Abstract
Key Points
This study investigated the influence of group III/IV muscle afferents on corticospinal excitability during cycling exercise and focused on GABAB neuron‐mediated inhibition as a potential underlying mechanism.
The study provides novel evidence to demonstrate that group III/IV muscle afferent feedback facilitates inhibitory intracortical neurons during whole body exercise.
Firing of these interneurons probably contributes to the development of central fatigue during physical activity.
Abstract
We investigated the influence of group III/IV muscle afferents in determining corticospinal excitability during cycling exercise and focused on GABAB neuron‐mediated inhibition as a potential underlying mechanism. Both under control conditions (CTRL) and with lumbar intrathecal fentanyl (FENT) impairing feedback from group III/IV leg muscle afferents, subjects (n = 11) cycled at a comparable vastus‐lateralis EMG signal (∼0.26 mV) before (PRE; 100 W) and immediately after (POST; 90 ± 2 W) fatiguing constant‐load cycling exercise (80% Wpeak; 221 ± 10 W; ∼8 min). During, PRE and POST cycling, single and paired‐pulse (100 ms interstimulus interval) transcranial magnetic stimulations (TMS) were applied to elicit unconditioned and conditioned motor‐evoked potentials (MEPs), respectively. To distinguish between cortical and spinal contributions to the MEPs, cervicomedullary stimulations (CMS) were used to elicit unconditioned (CMS only) and conditioned (TMS+CMS, 100 ms interval) cervicomedullary motor‐evoked potentials (CMEPs). While unconditioned MEPs were unchanged from PRE to POST in CTRL, unconditioned CMEPs increased significantly, resulting in a decrease in unconditioned MEP/CMEP (P < 0.05). This paralleled a reduction in conditioned MEP (P < 0.05) and no change in conditioned CMEP. During FENT, unconditioned and conditioned MEPs and CMEPs were similar and comparable during PRE and POST (P > 0.2). These findings reveal that feedback from group III/IV muscle afferents innervating locomotor muscle decreases the excitability of the motor cortex during fatiguing cycling exercise. This impairment is, at least in part, determined by the facilitating effect of these sensory neurons on inhibitory GABAB intracortical interneurons.
Keywords: sensory muscle afferents, intracortical inhibition, whole body exercise, central fatigue
Key Points
This study investigated the influence of group III/IV muscle afferents on corticospinal excitability during cycling exercise and focused on GABAB neuron‐mediated inhibition as a potential underlying mechanism.
The study provides novel evidence to demonstrate that group III/IV muscle afferent feedback facilitates inhibitory intracortical neurons during whole body exercise.
Firing of these interneurons probably contributes to the development of central fatigue during physical activity.
Introduction
Central fatigue encompasses processes within the CNS that reduce neural drive to the exercising muscle and lead to a decrease in voluntary muscle activation and, subsequently, performance (Taylor et al. 2016). Exercise‐induced impairments in the efficacy of the corticospinal motor pathway to relay neural signals from higher brain areas to the contracting muscle are considered a determinant of central fatigue and may occur at the motor cortical and/or motoneuronal level (McNeil et al. 2009, 2011b; Weavil et al. 2016; Sidhu et al. 2017).
It is becoming increasingly accepted that feedback from mechano‐ and metabosensitive group III/IV muscle afferents facilitates central fatigue during, and shortly after, single joint (Gandevia et al. 1996; Gandevia, 2001; Kennedy et al. 2014) and whole body exercise (Amann et al. 2009; Sidhu et al. 2014, 2017). However, the role of these sensory neurons in altering corticospinal excitability during such fatiguing exercise remains elusive. While earlier studies suggested no (Taylor et al. 2000) or some (Martin et al. 2008) effect of group III/IV muscle afferent feedback on corticospinal excitability during elbow flexor exercise, a significant role of these sensory neurons on motor cortical excitability was recently documented during strenuous cycling exercise (Sidhu et al. 2017). Specifically, when feedback from group III/IV muscle afferents was pharmacologically blocked via intrathecal fentanyl, the excitability of the corticospinal tract was increased in the absence of a change at the motoneuronal level (Sidhu et al. 2017). Of note, although this suggests a disfacilitating, or inhibitory, influence of group III/IV afferent feedback on the motor cortex, the underlying mechanism mediating this dampening effect is still unknown. Interestingly, a previous study documented a significantly shortened cortical silent period during intermittent quadriceps contractions performed with fentanyl blockade (Hilty et al. 2011). While this finding may suggest that group III/IV muscle afferents facilitate intracortical inhibitory networks, this hypothesis remains untested during locomotor exercise.
Long‐interval intracortical inhibition (LII), which is in part mediated by GABAB receptors (Werhahn et al. 1999), can be quantified using a paired‐pulse paradigm including non‐invasive transcranial magnetic stimulation (TMS; eliciting motor‐evoked potential; MEP) (McNeil et al. 2009). Typically, at stimulation intensities above motor threshold, a test stimulus delivered during the silent period produced by a preceding conditioning stimulus (at an interstimulus interval of 100–200 ms) results in a diminished MEP (conditioned response) compared to the MEP when the test stimulus is delivered alone (unconditioned response) (Valls‐Sole et al. 1992). Using this method, there is evidence, from single joint studies, that the conditioned MEP size progressively diminishes during sustained, fatiguing elbow flexor contractions (McNeil et al. 2009, 2011a). Furthermore, the authors employed a complementary paradigm whereby they substituted the test TMS with cervicomedullary stimulation (CMS; eliciting cervicomedullary motor‐evoked potential; CMEP) (Taylor, 2006) and demonstrated that the increase in LII was probably due to spinal contributions. A major consideration in the interpretation of these results is that fatigue‐induced increases in EMG (a surrogate for central motor drive) can influence centrally evoked responses (Lévénez et al. 2008; Weavil et al. 2015) and the magnitude of LII (Hammond & Vallence, 2007; McNeil et al. 2011c; Opie & Semmler, 2014). However, a reduction in both conditioned MEP and CMEP was apparent even when EMG was ‘clamped’ during single‐joint exercise (McNeil et al. 2011a). Regardless, neither the effect of fatiguing whole‐body exercise on LII nor the contributing role of group III/IV muscle afferent feedback are currently known.
Consequently, we assessed the effect of group III/IV muscle afferents on unconditioned and conditioned MEPs and CMEPs during constant EMG cycling performed before (PRE) and immediately after (POST) fatiguing cycling exercise. Based on previous findings (Hilty et al. 2011; Sidhu et al. 2017), we hypothesised that fatigue‐related feedback from these sensory neurons would facilitate LII pathways within the motor cortex.
Methods
Ethical approval
Eleven (9 male, 2 female) healthy, recreationally active subjects [maximal O2 consumption () = 46 ± 7 ml kg−1 min−1; peak power output (W peak) = 280 ± 12 W], with a mean age of 25 ± 3 years, body mass of 75 ± 11 kg and height of 177 ± 7 cm were recruited for the study. Written informed consent was obtained from each participant. All experimental procedures were approved by the University of Utah and Salt Lake City Veterans Affairs Medical Center Institutional Review Boards (IRB approval number: 62914) and conformed to the Declaration of Helsinki (study is not registered in a research database).
Torque and EMG readings
Knee extension force was measured using a calibrated linear strain gauge (MLP 300; Transducer Techniques, Temecula, CA, USA). Quadriceps torque was calculated by multiplying knee extension force with the lever arm (distance from the fibular head to the lateral malleolus). EMG was recorded with surface electrodes (Ag‐AgCl, 10 mm in diameter) placed over the muscle belly of the vastus lateralis (VL; a key locomotor muscle) in a tendon–muscle montage and ∼5 cm inter‐electrode distance (i.e. monopolar configuration to optimise measurement of centrally evoked potentials) (McNeil et al. 2011c). EMG electrode placement was recorded and marked for replication between sessions. EMG signals were amplified (1000×; Neurolog Systems, Digitimer Ltd, Welwyn Garden City, UK), band‐pass filtered (10–1000 Hz; NL‐844, Digitimer Ltd) and converted from analog to digital at a sampling rate of 2000 Hz using a 16‐bit Micro 1401 mk‐II and Spike 2 data collection software (Cambridge Electronic Design, Cambridge, UK) via custom written program scripts.
Cycle ergometer set up
Subjects were positioned on a bicycle ergometer (Velotron, Elite Model, Racer Mate, Seattle, WA, USA) with their feet fastened securely to the pedals and their hands holding onto a handle bar in front of them. A mouthpiece, connected to a metabolic cart (Innocor, Glamsbjerg, Denmark) to measure pulmonary ventilation and gas exchange, was mounted onto a bar to minimise head movements. Subjects’ optimal seat height and handle bar height were recorded in the first session and kept consistent throughout the study.
Experimental protocol
Each subject participated in a total of five sessions. Subjects were familiarised with the experimental procedures during three preliminary visits. During the first preliminary session, subjects were asked to perform a maximal exercise test [20 W + 25 W min−1] (Amann et al. 2004) on a bicycle ergometer (Velotron, Elite Model) for the determination of peak power output (W peak) and maximal oxygen consumption (). In the second preliminary session, subjects performed and practised all procedures (stimulations, maximal exercise test, quadriceps contractions) that were performed in the experimental sessions. This also allowed the experimenters to determine the approximate workload required during the continuous cycling phase after task failure was reached during the fatiguing bout. The purpose of this approach was to achieve a similar EMG to that obtained during the continuous cycling phase (i.e. fixed at 100 W) performed prior to the fatiguing bout. In the third preliminary session, subjects only practised the constant‐load cycling exercise (80% W peak, 221 ± 10 W) sustained to task failure (i.e. a pedal frequency of below 80% of the target rpm for more than 10 s).
During the final two sessions, subjects performed the cycling exercise either under control conditions (CTRL) or following intrathecal fentanyl administration through L3–L4 vertebral interspace (FENT). In both experimental sessions, subjects initially performed three maximal voluntary contractions (MVCs) of the right knee extensors (with a 1‐min rest in between contractions). Following this, optimal motor nerve stimulation (MNS) intensity was established for neuromuscular assessment of the quadriceps (see below for details) while subjects were seated on a custom‐made chair. Subjects were then moved to the cycle ergometer to exercise at 100 W for 2 min. The cycling EMG versus crank angle relationship was quantified during exercise and was used to determine the optimal crank position for stimulations during cycling. Following this, during brief 100 W cycling bouts (with sufficient rest in between to minimise the occurrence of fatigue), optimal CMS and TMS intensities were set to measure LII. This was followed by arm cycling exercise. Arm cycling was repeated after fentanyl administration in FENT (to evaluate the potential for a cephalad movement of fentanyl sufficient to directly bind to brain opioid receptors) (Amann et al. 2010) and after ‘rest’ in CTRL. Two sets of stimulations were elicited during a 3‐min 100‐W cycling bout immediately prior (PRE) to fatiguing cycling exercise and during the 3‐min cycling bout immediately after fatiguing exercise (POST). The transition time from PRE to the start of fatiguing cycling and from the end of fatiguing cycling to POST was < 10 s. VL EMG during POST was matched to that during PRE (CTRL: 95 ± 7 W; FENT: 85 ± 8 W). Three minutes after POST was completed, neuromuscular assessment of the quadriceps was repeated (Fig. 1 A).
Figure 1. Schematic illustration of stimulations elicited during exercise.
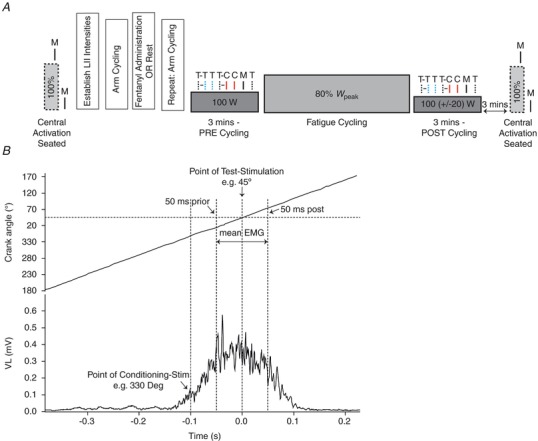
A, femoral motor nerve stimulation (M = MNS) delivered during maximum voluntary quadriceps contraction (MVC) and at rest was used to calculate voluntary activation (VA). Two sets of stimulations were elicited during the cycling exercise performed before (PRE) and immediately after (POST) the fatiguing trial. Note, the workload during POST was reduced to match the quadriceps EMG during PRE. Six types of stimulations were used in each set: paired stimuli in which the conditioning transcranial magnetic stimulation (T = TMS) pulse (CS) preceded the test TMS pulse (TST) or test cervicomedullary (C = CMS) pulse (TSC) by 100 ms (CS‐TST, dotted black and dotted blue line; or CS‐TSC, dotted black and solid red line), single TMS or CMS test pulse (TST, dotted blue line; or TSC, solid red line), single conditioning TMS pulse (CS, dotted black line) and M (solid black line). Stimulations were delivered in a random order and at the individually determined optimal crank position. B, the crank angle of the cycle ergometer was monitored continuously via a calibrated linear encoder mounted near the crank shaft. The point for overlaying and averaging was taken as the top dead centre of the 0–360° crank cycle (i.e. 0°). The corresponding point on the crank angle relative to the peak occurrence of the EMG was determined and CS was delivered 100 ms prior to this point, such that the test stimulus eliciting the conditioned or unconditioned response would always occur at peak EMG.
Stimulations
Three forms of stimulation were used during the study: (1) electrical MNS, (2) TMS and (3) CMS.
Motor nerve stimulation
The position of the MNS electrode on the femoral nerve (located in the femoral triangle), which elicited the highest compound muscle action potential (M‐wave) in VL and quadriceps twitch, was determined by delivering low‐intensity single pulse stimuli (200 μs pulse width; 100–150 mA) using a cathode probe (with the anode fixed between the greater trochanter and iliac crest) and a constant current stimulator (Model DS7AH, Digitimer Ltd). Once this position was established, the cathode electrode was fixed in that location. Thereafter, the stimulation intensity was increased in 20 mA increments until the size of the M‐wave demonstrated no further increase (i.e. maximal M‐wave; M max) at rest. The stimulation intensity was set at 130% of M max intensity at rest (257 ± 20 mA) and checked for supramaximality during a 50% quadriceps MVC. If the M‐wave size increased during the 50% MVC, the intensity was increased further to ensure a plateau. The supramaximality of this intensity was also checked during a brief (∼30 s) cycling bout at 100 W by increasing the stimulation intensity in 20 mA increments above that determined in the seated position. This was performed to ensure that a true plateau was also attained in the stimulus–response curve during cycling (265 ± 17 mA).
Transcranial and cervicomedullary stimulations
A double cone coil (diameter 130 mm) attached via a BiStim unit to two Magstim 200 stimulators (The Magstim Company Ltd, Dyfed, UK) was used to elicit MEPs in the VL. Initially, optimal coil position (posterior to anterior direction of current flow in the motor cortex) to preferentially activate the portion of the left motor cortex with the largest representation for the quadriceps (position relative to vertex: ∼2–3 cm lateral) was determined. This location was marked directly on the scalp for accurate placement throughout the session. Stimulation intensities were set during brief 100 W cycling bouts at a constant speed of 80 r.p.m. One stimulator unit delivered the conditioning stimulus (CS) and the other delivered the test stimulus (TST). The intensity of the CS (50–80% stimulator output) was set to produce a silent period of ∼150–200 ms in the cycling EMG burst. In half of the paired‐pulse stimulations, TST was replaced by CMS (i.e. TSC) (Fig. 1 A). An electrical percutaneous stimulator (D‐185 mark IIa, Digitimer Ltd) was used to activate the cervicomedullary junction at the back of the neck to elicit CMEPs in the VL. This was achieved by passing a high‐voltage pulse (100 μs pulse width) between a set of self‐adhesive electrodes attached to the skin in the groove between the mastoid processes and the occiput (cathode on the left, contralateral to the right limb muscle). The stimulation intensity (438 ± 18 V) of TSC was set to produce an unconditioned CMEP of approximately 50–80% M max (note: due to the depth at which the descending tracts are located beneath the skull, in some subjects it was not possible to achieve this target at a tolerable stimulation intensity). This was then preceded, 100 ms prior, by a CS that evoked a conditioned CMEP of ∼20% M max. The TST intensity was then set to elicit a conditioned MEP response that was approximately equivalent in size to the conditioned CMEP response (i.e. ∼20% M max).
Neuromuscular assessment of quadriceps function
Subjects were seated comfortably on a custom‐built chair with full back support, such that the hip and knee were at approximately 120° and 90° of flexion, respectively. A cuff attached to the strain gauge was fixed ∼2 cm above the lateral malleolus of the right leg. Three sets of contractions, separated by 1 min, were performed during each assessment (Fig. 1 A). In each set, subjects performed a quadriceps MVC (∼3–5 s) during which MNS was delivered to evoke a superimposed twitch (SIT), followed by another MNS to evoke a potentiated resting twitch (RT). Voluntary activation (VA; %) was assessed by expressing SIT as a percentage of RT: VA = (1 − SIT/RT) × 100 (Merton, 1954).
Optimal crank position for stimulations during cycling
The crank angle of the cycle ergometer was monitored continuously via a calibrated linear encoder mounted near the crank shaft (Weavil et al. 2015). During a 100 W cycling bout, the VL EMG (rectified) versus crank angle relationship was obtained using waveform averaging to characterise the muscle activity versus crank angle relationship (Fig. 1 B). The point for overlaying and averaging was taken as the top dead centre (i.e. 0°) on the 0–360° crank cycle. The corresponding point on the crank angle relative to the peak occurrence of the EMG was determined and the point of delivering the conditioning stimulus was set at 100 ms prior to the peak EMG point, such that the stimulus eliciting the test response (conditioned or unconditioned) occurred at peak EMG (289 ± 41°; Fig. 1 B). This position was kept consistent within each subject throughout the study.
Set of stimulations during cycling
Two sets of responses were elicited during PRE and POST. Six patterns of stimulations were employed in each set: paired stimuli in which the CS preceded the TST or TSC by 100 ms (CS‐TST or CS‐TSC; producing a conditioned MEP or CMEP response, respectively), single test stimulus (TST or TSC; producing an unconditioned MEP or CMEP, respectively), single conditioning stimulus (CS; producing a silent period; SP) and MNS (M; producing an M max) (Fig. 1 A). The order of stimulation pattern and pedal revolution during which stimulations were delivered was randomised (Sidhu et al. 2012). All stimulations during cycling were elicited at the optimal position determined at the start of the session (see ‘Optimal crank position for stimulations during cycling’). Each stimulation was separated by at least five full pedal revolutions.
Intrathecal fentanyl
Subjects were seated in a forward flexed sitting position and 1 ml of fentanyl (0.025 mg ml−1) was delivered at the vertebral interspace L3–L4 as previously described (Amann et al. 2009). Data collection was completed within 60 min from the time fentanyl was administered.
Arm cycling
Migration of fentanyl to the brain would negate our ability to speculate on cortical effects of group III/IV afferents because opioid receptors are widely distributed throughout the brain, including various areas known to be involved in the regulation of motor function and behaviour (Bruijnzeel, 2009). To exclude the possibility of direct cortical effects, we utilised the fact that binding of fentanyl (applied intrathecally at the lumbar level) on medullary opioid receptors decreases the ventilatory response to arm exercise (Amann et al. 2010). Fentanyl had no effect on the ventilatory response to arm cycling in any subject (Table 1), suggesting only local (i.e. probably below T2) effects of the drug. During the preliminary sessions, participants were familiarised with the upper body ergometry (arm cycling; Monark 881E, Sweden). During both CTRL and FENT sessions, subjects performed constant‐load arm cycling (15 W and 30 W, 3 min each) twice (before and after fentanyl administration in FENT and before and after a 10‐min rest in CTRL). There was a 2‐min break between each workload and the target cadence was set at 60 r.p.m. (Amann et al. 2010). Variables including breathing frequency (f R; breaths min−1) and tidal volume (V T; litres) were assessed and averaged over the final minute of each workload.
Table 1.
Cardioventilatory response during the final minute of 15 W and 30 W arm cycling
| 15 W | 30 W | |||||||
|---|---|---|---|---|---|---|---|---|
| CTRL | FENT | CTRL | FENT | |||||
| Pre | Post | Pre | Post | Pre | Post | Pre | Post | |
| HR (beats/min) | 127 ± 7 | 125 ± 6 | 132 ± 7 | 123 ± 7 | 145 ± 5 | 140 ± 5 | 148 ± 7 | 142 ± 10 |
| V E (l/min) | 42 ± 3 | 39 ± 2 | 40 ± 1 | 34 ± 2 | 57 ± 3 | 55 ± 3 | 54 ± 2 | 51 ± 3 |
| f R (breaths/min) | 27 ± 2 | 26 ± 2 | 27 ± 2 | 26 ± 2 | 29 ± 2 | 29 ± 2 | 30 ± 2 | 28 ± 2 |
| V T (litres) | 1.6 ± 0.1 | 1.6 ± 0.1 | 1.6 ± 0.1 | 1.4 ± 0.1 | 2.0 ± 0.1 | 2.0 ± 0.1 | 1.9 ± 0.1 | 2.0 ± 0.2 |
| (l/min) | 1.1 ± 0.1 | 1.2 ± 0.1 | 1.1 ± 0.1 | 1.1 ± 0.05 | 1.5 ± 0.1 | 1.6 ± 0.1 | 1.4 ± 0.1 | 1.5 ± 0.1 |
| (l/min) | 1.1 ± 0.1 | 1.0 ± 0.1 | 1.0 ± 0.1 | 0.9 ± 0.1 | 1.6 ± 0.1 | 1.5 ± 0.1 | 1.4 ± 0.1 | 1.4 ± 0.1 |
| V E/ | 38 ± 3 | 31 ± 1 | 34 ± 2 | 29 ± 2 | 36 ± 2 | 34 ± 2 | 35 ± 1 | 31 ± 2 |
| V E/ | 37 ± 1 | 36 ± 1 | 35 ± 1 | 35 ± 1 | 34 ± 1 | 35 ± 1 | 34 ± 1 | 33 ± 1 |
| (Torr) | 92 ± 1 | 89 ± 2 | 91 ± 1 | 87 ± 2 | 93 ± 2 | 92 ± 1 | 93 ± 1 | 90 ± 2 |
| (Torr) | 32 ± 1 | 32 ± 1 | 32 ± 1 | 32 ± 1 | 33 ± 1 | 33 ± 1 | 31 ± 1 | 33 ± 1 |
HR, heart rate; V E, minute ventilation; f R, breathing frequency; V T, tidal volume; , oxygen consumption; , carbon dioxide production; V E/, ventilatory equivalent for oxygen; V E/, ventilatory equivalent for carbon dioxide; , end‐tidal ; , end‐tidal . Data are given as means ± SEM.
Data analysis
All neurophysiological data were analysed offline using Spike 2 software (Cambridge Electronic Design). Areas of MEPs, CMEPs (both conditioned and unconditioned) and M max were measured between cursors placed to encompass all phases of evoked potentials in VL. LII was quantified by expressing conditioned MEPs or CMEPs as a percentage of the unconditioned MEP or CMEP, respectively (i.e. LIITMS and LIICMS; NB: an increase in long‐interval inhibition is demonstrated by a reduction in the conditioned/unconditioned ratio). The area of each unconditioned and conditioned MEP and CMEP was normalised to that of M max elicited in the same set to account for activity‐dependent changes in muscle sarcolemma excitability. To quantify excitability changes at the cortical level, MEPs were expressed relative to CMEPs (i.e. MEP/CMEP; %). The duration of the silent period (SP) was considered the time interval from the CS pulse to the return of the cycling EMG, as determined by an automated script written to detect EMG exceeding ± 2 SD of EMG obtained during revolutions without stimulations for at least 50 ms (Goodall et al. 2010). The cycling EMG signal was rectified and waveform average analysis was performed on a 10 s segment just prior to the stimulation set during PRE and POST as well as at the start and termination of fatiguing exercise. The reference point for overlaying and averaging the EMG signal was taken as the same point on the crank angle that was used to elicit stimulations. Average EMG was measured across a 100 ms window (50 ms before and 50 ms after the reference point). Pre‐and post‐exercise MVC, VA, RT and M max area represent the average from the three sets performed.
Statistical analysis
Normality of the data was confirmed by a Shapiro–Wilk W test. The following repeated‐measures ANOVAs were performed. (1) Two‐way repeated‐measures ANOVAs were used to examine the effect of fentanyl blockade on ventilatory responses during arm‐cycling exercise. (2) Two‐way repeated‐measures ANOVAs were used to compare the pre‐ to post‐exercise changes in MVC, VA, RT and M max area. (3) Two‐way repeated‐measures ANOVAs were used to compare the changes in unconditioned MEP (%M max), unconditioned CMEP (%M max), conditioned MEP (%M max), conditioned CMEP (%M max), LIITMS, LIICMS, MEP/CMEP, cycling EMG, M max, SP and cardioventilatory responses from PRE to POST. If the data did not conform to the assumption of sphericity, the P‐value was Greenhouse–Geisser corrected. When ANOVA revealed a significant interaction or a main effect, Bonferroni post hoc tests were performed and corrected for the number of comparisons. Data are presented as mean ± SD in the text and, for clarity purposes, as mean ± SEM in figures and tables. Statistical significance was set at P ≤ 0.05.
Results
The cardioventilatory response to arm cycling exercise was similar during CTRL and FENT, suggesting that the drug did not migrate to the cardiovascular and ventilatory control centres within the brainstem (Table 1). A direct effect of fentanyl on brain opioid receptors can be excluded.
Effect of group III/IV muscle afferents on locomotor muscle fatigue and VL EMG
Cycling time to task failure was similar between the two sessions (∼8.1 min; P = 0.70). There was no interaction (F 1,10 = 0.003; P = 0.96) nor main effect of time (F 1,10 = 1.893; P = 0.20) or session (F 1,10 = 0.061; P = 0.81) on M max (group mean area across time and sessions: 0.06 ± 0.02 mV.s). While there was no interaction (F 1,10 < 0.600; P > 0.45) or main effect of session (F 1,10 < 2.386; P > 0.15) on MVC and VA, there was a main effect of time on both variables (F 1,10 > 14.400; P < 0.01; Table 2). MVC was diminished following exercise in both CTRL and FENT by 11 ± 3% and 15 ± 4%, respectively (P < 0.01). VA was diminished after CTRL by 8 ± 4% (P = 0.02), but not after FENT (P = 0.08) (Table 2). While there was no interaction effect (F 1,10 = 1.783; P = 0.21), there was a main effect of time (F 1,10 = 26.881; P < 0.001) and session (F 1,10 = 7.822; P = 0.02) on RT. RT was reduced after exercise in both conditions (P < 0.001), but the reduction was larger in FENT compared to CTRL (45 ± 8% vs. 30 ± 4%; P = 0.01; Table 2). There was no interaction (F 1,10 = 1.568; P = 0.24) or main effect of session (F 1,10 = 0.050; P = 0.83) on cycling EMG during the fatiguing constant‐load cycling exercise, but there was a main effect of time (F 1,10 = 9.309; P = 0.01). Cycling EMG increased during FENT by 13 ± 5% (start: 0.43 ± 0.13 mV; end: 0.48 ± 0.14 mV; P = 0.01) and during CTRL by 8 ± 3% (start: 0.43 ± 0.17 mV; end: 0.46 ± 0.17 mV; P = 0.04).
Table 2.
Exercise‐induced quadriceps fatigue
| CTRL | FENT | |||||
|---|---|---|---|---|---|---|
| Pre | Post | % Change | Pre | Post | % Change | |
| MVC (Nm) | 231 ± 13 | 204 ± 10 * | 11 ± 3 | 228 ± 14 | 193 ± 15 * | 15 ± 4 |
| RT (Nm) | 62 ± 7 | 42 ± 3 * | 30 ± 4 | 58 ± 4 | 30 ± 3 *† | 45 ± 8 † |
| VA (%) | 93 ± 1 | 85 ± 3 * | 8 ± 4 | 93 ± 1 | 89 ± 1 | 5 ± 2 |
Pre‐ to post‐exercise changes in maximal voluntary contraction torque (MVC; A), resting twitch torque (RT; B) and voluntary quadriceps activation (VA, C). *Significantly different from pre‐exercise. †Significantly different across sessions.
Effects of group III/IV muscle afferents on centrally evoked responses
As intended, cycling EMG was matched during PRE and POST in both CTRL (0.27 ± 0.12 and 0.24 ± 0.10 mV, respectively; P = 0.10) and FENT (0.26 ± 0.10 and 0.26 ± 0.08 mV, respectively; P = 0.92). Unconditioned MEP and CMEP (% M max) were also matched at PRE in CTRL (80.2 ± 17.2 and 68.8 ± 16.3, respectively; P = 0.10; Fig. 2 A, B) and FENT (79.7 ± 28.2 and 73.6 ± 18.8, respectively; P = 0.60; Fig. 2 A, B). Similarly, conditioned MEP and CMEP (% M max) were matched at PRE in both CTRL (30.8 ± 29.7 and 23.3 ± 30.2, respectively; P = 0.40; Figs 3 A, C and 4 A, B) and FENT (17.2 ± 23.3 and 11.0 ± 14.8, respectively; P = 0.60; Figs 3 E, G and 4 A, B).
Figure 2. Unconditioned responses during cycling exercise performed before (PRE) and immediately after (POST) a fatiguing exercise trial.
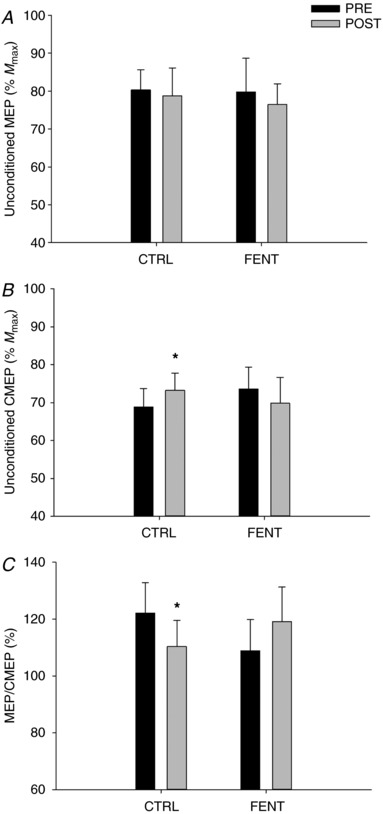
A, unconditioned motor‐evoked potentials (MEP normalised to M max); B, unconditioned cervicomedullary motor‐evoked potentials (CMEP normalised to M max); and C, unconditioned MEP/unconditioned CMEP. *Significantly different from ‘PRE’.
Figure 3. Representative raw traces.
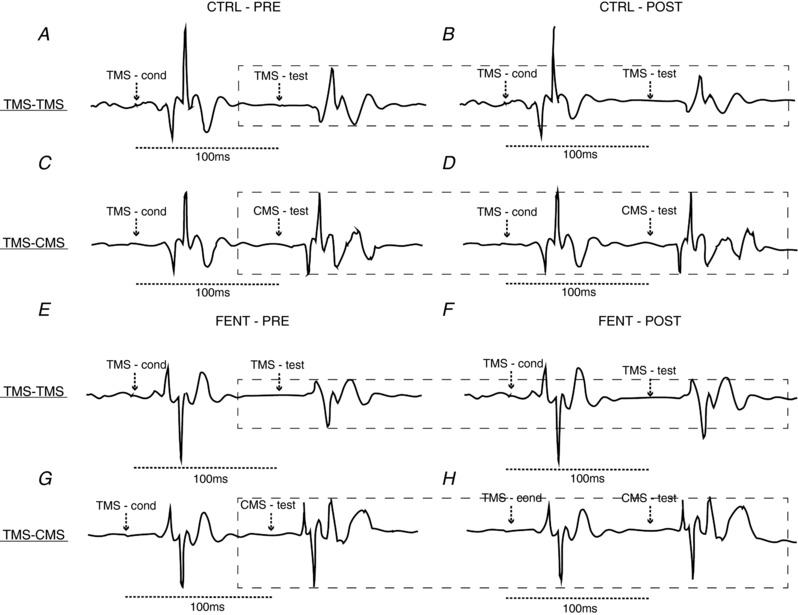
Conditioned MEPs (A, B, E, F) and conditioned CMEPs (C, D, G, H) during PRE and POST under control conditions (CTRL; A–D) and with attenuated muscle afferent feedback (FENT; E–H). The dashed box surrounds the amplitude of the MEP and CMEP in the silent period following conditioning TMS at PRE. The arrows indicate the timing of the conditioning and test stimuli. In this subject, while the conditioned MEP is reduced in size at POST compared to PRE during CTRL (A, B), this effect was not evident during the exercise performed with afferent blockade (i.e. FENT; E and F).
Figure 4. Conditioned responses during cycling exercise performed before (PRE) and immediately after (POST) a fatiguing exercise trial.
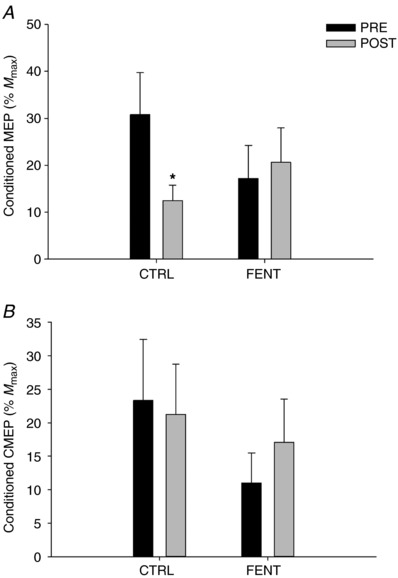
A, group mean conditioned motor‐evoked potentials (MEP normalised to M max); and B, conditioned cervicomedullary motor‐evoked potentials (CMEP normalised to M max). *Significantly different from ‘PRE’.
There was no interaction (F 1,10 < 2.488; P > 0.10) or main effect of session (F 1,10 < 1.223; P > 0.12) and time (F 1,10 < 2.588; P > 0.13) on unconditioned MEP (%M max; Fig. 2 A), conditioned CMEP (%M max; Fig. 4 B), LIICMS (group mean across session and time: 30.5 ± 34.4%), cycling EMG or M max. There was, however, an interaction effect (F 1,10 > 5.688; P < 0.01) on unconditioned CMEP (%M max; Fig. 2 B), conditioned MEP (%M max; Fig. 4 A), MEP/CMEP (Fig. 2 C), LIITMS and SP. Unconditioned CMEP (%M max) was increased by 7 ± 2% (P = 0.01) at POST compared to PRE during CTRL, but not during FENT (P = 0.33) (Fig. 2 B). Conditioned MEP (%M max; Fig. 4 A) and LIITMS (PRE: 40.2 ± 35.9, POST: 21.5 ± 31.2) were decreased by 45 ± 12% and 30 ± 12%, respectively (P < 0.05; Fig. 5 A) POST compared to PRE during CTRL, but did not change during FENT (P > 0.28; Fig. 5 A). Unconditioned MEP/CMEP was decreased POST compared to PRE during CTRL by 9 ± 4% (P = 0.04; Fig. 2 C), but not during FENT (P = 0.23; Fig. 2 C). SP did not change during CTRL (PRE: 180.6 ± 30.8 ms, POST: 184.2 ± 43.7 ms; P = 0.56), but was decreased POST compared to PRE during FENT by 10 ± 3% (PRE: 193.6 ± 31.5 ms, POST: 174.4 ± 36.0 ms; P = 0.02).
Figure 5. Long‐interval inhibition (LII) during cycling exercise performed before (PRE) and immediately after (POST) a fatiguing exercise trial.
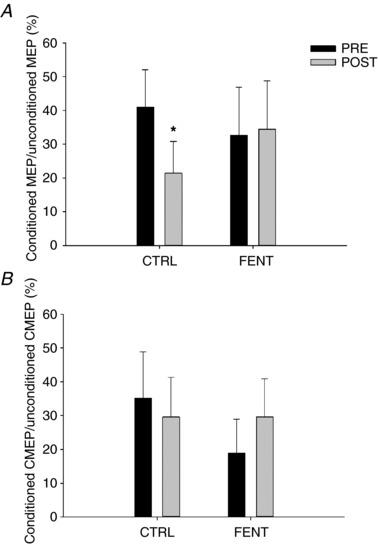
A, group mean conditioned motor‐evoked potentials (MEP) as a percentage of unconditioned MEP (LIITMS); and B, group mean conditioned cervicomedullary motor‐evoked potentials (CMEP) as a percentage of unconditioned CMEP (LIICMS). *Significantly different from ‘PRE’.
Discussion
This study used a pharmacological approach to determine the influence of fatigue‐related feedback from group III/IV muscle afferents on attenuating corticospinal excitability during locomotor exercise and focused on intracortical inhibition as a potential mechanism underlying this effect. The findings indicate that firing of these muscle afferents during fatiguing cycling exercise inhibits, or disfacilitates, the motor cortex. Paired cortical and spinal cord stimulations suggest that this impact is, at least in part, determined by the group III/IV‐mediated activation of inhibitory intracortical GABAB neurons.
Fatigue‐related group III/IV muscle afferent firing influences corticospinal excitability during locomotor exercise
Progressive increases in EMG, as occurs with the development of fatigue during submaximal constant‐load exercise, facilitate centrally evoked motor potentials and can therefore mask potentially disfacilitating or inhibitory influences on the excitability of the corticospinal pathway (Rothwell, 2009; Weavil et al. 2015, 2016). To circumvent this issue, we investigated fatigue‐related modulations in MEPs and CMEPs and the role of group III/IV muscle afferents on these modulations during cycling exercise performed at a given locomotor muscle EMG. Specifically, short cycling bouts implemented prior to the start (PRE) of the fatiguing exercise and immediately after (POST) were matched for EMG by appropriately titrating (i.e. reducing) the workload during POST. Using this approach under CTRL conditions, the excitability of spinal motoneurons increased from PRE to POST, as shown by an increase in unconditioned CMEP (Fig. 2 B), while that of motor cortical cells decreased, as shown by a decrease in unconditioned MEP/CMEP (Fig. 2 C). Interestingly, these effects were no longer evident when the exercise was performed with attenuated neural feedback from the locomotor muscles (Fig. 2 B, C), suggesting that fatigue‐related group III/IV muscle afferent feedback facilitates knee extensor motoneurons, but inhibits, or disfacilitates, the motor cortex.
As fatigue alters motor cortical and motoneuronal responsiveness to synaptic input and/or the efficacy of the corticomotoneuronal synapse to relay neural drive, matching EMG during POST to that observed during PRE only ensures similar motoneuronal activity, but does not necessarily lead to a comparable level of voluntary descending drive during both exercise bouts. To address this potentially confounding scenario, we added the paired pulse LII paradigm which includes a conditioning TMS that causes a brief pause (i.e. silent period, ∼200 ms) in voluntary descending drive (Fuhr et al. 1991; Inghilleri et al. 1993). Taking advantage of this phenomenon, the current study demonstrated that, under control conditions, fatigue during locomotor exercise decreases the size of MEPs evoked during the silent period (i.e. conditioned MEPs; Fig. 4 A) with no effect on conditioned CMEPs (Fig. 4 B), indirectly suggesting that fatigue compromises cortical excitability. Interestingly, fentanyl blockade had no effect on the motoneurone pool (Fig. 4B), but prevented the decrease in conditioned MEP (Fig. 4 A) further suggesting that group III/IV muscle afferents inhibit/disfacilitate the motor cortex.
Although, the inhibitory effect of fatigue on the motor cortex was reflected in the modulation of both unconditioned (Fig. 2) and conditioned (Fig. 4) responses, the outcomes of the two paradigms also feature considerable differences. Specifically, the significant fatigue‐induced decrease in conditioned MEPs (Fig. 4 A) and the lack of change in conditioned CMEPs (Fig. 4 B) in CTRL differs from the unchanged unconditioned MEPs (Fig. 2 A) and the increase in unconditioned CMEPs (Fig. 2 B). This might indicate, despite similar ongoing EMG at PRE and POST, a greater facilitation of the motor pathway secondary to a higher descending drive (Weavil et al. 2015) during POST, a circumstance that could have masked the impact of fatigue on corticospinal excitability to be reflected in the unconditioned responses. Finally, although speculative, the observation that group III/IV muscle afferents facilitate unconditioned CMEPs (Fig. 2 B) without affecting conditioned CMEPs (Fig. 4 B) could mean that some degree of descending drive is required for neural feedback to facilitate motoneurons.
There are several variations in the fatigue‐induced modulation of unconditioned MEPs and CMEPs in this investigation compared to earlier locomotor studies. First, under control conditions, unconditioned CMEPs were previously demonstrated to be similar at the start of exhaustive exercise and at task failure (Weavil et al. 2016; Sidhu et al. 2017), whereas the current study suggests a fatigue‐induced facilitation of motoneurons (Fig. 2 B). This discrepancy is probably explained by the fact that excitability in previous studies was quantified immediately upon exhaustion (i.e. task failure), a point at which mechano‐ and metabosensitive afferent feedback and associated inhibitory consequences are larger than at the later time point assessed in the present study, i.e. at a lower workload for 3 min after exhaustion (Fig. 1). Second, in our previous work (Sidhu et al. 2017), unconditioned MEPs were greater at exhaustion compared to the start of the cycling exercise performed with afferent blockade, an effect that was not apparent in the present study (Fig. 2 A). To appreciate this difference, it needs to be recognised that corticospinal excitability in our previous work was quantified in the face of the ‘normally’ occurring voluntary background EMG, which is larger at exhaustion compared to the start of exercise, while, in the present study, EMG was held consistent when corticospinal excitability was quantified. Given the facilitating effect of cycling EMG on both MEPs and CMEPs (Weavil et al. 2015), it is possible that, once the group III/IV‐mediated inhibitory effect was minimised with fentanyl, the greater EMG in our previous study accounted for the increase in MEP, a phenomenon that was not evident in the current study (Fig. 2 A) where EMG was controlled.
Group III/IV muscle afferents influence intracortical inhibition during locomotor exercise
Our findings suggest that the group III/IV‐mediated inhibition of the motor cortex results from the facilitating effect of these sensory neurons on intracortical inhibitory interneurons (Fig. 5). Specifically, using a paired TMS pulse technique with an inter‐stimulus interval of 100 ms (i.e. the LII paradigm) (Valls‐Sole et al. 1992; Sanger et al. 2001), we observed a decrease in LIITMS from PRE to POST during CTRL (Fig. 5 A), a change that has been documented to reflect increased excitability of GABAB receptor‐sensitive inhibitory interneurons (Werhahn et al. 1999; McDonnell et al. 2007). As LIICMS (i.e. substituting the second motor cortical stimulus with CMS) remained unaltered with fatigue (Fig. 5 B), the decrease in LIITMS was probably independent of spinal mechanisms. Importantly, pharmacological attenuation of group III/IV muscle afferents had no effect on the unchanged LIICMS from PRE to POST, but prevented the decrease in LIITMS (Fig. 5 A, B). Taken together, these findings suggest that the cortical depression during fatiguing locomotor exercise is, at least in part, determined by the facilitating effect of group III/IV muscle afferents on GABAB‐mediated inhibitory cortical neurons. An increased excitability of low‐threshold intracortical inhibitory interneurons (i.e. GABAA) might also contribute to the decrease in cortical excitability during cycling exercise (Sidhu et al. 2013), although the role of muscle afferents in this modulation is unknown.
Although the TMS‐evoked SP is recognised to reflect both spinal and cortical processes, the latter component, traditionally after 100 ms, has been thought to be determined by GABAB receptor‐mediated intracortical inhibition (Inghilleri et al. 1993; Siebner et al. 1998). A more recent study has now suggested that the spinal component of the SP may actually extend up to 150 ms (Yacyshyn et al. 2016). Importantly, however, the average SP in the current study was 180 ms during cycling exercise, supporting the argument that the afferent‐mediated changes observed at this latency are probably of cortical origin. Regardless, similar to the outcome in recent studies (Hilty et al. 2011; Sidhu et al. 2017), we demonstrate that relative to CTRL, the SP was significantly reduced when the same exercise was performed with afferent blockade. This outcome provides further support to the presented LII findings suggesting that group III/IV muscle afferents enhance intracortical inhibition. However, it should be noted that both previous studies (Hilty et al. 2011; Sidhu et al. 2017) measured SP after exercise in an isometric context and the current study is, in fact, the first to report SP during uninterrupted locomotor exercise.
The changes in excitability of cortical and spinal GABAB neurons with exhaustive locomotor exercise differ from that observed during single‐joint exercise. Specifically, conditioned MEP and CMEP are reduced to a similar extent as fatigue develops during isometric maximal and submaximal elbow flexions (McNeil et al. 2009, 2011a) suggesting that the fatigue‐related decrease in LII typically observed during single joint exercise (Taylor et al. 1996; Sacco et al. 1997; Benwell et al. 2007) is mainly a consequence of spinal mechanisms (i.e. impaired motoneuron excitability). Although this study cannot offer a direct explanation for the different LII responses during fatiguing whole body compared to single‐joint exercise, differences in exercise modality and contractile regime may account for some of this discrepancy (Martin et al. 2006; Sidhu et al. 2013; Amann et al. 2015; Goodall et al. 2018).
While methodological studies suggest that increased LII is mediated via GABAB receptors (Werhahn et al. 1999), it should be acknowledged that extrasynaptic GABAA receptors can also tonically inhibit corticospinal neurons by responding to ambient levels of GABA (Lee & Maguire, 2014). In addition, the influence of other neurotransmitters, such as dopamine and glutamate, on LII cannot be excluded (Salavati et al. 2018) and may have contributed to the suppressed firing of neurons in the motor cortex, independent of GABA receptor‐mediated mechanisms.
Finally, it is important to emphasise that the visual differences in conditioned CMEP (Fig. 4 B) and conditioned CMEP/unconditioned CMEP (Fig. 5 B) at PRE between CTRL and FENT are not statistically significant and probably result from the naturally occurring between‐day variations in the amount of motoneurons activated with CMS and/or differences in EMG electrode placements.
Effect of group III/IV muscle afferents on the development of fatigue
Peripheral locomotor muscle fatigue was greater when the exercise was performed with attenuated feedback from group III/IV muscle afferents (Table 2). This finding may be explained by compromised muscle O2 transport secondary to the attenuated ventilatory and circulatory response during exercise performed with reduced neural feedback from the locomotor musculature (Amann et al. 2010, 2011). Importantly, however, the smaller pre‐ to post‐exercise reduction in VA during FENT compared to CTRL (Table 2) confirms previous observations (Sidhu et al. 2017) and further emphasises the critical role of group III/IV muscle afferent feedback in determining central fatigue during locomotor exercise.
Summary
Feedback from group III/IV muscle afferents innervating locomotor muscle decreases the excitability of the motor cortex during fatiguing cycling exercise. This impairment is, at least in part, determined by the facilitating effect of these sensory neurons on inhibitory GABAB receptor‐mediated interneurons at the cortical level. The outcome of this study has implications for our current understanding of exercise limitations in healthy humans and clinical populations particularly prone to altered afferent feedback.
Additional information
Conflict of interest
The authors have no conflicts of interest to declare.
Author contributions
SS and MA conceived and designed the study. SS, JW, EW, TT, JJ, DR, RR, CM and MA executed this study. SS analysed the data, prepared the figures and drafted the manuscript. SS and MA interpreted the data and prepared the manuscript. All authors edited the manuscript, revised it for important intellectual content and approved the final version.
Funding
This study was supported by the National Heart, Lung, and Blood Institute (HL‐116579), Veteran Affairs Merit Grant (E6910R), and the American Heart Association Postdoctoral Fellowship (14POST17770016).
Biography
Simranjit Sidhu received her PhD in neuroscience at the University of Queensland (Australia) in 2012 and completed her postdoctoral fellowship at the University of Utah (USA) under the mentorship of Markus Amann in 2015. She is currently a Lecturer at the University of Adelaide (Australia). Her research interests lie in the field of integrative human neurophysiology with a focus on determining the mechanisms of exercise‐related fatigue.

Edited by: Scott Powers & Paul Greenhaff
References
- Amann M, Blain GM, Proctor LT, Sebranek JJ, Pegelow DF & Dempsey JA (2010). Group III and IV muscle afferents contribute to ventilatory and cardiovascular response to rhythmic exercise in humans. J Appl Physiol 109, 966–976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amann M, Proctor LT, Sebranek JJ, Pegelow DF & Dempsey JA (2009). Opioid‐mediated muscle afferents inhibit central motor drive and limit peripheral muscle fatigue development in humans. J Physiol 587, 271–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amann M, Runnels S, Morgan DE, Trinity JD, Fjeldstad AS, Wray DW, Reese VR & Richardson RS (2011). On the contribution of group III and IV muscle afferents to the circulatory response to rhythmic exercise in humans. J Physiol 589, 3855–3866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amann M, Sidhu SK, Weavil JC, Mangum TS & Venturelli M (2015). Autonomic responses to exercise: group III/IV muscle afferents and fatigue. Auton Neurosci 188, 19–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amann M, Subudhi A & Foster C (2004). Influence of testing protocol on ventilatory thresholds and cycling performance. Med Sci Sports Exerc 36, 613–622. [DOI] [PubMed] [Google Scholar]
- Benwell NM, Mastaglia FL & Thickbrrom GW (2007). Differential changes in long‐interval intracortical inhibition and silent period duration during fatiguing hand exercise. Exp Brain Res 179, 255–262. [DOI] [PubMed] [Google Scholar]
- Bruijnzeel AW (2009). κ‐Opioid receptor signaling and brain reward function. Brain Res Rev 62, 127–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuhr P, Agostino R & Hallett M (1991). Spinal motor neuron excitability during the silent period after cortical stimulation. Electroencephalogr Clin Neurophysiol 81, 257–262. [DOI] [PubMed] [Google Scholar]
- Gandevia SC (2001). Spinal and supraspinal factors in human muscle fatigue. Physiol Rev 81, 1725–1789. [DOI] [PubMed] [Google Scholar]
- Gandevia SC, Allen GM, Butler JE & Taylor JM (1996). Supraspinal factors in human muscle fatigue: evidence for suboptimal output from the motor cortex. J Physiol 490, 529–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodall S, Howatson G & Thomas K (2018). Modulation of specific inhibitory networks in fatigued locomotor muscles of healthy males. Exp Brain Res 236, 463–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodall S, Ross EZ & Romer LM (2010). Effect of graded hypoxia on supraspinal contributions to fatigue with unilateral knee‐extensor contractions. J Appl Physiol 109, 1842–1851. [DOI] [PubMed] [Google Scholar]
- Hammond G & Vallence AM (2007). Modulation of long‐interval intracortical inhibition and the silent period by voluntary contraction. Brain Res 1158, 63–70. [DOI] [PubMed] [Google Scholar]
- Hilty L, Lutz K, Maurer K, Rodenkirch T, Spengler CM, Boutellier U, Jancke L & Amann M (2011). Spinal opioid receptor‐sensitive muscle afferents contribute to the fatigue‐induced increase in intracortical inhibition in healthy humans. Exp Physiol 96, 505–517. [DOI] [PubMed] [Google Scholar]
- Inghilleri M, Berardelli A, Cruccu G & Manfredi M (1993). Silent period evoked by transcranial stimulation of the human cortex and cervicomedullary junction. J Physiol 466, 521–534. [PMC free article] [PubMed] [Google Scholar]
- Kennedy DS, McNeil CJ, Gandevia SC & Taylor JL (2014). Fatigue‐related firing of distal muscle nociceptors reduces voluntary activation of proximal muscles of the same limb. J Appl Physiol (1985) 116, 385–394. [DOI] [PubMed] [Google Scholar]
- Lee V & Maguire J (2014). The impact of tonic GABAA receptor‐mediated inhibition on neuronal excitability varies across brain region and cell type. Front Neural Circuits 8, 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lévénez M, Garland SJ, Klass M & Duchateau J (2008). Cortical and spinal modulation of antagonist coactivation during a submaximal fatiguing contraction in humans. J Neurophysiol 99, 554–563. [DOI] [PubMed] [Google Scholar]
- Martin PG, Smith JL, Butler JE, Gandevia SC & Taylor JL (2006). Fatigue‐sensitive afferents inhibit extensor but not flexor motoneurons in humans. J Neurosci 26, 4796–4802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin PG, Weerakkody N, Gandevia SC & Taylor JL (2008). Group III and IV muscle afferents differentially affect the motor cortex and motoneurones in humans. J Physiol 586, 1277–1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonnell MN, Thompson PD & Ridding MC (2007). The effect of cutaneous input on intracortical inhibition in focal task‐specific dystonia. Mov Disord 22, 1286–1292. [DOI] [PubMed] [Google Scholar]
- McNeil CJ, Giesebrecht S, Gandevia SC & Taylor JL (2011a). Behaviour of the motoneurone pool in a fatiguing submaximal contraction. J Physiol 589, 3533–3544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNeil CJ, Giesebrecht S, Khan SI, Gandevia SC & Taylor JL (2011b). The reduction in human motoneurone responsiveness during muscle fatigue is not prevented by increased muscle spindle discharge. J Physiol 589, 3731–3738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNeil CJ, Martin PG, Gandevia SC & Taylor JL (2009). The response to paired motor cortical stimuli is abolished at a spinal level during human muscle fatigue. J Physiol 587, 5601–5612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNeil CJ, Martin PG, Gandevia SC & Taylor JL (2011c). Long‐interval intracortical inhibition in a human hand muscle. Exp Brain Res 209, 287–297. [DOI] [PubMed] [Google Scholar]
- Merton PA (1954). Voluntary strength and fatigue. J Physiol 123, 553–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opie GM & Semmler JG (2014). Modulation of short‐ and long‐interval intracortical inhibition with increasing motor evoked potential amplitude in a human hand muscle. Clin Neurophysiol 125, 1440–1450. [DOI] [PubMed] [Google Scholar]
- Rothwell JC (2009). The fatigued spinal cord. J Physiol 587, 5517–5518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacco P, Thickbroom GW, Thompson PD & Mastaglia FL (1997). Changes in corticomotor excitation and inhibition during prolonged submaximal muscle contractions. Muscle Nerve 20, 1158–1166. [DOI] [PubMed] [Google Scholar]
- Salavati B, Rajji TK, Zomorrodi R, Blumberger DM, Chen R, Pollock BG & Daskalakis ZJ (2018). Pharmacological manipulation of cortical inhibition in the dorsolateral prefrontal cortex. Neuropsychopharmacology 43, 354–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger TD, Garg RR & Chen R (2001). Interactions between two different inhibitory systems in the human motor cortex. J Physiol 530, 307–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidhu SK, Cresswell AG & Carroll TJ (2012). Motor cortex excitability does not increase during sustained cycling exercise to volitional exhaustion. J Appl Physiol 113, 401–409. [DOI] [PubMed] [Google Scholar]
- Sidhu SK, Cresswell AG & Carroll TJ (2013). Corticospinal responses to sustained locomotor exercises: moving beyond single‐joint studies of central fatigue. Sports Med 43, 437–449. [DOI] [PubMed] [Google Scholar]
- Sidhu SK, Weavil JC, Mangum TS, Jessop JE, Richardson RS, Morgan DE & Amann M (2017). Group III/IV locomotor muscle afferents alter motor cortical and corticospinal excitability and promote central fatigue during cycling exercise. Clin Neurophysiol 128, 44–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidhu SK, Weavil JC, Venturelli M, Garten RS, Rossman MJ, Richardson RS, Gmelch BS, Morgan DE & Amann M (2014). Spinal μ‐opioid receptor‐sensitive lower limb muscle afferents determine corticospinal responsiveness and promote central fatigue in upper limb muscle. J Physiol 592, 5011–5024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siebner HR, Dressnandt J, Auer C & Conrad B (1998). Continuous intrathecal baclofen infusions induced a marked increase of the transcranially evoked silent period in a patient with generalized dystonia. Muscle Nerve 21, 1209–1212. [DOI] [PubMed] [Google Scholar]
- Taylor JL (2006). Stimulation at the cervicomedullary junction in human subjects. J Electromyogr Kinesiol 16, 215–223. [DOI] [PubMed] [Google Scholar]
- Taylor JL, Amann M, Duchateau J, Meeusen R & Rice CL (2016). Neural contributions to muscle fatigue: from the brain to the muscle and back again. Med Sci Sports Exerc 48, 2294–2306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor JL, Butler JE, Allen GM & Gandevia SC (1996). Changes in motor cortical excitability during human muscle fatigue. J Physiol 490, 519–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor JL, Petersen N, Butler JE & Gandevia SC (2000). Ischaemia after exercise does not reduce responses of human motoneurones to cortical or corticospinal tract stimulation. J Physiol 525, 793–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valls‐Sole J, Pascual‐Leone A, Wassermann EM & Hallett M (1992). Human motor evoked responses to paired transcranial magnetic stimuli. Electroencephalogr Clin Neurophysiol 85, 355–364. [DOI] [PubMed] [Google Scholar]
- Weavil JC, Sidhu SK, Mangum TS, Richardson RS & Amann M (2015). Intensity‐dependent alterations in the excitability of cortical and spinal projections to the knee extensors during isometric and locomotor exercise. Am J Physiol Regul Integr Comp Physiol 308, R998–R1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weavil JC, Sidhu SK, Mangum TS, Richardson RS & Amann M (2016). Fatigue diminishes motoneuronal excitability during cycling exercise. J Neurophysiol 116, 1743–1751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werhahn KJ, Kunesch E, Noachtar S, Benecke R & Classen J (1999). Differential effects on motorcortical inhibition induced by blockade of GABA uptake in humans. J Physiol 517, 591–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yacyshyn AF, Woo EJ, Price MC & McNeil CJ (2016). Motoneuron responsiveness to corticospinal tract stimulation during the silent period induced by transcranial magnetic stimulation. Exp Brain Res 234, 3457–3463. [DOI] [PubMed] [Google Scholar]


