Abstract
Background
The combination of PD‐1 inhibitors and cytotoxic drugs is reported to enhance anti‐tumor activity in non‐small cell lung cancer; however, the underlying synergistic mechanisms remain uncertain. This retrospective case series was designed to investigate objective response and survival rates of salvage chemotherapy following nivolumab and explore the immunohistochemical profiles of tumor‐infiltrating immune cells.
Methods
The medical records of 37 patients administered nivolumab were retrospectively reviewed. Overall response rate and progression‐free survival were compared among three groups: salvage chemotherapy following nivolumab, nivolumab therapy alone, and chemotherapy preceding nivolumab.
Results
Eight cases met the study criteria. Salvage chemotherapy following nivolumab improved the overall response rate to 62.5% (95% confidence interval [CI] 34.4–90.6%; P = 0.004) and median progression‐free survival to six months (95% CI 4.6–7.4; P = 0.016), compared to nivolumab alone and preceding chemotherapy. The response to salvage chemotherapy was not associated with tumor PD‐L1 expression. A partial response was achieved in four cases with ≤ 5% and ≤ 2.9 cells/mm2 of PD‐1+ immune cells, whereas stable disease and progressive disease were observed in three cases with ≥ 30% and ≥ 12.7 cells/mm2. Responders had fewer PD‐1+ immune cells than non‐responders (percentage P = 0.028; density P = 0.034).
Conclusion
Salvage chemotherapy following nivolumab improved anti‐tumor activity regardless of tumor PD‐L1 status, but nivolumab following chemotherapy did not. The presence of few PD‐1+ tumor‐infiltrating immune cells may serve as a potential predictor of response to salvage chemotherapy. Further studies involving a large cohort are needed to clarify how nivolumab re‐sensitizes the tumor immune microenvironment to chemotherapy.
Keywords: Exhausted T cell, immunotherapy, PD‐1, salvage chemotherapy
Introduction
PD‐1 inhibitors, such as nivolumab, have emerged as breakthrough therapy for non‐small cell lung cancer (NSCLC). We have reported the drastic regression of lung cancer cases treated with chemotherapy following nivolumab, which is known as salvage chemotherapy.1 Several recent studies have also shown that the combination therapy of PD‐1 inhibitors with anti‐cancer drugs, including salvage chemotherapy, produces high anti‐tumor activity in some patients.2, 3, 4, 5, 6, 7 Salvage chemotherapy showed a high objective response rate of 38% (95% confidence interval [CI] 31.3–44.7%) in a total of 200 cases obtained from four retrospective studies.2, 3, 4, 5
However, the mechanism that underlies this immunochemotherapy and whether optimal patients can be selected in advance are unknown. The evaluation of salvage chemotherapy may provide clues to these issues, because the anti‐tumor effects of PD‐1 inhibitors and chemotherapy are considered separately. This preliminary case series was designed to investigate the response rate and survival according to the sequence of chemotherapy and nivolumab, and to explore the tumor immune microenvironment using tissue samples taken at initial diagnosis.
Methods
Study design
This retrospective, single‐institute, exploratory case series was conducted at Sasebo City General Hospital, which is officially authorized as a regional core cancer center. Patients were included in the present study based on the following two criteria: they had recurrent or advanced NSCLC, and underwent salvage chemotherapy following nivolumab between April 2016 and December 2017. Lung cancer stage was determined according to the 8th edition of the Union for International Cancer Control classification. The therapy regimens were decided by discussion between the physicians and patients. The patients received 3 mg/kg of nivolumab intravenously every two weeks. All patients were followed‐up until 1 June 2018. Patients were excluded from the analysis if they had any other neoplasms requiring concurrent treatment, if their lung cancer harbored EGFR mutations or ALK rearrangements, and if they had received other immune checkpoint inhibitors.
The institutional review board of Sasebo City General Hospital approved the study protocol (approval number 2018‐A012). Informed consent was obtained from the patients by opt‐out on the hospital website, in accordance with the ethical guidelines presented by the Japanese Ministry of Health, Labor and Welfare.8 This study was registered with the University Hospital Medical Information Network in Japan (registry number: UMIN000032667). Three cases in the study population were previously published as case reports.1, 9
Data collection
The records of patients receiving nivolumab were extracted from the database of the institutional multidisciplinary medical team for immune checkpoint inhibitors. Demographics, histology, molecular profiling, imaging, and treatment history were reviewed. A systemic follow‐up survey of the lesions was performed by physical examination, chest radiography, and blood tests, including serum tumor markers, at least once a month. Computed tomography scans of the chest and upper abdomen, brain magnetic resonance imaging, and positron emission tomography were routinely conducted as scheduled for outpatient follow‐up. Two experts, blinded to the clinical data, separately assessed the best tumor responses according to Response Evaluation Criteria in Solid Tumors version 1.1. In cases of a discrepancy, the experts reached agreement by means of re‐analysis and discussion. Treatment‐related adverse events were assessed according to Common Terminology Criteria for Adverse Events version 4.0.
Immunohistochemistry
Immunohistochemical findings of formalin‐fixed, paraffin‐embedded tumor specimens taken at initial diagnosis, including PD‐L1 expression on cancer cells, CD8+ T cells, PD‐1+ immune cells, and FOXP3+ regulatory T cells, were examined. The antibody clones used were as follows: PD‐L1 (22C3, Dako, Santa Clara, CA, USA); CD8 (4B11, Leica Biosystems, Nussloch, Germany); PD‐1 (SP269, Spring Bioscience, Pleasanton, CA, USA); and FOXP3 (236A/E7, Abcam, Cambridge, UK). Positivity of PD‐L1 for tumor cells and of CD8, PD‐1, and FOXP3 for tumor‐infiltrating immune cells was expressed as a percentage of positive cells in the four representative areas. Additionally, the number of PD‐1+ immune cells was expressed as the density of positive cells in the four representative areas. Two independent experts blinded to the clinical data evaluated the immunohistochemical findings. If the assessments differed, the experts re‐evaluated the specimen and reached a consensus.
Statistical analyses
The overall response rate (ORR) was expressed with 95% confidence interval (CI), and differences between groups were statistically evaluated by Fisher's exact test. Progression‐free survival (PFS) from the initiation of each therapy was estimated using the Kaplan–Meier method, with differences analyzed by the log‐rank test. Responders to salvage chemotherapy were defined as those with a complete response (CR) or partial response (PR), while non‐responders were those with stable disease (SD) or progressive disease (PD). The differences in immunohistochemical findings between the responders and non‐responders were assessed using the Mann–Whitney U test. Data were analyzed using the SAS software program (SAS Institute Inc., Cary, NC, USA), and a two‐tailed P value < 0.05 was considered significant.
Results
Patient characteristics
A total of 37 patients received nivolumab, including three who achieved CR and five PR as their best response (Fig 1). The ORR of nivolumab therapy in the 37 patients was 21.6% (95% CI 8.4–34.8%). As of June 2018, 9 patients were still being treated with nivolumab, and 18 did not receive salvage chemotherapy but best supportive care as a result of disease exacerbation or adverse events. Of the remaining 10 patients, 2 were excluded because of the presence of concurrent uterine cancer or EGFR mutation. Thus, the data of eight patients were analyzed in the present study. The patients’ characteristics are summarized in Table 1.
Figure 1.
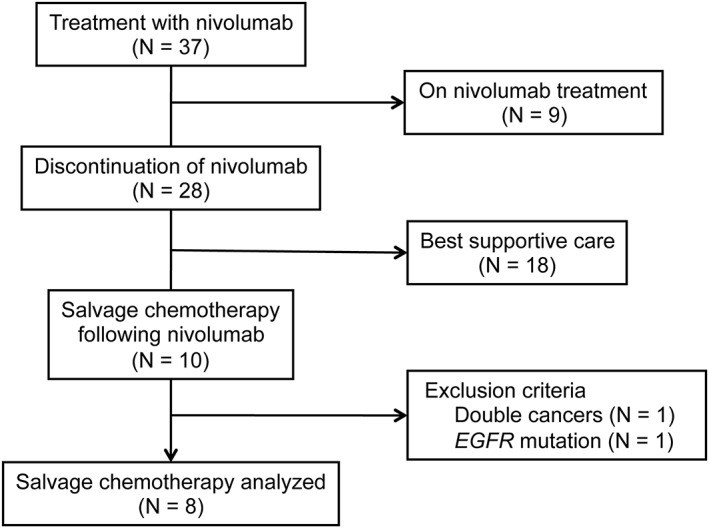
Study flow diagram.
Table 1.
Patient characteristics (n = 8)
| Variable | Number |
|---|---|
| Age, years | |
| Median (range) | 69 (62–75) |
| Gender | |
| Male | 6 (75%) |
| Female | 2 (25%) |
| ECOG performance status | |
| 0 | 1 (12.5%) |
| 1 | 7 (87.5%) |
| Smoking status | |
| Former smoker | 8 (100%) |
| Never‐smoker | 0 (0%) |
| Histologic subtype | |
| Adenocarcinoma | 2 (25%) |
| Squamous cell carcinoma | 4 (50%) |
| Undifferentiated carcinoma | 2 (25%) |
| Stage | |
| III | 1 (12.5%) |
| IV | 5 (62.5%) |
| Recurrence | 2 (25%) |
| Regimen of preceding chemotherapy before nivolumab | |
| Platinum doublet | 5 (62.5%) |
| S‐1 | 2 (25%) |
| Docetaxel | 1 (12.5%) |
| Number of nivolumab administrations | |
| Median (range) | 7 (2–10) |
| Period between the last nivolumab and first salvage chemotherapy, days | |
| Median (range) | 21 (20–69) |
| Line of salvage chemotherapy | |
| Third‐line | 3 (37.5%) |
| Fourth‐line | 3 (37.5%) |
| Fifth‐line | 2 (25%) |
| Regimen of salvage chemotherapy | |
| S‐1 | 5 (62.5%) |
| Carboplatin + albumin‐bound paclitaxel | 2 (25%) |
| Docetaxel + ramucirumab | 1 (12.5%) |
ECOG, Eastern Cooperative Oncology Group.
All patients received platinum doublet therapy as first‐line chemotherapy. The median number of nivolumab administrations was 7, and the median period between the last nivolumab and first salvage chemotherapy administration was 21 days. Three patients underwent salvage chemotherapy as third‐line therapy, and five as fourth‐line or higher (nivolumab was included as a line of therapy). Five patients received single‐agent S‐1 and three received combination therapy including taxanes as salvage chemotherapy. Two patients developed grade 3 anemia after exposure to S‐1 and grade 3 pneumonitis after treatment with docetaxel/ramucirumab.
Changes in overall response rate (ORR) and progression‐free survival (PFS) by consecutive therapy
Overall response rate and PFS were compared among three groups: salvage chemotherapy following nivolumab, nivolumab therapy alone, and chemotherapy preceding nivolumab. As shown in Table 2, the ORR after salvage chemotherapy was 62.5% (5/8, 95% CI 34.4–90.6%), whereas the ORRs of nivolumab therapy alone or preceding chemotherapy were both 0% (0/8, 95% CI 0–21.0%). The ORR after salvage chemotherapy was significantly higher than in the other groups (P = 0.004). The median PFS after salvage chemotherapy was 6 months (95% CI 4.6–7.4) compared to 3.5 (95% CI 2.0–5.0) and 3.6 (95% CI 2.6–4.7) months after nivolumab therapy and preceding chemotherapy, respectively (Fig 2). PFS was significantly longer after salvage chemotherapy (P = 0.016).
Table 2.
Relationship between response to salvage chemotherapy and immunologic profiles of lung cancer
| Case | Preceding chemotherapy | Nivolumab Response | Salvage chemotherapy | Histologic subtype | PD‐L1+ tumor cells | CD8+ T cells | PD‐1+ immune cells | FOXP3+ Tregs | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Regimen | Response | Regimen | Response | % | /mm2 | ||||||
| Case 1 | DTX | SD | PD | S‐1 | PR | UN | 95% | 60% | < 1% | 1.3 | 30% |
| Case 2 | S‐1 | PD | SD | CBDCA/nab‐PTX | PR | SCC | 10% | 40% | 5% | 2.9 | 5% |
| Case 3 | CDDP/PEM/BEV | SD | PD | S‐1 | PR | AD | < 1% | 30% | < 1% | 1.5 | 5% |
| Case 4 | CDDP/GEM | PD | PD | DTX/RAM | PR | SCC | < 1% | 50% | < 1% | 0.6 | 20% |
| Case 5 | S‐1 | PD | PD | CBDCA/nab‐PTX | PR | AD | NA | NA | NA | NA | NA |
| Case 6 | CBDCA/nab‐PTX | SD | PD | S‐1 | SD | SCC | 20% | 50% | 30% | 12.7 | 15% |
| Case 7 | CBDCA/PTX | PD | PD | S‐1 | SD | UN | < 1% | 60% | 50% | 24 | 20% |
| Case 8 | NDP/DTX | PD | PD | S‐1 | PD | SCC | 70% | 60% | 60% | 33.9 | 15% |
AD, adenocarcinoma; BEV, bevacizumab; CBDCA, carboplatin; CDDP, cisplatin; DTX, docetaxel; GEM, gemcitabine; NA; not available; nab‐PTX, albumin‐bound PTX; NDP, nedaplatin; PD, progressive disease; PEM, pemetrexed; PR, partial response; PTX, paclitaxel; RAM, ramucirumab; SCC, squamous cell carcinoma; SD, stable disease; Tregs, regulatory T cells; UN, undifferentiated carcinoma.
Figure 2.
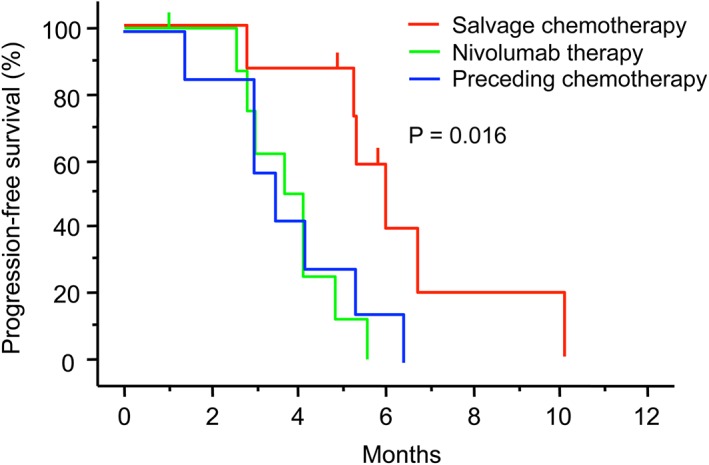
Kaplan–Meier estimates of progression‐free survival according to consecutive therapies. The vertical bars indicate censored cases. Progression‐free survival after salvage chemotherapy, nivolumab alone, and preceding chemotherapy (P = 0.016 by log‐rank test).
Tumor immune microenvironment according to response to salvage chemotherapy
The relationship between objective response to salvage chemotherapy and the profiles of the tumor immune microenvironment was explored (Table 2, Fig 3). One of eight patients was excluded from immunohistochemical analysis because of an inadequate tissue specimen. The objective response to salvage chemotherapy was not associated with the level of tumor PD‐L1 expression or the degree of tumor‐infiltrating CD8+ T and FOXP3+ regulatory T cells. PD‐1+ immune cells were mainly composed of lymphocytes, and the positivity of PD‐1 on immune cells ranged from < 1% to 60%. Four responders had ≤ 5% of PD‐1+ immune cells, while three non‐responders had ≥ 30% of PD‐1+ immune cells. Responders had a lower percentage of PD‐1+ immune cells than non‐responders (P = 0.028). The number of PD‐1+ immune cells ranged from 0.6 to 33.9 cells/mm2. Responders had ≤ 2.9 cells/mm2 PD‐1+ immune cells, while non‐responders had ≥ 12.7 cells/mm2 PD‐1+ cells. Responders had less PD‐1+ immune cells than non‐responders (P = 0.034).
Figure 3.
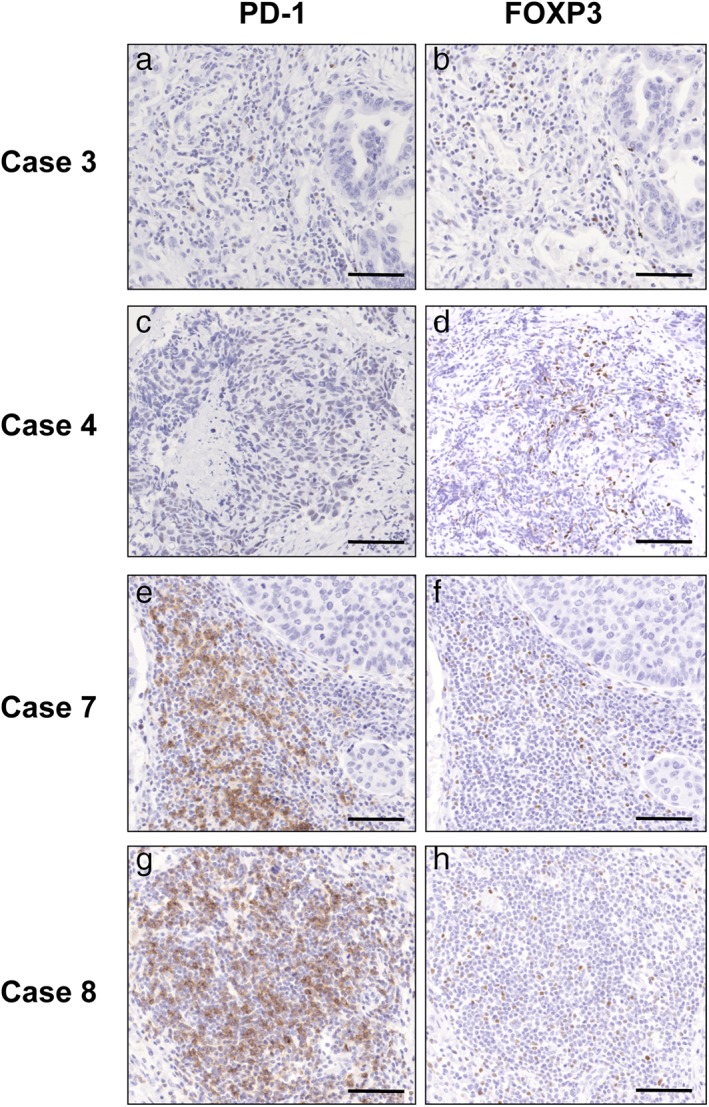
Immunohistochemical examination of the tumor‐infiltrating immune cells in four representative cases. Case 3 of adenocarcinoma and case 4 of squamous‐cell carcinoma show a partial response to salvage chemotherapy, case 7 of undifferentiated carcinoma shows stable disease, and case 8 of squamous‐cell carcinoma shows progressive disease. (a,c,e,g) PD‐1+ immune cells; (b,d,f,h) FOXP3+ regulatory T cells (scale bars 100 μm).
Discussion
This exploratory case series provides two important conclusions. Salvage chemotherapy following nivolumab revived anti‐tumor activity regardless of tumor PD‐L1 expression, but nivolumab following chemotherapy did not. The presence of few PD‐1+ immune cells in the tumor may be a candidate therapeutic predictor of response to salvage chemotherapy.
The sequence of chemotherapy following nivolumab improved anti‐tumor activity regardless of tumor PD‐L1 expression, but the reverse sequence did not. Similar to these findings, four retrospective studies of salvage chemotherapy, including a case–control study, showed a high ORR of 38% and median PFS of approximately four months in previously treated patients with NSCLC.2, 3, 4, 5 In the case–control study, the odds ratio for achieving an objective response to salvage chemotherapy was more than three times higher.2 However, previous clinical studies did not fully identify whether the anti‐tumor activity of salvage chemotherapy was associated with the sequence of PD‐1 inhibitors.
The sequence and timing of chemotherapy for PD‐1 blockade are critical issues. Although chemotherapeutic agents have immunomodulatory effects,10, 11 subset analysis of a phase III trial of first‐line pembrolizumab showed that lung cancer patients who received second‐line chemotherapy following pembrolizumab had superior PFS compared to those who received second‐line PD‐1 inhibitors following chemotherapy.12 In a preclinical model with melanoma, PD‐1 blockade followed three days later with chemotherapy regressed the tumor and prolonged survival compared to chemotherapy alone, PD‐1 blockade alone, and concurrent administration of these agents.13 Although tumor growth in PD‐1 knockout mice was similar to that of wild type mice, chemotherapy significantly suppressed tumor growth in PD‐1 knockout mice.13 Collectively, these findings (including ours) suggest that preceding nivolumab could synergize with salvage chemotherapy, even without tumor PD‐L1 expression or an evident objective response to nivolumab.
The presence of few PD‐1+ tumor‐infiltrating immune cells may be a candidate therapeutic predictor of salvage chemotherapy following nivolumab. PD‐1‐expressing CD8+ cells consist of different populations that respond differentially to PD‐1 blockade. PD‐1 inhibitors can activate partially exhausted cytotoxic T cells and shrink the tumors, but not deeply exhausted T cells.14 High PD‐1‐expressing CD8+ cells secret less cytotoxic cytokines in vitro,15, 16 but nivolumab does not subsequently restore the release of cytokines.17 Although a subset of effector T cells differentiated into effector memory T cells for durable effects of PD‐1 inhibitors,13, 14 the deeply exhausted T cells were not able to differentiate into effector memory T cells.18 In a retrospective cohort study of lung cancer, a low ratio of PD‐1+ cells to CD8+ cells in the tumor, but not the actual number of PD‐1+ cells, conferred better ORR and PFS after nivolumab therapy.19 In the present study, a low ratio of PD‐1+ cells to immune cells and a small number of PD‐1+ cells were not associated with ORR after nivolumab therapy, but conferred better ORR after salvage chemotherapy. Although the reason for this discrepancy is unclear, recent studies have raised a fundamental question concerning the conflicting roles of PD‐1 on T cells: can PD‐1 expression be considered a reflection of tumor‐specific T cell activation as well as T cell dysfunction in the dynamic process of the tumor immune microenvironment?20 High PD‐1‐expressing CD8+ cells may be deeply exhausted and irreversibly dysfunctional to PD‐1 blockade followed by salvage chemotherapy.
Another important finding of our study was the coexistence of FOXP3+ Tregs with CD8+ T cells within the tumor. We reported that a case of lung cancer regressed spontaneously and subsequently relapsed with an increase in the number of Tregs in the circulation.21, 22 We also reported that lung cancer cases showing a remarkable response to nivolumab contained < 5% Tregs in the tumor.23, 24 Tregs inhibited nivolumab‐stimulated release of interferon‐γ from effector T cells in vitro.25 Although PD‐1 blockade did not affect the function of Tregs,26 several anti‐cancer drugs are known to reduce the number of Tregs and restore the activity of effector T cells.27 In addition, the selective depletion of Tregs by the chemokine receptor 4 antagonist mogamulizumab has been suggested to revive sensitivity to chemotherapy.28 These findings encourage further investigation to define whether Tregs may affect, at least in part, the efficacy of nivolumab and subsequent chemotherapy.
Several limitations of this study need to be acknowledged. First, most of the study cases received late‐line chemotherapy, suggesting that they may have had indolent tumors or better clinical conditions. In order to reduce the effect of selection bias, we used a self‐controlled case series method: the individuals acted as their own control, and the efficacy of salvage chemotherapy was compared to that of previous therapies within the individuals. Second, the sample cases incidentally consisted of patients without an objective response to nivolumab. This is mainly because the responders to nivolumab remain on the treatment. It is poorly documented whether the underlying mechanisms of the innate primary resistance to nivolumab differ from those of acquired resistance.14 Nevertheless, a retrospective cohort study suggests that the response to salvage chemotherapy seems similar in the presence or absence of the response to PD‐1 blockade.5
The third limitation is that the tumor immune microenvironment was evaluated using tissue specimens taken at initial diagnosis. In clinical practice, re‐biopsy of tumor tissue for salvage chemotherapy is difficult. However, it is well known that immunotherapy can alter the characteristics of the tumor immune microenvironment.29 Additionally, monitoring the phenotype and frequency of blood‐circulating immune cells may be useful to clarify the mechanisms underlying immunotherapy and salvage chemotherapy.30 In patients with melanoma receiving salvage chemotherapy, the preexistence or increase of blood‐circulating CX3CR1+ CD8+ effector memory T cells, which frequently expressed PD‐1, was associated with anti‐tumor activity.13 The present preliminary findings warrant prospective clinical trials in a large cohort undergoing re‐biopsy of tumor tissue or monitoring of blood‐circulating immune cells before and during salvage chemotherapy.
In conclusion, to the best of our knowledge, this article is the first to report that the sequence of chemotherapy following nivolumab could enhance anti‐tumor activity regardless of tumor PD‐L1 expression, but not the reverse sequence. Furthermore, the presence of few PD‐1+ tumor‐infiltrating immune cells may serve as a potential predictor of response to salvage chemotherapy. These findings may provide insight into a better approach for immune escape in lung cancer. Further studies are required to elucidate how nivolumab renders the tumor immune microenvironment more susceptible to chemotherapy specifically.
Disclosure
No authors report any conflicts of interest.
References
- 1. Ogawara D, Soda H, Iwasaki K et al Remarkable response of nivolumab‐refractory lung cancer to salvage chemotherapy. Thorac Cancer 2018; 9: 175–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Leger PD, Rothschild S, Castellanos E, Narayana R, York SJ, Horn L. Response to salvage chemotherapy following exposure to immune checkpoint inhibitors in patients with non‐small cell lung cancer. J Clin Oncol 2017; 35 (15 Suppl): Abstract 9084. [Google Scholar]
- 3. Schvartsman G, Peng A, Bis G et al Response rates to single‐agent chemotherapy after exposure to immune checkpoint inhibitors in advanced non‐small cell lung cancer. Lung Cancer 2017; 112: 90–5. [DOI] [PubMed] [Google Scholar]
- 4. Grigg C, Reuland BD, Sacher AG et al Clinical outcomes of patients with non‐small cell lung cancer (NSCLC) receiving chemotherapy after immune checkpoint blockade. J Clin Oncol 2017; 35 (15 Suppl): Abstract 9082. [Google Scholar]
- 5. Park SE, Lee SH, Ahn JS, Ahn MJ, Park K, Sun JM. Increased response rates to salvage chemotherapy administered after PD‐1/PD‐L1 inhibitors in patients with non‐small cell lung cancer. J Thorac Oncol 2018; 13: 106–11. [DOI] [PubMed] [Google Scholar]
- 6. Gandhi L, Rodríguez‐Abreu D, Gadgeel S et al Pembrolizumab plus chemotherapy in metastatic non‐small‐cell lung cancer. N Engl J Med 2018; 378: 2078–92. [DOI] [PubMed] [Google Scholar]
- 7. Borghaei H, Hellmann MD, Paz‐Ares LG et al Nivolumab (Nivo) + platinum doublet chemotherapy (chemo) vs chemo as first‐line (1L) treatment (Tx) for advanced non‐small cell lung cancer (NSCLC) with <1% tumor PD‐L1 expression: Results from CheckMate 227. J Clin Oncol 2018; 36: Abstract 9001. [Google Scholar]
- 8. The Ministry of Health, Labor and Welfare in Japan . Ethical Guideline for Medical and Health Research Involving Human Subjects, 2015. [Cited 10 Jun 2018.] Available from URL: http://www.mhlw.go.jp/file/06-Seisakujouhou-10600000-Daijinkanboukouseikagakuka/0000080278.pdf
- 9. Ogawara D, Soda H, Ikehara S et al Nivolumab infusion reaction manifesting as plantar erythema and pulmonary infiltrate in a lung cancer patient. Thorac Cancer 2017; 8: 706–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bracci L, Schiavoni G, Sistigu A, Belardelli F. Immune‐based mechanisms of cytotoxic chemotherapy: Implications for the design of novel and rationale‐based combined treatments against cancer. Cell Death Differ 2014; 21: 15–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Parra ER, Villalobos P, Behrens C et al Effect of neoadjuvant chemotherapy on the immune microenvironment in non‐small cell lung carcinomas as determined by multiplex immunofluorescence and image analysis approaches. J Immunother Cancer 2018; 6: 48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Brahmer JR, Rodríguez‐Abreu D, Robinson AG et al Progression after the next line of therapy (PFS2) and updated OS among patients (pts) with advanced NSCLC and PD‐L1 TPS ≥50% enrolled in KEYNOTE‐024. J Clin Oncol 2017; 35: Abstract 9000. [Google Scholar]
- 13. Yan Y, Cao S, Liu X et al CX3CR1 identifies PD‐1 therapy‐responsive CD8+ T cells that withstand chemotherapy during cancer chemoimmunotherapy. JCI Insight 2018; 3: e97828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Jenkins RW, Barbie DA, Flaherty KT. Mechanisms of resistance to immune checkpoint inhibitors. Br J Cancer 2018; 118: 9–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kansy BA, Concha‐Benavente F, Srivastava RM et al PD‐1 status in CD8+ T cells associates with survival and anti‐PD‐1 therapeutic outcomes in head and neck cancer. Cancer Res 2017; 77: 6353–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wei F, Zhong S, Ma Z et al Strength of PD‐1 signaling differentially affects T‐cell effector functions. Proc Natl Acad Sci U S A 2013; 110: e2480–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Thommen DS, Schreiner J, Müller P et al Progression of lung cancer is associated with increased dysfunction of T cells defined by coexpression of multiple inhibitory receptors. Cancer Immunol Res 2015; 3: 1344–55. [DOI] [PubMed] [Google Scholar]
- 18. Pauken KE, Sammons MA, Odorizzi PM et al Epigenetic stability of exhausted T cells limits durability of reinvigoration by PD‐1 blockade. Science 2016; 354: 1160–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Mazzaschi G, Madeddu D, Falco A et al Low PD‐1 expression in cytotoxic CD8+ tumor‐infiltrating lymphocytes confers an immune‐privileged tissue microenvironment in NSCLC with a prognostic and predictive value. Clin Cancer Res 2018; 24: 407–19. [DOI] [PubMed] [Google Scholar]
- 20. Simon S, Labarriere N. PD‐1 expression on tumor‐specific T cells: Friend or foe for immune therapy? Oncoimmnology 2018; 7: e1364828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Nakamura Y, Noguchi Y, Satoh E et al. Spontaneous remission of a non‐small cell lung cancer possibly caused by anti‐NY‐ESO‐1 immunity. Lung Cancer 2009; 65: 119–22. [DOI] [PubMed] [Google Scholar]
- 22. Isobe M, Eikawa S, Uenaka A et al Correlation of high and decreased NY‐ESO‐1 immunity to spontaneous regression and subsequent recurrence in a lung cancer patient. Cancer Immun 2009; 9: e8. [PMC free article] [PubMed] [Google Scholar]
- 23. Okamura K, Fukuda Y, Soda H et al. Pulmonary pleomorphic carcinoma with few PD‐1‐positive immune cells and regulatory T cells that showed a complete response to nivolumab. Thorac Cancer 2018; 9: 193–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Suyama T, Fukuda Y, Soda H et al. Successful treatment with nivolumab for lung cancer with low expression of PD‐L1 and prominent tumor‐infiltrating B cells and immunoglobulin G. Thorac Cancer 2018; 9: 750–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wang C, Thudium KB, Han M et al. In vitro characterization of the anti‐PD‐1 antibody nivolumab, BMS‐936558, and in vivo toxicology in non‐human primates. Cancer Immunol Res 2014; 2: 846–56. [DOI] [PubMed] [Google Scholar]
- 26. Toor SM, Syed Khaja AS, Alkurd I, Elkord E. In‐vitro effect of pembrolizumab on different T regulatory cell subsets. Clin Exp Immunol 2018; 191: 189–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Zheng Y, Dou Y, Duan L et al. Using chemo‐drugs or irradiation to break immune tolerance and facilitate immunotherapy in solid cancer. Cell Immunol 2015; 294: 54–9. [DOI] [PubMed] [Google Scholar]
- 28. Kurose K, Ohue Y, Isobe M et al Reviving chemotherapy sensitivity after anti‐CCR4 mAb (mogamulizumab) treatment in lung cancer patients. J Thorac Oncol 2017; 12 (11 Suppl 2): S2420–1 (Abstract P2.07‐015). [Google Scholar]
- 29. Wei SC, Levine JH, Cogdill AP et al. Distinct cellular mechanisms underlie anti‐CTLA‐4 and anti‐PD‐1 checkpoint blockade. Cell 2017; 170: 1120–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Tunger A, Kießler M, Wehner R et al Immune monitoring of cancer patients prior to and during CTLA‐4 or PD‐1/PD‐L1 inhibitor treatment. Biomedicine 2018; 6: e26. [DOI] [PMC free article] [PubMed] [Google Scholar]


