophages were CD11b+ and CD68+ and M2 macrophages were CD11b+ and CD206+.
In vivo strength of anterior crural muscles
Stimulating nerve cuffs were constructed and placed around the common peroneal nerve of the left leg as previously described (Warren et al. 1998). During each strength test, the proximal end of the nerve cuff was externalized in the dorsal cervical region and connected to a stimulator. While under anaesthesia, peak isometric torque of the left anterior crural muscles was measured using a miniature dynamometer as previously described (Warren et al. 1999a, 2007); the TA muscle contributes 89% of the isometric torque produced by the anterior crural muscles in the uninjured condition (Warren et al. 1998). Strength was assessed in each mouse immediately prior to injury, immediately post‐injury, and at 3, 7, 14, 21, 28, 35 and 42 days post‐injury.
Oestradiol assay
Oestradiol was precipitated from blood samples, reconstituted in 95% acetonitrile, then extracted using solid phase extraction columns (Strata‐X‐SPE columns, Phenomenex; Torrance, CA, USA) on a 12‐port manifold (AH0‐6023, Phenomenex). The final eluate was collected, lyophilized and stored at –20°C. Blood samples were comprised of plasma or serum; oestradiol levels did not differ between these sample types and therefore results were combined.
LC‐MS/MS for oestradiol was performed using modified protocols (Wooding et al. 2013; Torres et al. 2018) on a micro LC column (2.7 μm, C18 90 Å, Halo Fused core, 100 × 0.3 mm, Eksigent; SCIEX, Framingham, MA, USA) in series with an AB SCIEX QTRAP 5500 mass spectrometer (Thermo Fisher Scientific). The mass spectrometer was operated in negative ionization mode using MRM scans with a collision energy of −55 V, source temperature of 295°C, with nitrogen as the collision and ion source gas. The scan window was 240 ms and target scan time was 55 ms. The oestradiol molecule was identified by a m/z of 271.2 Da. LC‐MS/MS results were analysed by peak area of the chromatograph using MultiQuant™ (SCIEX).
Statistical analysis
All qPCR data, including those of the gene expression array, were analysed with REST (Relative Expression Software Tool) software to determine differences between OVX+placebo and OVX+E2 groups (Pfaffl et al. 2002). REST relies on a non‐parametric, specialized randomization statistical test and its error term is calculated using a Taylor series algorithm. For the array data, initially, genes whose expression was altered by injury in either group were identified. Expression of those genes were then compared between OVX+placebo and OVX+E2 groups at each time point post‐injury. For immunoblotting, myeloperoxidase assay and quantifications of cells by fluorescence‐activated cell sorting, two‐way ANOVAs (oestradiol condition × time) were used to determine the effect of oestradiol treatment and time post‐injury. If a significant main effect or interaction existed, Holm–Sidak post hoc tests were applied. Comparisons of uninjured muscle from OVX+E2 and OVX mice were done using an independent Student's t test. Muscle strength data were analysed using a two‐way repeated measures ANOVA (oestradiol condition × time) with time as the repeated factor. Because a significant interaction occurred, user‐defined contrasts with a Benjamini–Hochberg false discovery rate correction were used as post hoc tests. Circulating oestradiol levels for OVX and OVX+E2 mice were compared with ovary‐intact mice using independent t tests. With the exception of the qPCR data, all statistical testing was conducted using SPSS Statistics v. 24 (IBM Corp. Armonk, NY, USA) and values are reported as means (SD). An overall α level of 0.05 was used in all analyses.
Results
Circulating oestradiol levels
Mean circulating oestradiol in OVX (n = 10) and OVX+E2 (n = 11) mice was 5.9 (4.9) and 28.4 (15.1) pg ml−1, respectively. For comparison, the level in ovary‐intact mice (n = 13) was 13.7 (10.8) pg ml−1. The values for OVX and OVX+E2 mice were significantly lower and greater, respectively, from that for ovary‐intact mice (P ≤ 0.047).
Gene expression of chemokines, cytokines and receptors in injured muscles
Genes encoding inflammatory chemokines, cytokines and their receptors were screened to determine which were altered as a result of injury and by oestradiol. Muscle injury altered the expression of 68 of the 84 genes on the gene array (Table 1). Of the 68 genes altered, expression of 20 was modulated by the presence of oestradiol (Table 1); eight genes were significantly up‐regulated (1.2‐ to 3.0‐fold) and 12 were down‐regulated by oestradiol (0.28‐ to 0.85‐fold). Of the eight oestradiol‐modulated genes at 1 day post‐injury, six were up‐regulated (Ccl2, Ccl6, Ccl24, Ccr1, Cxcl1 and Cxcl5; Table 1); these are mostly chemokines that modulate neutrophil or macrophage activities. Of the 14 oestradiol‐modulated genes at 3 days post‐injury, 11 were down‐regulated (Ccl5, Ccl22, Ccr3, Ccr7, Cxcl9, Cxcl10, Ifng, Il5rα, Il‐10rα, Il‐15 and Il‐17b; Table 1); these are mediators that are primarily pro‐inflammatory in nature or modulate T‐cell migration.
Quantitative PCR using TaqMan methodology confirmed that gene expression of the neutrophil chemoattractants, Cxcl1 and Cxcl5, was greater in TA muscle from OVX+E2 than OVX+placebo mice at 1 and 2 days post‐injury (Fig. 1 A and B). The expression of Ccl2, the strong monocyte chemoattractant and activation factor, however, was not found to differ significantly between muscles from OVX+placebo and OVX+E2 mice at any time point post‐injury (Fig. 1 C). Confirming the gene array results, the expression of the chemoattractant for monocytes and macrophages (Spp1) was higher at 3 and 4 days post‐injury in TA muscles from OVX+E2 mice as compared to muscles from OVX+placebo mice (Fig. 1 D).
Figure 1. Effect of oestradiol on gene expression of inflammatory chemokines Cxcl1 (A), Cxcl5 (B), Ccl2 (C) and Spp1 (D) in injured skeletal muscle.
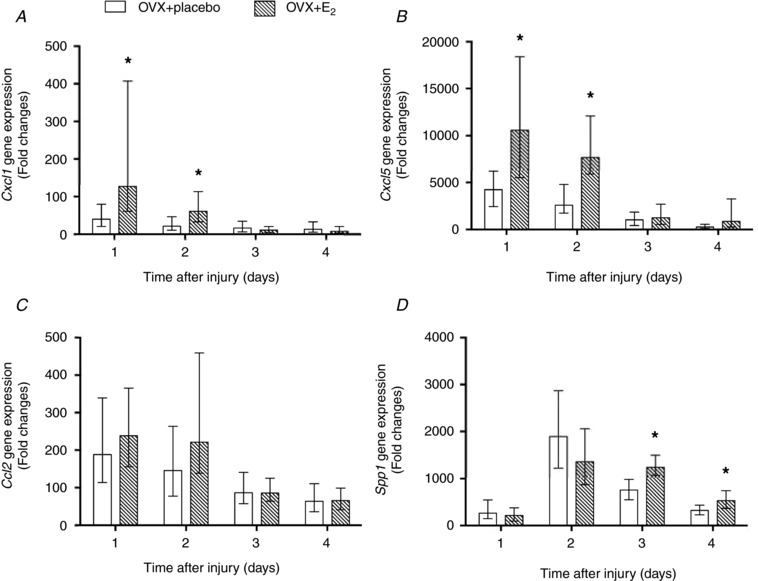
TA muscles from OVX+placebo and OVX+E2 mice at 1, 2, 3 and 4 days post‐injury were analysed for mRNA transcripts using quantitative TaqMan PCR. Data are presented as fold expression relative to control (uninjured, OVX+placebo muscle). Data are means ± SEM, with the SEM being calculated via a complex Taylor‐series algorithm (Pfaffl et al. 2002). *Significantly different from OVX+placebo at the corresponding time point (n = 5 per condition per time point).
Gene expression of leukocyte cell surface markers in injured muscles
Gene expression of cell surface markers for neutrophils (Ly6g), M1 macrophages (Cd68), M2a/M2c macrophages (Cd206) and M2c macrophages (Cd163) was affected by oestradiol (Fig. 2), although the pan‐leukocyte marker (Cd11b) was not (P ≥ 0.33; data not shown). Ly6g expression in injured muscle of OVX+E2 mice was 10‐ to 18‐fold that of OVX+placebo mice 1–4 days post‐injury (Fig. 2 A). Similarly, Cd163 expression in injured muscle of OVX+E2 mice was 2‐ to 5‐fold that of OVX+placebo mice (Fig. 2 D).
Figure 2. Effect of oestradiol on gene expression of phagocyte cell surface markers Ly6g (A), Cd68 (B), Cd206 (C) and Cd163 (D) in injured skeletal muscle.
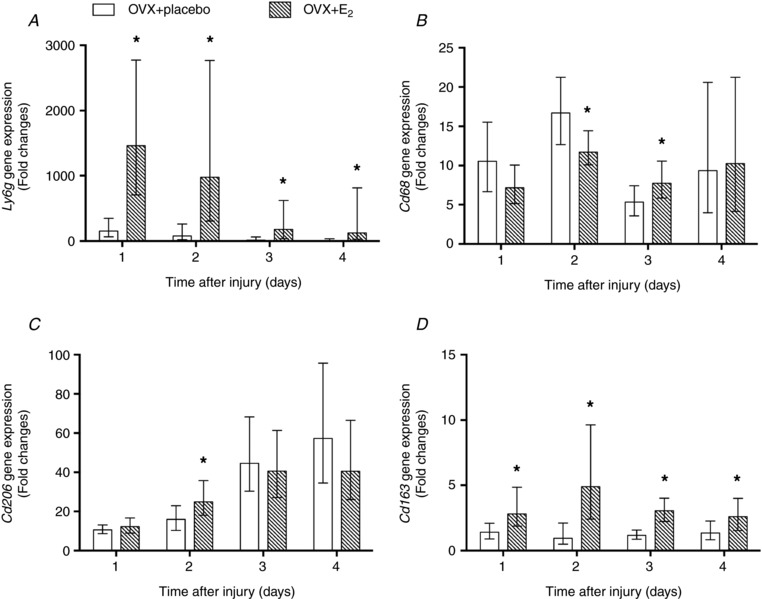
TA muscles from OVX+placebo and OVX+E2 mice at 1, 2, 3 and 4 days post‐injury were analysed for mRNA transcripts using quantitative TaqMan PCR. Data are presented as fold expression relative to control (uninjured, OVX+placebo muscle). Data are means ± SEM, with the SEM being calculated via a complex Taylor‐series algorithm (Pfaffl et al. 2002). *Significantly different from OVX+placebo at the corresponding time point (n = 5 per condition per time point).
LY6G/C and CD163 protein content in injured muscles
To determine the extent that changes in gene expression represented changes in protein content, we measured protein levels of cell surface markers for neutrophils and M2c macrophages in the injured muscles. Figure 3 shows representative immunoblotting of LY6G/C and CD163 protein in injured TA muscles from OVX and OVX+E2 mice and quantitation of such blots. LY6G/C content was 60–102% greater in injured TA muscle from OVX+E2 compared to OVX mice (Fig. 3 A), consistent with our gene expression results. However, CD163 protein content in injured muscle did not differ significantly between OVX and OVX+E2 groups (P = 0.064; Fig. 3 B).
Figure 3. Effect of oestradiol on protein content of phagocyte cell surface markers in injured TA muscle.
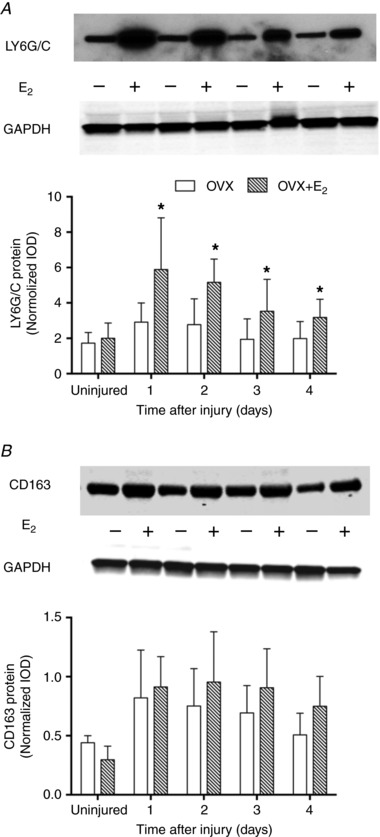
Representative immunoblot and semi‐quantitative analysis for neutrophil‐specific LY6G/C (A) and M2c macrophage‐specific CD163 (B) in uninjured muscle and at 1, 2, 3 and 4 days post‐injury. Data are means (SD). *Significantly different from OVX at the corresponding time point (n = 5 per condition per time point).
Myeloperoxidase enzyme activity in injured muscles
To support the LY6G/C protein content results, we determined how oestradiol affected myeloperoxidase activity, an indicator of neutrophil activity, in TA muscles. Compared to OVX mice, injured muscles from OVX+E2 mice had 15–56% greater myeloperoxidase activity (Fig. 4).
Figure 4. Effect of oestradiol on myeloperoxidase enzyme activity in injured TA muscle.
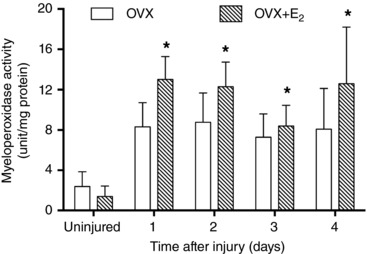
Myeloperoxidase enzyme activity, indicative of neutrophil cell function, was measured in uninjured muscle and at 1, 2, 3 and 4 days post‐injury. Data are means (SD). *Significantly different from OVX at the corresponding time point (n = 5 per condition per time point).
FACS analyses of phagocytes in injured muscles
To quantify phagocytes infiltrated into muscle before and after injury, we measured neutrophils and sub‐populations of macrophages (M1 and M2a/M2c) by FACS analysis. Figure 5 shows representative FACS plots of three populations of CD45+ cells that were separated to identify neutrophils from TA muscles of OVX and OVX+E2 mice. The proportion of CD45+ cells that were neutrophils (CD11b+LY6G+ cells) was 12–61% greater in injured TA muscles from OVX+E2 than OVX mice (Fig. 6 A). The proportions of CD45+ cells in TA muscles that were M1 (CD11b+CD68+ cells) or M2a/M2c (CD11b+CD206+ cells) macrophages were not affected by the oestradiol condition (P ≥ 0.41; Fig. 6 B and C). Anti‐CD163 antibody for FACS was not available, limiting our ability to quantify infiltrated M2c macrophages in injured muscle.
Figure 5. Representative plots of fluorescence activated cell sorting (FACS) of CD45+ cells in uninjured and injured TA muscles.
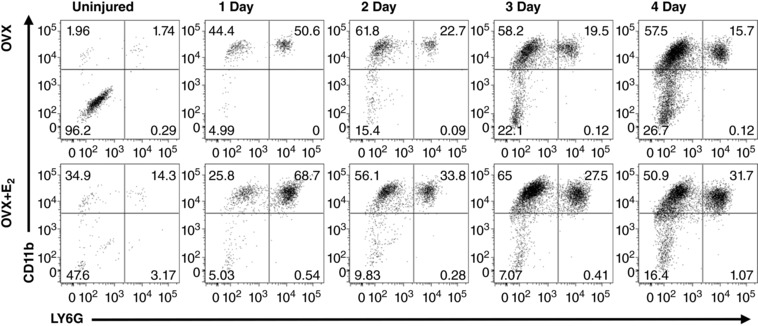
TA muscles from OVX mice (top panels) and OVX+E2 mice (bottom panels) were analysed for CD45+ cell populations repartitioned by FACS to identify neutrophils. CD11b+LY6G− (leukocytes such as monocytes and macrophages; upper left quadrant of each panel), CD11b+LY6G+ (neutrophils; upper right quadrant of each panel) and CD11b−LY6G− (e.g. erythrocytes, fibroblasts, adipocytes, endothelial cells; lower left of each panel) are shown in representative FACS analysis dot plots for each time point.
Figure 6. Effect of oestradiol on the infiltration of neutrophils and macrophages in injured TA muscles.
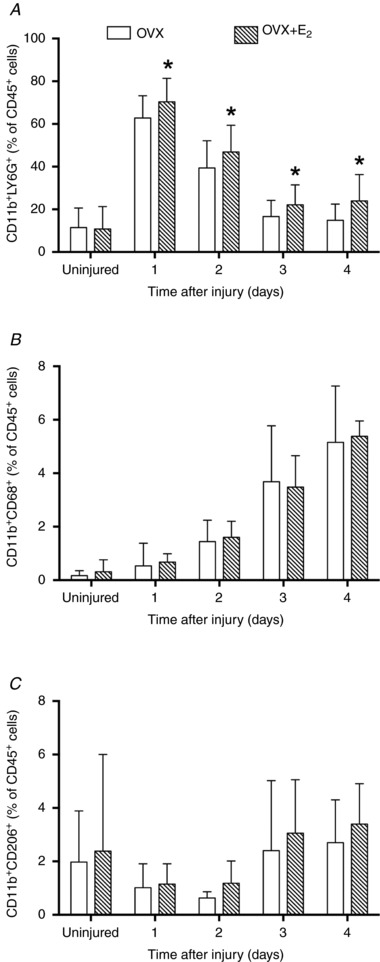
Neutrophils (CD11b+LY6G+ cells), M1 macrophages (CD11b+CD68+ cells) or M2a/M2c macrophages (CD11b+CD206+ cells) were sorted by FACS and presented as a percentage of total CD45+ cells in uninjured muscle and at 1, 2, 3 and 4 days post‐injury. Data are means (SD). *Significantly different from OVX at the corresponding time point (n = 5–6 per condition per time point).
Recovery of strength following injury
Isometric strength of the anterior crural muscles before injury did not differ significantly (P = 0.27) between OVX+placebo and OVX+E2 mice (2.31 (0.32) vs. 2.42 (0.15) mN m, respectively; Fig. 7). The loss of strength immediately after injury also did not differ between the two groups (79 (9)% vs. 76 (6)% decrement). Strength recovery was identical for OVX+placebo and OVX+E2 mice out to 7 days post‐injury. However, strength recovery in OVX+E2 mice accelerated over the second week post‐injury; strength in OVX+E2 mice was 13–24% greater than that in OVX+placebo mice from 14 days post‐injury and on. The OVX+placebo mice never caught up with the OVX+E2 mice even after 6 weeks of recovery.
Figure 7. Effect of oestradiol on recovery of muscle strength after injury.
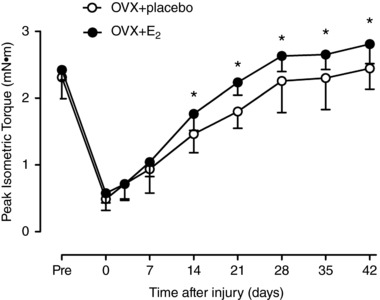
Maximal isometric torque of the left anterior crural muscles in OVX+placebo and OVX+E2 mice was measured immediately before and after a freeze injury to the TA muscle and re‐measured 3, 7, 14, 21, 28, 35 and 42 days post‐injury. Data are means (SD). *Significantly different from OVX+placebo at the corresponding time point (n = 12–13 per group).
Discussion
Our findings, in general, argue for a strong modulation of inflammation by oestradiol following a traumatic injury to skeletal muscle. Gene expression of several chemokines and cytokines was markedly altered by oestradiol after injury and many of these changes are associated with activation or recruitment of neutrophils, macrophages and/or monocytes. These changes in gene expression were in turn associated with large increases in expression of the neutrophil cell surface marker Ly6g and more modest increases in the anti‐inflammatory (M2) macrophage cell surface markers Cd163 and Cd206 over the first 4 days post‐injury. These oestradiol‐induced increases in gene expression were accompanied by increases at the protein level for the LY6G/C (but not CD163) cell surface marker as determined by immunoblot. The changes observed at the gene and protein levels are indicative of oestradiol‐enhanced migration of neutrophils into the injured muscle. This was confirmed by the increased neutrophil cell counts observed in injured muscles from oestradiol‐treated mice as indicated by FACS and indirectly by the myeloperoxidase activity assays. Finally, the modulation of inflammatory processes by oestradiol was associated with improved recovery of strength in injured muscles from oestradiol‐treated mice.
For the 14 rodent studies mentioned in the introduction that studied the effect of female sex or oestradiol on the inflammatory response in injured muscle, seven found oestradiol or the female sex to reduce the response (St Pierre Schneider et al. 1999; Tiidus & Bombardier, 1999; Stupka & Tiidus, 2001; Tiidus et al. 2001, 2005b; Enns et al. 2008; Iqbal et al. 2008) but four studies found the contrary (Tiidus et al. 2005a; Schneider et al. 2012; Fulkerson et al. 2015; Fearing et al. 2016). The present study's findings agree with that of the latter group. The explanation for the disparate findings is not clear. The explanation does not appear to be due to the type of injury induced (i.e. exercise‐ or eccentric contraction‐induced vs. ischaemia–reperfusion vs. toxin injection vs. freeze), whether mice or rats were used, the particular muscle that was injured, time of assessment after injury or the measure used to assess inflammation (i.e. histology vs. myeloperoxidase activity vs. gene expression vs. FACS).
A possible explanation for the discrepant observations among the animal studies may be related to the oestradiol dosage/level used. Oestradiol has been found to affect inflammation in a number of tissues in addition to skeletal muscle, including lung, reproductive, neural and several others (Straub, 2007). It appears that for inflammatory responses in most tissues without a strong B‐cell involvement, oestrogens generally stimulate inflammatory processes at relatively low concentrations typical of a non‐pregnant, ovary‐intact animal but suppress them at higher concentrations such as those seen during pregnancy (Straub, 2007).
Unfortunately, it is not clear what constitutes the dividing line between low and high levels of oestrogen. This is further complicated by the insensitivity and inaccuracy of essentially all ELISAs that are commonly used to determine circulating oestradiol levels (Rosner et al. 2013; Newman & Handelsman, 2014; Ketha et al. 2015). These assays are only considered appropriate for assays of serum oestradiol levels typical of healthy premenopausal women (Newman & Handelsman, 2014). Lower serum levels, such as those observed in postmenopausal women, men, children and intact rodents tend to fall well below the low end of levels in premenopausal women (∼100 pg ml−1) (Sherman & Korenman, 1975; Lee et al. 1988; Teirmaa et al. 1998). Of the 14 rodent studies previously mentioned, measurement of serum oestradiol level was not attempted in eight studies (St Pierre Schneider et al. 1999; Tiidus & Bombardier, 1999; Schneider et al. 2002, 2012; Iqbal et al. 2008; Velders et al. 2012; Fulkerson et al. 2015; Fearing et al. 2016), though it should have been in five because those studies used oestradiol supplementation (Tiidus & Bombardier, 1999; Iqbal et al. 2008; Schneider et al. 2012; Velders et al. 2012; Fulkerson et al. 2015). For the six studies where serum oestradiol was measured, all six reported serum levels for oestradiol‐treated rodents that appear to be above, many well above, the physiological range for intact female rodents that are not pregnant. Five of these six studies used oestradiol supplementation to study oestradiol effects on inflammation; four found oestradiol to inhibit inflammation (Stupka & Tiidus, 2001; Tiidus et al. 2001, 2005b; Enns et al. 2008) while one found oestradiol to enhance inflammation (Tiidus et al. 2005a). These observations tend to support the claim from the Straub review (Straub, 2007) that high levels of oestrogen inhibit inflammation in most tissues. Mean circulating oestradiol levels for OVX+E2 and intact female mice in the present study were numerically similar (i.e. 28 vs. 14 pg ml−1). Peak circulating oestradiol levels in pregnant mice are 3‐ to 4‐ fold greater than in non‐pregnant mice and constitute ‘high’ levels (Barkley et al. 1979; Zhang et al. 1999). Presumably, the levels we measured in OVX+E2 mice would constitute ‘low’ levels according to Straub and thus it would make sense that oestradiol was observed to enhance inflammatory processes in our study. Obviously, the oestradiol level in an OVX animal would constitute ‘too low’ a level to modulate inflammation.
If low levels of oestrogen enhance inflammatory responses in injured muscle, one might wonder how strength recovered more robustly in our OVX+E2 mice than in OVX+placebo mice. Based on older reviews of the exercise‐induced muscle injury literature (Armstrong et al. 1991; Faulkner et al. 1993), one might assume that inflammation exacerbates the initial muscle injury resulting in what is referred to as a secondary injury. However, our recent systematic review and meta‐analysis indicates that there is minimal evidence for a secondary injury, at least one that results in an additional strength loss (Warren et al. 2017). Furthermore, we have demonstrated in three previous studies that elimination of the cytokine tumour necrosis factor α, or the chemokine monocyte chemoattractant protein‐1 (CCL2) and/or their receptors can impair the strength recovery of injured muscle (Warren et al. 2002, 2004, 2005). Presumably, this is because of the essential nature of these inflammatory processes, which are needed to remove damaged tissue before regeneration can begin. It is possible that a quicker and more substantial inflammation, such as that induced by oestradiol, might speed up the degeneration and regeneration processes. It is likely that enhanced inflammatory processes do not result in a secondary injury because they are constrained to the tissue portions initially injured.
The time course for the oestradiol effect on strength recovery that we observed in the present study was very similar to those in our previous studies where we manipulated inflammatory processes after injury by eliminating cytokines and/or their receptors (Warren et al. 2002, 2004, 2005). In both the present and previous studies, the manipulation of inflammatory processes was not associated with an altered strength recovery until approximately 2 weeks post‐injury despite the largest effects on inflammation being manifested in the first few days post‐injury. These observations argue for oestradiol's beneficial effect on strength recovery being manifested by its effect on inflammation. However, we cannot make a cause‐and‐effect claim that the oestradiol‐increased inflammation is responsible for the enhanced strength recovery. An oestradiol effect on satellite cell number and/or myoblasts is an equally plausible explanation for the oestradiol‐enhanced strength recovery (Velders & Diel, 2013; Mangan et al. 2014). However, these two potential mechanisms for explaining oestradiol's beneficial effect on strength recovery may be intertwined. There is strong evidence for inflammatory processes modulating muscle regenerative processes, including effects on myogenesis (Chazaud et al. 2009) and overall cellular regenerative responses of mesenchymal cells and satellite cells (Heredia et al. 2013). Thus, a beneficial effect of oestradiol on strength recovery may be manifested indirectly by its effect on inflammatory processes, which in turn enhance myogenic processes during regeneration. Studies are currently ongoing in attempting to tease out the possible mechanisms for the oestradiol‐enhanced strength recovery and will need to be combined with muscle histological assessments (e.g. regenerating fibre size, fibrotic area) before the oestradiol effect on strength recovery can be fully explained.
In conclusion, chronic administration of oestradiol to ovariectomized mice such that serum levels are comparable to those of intact female mice results in enhanced inflammation and a faster, more complete strength recovery following traumatic skeletal muscle injury. The oestradiol effect on inflammatory processes is primarily that associated with neutrophils and to a lesser extent anti‐inflammatory (M2) macrophages. Additional research is needed to confirm these findings. When an animal is supplemented with oestradiol, attention needs to be paid to the serum level that results. Low physiological levels, but above that of ovariectomized animals, may elicit results opposite those of studies using high physiological levels (e.g. as during pregnancy) or supraphysiological levels. As a final point, in studies where oestradiol is supplemented, assessment of circulating oestradiol levels using a reliable and valid methodology (i.e. preferably LC‐MS/MS) should be mandatory. Much of the uncertainty about oestradiol's effect on inflammation can be attributed to the uncertainty of the oestradiol levels used during those studies.
Additional information
Competing interests
The authors do not have competing interests.
Author contributions
This work was performed in the laboratory of D.A.L. G.L., D.A.L. and G.L.W.: conception and design of research; G.L., S.A.N., T.L.M., S.M.G., S.C., M.K., D.A.L. and G.L.W. performed acquisition, analysis or interpretation of the data; G.L., S.A.N., T.L.M., S.M.G., S.C., M.K., D.A.L. and G.L.W. drafted or revised the manuscript. All authors have read and approved the final version of this manuscript and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All persons designated as authors qualify for authorship, and all those who qualify for authorship are listed.
Funding
Our research has been supported by NIH Grants R01‐AG031743 (D.A.L. and G.L.W.), T32‐AR050938 (G.L.), T32‐AR007612 (S.A.N.), T32‐AG0299796 (T.L.M.), and a grant from the Office of the Vice President for Research, University of Minnesota (D.A.L.).
Acknowledgements
We acknowledge Matthew Jergenson, Angela Greising, Natalie Wall and Dr Shaojuan Lai for assistance with surgery, Yi Ren and Dr Robert Arpke for assistance with FACS, Drs Angus Lindsay, Ebbing De Jong and Bruce Witthuhn for assistance with LC‐MS/MS and Dr John Hughes (School of Public Health) for bio‐statistical consultation.
Biography
Gengyun Le is a postdoctoral fellow supported by a National Institutes of Health T32 training grant at the University of Minnesota Twin Cities. She received her PhD in Physiology at the University of Hong Kong and joined D.A.L.’s lab in 2014, focusing on investigating how oestrogens influence inflammatory responses following skeletal muscle injury. She is particularly interested in how oestradiol‐sensitive inflammatory chemokines impact recovery of muscle strength after injury in females, as well as understanding the underlying cellular and molecular mechanisms.

Edited by: Scott Powers & Troy Hornberger
Linked articles This article is highlighted in a Perspectives article by Tiidus. To read this article, visit https://doi.org/10.1113/JP276870.
References
- Apolloni S, Fabbrizio P, Amadio S & Volonte C (2016). Actions of the antihistaminergic clemastine on presymptomatic SOD1‐G93A mice ameliorate ALS disease progression. J Neuroinflammation 13, 191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong RB, Warren GL & Warren JA (1991). Mechanisms of exercise‐induced muscle fibre injury. Sports Med 12, 184–207. [DOI] [PubMed] [Google Scholar]
- Baltgalvis KA, Greising SM, Warren GL & Lowe DA (2010). Estrogen regulates estrogen receptors and antioxidant gene expression in mouse skeletal muscle. PLoS One 5, e10164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barkley MS, Geschwind II & Bradford GE (1979). The gestational pattern of estradiol, testosterone and progesterone secretion in selected strains of mice. Biol Reprod 20, 733–738. [DOI] [PubMed] [Google Scholar]
- Chazaud B, Brigitte M, Yacoub‐Youssef H, Arnold L, Gherardi R, Sonnet C, Lafuste P & Chretien F (2009). Dual and beneficial roles of macrophages during skeletal muscle regeneration. Exerc Sport Sci Rev 37, 18–22. [DOI] [PubMed] [Google Scholar]
- Clarkson PM & Hubal MJ (2001). Are women less susceptible to exercise‐induced muscle damage? Curr Opin Clin Nutr Metab Care 4, 527–531. [DOI] [PubMed] [Google Scholar]
- Dieli‐Conwright CM, Spektor TM, Rice JC, Sattler FR & Schroeder ET (2009). Hormone therapy attenuates exercise‐induced skeletal muscle damage in postmenopausal women. J Appl Physiol (1985) 107, 853–858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enns DL, Iqbal S & Tiidus PM (2008). Oestrogen receptors mediate oestrogen‐induced increases in post‐exercise rat skeletal muscle satellite cells. Acta Physiol (Oxf) 194, 81–93. [DOI] [PubMed] [Google Scholar]
- Enns DL & Tiidus PM (2010). The influence of estrogen on skeletal muscle: sex matters. Sports Med 40, 41–58. [DOI] [PubMed] [Google Scholar]
- Faulkner JA, Brooks SV & Opiteck JA (1993). Injury to skeletal muscle fibers during contractions: conditions of occurrence and prevention. Phys Ther 73, 911–921. [DOI] [PubMed] [Google Scholar]
- Fearing CM, Melton DW, Lei X, Hancock H, Wang H, Sarwar ZU, Porter L, McHale M, McManus LM & Shireman PK (2016). Increased adipocyte area in injured muscle with aging and impaired remodeling in female mice. J Gerontol A Biol Sci Med Sci 71, 992–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulkerson ND, Nicholas J & St Pierre Schneider B (2015). Estrogen modulates 7/4 antigen distribution within eccentrically contracted injured skeletal muscle. Biotech Histochem 90, 294–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heredia JE, Mukundan L, Chen FM, Mueller AA, Deo RC, Locksley RM, Rando TA & Chawla A (2013). Type 2 innate signals stimulate fibro/adipogenic progenitors to facilitate muscle regeneration. Cell 153, 376–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iqbal S, Thomas A, Bunyan K & Tiidus PM (2008). Progesterone and estrogen influence postexercise leukocyte infiltration in overiectomized female rats. Appl Physiol Nutr Metab 33, 1207–1212. [DOI] [PubMed] [Google Scholar]
- Jutila MA, Kroese FG, Jutila KL, Stall AM, Fiering S, Herzenberg LA, Berg EL & Butcher EC (1988). Ly‐6C is a monocyte/macrophage and endothelial cell differentiation antigen regulated by interferon‐gamma. Eur J Immunol 18, 1819–1826. [DOI] [PubMed] [Google Scholar]
- Kendall B & Eston R (2002). Exercise‐induced muscle damage and the potential protective role of estrogen. Sports Med 32, 103–123. [DOI] [PubMed] [Google Scholar]
- Ketha H, Girtman A & Singh RJ (2015). Estradiol assays – The path ahead. Steroids 99, 39–44. [DOI] [PubMed] [Google Scholar]
- Lee SJ, Lenton EA, Sexton L & Cooke ID (1988). The effect of age on the cyclical patterns of plasma LH, FSH, oestradiol and progesterone in women with regular menstrual cycles. Hum Reprod 3, 851–855. [DOI] [PubMed] [Google Scholar]
- MacIntyre DL, Reid WD, Lyster DM & McKenzie DC (2000). Different effects of strenuous eccentric exercise on the accumulation of neutrophils in muscle in women and men. Eur J Appl Physiol 81, 47–53. [DOI] [PubMed] [Google Scholar]
- MacNeil LG, Baker SK, Stevic I & Tarnopolsky MA (2011). 17β‐estradiol attenuates exercise‐induced neutrophil infiltration in men. Am J Physiol Regul Integr Comp Physiol 300, R1443–R1451. [DOI] [PubMed] [Google Scholar]
- Mangan G, Bombardier E, Mitchell AS, Quadrilatero J & Tiidus PM (2014). Oestrogen‐dependent satellite cell activation and proliferation following a running exercise occurs via the PI3K signalling pathway and not IGF‐1. Acta Physiol (Oxf) 212, 75–85. [DOI] [PubMed] [Google Scholar]
- McHale MJ, Sarwar ZU, Cardenas DP, Porter L, Salinas AS, Michalek JE, McManus LM & Shireman PK (2012). Increased fat deposition in injured skeletal muscle is regulated by sex‐specific hormones. Am J Physiol Regul Integr Comp Physiol 302, R331–R339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran AL, Nelson SA, Landisch RM, Warren GL & Lowe DA (2007). Estradiol replacement reverses ovariectomy‐induced muscle contractile and myosin dysfunction in mature female mice. J Appl Physiol (1985) 102, 1387–1393. [DOI] [PubMed] [Google Scholar]
- Newman JD & Handelsman DJ (2014). Challenges to the measurement of oestradiol: comments on an Endocrine Society position statement. Clin Biochem Rev 35, 75–79. [PMC free article] [PubMed] [Google Scholar]
- Pfaffl MW, Horgan GW & Dempfle L (2002). Relative expression software tool (REST) for group‐wise comparison and statistical analysis of relative expression results in real‐time PCR. Nucleic Acids Res 30, e36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulli B, Ali M, Forghani R, Schob S, Hsieh KL, Wojtkiewicz G, Linnoila JJ & Chen JW (2013). Measuring myeloperoxidase activity in biological samples. PLoS One 8, e67976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosner W, Hankinson SE, Sluss PM, Vesper HW & Wierman ME (2013). Challenges to the measurement of estradiol: an Endocrine Society position statement. J Clin Endocrinol Metab 98, 1376–1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider BS, Sannes H, Fine J & Best T (2002). Desmin characteristics of CD11b‐positive fibers after eccentric contractions. Med Sci Sports Exerc 34, 274–281. [DOI] [PubMed] [Google Scholar]
- Schneider BS, Vigil SA & Moonie S (2012). Body weight and leukocyte infiltration after an acute exercise‐related muscle injury in ovariectomized mice treated with estrogen and progesterone. Gen Comp Endocrinol 176, 144–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman BM & Korenman SG (1975). Hormonal characteristics of the human menstrual cycle throughout reproductive life. J Clin Invest 55, 699–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St Pierre Schneider B, Correia LA & Cannon JG (1999). Sex differences in leukocyte invasion in injured murine skeletal muscle. Res Nurs Health 22, 243–250. [DOI] [PubMed] [Google Scholar]
- Straub RH (2007). The complex role of estrogens in inflammation. Endocr Rev 28, 521–574. [DOI] [PubMed] [Google Scholar]
- Stupka N, Lowther S, Chorneyko K, Bourgeois JM, Hogben C & Tarnopolsky MA (2000). Gender differences in muscle inflammation after eccentric exercise. J Appl Physiol (1985) 89, 2325–2332. [DOI] [PubMed] [Google Scholar]
- Stupka N, Tarnopolsky MA, Yardley NJ & Phillips SM (2001). Cellular adaptation to repeated eccentric exercise‐induced muscle damage. J Appl Physiol (1985) 91, 1669–1678. [DOI] [PubMed] [Google Scholar]
- Stupka N & Tiidus PM (2001). Effects of ovariectomy and estrogen on ischemia‐reperfusion injury in hindlimbs of female rats. J Appl Physiol (1985) 91, 1828–1835. [DOI] [PubMed] [Google Scholar]
- Teirmaa T, Luukkaa V, Rouru J, Koulu M & Huupponen R (1998). Correlation between circulating leptin and luteinizing hormone during the menstrual cycle in normal‐weight women. Eur J Endocrinol 139, 190–194. [DOI] [PubMed] [Google Scholar]
- Tiidus PM (2003). Influence of estrogen on skeletal muscle damage, inflammation, and repair. Exerc Sport Sci Rev 31, 40–44. [DOI] [PubMed] [Google Scholar]
- Tiidus PM & Bombardier E (1999). Oestrogen attenuates post‐exercise myeloperoxidase activity in skeletal muscle of male rats. Acta Physiol Scand 166, 85–90. [DOI] [PubMed] [Google Scholar]
- Tiidus PM, Deller M, Bombardier E, Gul M & Liu XL (2005a). Estrogen supplementation failed to attenuate biochemical indices of neutrophil infiltration or damage in rat skeletal muscles following ischemia. Biol Res 38, 213–223. [DOI] [PubMed] [Google Scholar]
- Tiidus PM, Deller M & Liu XL (2005b). Oestrogen influence on myogenic satellite cells following downhill running in male rats: a preliminary study. Acta Physiol Scand 184, 67–72. [DOI] [PubMed] [Google Scholar]
- Tiidus PM, Holden D, Bombardier E, Zajchowski S, Enns D & Belcastro A (2001). Estrogen effect on post‐exercise skeletal muscle neutrophil infiltration and calpain activity. Can J Physiol Pharmacol 79, 400–406. [PubMed] [Google Scholar]
- Torres MJ, Kew KA, Ryan TE, Pennington ER, Lin CT, Buddo KA, Fix AM, Smith CA, Gilliam LA, Karvinen S, Lowe DA, Spangenburg EE, Zeczycki TN, Shaikh SR & Neufer PD (2018). 17β‐Estradiol directly lowers mitochondrial membrane microviscosity and improves bioenergetic function in skeletal muscle. Cell Metab 27, 167–179.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velders M & Diel P (2013). How sex hormones promote skeletal muscle regeneration. Sports Med 43, 1089–1100. [DOI] [PubMed] [Google Scholar]
- Velders M, Schleipen B, Fritzemeier KH, Zierau O & Diel P (2012). Selective estrogen receptor‐β activation stimulates skeletal muscle growth and regeneration. FASEB J 26, 1909–1920. [DOI] [PubMed] [Google Scholar]
- Warren GL, Call JA, Farthing AK & Baadom‐Piaro B (2017). Minimal evidence for a secondary loss of strength after an acute muscle injury: A systematic review and meta‐analysis. Sports Med 47, 41–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren GL, Hulderman T, Jensen N, McKinstry M, Mishra M, Luster MI & Simeonova PP (2002). Physiological role of tumor necrosis factor α in traumatic muscle injury. FASEB J 16, 1630–1632. [DOI] [PubMed] [Google Scholar]
- Warren GL, Hulderman T, Mishra D, Gao X, Millecchia L, O'Farrell L, Kuziel WA & Simeonova PP (2005). Chemokine receptor CCR2 involvement in skeletal muscle regeneration. FASEB J 19, 413–415. [DOI] [PubMed] [Google Scholar]
- Warren GL, Ingalls CP & Armstrong RB (1998). A stimulating nerve cuff for chronic in vivo measurements of torque produced about the ankle in the mouse. J Appl Physiol (1985) 84, 2171–2176. [DOI] [PubMed] [Google Scholar]
- Warren GL, Ingalls CP, Shah SJ & Armstrong RB (1999a). Uncoupling of in vivo torque production from EMG in mouse muscles injured by eccentric contractions. J Physiol 515, 609–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren GL, Lowe DA & Armstrong RB (1999b). Measurement tools used in the study of eccentric contraction‐induced injury. Sports Med 27, 43–59. [DOI] [PubMed] [Google Scholar]
- Warren GL, O'Farrell L, Summan M, Hulderman T, Mishra D, Luster MI, Kuziel WA & Simeonova PP (2004). Role of CC chemokines in skeletal muscle functional restoration after injury. Am J Physiol Cell Physiol 286, C1031–C1036. [DOI] [PubMed] [Google Scholar]
- Warren GL, Summan M, Gao X, Chapman R, Hulderman T & Simeonova PP (2007). Mechanisms of skeletal muscle injury and repair revealed by gene expression studies in mouse models. J Physiol 582, 825–841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wooding KM, Barkley RM, Hankin JA, Johnson CA, Bradford AP, Santoro N & Murphy RC (2013). Mechanism of formation of the major estradiol product ions following collisional activation of the molecular anion in a tandem quadrupole mass spectrometer. J Am Soc Mass Spectrom 24, 1451–1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Fishman MC & Huang PL (1999). Estrogen mediates the protective effects of pregnancy and chorionic gonadotropin in a mouse model of vascular injury. Arterioscler Thromb Vasc Biol 19, 2059–2065. [DOI] [PubMed] [Google Scholar]


