Abstract
Background
Long non‐coding RNAs (lncRNAs) participate in many biological dynamics and play significant roles in gene regulation. LncRNA expression is altered in many cancers; however, the expressions and functions of lncRNA genes in lung adenocarcinoma (LAD) remain unknown.
Methods
LncRNA and messenger RNA (mRNA) expression in LAD without lymphatic metastasis versus paired adjacent non‐tumor (ANT) lung tissues and LAD with versus without lymphatic metastasis were analyzed using Human LncRNA Arraystar V3.0. The expression levels of four downregulated and four upregulated lncRNAs were verified using quantitative real‐time PCR in cells and tissue specimens.
Results
In this study, 949 lncRNAs and 681 mRNAs had differential expression in LAD without lymphatic metastasis compared to ANT lung tissues, while 2740 lncRNAs and 1714 mRNAs were differentially expressed in LAD with lymphatic metastasis compared to LAD without lymphatic metastasis. The expression patterns of selected lncRNAs (LINC00113, AC005009.1, ARHGAP22‐IT1, AC009411.1, SRGAP3‐AS2, EGFEM1P, FAM66E, and HLA‐F‐AS1) were consistent with microarray data. Differentially expressed mRNA genes were enriched in crucial Gene Ontology terms and pathways.
Conclusion
Our results revealed differentially expressed lncRNAs in LAD, suggesting lncRNAs may be potential indicators for LAD diagnosis and therapy.
Keywords: Gene ontology, long non‐coding RNA, lung adenocarcinoma, lymphatic metastasis, microarray
Introduction
Long non‐coding RNAs (lncRNAs) have increasingly drawn attention among diverse non‐protein‐coding transcripts. LncRNAs refer to transcripts longer than 200 nucleotides without protein‐coding capacity. The number of lncRNA genes in humans is estimated to surpass protein‐coding genes, ranging from 20 000 to more than 100 000. LncRNAs are reported to implement various functions in many biological phenomena, from transcription regulation trans and in cis to the regulation of proteins or RNA molecules, such as messenger RNA (mRNA) expression modulation, which suggests a co‐regulated network. Despite a number of differences, such as the sense (mRNA) and antisense (lncRNA) directions of promoters and other divergent features, lncRNAs and mRNAs are actually alike.1 Nevertheless, the biological relevance and functional roles of a large proportion of lncRNAs and mRNAs are still poorly characterized.
Cancer causes 8 million deaths per year worldwide, with more than 1.6 million resulting from lung cancer.2, 3 In the United States in 2017 there were an estimated 222 500 new cases and 155 870 deaths related to the lung and bronchus, with 234 030 new cases and 154 050 deaths expected in 2018.4, 5 Lung cancer accounts for one in five cancer‐related deaths, making it the leading cause of mortality.6 The therapy applied and prognosis of lung cancer depends upon the different subtype and pathologic alterations. Lung cancer is mainly comprised of non‐small‐cell lung cancer (adenocarcinoma and squamous carcinoma) and small‐cell lung cancer.7 Lung adenocarcinoma (LAD), accounting for more than 40% of lung malignancies, is the most familiar subtype and one of the most difficult to treat in a clinical situation. Moreover, a metastatic signature tends to be associated with poorer prognosis, and the presence of lymphatic metastasis is a significant predictor of a poor outcome.
Herein, we analyzed lncRNA and mRNA expression patterns in LAD to determine the differentially expressed lncRNAs and mRNAs in LAD with or without lymphatic metastasis to examine the significance between them.
Methods
Cell lines and reagents
BEAS‐2B bronchial epithelial cells, H1299, Calu1, and SPC‐A1 and A549 human LAD cells were acquired from American Type Culture Collection (Manassas, VA, USA). The BEAS‐2B bronchial epithelial cells were cultivated in LHC‐9 medium, while the H1299, Calu1, and SPC‐A1 cell lines were cultivated in 10% fetal bovine serum (FBS) supplemented with RPMI‐1640 medium and A549 in 10% FBS supplemented Ham's F12 at 37°C 5% CO2.
Tissue collection
Fresh tissues were acquired from patients pathologically identified with LAD during surgery at the Affiliated Hospital of Weifang Medical University. Twenty LAD tissue specimens and corresponding adjacent non‐tumor (ANT) lung tissues from LAD patients with lymphatic metastasis and 20 specimens from LAD patients without lymphatic metastasis were collected, and three of each group were used in microarrays. None of the patients had been administered chemotherapy or radiotherapy before surgery. Patients provided informed consent for the use of tissue specimens and the hospital ethics committee approved the study. The clinical characteristics of the enrolled patients are listed in Table 1.
Table 1.
Clinical characteristics of the enrolled patients (n = 6)
| Sample no. | 1 | 2 | 3 | 4 | 5 | 6 |
|---|---|---|---|---|---|---|
| Gender | Female | Male | Female | Male | Female | Female |
| Age (years) | 63 | 48 | 48 | 57 | 61 | 43 |
| Smoking status | No | Yes | Yes | Yes | No | Yes |
| Tumor size (cm) | 2.5 × 2 × 1 | 2.5 × 2 × 1.5 | 2.1 × 1.5 × 1 | 5 × 4 × 2.5 | 3 × 3 × 2.5 | 1.5 × 1.5 × 2 |
| Tumor differentiation | Moderate | Moderate | Well | Moderate | Poorly | Moderate |
| Lymphatic metastasis | Negative | Negative | Negative | Positive | Positive | Positive |
Long non‐coding RNA (LncRNA) microarray
Microarray analysis of three pairs of LAD tissues and corresponding ANT lung tissues from patients with and without lymphatic metastasis was conducted. Microarrays were performed using Human LncRNA Array V3.0 (Arraystar, Rockville, Maryland, USA), to profile human lncRNAs and mRNAs. Microarray analysis was conducted by KangChen Bio‐tech (Shanghai, China). The Human LncRNA Array was designed with probes of transcriptional specificity and provided an overview of lncRNAs and mRNAs of the human genome.
Data analysis
Differentially expressed lncRNAs and mRNAs were filtered by fold change. Hierarchical clustering and scatter plots were used to visualize differences in lncRNA and mRNA expression. Gene ontology (GO) analysis was conducted using standard enrichment calculations. Pathway analysis was conducted based on data from the Kyoto Encyclopedia of Genes and Genomes database (KEGG, http://www.genome.jp/kegg).
Quantitive real‐time PCR (qRT‐PCR)
We made a random selection of four downregulated and four upregulated lncRNAs to verify the results of microarray analysis and then tested their expression using quantitative real‐time (qRT)‐PCR (≥ 2‐fold, P < 0.05). Twenty pairs each of LAD tissues without and without lymphatic metastasis and corresponding ANT lung tissues were used for validation. We extracted and reverse‐transcribed the total RNA of specimens and LAD cells into corresponding complementary DNA using a Reverse Transcription Kit (Promega, Madison, WI, USA), following the manufacturer's instructions. Standard qRT‐PCR was then performed with an Applied Biosystems 7500 FAST Real‐Time PCR System (Thermo Fisher Scientific, Waltham, MA, USA) using the following process: initialization of 30 seconds at 95°C, 35 PCR cycles including denaturation of 5 seconds at 95°C, annealing of 30 seconds at 63°C, and elongation of 30 seconds at 72°C for each cycle. The specific qRT‐PCR primer sequences are shown in Table 2. All analyses were performed in triplicate. The results were normalized and analyzed using the 2‐ΔΔCT method, with glyceraldehyde 3‐phosphate dehydrogenase (GAPDH) as an internal control.
Table 2.
Primer sequences for quantitative real‐time PCR
| Gene symbol | Forward sequence (5′‐3′) | Reverse sequence (5′‐3′) |
|---|---|---|
| LINC00113 | TGGCAATTACCTGGAGGAATGTC | AGGAAGTGAGTCTGATTGAAGCG |
| AC005009.1 | CCTACAGTCCTCCAGGAAGTCG | CCAGGAAGGATTCAAGGTGACC |
| ARHGAP22‐IT1 | ATGGTGGAGGTGGAGACAGTAC | TCTCAGAGGTGGCATGGAAGG |
| AC009411.1 | CCGTGTCTCCTTATCATCTTCCAG | GAGGTGGCGATCAGGATATGC |
| SRGAP3‐AS2 | CGTCATCCACACCACAGATCAG | TTGTTGGAGCCATCACAGCAC |
| EGFEM1P | AACAGTGCAGCAGTGACAGG | CCTCTCATTCTCTTGGACGATGG |
| FAM66E | AGGTCCGTCAGTCAGTCTTCC | TGGTTCTGTGGCTCCTGAGG |
| HLA‐F‐AS1 | TCCTAGTGGTCTCTGCTCTTCC | CCTCCTCTAACATGGTCCAATCTC |
| GAPDH | GTTGGAGGTCGGAGTCAACGG | GAGGGATCTCGCTCCTGGAGGA |
Statistical analysis
SPSS version 22.0 (IBM Corp., Armonk, NY, USA) was used to analyze the results, which were demonstrated as the means ± standard deviation of three independent trials. The significance between groups was evaluated by analysis of variance. Statistical significance was defined as P < 0.05.
Results
Differentially expressed lncRNAs and messenger RNAs
In order to illuminate the patterns of differentially expressed lncRNAs and mRNAs, we divided tissue specimens into two comparisons: one containing three LAD tissue samples without lymphatic metastasis (group‐Exp1) versus paired ANT lung tissues (group‐Ctrl), and the other with three LAD tissue samples with lymphatic metastasis (group‐Exp2) versus LAD samples without lymphatic metastasis (group‐Exp1). The high throughput analysis of lncRNAs and mRNAs for these two comparisons revealed a slight difference in the differentially expressed genes between the samples. Quantile normalization and subsequent data processing was conducted using Agilent Gene Spring GX software version 11.5.1 (Agilent Technologies, Santa Clara, CA, USA), using the lncRNA (Fig 1a,b) and mRNA (Fig 1c,d) signatures in the comparative groups.
Figure 1.

Long non‐coding RNA (lncRNA) and messenger RNA (mRNA) expression profiles. (a) Hierarchical clustering of lncRNA profiles in lung adenocarcinoma (LAD) without lymphatic metastasis (group‐Exp1) compared to adjacent non‐tumor (ANT) lung tissues (group‐ctrl), and (b) in LAD with lymphatic metastasis (group‐Exp2) versus LAD without lymphatic metastasis (group‐Exp1). (c) Hierarchical clustering of mRNA profiles in group‐Exp1 compared to group‐ctrl, and (d) in group‐Exp2 versus group‐Exp1. The expression level increases from green to red (≥ 2‐fold, P < 0.05).
The results of the comparison between LAD samples without lymphatic metastasis and ANT lung tissues revealed that 949 lncRNAs (450 upregulated, 499 downregulated) (Fig 2a) and 681 mRNAs (112 upregulated, 569 downregulated) were differentially expressed (≥ 2‐fold, P < 0.05) (Fig 2b). The results of comparison between LAD samples with and without lymphatic metastasis revealed that 2740 lncRNAs (1208 upregulated, 1532 downregulated) (Fig 2c) and 1714 mRNAs (400 upregulated, 1314 downregulated) were differentially expressed (≥ 2‐fold, P < 0.05) (Fig 2d).
Figure 2.
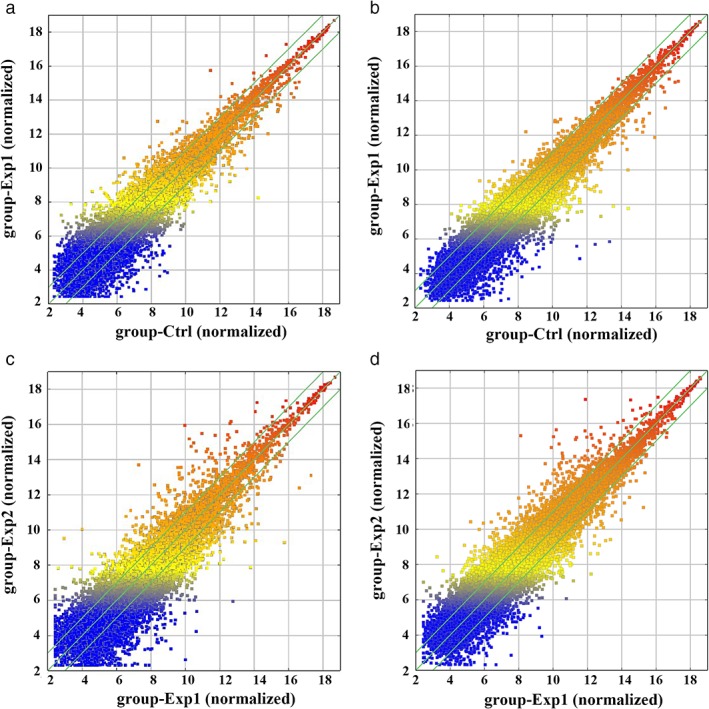
Long non‐coding RNA (lncRNA) and messenger RNA (mRNA) differential expression characteristics. A scatter plot shows the differences between (a) lncRNA and (b) mRNA expression in lung adenocarcinoma (LAD) without lymphatic metastasis (group‐Exp1) versus adjacent non‐tumor (ANT) lung tissues (group‐ctrl). A scatter plot of the differences in (c) lncRNA and (d) mRNA expression in LAD with lymphatic metastasis (group‐Exp2) versus LAD without lymphatic metastasis (group‐Exp1). The relative expression level increases from blue to red (≥ 2‐fold, P < 0.05).
Validation of microarray results by qRT‐PCR
To verify the microarray data, we performed qRT‐PCR to assess the levels of eight lncRNAs (4 downregulated, 4 upregulated genes; ≥ 2‐fold, P < 0.05). In qRT‐PCR of LAD samples without and without lymphatic metastasis and ANT lung tissues, the levels of SRGAP3‐AS2, EGFEM1P, FAM66E, and HLA‐F‐AS1 were downregulated, while the levels of LINC00113, AC005009.1, ARHGAP22‐IT1 and AC009411.1 were upregulated. ARHGAP22‐IT1 had a maximum log2 fold change (≥ 2‐fold, P < 0.05). The expression signatures of selected lncRNAs in the specimens were consistent with microarray data (Fig 3a). In the qRT‐PCR of LAD cells versus BEAS‐2B cells, parallel characteristics were identified. The expression of four upregulated genes, LINC00113, AC005009.1, ARHGAP22‐IT1, and AC009411.1, in SPC‐A1, A549, H1299, and Calu1 cells was higher than in BEAS‐2B cells (Fig 3b). Meanwhile, expression of the four downregulated genes SRGAP3‐AS2, EGFEM1P, FAM66E, and HLA‐F‐AS1, was lower in SPC‐A1, A549, H1299, and Calu1 compared to BEAS‐2B cells (Fig 3c).
Figure 3.

Validation of differentially expressed long non‐coding RNA (lncRNA) genes. (a) Comparison of lncRNA gene expression using microarray and quantitative real‐time PCR (qRT‐PCR) (log2 scaled) ( ) Microarray and (
) Microarray and ( ) qRT‐PCR. (b) Expression of upregulated genes in referred cell lines by qRT‐PCR (
) qRT‐PCR. (b) Expression of upregulated genes in referred cell lines by qRT‐PCR ( ) LINC00113, (
) LINC00113, ( ) AC005009.1, (
) AC005009.1, ( ) ARHGAP22‐IT1, and (
) ARHGAP22‐IT1, and ( ) AC009411.1. (c) Expression of downregulated genes in referred cell lines by qRT‐PCR (
) AC009411.1. (c) Expression of downregulated genes in referred cell lines by qRT‐PCR ( ) SRGAP3‐AS2, (
) SRGAP3‐AS2, ( ) EGFEM1P, (
) EGFEM1P, ( ) FAM66E and (
) FAM66E and ( ) HLA‐F‐AS1. (Triplicate assays were performed; P < 0.05).
) HLA‐F‐AS1. (Triplicate assays were performed; P < 0.05).
Gene Ontology analysis
Gene Ontology analysis defines the functions and description of genes and their products, and covers three aspects: biological process (BP), cellular component (CC), and molecular function (MF) (http://www.geneontology.org). We conducted GO analysis to identify enrichment of mRNAs. A GO term with a low P value indicated high significance (P ≤ 0.05).
The results showed that upregulated mRNA genes in LAD without lymphatic metastasis compared to ANT lung tissues were mainly involved in leukocyte migration, digestion, leukocyte chemotaxis, and regulation of system and multicellular organism processes. These genes were found in the cornified envelope, ruffle membrane, Golgi lumen, lateral plasma membrane, and cell leading edge. The MF of these genes included diacylglycerol kinase, voltage‐gated channel, NAD+ kinase and antiporter activities, and glycosaminoglycan binding (Fig 4a). Meanwhile, the downregulated genes in LAD without lymphatic metastasis compared to ANT lung tissues were involved in BP, including regulation of the multicellular organism process, response to external stimulus, and cellular component movement. We found high GO enrichment in motile cilium, ciliary cytoplasm, axoneme, cilium, cell projection cytoplasm, and other parts. The MF included protein binding, cytokine receptor activity, and microtubule motor activity (Fig 4b).
Figure 4.
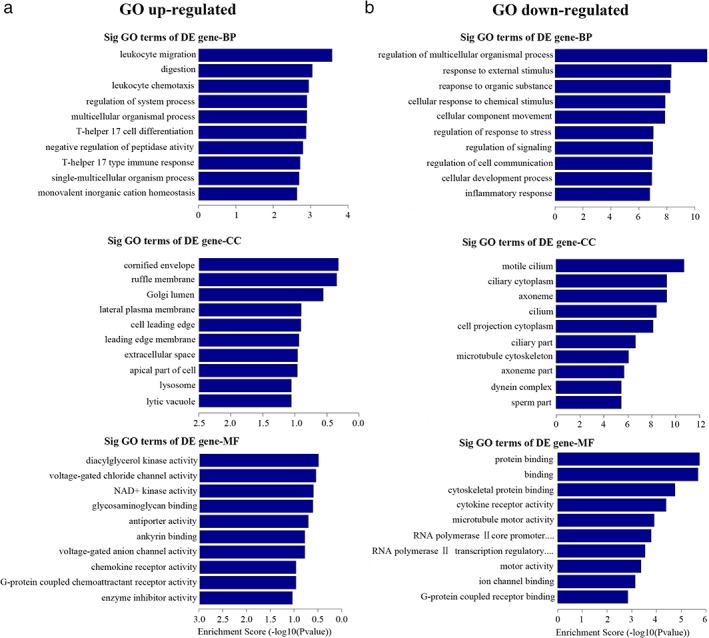
The enrichment Gene Ontology (GO) terms of differentially expressed messenger RNA (mRNA) genes in lung adenocarcinoma (LAD) without lymphatic metastasis compared to adjacent non‐tumor (ANT) lung tissues. Biological process (BP), cellular component (CC) and molecular function (MF) of (a) upregulated and (b) downregulated mRNAs (P < 0.05).
In the comparison of LAD with and without lymphatic metastasis, the GO terms with the most significant enrichment of upregulated mRNA genes were regulation of macromolecule metabolic, cellular process, and cellular responses to organic substance in BP. They were encountered in the cytoplasm and intracellular parts of CC. In regard to MF, various types of binding occurred, such as protein and ion binding (Fig 5a). Downregulated genes were found most frequently during cell‐cell adhesion, branching involved in the salivary gland, and homophilic cell adhesion in BP; and in mostly extracellular regions during CC. The prominently enriched GO terms of MF were glycosaminoglycan, heparin, and sulfur compound binding (Fig 5b).
Figure 5.

The enrichment Gene Ontology (GO) terms of differentially expressed (DE) messenger RNA (mRNA) genes in lung adenocarcinoma (LAD) with and without lymphatic metastasis. Biological process (BP), cellular component (CC), and molecular function (MF) of (a) upregulated and (b) downregulated mRNAs (P < 0.05).
Pathway analysis
We analyzed differentially expressed mRNAs based on KEGG, and the results demonstrated that six pathways were involved with upregulated genes in LAD without lymphatic metastasis compared to ANT lung tissues. The significant pathways containing more upregulated mRNAs were carbohydrate digestion and absorption, starch and sucrose metabolism, and African trypanosomiasis (Fig 6a). Meanwhile, 35 pathways corresponded to downregulated genes, including apoptosis, the MAPK and FoxO signaling pathways, and cytokine‐cytokine receptor interaction (Fig 6b).
Figure 6.
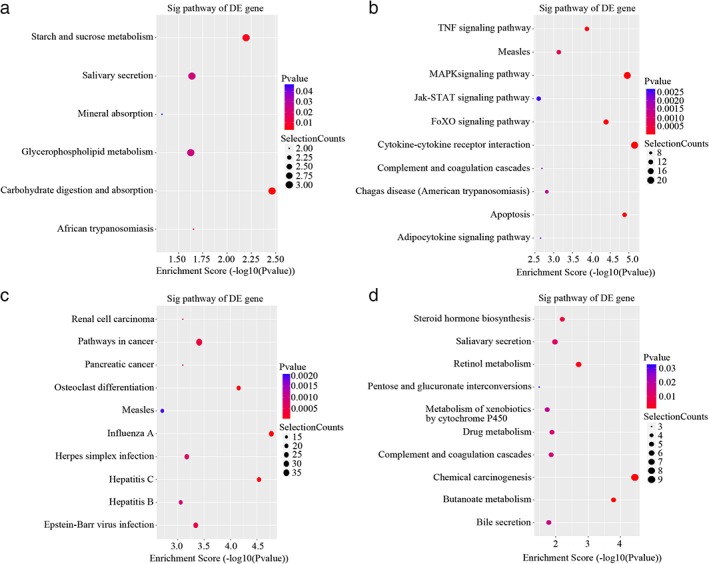
Kyoto Encyclopedia of Genes and Genomes pathway analysis. Significant enrichment pathways of differentially expressed (DE) (a) upregulated and (b) downregulated mRNAs in lung adenocarcinoma (LAD) without lymphatic metastasis compared to adjacent non‐tumor (ANT) lung tissues. Significant enrichment pathways of DE (c) upregulated and (d) downregulated mRNAs in LAD with and without lymphatic metastasis (P < 0.05).
On the other hand, the analysis between LAD with and without lymphatic metastasis showed that 37 pathways of upregulated mRNAs are significantly enriched, including Influenza A, Hepatitis C, osteoclast differentiation, pathways in cancer, and Epstein–Barr virus infection (Fig 6c). The high‐enrichment of 12 significant pathways of downregulated transcripts include chemical carcinogenesis, Butanoate and retinol metabolism, steroid hormone biosynthesis, and salivary secretion (Fig 6d).
Discussion
Over the past several years, lncRNAs have been extensively examined to determine their role in biological processes.8 LncRNAs function in numerous cellular contexts, including cell‐cell signaling and transcription regulation.9 Many lncRNAs show specific expression patterns from embryogenesis and organogenesis to tumorigenesis.10 Herriges et al. examined transcription factor expression in the foregut and found that lung endoderm development may be regulated by lncRNAs.11 Niland et al. reported that lncRNAs may link to certain human neurological disorders.12 Zhang et al. analyzed the expression patterns of lncRNA genes in skeletal muscle compared to brown adipose tissue.13
LncRNAs are also involved in carcinogenesis; as several cancer risk loci are transcribed into lncRNAs, a large number of cellular processes are therefore affected.14 Many studies have proved that the abnormal regulation of certain lncRNAs can result in the occurrence of malignancies and tumor development. Lu et al. found that LncRNA BC032469 can promote the proliferation of gastric cancer cells.15 Metastasis‐associated lung adenocarcinoma transcript 1 (Malat1) is a tumorigenic lncRNA with a strong correlation to various types of cancers.16 Upregulation of oncogenic lncRNA HOTAIR is clinically and functionally relevant to the development of breast, esophagus, and liver cancers.17, 18, 19
LncRNA expression is altered in numerous kinds of cancers compared to corresponding adjacent noncancerous tissues.20, 21 Xiong et al. found differentially expressed lncRNAs in colorectal cancer cells with 5‐FU‐based chemoradiation resistance compared to the corresponding parental cells.22 Huang et al. conducted microarray and identified significantly deregulated lncRNAs/circular RNAs and mRNAs in bladder cancer compared to ANT tissues.23
The correlation between lncRNAs and LAD has not been definitively determined. Several lncRNAs have been proposed as oncogenic in LAD, including LINC00707, LINC00319, and lncRNA MIR31HG.24, 25, 26 However, whether lncRNAs are related to the occurrence of LAD, and whether differentially expressed lncRNAs could be considered significant with the presence or absence of lymphatic metastasis has not yet been determined.
Many studies have used GO analysis to determine differentially expressed RNAs, both coding and non‐coding RNAs. GO can analyze different RNAs or annotate interaction in co‐expressed lncRNAs and coding RNAs. Yang et al. reported that the top enrichment term of coding genes was catalytic activity in trichostatin‐induced apoptosis liver cancer cells.27 GO analysis of lncRNAs in increasingly being applied develop and the results vary with different diseases. Lv et al. evaluated dysregulated lncRNAs in triple negative versus non‐triple negative breast cancer and discovered via GO analysis that lncRNAs are involved in many cellular and metabolic processes, as well as in diverse binding and ligase activities.28 Peng et al. analyzed aberrant lncRNA genes in LAD and found that the top GO terms are mainly enriched in cell adhesion, parts of the extracellular region, and cytoskeletal protein binding.29
In our comparison of LAD with and without lymphatic metastasis, we found that cell‐cell adhesion was downregulated in BP of GO, and when altered may facilitate the migration and metastasis of tumor cells. Upregulated genes in this comparison were enriched in mostly intracellular rather than extracellular regions where the downregulated genes were found. In the comparison of LAD without lymphatic metastasis compared to ANT lung tissues, some cell‐biological properties were found upregulated in KEGG pathway analysis, such as carbohydrate digestion and absorption” and starch and sucrose metabolism. These energy metabolism processes are reported to influence cell cycle and growth, but can also be deregulated by cancer cells.30 One of the pathways enriched by downregulated lncRNAs was apoptosis. Tumor cells evolve using various strategies to circumvent apoptosis and resist normal cell death, gaining unlimited proliferation as a result. Herein, we posited that the downregulation of apoptosis‐related genes might be associated with the development of LAD. Our pathway analysis showed that aberrant mRNAs were enriched in processes closely related to other cancer biology that encompassed the FoxO signaling pathway. As a subfamily of the forkhead transcription factor family, Forkhead box O (FOXO) is crucial to cellular consequences, and is also considered a cancer suppressor in different malignancies.31 Wang et al. found several enriched KEGG pathways in LAD among significantly upregulated genes, including the cell cycle and various metabolism‐related pathways, and downregulated genes, such as Rap1 signaling pathway.32 Sand et al. identified the highest enrichment scores in KEGG pathway analysis in basal cell carcinoma samples compared to control samples, including focal adhesion, ECM receptor interaction, and the PI3K‐Akt signaling pathway that regulates the cell cycle.33
Our microarray, GO, and pathway analyses of differentially expressed lncRNAs provide lncRNA and mRNA profiles in LAD with and without lymphatic metastasis. Our results may be used as a basis for gaining insight into the correlation of lncRNAs with lymphatic metastasis in LAD patients, and subsequently, prospective diagnosis biomarkers and specific treatment options based on these diverse pathological patterns. Further research using a larger cohort is warranted to validate our results.
Disclosure
No authors report any conflict of interest.
Acknowledgments
This study was supported by the National Natural Science Foundation of China (81641111, 81702932 and 81402389) and the Natural Science Foundation of Shandong Province (ZR2015HL065).
Zhiyi Yang and Hongli Li contributed equally to this work.
Contributor Information
Zhiyi Yang, Email: onesheeptwo@163.com.
Chonggao Yin, Email: ycglihongli@163.com.
References
- 1. Quinn JJ, Chang HY. Unique features of long non‐coding RNA biogenesis and function. Nat Rev Genet 2016; 17: 47–62. [DOI] [PubMed] [Google Scholar]
- 2. Torre LA, Siegel RL, Jemal A. Lung cancer statistics. Adv Exp Med Biol 2016; 893: 1–19. [DOI] [PubMed] [Google Scholar]
- 3. Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. (Published erratum appears in CA Cancer J Clin 2014; 64: 364.). CA Cancer J Clin 2014; 64: 9–29.24399786 [Google Scholar]
- 4. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin 2017; 67: 7–30. [DOI] [PubMed] [Google Scholar]
- 5. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin 2018; 68: 7–30. [DOI] [PubMed] [Google Scholar]
- 6. Rosenbaum JN, Bloom R, Forys JT et al Genomic heterogeneity of ALK fusion breakpoints in non‐small‐cell lung cancer. Mod Pathol 2018; 31: 791–808. [DOI] [PubMed] [Google Scholar]
- 7. Li H, Yin C, Zhang B et al PTTG1 promotes migration and invasion of human non‐small cell lung cancer cells and is modulated by miR‐186. Carcinogenesis 2013; 34: 2145–55. [DOI] [PubMed] [Google Scholar]
- 8. Kopp F, Mendell JT. Functional classification and experimental dissection of long noncoding RNAs. Cell 2018; 172: 393–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Geisler S, Coller J. RNA in unexpected places: Long non‐coding RNA functions in diverse cellular contexts. Nat Rev Mol Cell Biol 2013; 14: 699–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zhu JG, Shen YH, Liu HL et al Long noncoding RNAs expression profile of the developing mouse heart. J Cell Biochem 2014; 115: 910–8. [DOI] [PubMed] [Google Scholar]
- 11. Herriges MJ, Swarr DT, Morley MP et al Long noncoding RNAs are spatially correlated with transcription factors and regulate lung development. Genes Dev 2014; 28: 1363–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Niland CN, Merry CR, Khalil AM. Emerging roles for long non‐coding RNAs in cancer and neurological disorders. Front Genet 2012; 3: 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zhang J, Cui X, Shen Y et al Distinct expression profiles of LncRNAs between brown adipose tissue and skeletal muscle. Biochem Biophys Res Commun 2014; 443: 1028–34. [DOI] [PubMed] [Google Scholar]
- 14. Cheetham SW, Gruhl F, Mattick JS, Dinger ME. Long noncoding RNAs and the genetics of cancer. Br J Cancer 2013; 108: 2419–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lü MH, Tang B, Zeng S et al Long noncoding RNA BC032469, a novel competing endogenous RNA, upregulates hTERT expression by sponging miR‐1207‐5p and promotes proliferation in gastric cancer. Oncogene 2016; 35: 3524–34. [DOI] [PubMed] [Google Scholar]
- 16. Li S, Ma F, Jiang K, Shan H, Shi M, Chen B. Long non‐coding RNA metastasis associated lung adenocarcinoma transcript 1 promotes lung adenocarcinoma by directly interacting with specificity protein 1. Cancer Sci 2018; 109: 1346–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Li X, Wu Z, Mei Q et al Long non‐coding RNA HOTAIR, a driver of malignancy, predicts negative prognosis and exhibits oncogenic activity in oesophageal squamous cell carcinoma. Br J Cancer 2013; 109: 2266–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sorensen KP, Thomassen M, Tan Q et al Long non‐coding RNA HOTAIR is an independent prognostic marker of metastasis in estrogen receptor‐positive primary breast cancer. Breast Cancer Res Treat 2013; 142: 529–36. [DOI] [PubMed] [Google Scholar]
- 19. Li H, An J, Wu M et al LncRNA HOTAIR promotes human liver cancer stem cell malignant growth through downregulation of SETD2. Oncotarget 2015; 6: 27847–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Han L, Zhang K, Shi Z et al LncRNA profile of glioblastoma reveals the potential role of lncRNAs in contributing to glioblastoma pathogenesis. Int J Oncol 2012; 40: 2004–12. [DOI] [PubMed] [Google Scholar]
- 21. Li JP, Liu LH, Li J et al Microarray expression profile of long noncoding RNAs in human osteosarcoma. Biochem Biophys Res Commun 2013; 433: 200–6. [DOI] [PubMed] [Google Scholar]
- 22. Xiong W, Jiang YX, Ai YQ et al Microarray analysis of long non‐coding RNA expression profile associated with 5‐fluorouracil‐based chemoradiation resistance in colorectal cancer cells. Asian Pac J Cancer Prev 2015; 16: 3395–402. [DOI] [PubMed] [Google Scholar]
- 23. Huang M, Zhong Z, Lv M, Shu J, Tian Q, Chen J. Comprehensive analysis of differentially expressed profiles of lncRNAs and circRNAs with associated co‐expression and ceRNA networks in bladder carcinoma. Oncotarget 2016; 7: 47186–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ma T, Ma H, Zou Z et al The long intergenic noncoding RNA 00707 promotes lung adenocarcinoma cell proliferation and migration by regulating Cdc42. Cell Physiol Biochem 2018; 45: 1566–80. [DOI] [PubMed] [Google Scholar]
- 25. Zhang ZW, Chen JJ, Xia SH et al Long intergenic non‐protein coding RNA 319 aggravates lung adenocarcinoma carcinogenesis by modulating miR‐450b‐5p/EZH2. Gene 2018; 650: 60–7. [DOI] [PubMed] [Google Scholar]
- 26. Qin J, Ning H, Zhou Y, Hu Y, Yang L, Huang R. LncRNA MIR31HG overexpression serves as poor prognostic biomarker and promotes cells proliferation in lung adenocarcinoma. Biomed Pharmacother 2018; 99: 363–8. [DOI] [PubMed] [Google Scholar]
- 27. Yang H, Zhong Y, Xie H et al Induction of the liver cancer‐down‐regulated long noncoding RNA uc002mbe.2 mediates trichostatin‐induced apoptosis of liver cancer cells. Biochem Pharmacol 2013; 85: 1761–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lv M, Xu P, Wu Y et al LncRNAs as new biomarkers to differentiate triple negative breast cancer from non‐triple negative breast cancer. Oncotarget 2016; 7: 13047–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Peng Z, Wang J, Shan B et al Genome‐wide analyses of long noncoding RNA expression profiles in lung adenocarcinoma. Sci Rep 2017; 7: 15331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hanahan D, Weinberg RA. Hallmarks of cancer: The next generation. Cell 2011; 144: 646–74. [DOI] [PubMed] [Google Scholar]
- 31. Farhan M, Wang H, Gaur U, Little PJ, Xu J, Zheng W. FOXO signaling pathways as therapeutic targets in cancer. Int J Biol Sci 2017; 13: 815–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wang X, Li G, Luo Q, Xie J, Gan C. Integrated TCGA analysis implicates lncRNA CTB‐193M12.5 as a prognostic factor in lung adenocarcinoma. Cancer Cell Int 2018; 18: 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Sand M, Bechara FG, Sand D et al Long‐noncoding RNAs in basal cell carcinoma. Tumour Biol 2016; 37: 10595–608. [DOI] [PubMed] [Google Scholar]


