Abstract
Common gene fusion of the ALK gene is fusion of the ALK tyrosine kinase area and the 5’end of EML4. Seventeen EML4‐ALK fusion variants have been reported. Herein, we report a novel EML4‐ALK variant detected by next‐generation sequencing in a 36‐year‐old female lung adenocarcinoma patient who experienced disease progression after six months of alectinib treatment. Second generation sequencing revealed an EML4‐ALK fusion variant in which intron 19 of EML4 was fused to exon 20 of ALK. This is the first case of EML4‐ALK (E19: A20) fusion to be reported. Alectinib may show unsatisfactory therapeutic effects for this kind of ALK fusion.
Keywords: Alectinib, crizotinib, EML4‐ALK, lung cancer
Introduction
Lung cancer is the leading malignant tumor responsible for morbidity and mortality worldwide. According to the latest published data, 110 000 new cases of lung cancer are diagnosed and approximately 70 000 people die of this disease annually in the United States (US).1 Non‐small cell lung cancer (NSCLC) is the most common type, accounting for 85% of all cases and patients are often in advanced stages when diagnosed. The one‐year survival rate after treatment with a doublet platinum‐based regimen is < 40%.2 With developments in research on the tumor signal pathway, targeted therapy has received increasing attention. Approximately 5% of NSCLC patients are reported to have mesenchymal lymphoma gene fusion (ALK), with EML4‐ALK being the most common.3 Approximately 10 000 ALK‐positive patients are confirmed annually in the US, particularly non‐smoking young women and adenocarcinoma and EGFR wild‐type patients.4
The ALK gene inhibitor has been a great breakthrough in recent years, significantly extending the survival period of advanced NSCLC patients with ALK (+). Crizotinib, ceritinib, and alectinib have all been approved as first‐line‐therapy for advanced NSCLC patients harboring ALK activating mutations.5, 6, 7 However, almost all patients with EML4‐ALK fusion mutations initially responsive to ALK‐ tyrosine kinase inhibitors acquire resistance. With the exception of non‐ALK mutated resistance (ALK WT), another major reason for drug resistance of ALK‐tyrosine kinase inhibitors (TKIs) is that mutations exist in the ALK kinase region. The main reasons for crizotinib resistance are ALK amplification and L1196M mutation.8 G1202R mutation is the main reason for the resistance of second‐generation TKIs.9 Clinically, G1202R mutations are sensitive to lorlatinib, but the sensitivity of other drug resistance sites to lorlatinib requires further study.10 The choice of subsequent treatments after patients develop resistance to first and second generation TKIs therefore remains a major challenge.
Case report
A 36‐year‐old woman was admitted to our hospital in June 2017 because of right cervical lymph node enlargement, which had been present for a month. Positron emission tomography‐computed tomography (PET‐CT) showed right upper lobe lung cancer with multiple lymph node, liver, and bone metastases (Fig 1). A biopsy was performed on 7 July. The immunohistochemical markers were: TTF ‐ 1(+), Ventana ALK (D5F3) (+), HER‐2(+), PMS‐2(+). Lymph node metastatic lung adenocarcinoma was confirmed and right upper lobe adenocarcinoma (cT4N3M1c, IVb) was diagnosed. The results of cervical lymph node tissue gene detection (second‐generation sequencing technology) showed fusion of intron 19 of EML4 and exon 20 of ALK (EML4‐ALK [E19: A20]) (Fig 2), abundance 15.6%, which was confirmed by Sanger sequencing of EML4‐ALK fusion (Fig 3).
Figure 1.
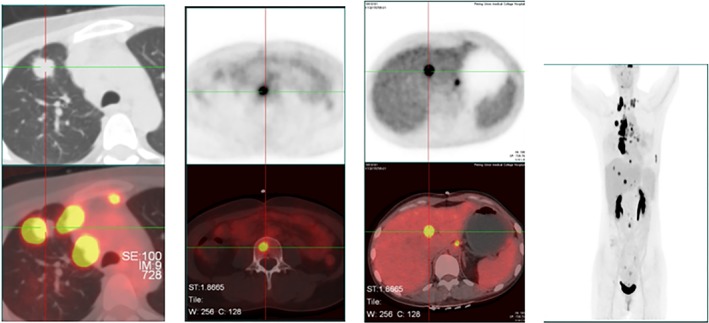
Positron emission tomography‐computed tomography baseline results 6 July 2017.
Figure 2.
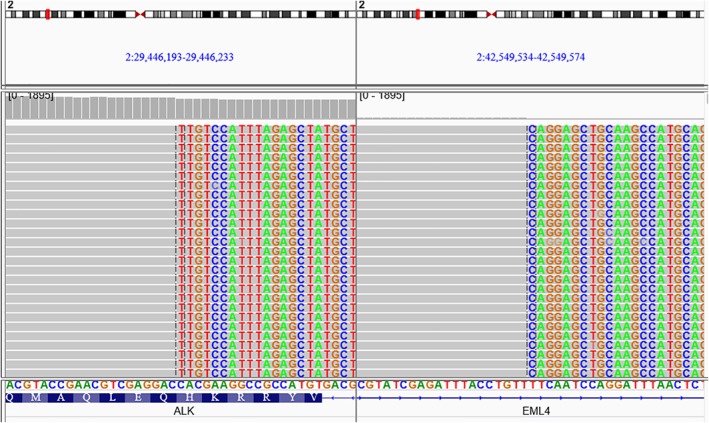
IGV screenshots (EML419‐ALK20) of the fusion sample.
Figure 3.
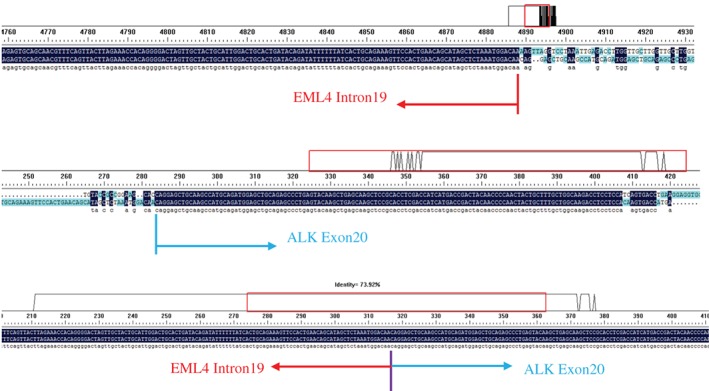
DNA Sanger sequencing results.
The patient was administered crizotinib 250 mg orally twice a day (b.i.d. per os) from 7 July 2017, but developed uncontrollable diarrhea after a week of treatment. Thus, alectinib 300 mg b.i.d. per os was administered instead, with no apparent adverse effects. On 16 August, PET‐CT revealed that the lesions in the upper lobe had significantly reduced. The metabolism had reduced and the liver and bone metastatic lesions had almost disappeared (Fig 4). The therapeutic efficacy was evaluated as a partial response (PR). We continued to use alectinib 300 mg b.i.d. per os. The PET‐CT examination on 20 October (after 3 months of alectinib treatment) indicated that metabolism in the upper lobe of the right lung was low and the liver and bone metastases were not obvious. The therapeutic efficacy was again evaluated as PR.
Figure 4.
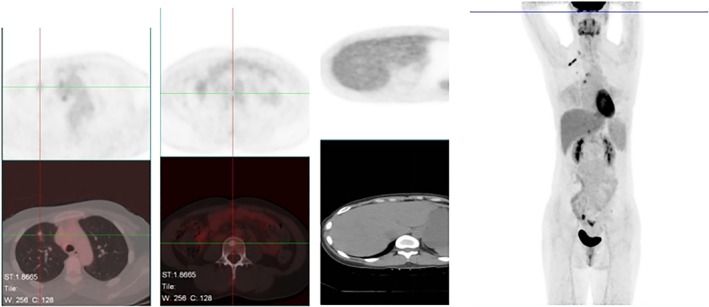
Results after one month of alectinib 300 mg orally twice per day, 16 Aug 2017.
On 8 February 2018 (after 6 months of alectinib), PET‐CT indicated soft tissue masses on both sides of the right second rib (2.1*1.6*1.6 cm and 2.1*1.9*2.1 cm) with significantly elevated metabolism (maximum standardized uptake value 23.4/21.4). Although the right lung lesion and the liver and bone metastases were no longer obvious, new tumor metastasis was considered (Fig 5). The therapeutic efficacy was evaluated as progressive disease (PD). We believed that the patient acquired drug resistance to alectinib and therefore performed another cervical lymph node biopsy on 16 February 2018. Gene sequencing showed a high abundance of EML4‐ALK (E19: A20) rearrangement (mutation abundance: 45.6%) and c‐MET copy amplification (CN = 2.9). We altered the targeted drug to crizotinib 250mg b.i.d. combined with local radiotherapy. No apparent side effects were observed in two months of treatment. On 19 April 2018, PET‐CT indicated that the intercostal soft tissue mass on the right side second rib was significantly smaller and the metabolism had decreased. Primary right upper lobe lesions and liver and bone metastases were no longer obvious (Fig 6). The therapeutic efficacy was evaluated as PR and the patient continues to be treated with crizotinib.
Figure 5.
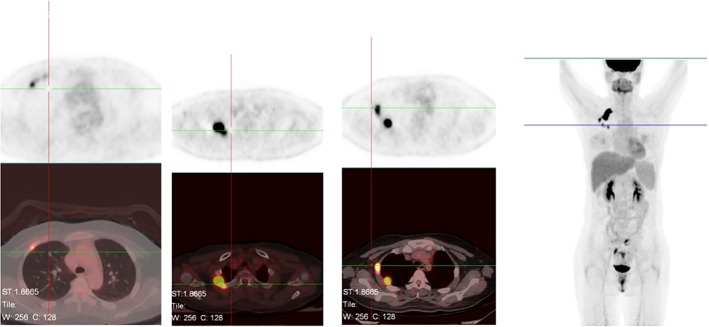
Results after six months alectinib 300 mg orally twice per day, 8 Feb 2018.
Figure 6.
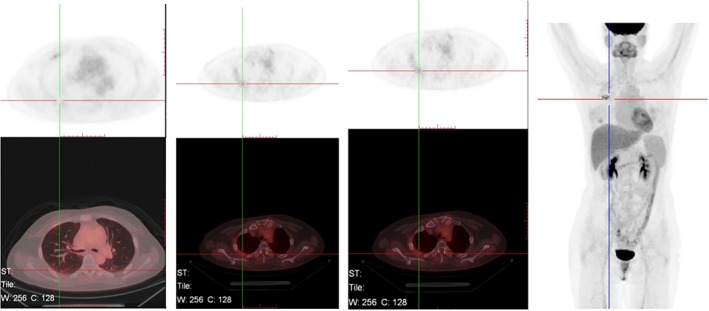
Results after two months of crizotinib 250 mg twice per day, 19 Apr 2018.
Discussion
Our patient is the first reported case of EML4‐ALK (E19: A20) fusion worldwide. No relevant reports were found in a database search including PubMed, Ovid, and Web of Science. This fusion variant is also not included in the Catalogue of Somatic Mutations in Cancer database (COSMIC v85, 8 May 2018).11 Since Soda et al. first discovered the ALK gene mutation EML4‐ALK (E13: A20) in lung adenocarcinoma tumor tissues through proteomics in 2007,12 at least 28 kinds of EML4‐ALK fusion variants have been reported.11 The most common are EML4 variant 1 (v1: exon 13 and ALK exon 20 fusion [E13: A20]) and variant 3 (v3a/b: exon 6 a/b and ALK exon 20 fusion [E6a/b: A20]), with incidence of > 60%. All variants retain the entire tyrosine kinase domain of ALK and the N‐terminal curly helix region of EML4, which is essential for the dimerization and constitutive activation of ALK.13
The results of the ALEX study were published in 2017: the median progression‐free survival (mPFS) period after alectinib treatment is 25.7 months, which is the longest mPFS of current first‐line treatment.7 In our case, PFS on alectinib treatment was only six months, far lower than the average data. The biopsy showed a significant increase in the abundance of EML4‐ALK mutations and activation of the MET bypass, which is consistent with research on the resistance mechanisms of alectinib, which include point mutations (including G1202R, I1171, V1180L10 and activation of hepatocyte growth factor‐MET bypass.14 In this case, the patient showed resistance to alectinib after a short time, but exhibited a good response to crizotinib, which can be explained MET bypass activation.14 Lin et al. studied the relationship between EML4‐ALK variant subtypes V1 and V3 and drug resistance and found that compared to the V1 variant, the V3 variant is more likely to develop drug resistance and to produce a G1202R drug‐resistant mutation, suggesting that the EML4‐ALK variant subtype may be related to the efficacy of targeted drugs.15 ALK expression at the protein level indicates functional and thus potential therapeutic relevance.16 Interestingly, our patient showed a significant increase in the abundance of EML4‐ALK fusion mutations, which may indicate that the EML4‐ALK (E19: A20) variant may show an unsatisfactory response specifically to alectinib. Although ours is presently the only reported case of EML4‐ALK (E19: A20), our experience shows that it is necessary to explicitly identify the subtype of variants in ALK‐positive patients, which may subsequently identify the patients who will benefit from treatment with ALK inhibitors.
Disclosure
No authors report any conflict of interest.
Acknowledgment
We thank the patient and her family for providing their information for this study.
References
- 1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin 2017; 67: 7–30. [DOI] [PubMed] [Google Scholar]
- 2. Ramalingam S, Belani CP. State‐of‐the‐art chemotherapy for advanced non‐small cell lung cancer. Semin Oncol 2004; 31 (1 Suppl 1): 68–74. [DOI] [PubMed] [Google Scholar]
- 3. Tsao AS, Scagliotti GV, Bunn PAJ et al Scientific advances in lung cancer 2015. J Thorac Oncol 2016; 11: 613–38. [DOI] [PubMed] [Google Scholar]
- 4. Shaw AT, Yeap BY, Mino‐Kenudson M et al Clinical features and outcome of patients with non‐small‐cell lung cancer who harbor EML4‐ALK. J Clin Oncol 2009; 27: 4247–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mok TS, Kim D, Wu Y et al LBA50 ‐ overall survival (OS) for first‐line crizotinib versus chemotherapy in ALK+ lung cancer: Updated results from PROFILE 1014. Annals of Oncology 2017; 28 (Suppl_5): v605–49. [Google Scholar]
- 6. Soria JC, Tan DSW, Chiari R et al First‐line ceritinib versus platinum‐based chemotherapy in advanced ALK‐rearranged non‐small‐cell lung cancer (ASCEND‐4): A randomised, open‐label, phase 3 study. (Published erratum appears in Lancet 2017; 389:908). Lancet 2017; 389: 917–29. [DOI] [PubMed] [Google Scholar]
- 7. Shaw AT, Peters S, Mok T, Gadgeel SM, Ahn JS, Ou SHI. Alectinib versus crizotinib in treatment‐naïve advanced ALK‐positive non‐small cell lung cancer (NSCLC): Primary results of the global phase III ALEX study. Paper presented at ASCO Annual Meeting; 2–6 Jun 2017, Chicago, IL, USA. Abstract LBA9008.
- 8. Wu J, Savooji J, Liu D. Second‐ and third‐generation ALK inhibitors for non‐small cell lung cancer. J Hematol Oncol 2016; 9: 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Friboulet L, Li N, Katayama R et al The ALK inhibitor ceritinib overcomes crizotinib resistance in non‐small cell lung cancer. Cancer Discov 2014; 4: 662–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gainor JF, Dardaei L, Yoda S et al Molecular mechanisms of resistance to first‐ and second‐generation ALK inhibitors in ALK‐rearranged lung cancer. Cancer Discov 2016; 6: 1118–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Catalogue of Somatic Mutations in Lung Cancer . Fusions: EML4‐ALK [Cited 29 May 2018.] Available from URL: https://cancer.sanger.ac.uk/cosmic/fusion/overview?fid=7134&gid=50
- 12. Soda M, Choi YL, Enomoto M et al Identification of the transforming EML4‐ALK fusion gene in non‐small‐cell lung cancer. Nature 2007; 448: 561–6. [DOI] [PubMed] [Google Scholar]
- 13. Sabir SR, Yeoh S, Jackson G, Bayliss R. EML4‐ALK variants: Biological and molecular properties, and the implications for patients. Cancers (Basel) 2017; 9: 118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Yoshimura Y, Kurasawa M, Yorozu K, Puig O, Bordogna W, Harada N. Antitumor activity of alectinib, a selective ALK inhibitor, in an ALK‐positive NSCLC cell line harboring G1269A mutation: Efficacy of alectinib against ALK G1269A mutated cells. Cancer Chemother Pharmacol 2016; 77: 623–8. [DOI] [PubMed] [Google Scholar]
- 15. Lin JJ, Zhu VW, Yoda S et al Impact of EML4‐ALK variant on resistance mechanisms and clinical outcomes in ALK‐positive lung cancer. J Clin Oncol 2018; 36: 1199–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Penzel R, Schirmacher P, Warth A. A novel EML4‐ALK variant: Exon 6 of EML4 fused to exon 19 of ALK. J Thorac Oncol 2012; 7: 1198–9. [DOI] [PubMed] [Google Scholar]


