Abstract
Aims
Hypertension (HTN) is a well-known contributor to cardiovascular disease, including heart failure (HF) and coronary artery disease, and is the leading risk factor for premature death world-wide. A J- or U-shaped relationship has been suggested between blood pressure (BP) and clinical outcomes in different studies. However, there is little information about the significance of BP on the outcomes of patients with coronary artery disease and left ventricular dysfunction. This study aimed to determine the relationship between BP and mortality outcomes in patients with ischaemic cardiomyopathy.
Methods and results
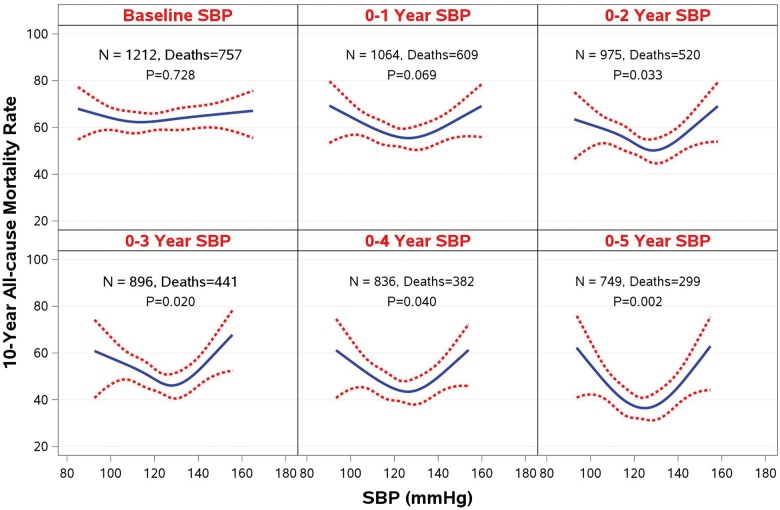
The influence of BP during a median follow-up of 9.8 years was studied in a total of 1212 patients with ejection fraction ≤35% and coronary disease amenable to coronary artery bypass grafting (CABG) who were randomized to CABG or medical therapy alone (MED) in the STICH (Surgical Treatment for Ischaemic Heart Failure) trial. Landmark analyses were performed starting at 1, 2, 3, 4, and 5 years after randomization, in which previous systolic BP values were averaged and related to subsequent mortality through the end of follow-up with a median of 9.8 years. Neither a previous history of HTN nor baseline BP had any significant influence on long-term mortality outcomes, nor did they have a significant interaction with MED or CABG treatment. The landmark analyses showed a progressive U-shaped relationship that became strongest at 5 years (χ2 and P-values: 7.08, P = 0.069; 8.72, P = 0.033; 9.86; P = 0.020; 8.31, P = 0.040; 14.52, P = 0.002; at 1, 2, 3, 4, and 5-year landmark analyses, respectively). The relationship between diastolic BP (DBP) and outcomes was similar. The most favourable outcomes were observed in the SBP range 120–130, and DBP 75–85 mmHg, whereas lower and higher BP were associated with worse outcomes. There were no differences in BP-lowering medications between groups.
Conclusion
A strong U-shaped relationship between BP and mortality outcomes was evident in ischaemic HF patients. The results imply that the optimal SBP might be in the range 120–130 mmHg after intervention, and possibly be subject to pharmacologic action regarding high BP. Further, low BP was a marker of poor outcomes that might require other interactions and treatment strategies.
Clinical Trial Registration
URL: http://www.clinicaltrials.gov. Unique identifier: NCT00023595.
Keywords: Heart failure , Coronary artery disease , Hypertension , Blood pressure , Coronary artery by-pass grafting, Survival , Surgery
Introduction
Hypertension (HTN) is the leading risk factor for premature death and disability worldwide, while being on 2nd and 4th place in ranking order of risk factors in the 2017, 2016, and 1990 evaluations of global burden of diseases, respectively.1,2 A large proportion of the adult population now suffers from this condition (25–40%).3 It has been estimated that HTN is responsible for about 40% of all myocardial infarctions. In the INTERHEART study,4 20–30% of all myocardial infarctions were attributable to HTN. In the recent world-wide PURE study, HTN was present in 32–49% of the study population,5 and in the GRACE registry 58% patients had a HTN history.6 In spite of these well-known and overwhelming data connecting HTN with coronary artery disease (CAD) and heart failure (HF), HTN has not been given any particular attention in clinical practice or in guidelines addressing CAD management.3,7–9 Likewise, the association between blood pressure (BP) and coronary artery bypass grafting (CABG) or percutaneous coronary intervention are rarely discussed.9–11 In the Secondary Prevention After Coronary Artery Bypass Graft Surgery scientific statement from the American Heart Association, HTN was addressed but with no specific recommendations other than that put forward by general HTN guidelines. ‘Admittedly, no clinical trials to date have specifically assessed BP targets after CABG with respect to clinical outcomes’.10 However, in an early study on the long-term effects of CABG, it was suggested that patients with a concomitant diagnosis of HTN did not benefit from surgical revascularization.12 Further, the most recent HTN guidelines do not contain specific recommendations for patients with CAD and left ventricular (LV) dysfunction.3,13
Whether there is a linear, J-, or U-shaped relation between BP levels and outcomes in HTN patients has been debated for decades.14–16 Although most of these studies have analysed primarily HTN populations, a few have specifically addressed patients with concomitant CAD. It has been proposed that a very low BP in CAD patients might endanger coronary perfusion and increase the risk of coronary events.17 Unrelated to the pathophysiology or treatment of HTN, low BP may be a consequence of the presence and extent of LV dysfunction, thereby a sign of increased risk. Thus, a U-shaped relationship between BP and outcomes has been noticed.18
Despite this seemingly convincing evidence relating BP to outcomes, how BP levels, the diagnosis of HTN, and the management of BP in the long-term should be factored in decision making regarding revascularization, and further management of patients with ischaemic HF is unclear. The population of patients included in the Surgical Treatment for Ischaemic Heart Failure (STICH) trial provides the opportunity to understand the role of HTN and BP measurements in the outcomes of patients with CAD and LV dysfunction who are considered and treated with surgical revascularization. Accordingly, the aim of the present study was to investigate how BP the previous diagnosis of HTN affect the mortality outcomes of patients with ischaemic HF with or without surgical revascularization. We hypothesized that a U-shaped relationship exists between BP and mortality outcomes, and that a high BP post-surgery diminishes the survival benefits of CABG.
Methods
The STICH trial (http://www.clinicaltrials.gov. Unique identifier NCT00023595) methodology has been described in detail previously.19–21 Briefly, patients ≥18 years of age with CAD amenable to CABG, and an ejection fraction (EF) ≤35% were randomized to CABG with guideline-directed medical therapy vs. medical therapy alone (MED). The primary endpoint was all-cause mortality, and the median follow-up time was 9.8 years (interquartile range 9.1–11.0 years). Trial sites were prompted by the Coordinating Center to implement guideline-recommended optimal medical therapy in both randomized arms. The study complied with the Declaration of Helsinki, and the locally appointed ethics committee approved the research protocol. Informed consent was obtained from the subjects or their legally authorized representatives.
Definitions
This study focused on two separate, albeit biologically related, variables: (i) diagnosis of HTN prior to entry into the study and (ii) BP at baseline and during follow-up, irrespective of the diagnosis of HTN. The diagnosis of HTN among study patients in this trial was reported by investigators at the time of randomization [the original study protocol defined HTN as treated or untreated systolic BP (SBP) ≥130 mmHg or diastolic BP (DBP) ≥85 mmHg (for diabetic patients DBP ≥80 mmHg) in repeated measurements]. Patients’ BP measured at the time of randomization was considered as the baseline BP for the purpose of this study. During follow-up, BP was recorded at each clinical visit at pre-specified time points, and these values were used in the analyses (see Statistical Methods section).
Statistical methods
Descriptive summaries of patient demographics and baseline clinical characteristics are presented as means and standard deviations for continuous variables, and as frequencies and percentages for categorical variables. Statistical comparisons of patient groups defined according to the presence or absence of a history of HTN were performed using the Wilcoxon rank-sum test for the continuous variables and the conventional χ2 test for categorical variables.
The relationships of history of HTN and of baseline BPs (systolic and diastolic) with all-cause mortality were examined using the Cox proportional hazards regression model.22 For the BP variables, we examined the shape and strength of their relationship with mortality using a flexible model-fitting approach involving cubic spline functions (cubic polynomials).23 These functions were graphically and statistically examined and, when relations were non-linear, their shape was characterized with the spline functions.
Because BP was measured at regular follow-up visits, and may change over time, we also performed analyses to take into account the post-baseline BP values, and assessed how the BP obtained over time were related to subsequent mortality. This was done in two ways: First, successive landmark analyses starting at 1, 2, 3, 4, and 5 years after randomization were performed. Each patient’s BP measurements obtained prior to the starting point of the landmark analysis were averaged, and the relationship of average BP with subsequent mortality was examined with the Cox model using cubic spline functions as described above. Second, we treated BP as a time-dependent covariate in the Cox model. Starting with the baseline BP, this method examined the relationship with mortality by updating an individual patient’s BP values each time a new measurement was obtained. On average, each patient had 8.5 BP measurements (median = 7 BP measurements), with minimum = 1 and maximum = 29 BP measurements.
To descriptively illustrate the added prognostic significance of BP when the post-baseline values are incorporated, we categorized patients into three categories: low BP (<110/60), high BP (>140/90), and normal BP (all values between the low and high thresholds) and examined the subsequent mortality in groups cross-classified according to baseline BP and the landmark average BP.
Finally, Cox model analyses were performed using (i) the baseline BP and (ii) landmark average BP to assess whether the effect of CABG compared with medical therapy differed according to BP, i.e. whether there was an interaction of treatment with BP. A plot of 10-year mortality rates comparing CABG vs. medical therapy as a function of average BP was produced for each of the landmark analyses.
All analyses were performed using SAS statistical software, version 9.4 (SAS Institute, Cary, NC, USA).
Results
Baseline characteristics
Of the 1212 patients included in the study, 728 (60.1%) reported a history of HTN. Patients with a history of HTN were slightly older, more likely to be female, and to have higher rates of previous stroke and risk factors for atherosclerosis (Table 1). Further, patients with a history of HTN had higher EF and smaller LV cavity size. However, the risk-at-randomization score (calculated for each patient with an equation derived from an independent data set using multiple variables with known power to predict the 5-year risk of death without CABG)20 was similar between the two groups. Baseline characteristics by baseline SBP and diastolic BP (DBP) tertiles are shown in Supplementary material online, Tables S1 and S2.
Table 1.
Baseline characteristics by patient hypertension status
| History of hypertension |
||||
|---|---|---|---|---|
| Baseline characteristics | All patients (n = 1212) | No (n = 484) | Yes (n = 728) | P-valuea |
| Age (years) | 60 ± 9 | 59 ± 9 | 61 ± 9 | <0.001 |
| Female | 148 (12) | 43 (9) | 105 (14) | 0.004 |
| White | 827 (68) | 326 (67) | 501 (69) | 0.592 |
| Medical history | ||||
| Stroke | 92 (8) | 25 (5) | 67 (9) | 0.009 |
| Diabetes | 478 (39) | 155 (32) | 323 (44) | <0.001 |
| History of MI | 934 (77) | 365 (75) | 569 (78) | 0.265 |
| Hyperlipidaemia | 730 (60) | 247 (51) | 483 (66) | <0.001 |
| Peripheral vascular disease | 184 (15) | 65 (13) | 119 (16) | 0.166 |
| Chronic renal insufficiency | 94 (8) | 31 (6) | 63 (9) | 0.155 |
| Atrial flutter/fibrillation | 153 (13) | 60 (12) | 93 (13) | 0.846 |
| Prior CABG | 36 (3) | 16 (3) | 20 (3) | 0.575 |
| NYHA heart failure class | 0.747 | |||
| I | 139 (11) | 51 (11) | 88 (12) | |
| II | 626 (52) | 254 (52) | 372 (51) | |
| III | 412 (34) | 163 (34) | 249 (34) | |
| IV | 35 (3) | 16 (3) | 19 (3) | |
| Diastolic blood pressure (mmHg) | 75 ± 11 | 73 ± 10 | 77 ± 11 | <0.001 |
| Systolic blood pressure (mmHg) | 121 ± 18 | 115 ± 15 | 125 ± 18 | <0.001 |
| BP Levels | <0.001 | |||
| Low: SBP <110 or DBP <60 | 234 (19) | 127 (26) | 107 (15) | |
| Normal | 836 (69) | 336 (69) | 500 (69) | |
| High: SBP >140 or DBP >90 | 142 (12) | 21 (4) | 121 (17) | |
| SBP Levels | <0.001 | |||
| Low: SBP <110 | 224 (18) | 121 (25) | 103 (14) | |
| Normal: SBP = (110–140) | 867 (72) | 345 (71) | 522 (72) | |
| High: SBP >140 | 121 (10) | 18 (4) | 103 (14) | |
| Three-vessel coronary disease | 733 (61) | 301 (62) | 432 (59) | 0.334 |
| LVEF (%) | 28 ± 9 | 27 ± 8 | 29 ± 9 | 0.009 |
| ESVI (mL/m2 ) | 83 ± 33 | 88 ± 36 | 80 ± 30 | 0.002 |
| EDVI (mL/m2 ) | 118 ± 39 | 124 ± 43 | 113 ± 36 | <0.001 |
| Moderate/severe mitral regurgitation | 220 (18) | 102 (21) | 118 (16) | 0.032 |
| Medications | ||||
| Beta-blocker | 1036 (85) | 406 (84) | 630 (87) | 0.199 |
| Digoxin | 245 (20) | 126 (26) | 119 (16) | <0.001 |
| ACE-I/ARB | 1085 (90) | 413 (85) | 672 (92) | <0.001 |
| Antiarrhythmic drug use | 128 (11) | 57 (12) | 71 (10) | 0.261 |
| Statin | 983 (81) | 392 (81) | 591 (81) | 0.934 |
| Diuretic (loop/thiazide) | 791 (65) | 321 (66) | 470 (65) | 0.549 |
| Diuretic (potassium sparing) | 556 (46) | 251 (52) | 305 (42) | <0.001 |
| Risk at randomization | 13 ± 9 | 12 ± 9 | 13 ± 9 | 0.411 |
Continuous variables are presented in the format of mean ± standard deviation, and categorical variables are presented with number (%).
P-values for categorical variables are based on Pearson χ2 test or MH-row mean scores difference, whereas P-values for continuous variables are based on Wilcoxon rank-sum test.
Outcomes in relation to diagnosis of hypertension and baseline blood pressure
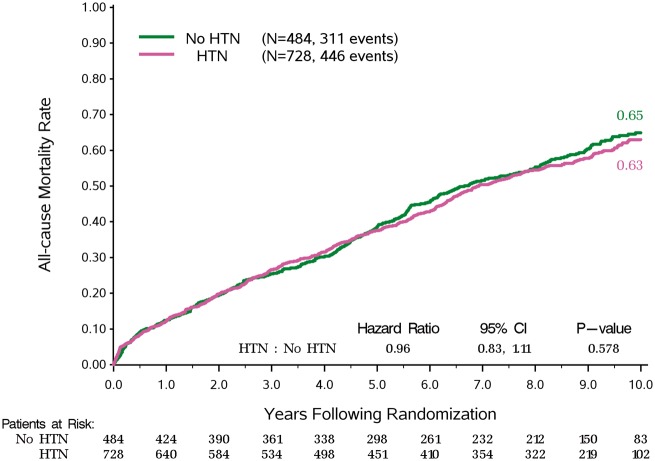
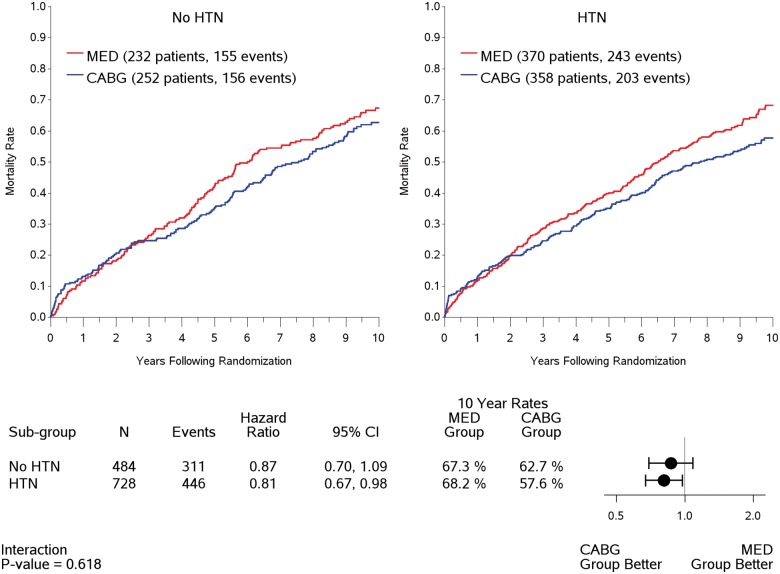
A prior diagnosis of HTN did not affect long-term mortality, hazard ratio 0.96, 95% confidence interval [0.83–1.11], P = 0.578 (Figure 1), or the effect of CABG treatment (interaction, P = 0.618) (Figure 2). Further, neither baseline SBP (P = 0.728) nor baseline DBP (P = 0.124) affected outcomes or the treatment effect of surgical revascularization.
Figure 1.
Kaplan–Meier rates of all-cause mortality for patients with or without a history of hypertension.
Figure 2.
Treatment effect on Kaplan–Meier rates of all-cause mortality by hypertension status.
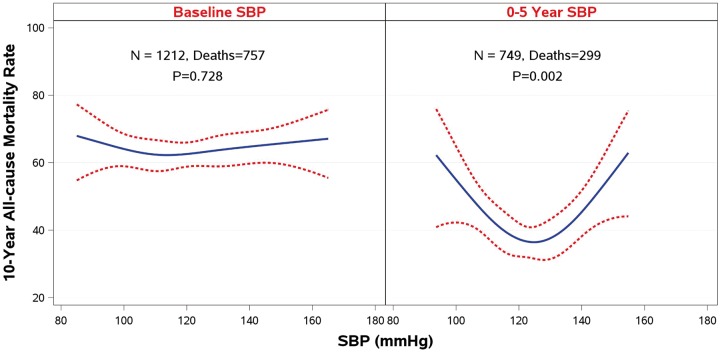
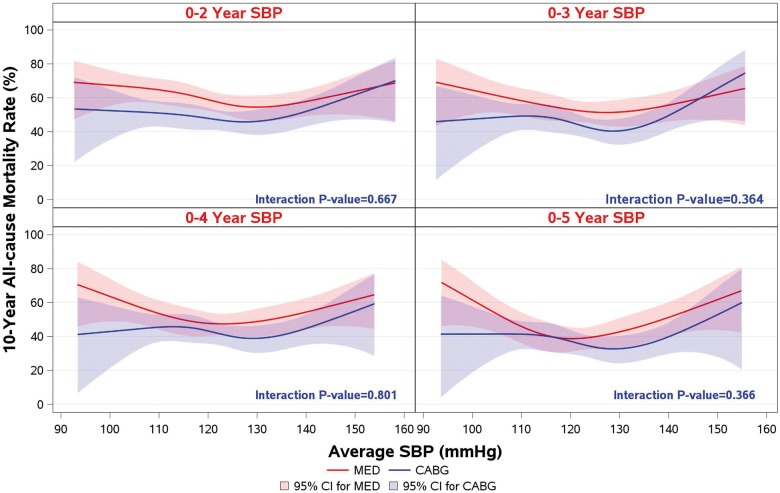
However, when 10-year clinical outcomes (all-cause mortality) were analysed with SBP and DBP as time-dependent covariates in a Cox model, there was a statistically significant relationship between BP and outcomes (Supplementary material online, Table S3). This relationship is best described by the results of the landmark analyses performed at 1, 2, 3, 4, and 5 years after randomization. Although no relationship was observed between baseline SBP and survival, the landmark analyses showed an increasingly stronger U-shaped relationship as the follow-up time progressed (Figure 3). A similar relationship was observed for DBP and survival (Supplementary material online, Figure S1). At the 5-year landmark analysis, the mortality risk during the subsequent follow-up was the lowest (around 40%) in the SBP range of 120–130, and DBP 75–85 mmHg, and the risk increased to over 60% in the lowest as well as in the highest SBP range (Figure 3).
Figure 3.
A landmark analysis on the relationship of systolic blood pressure and 10-year all-cause mortality rate.
Blood pressure during follow-up and treatment effect of coronary artery bypass grafting
Blood pressure during follow-up tended to increase in both treatment groups (Supplementary material online, Figures S2 and S3). Using the same landmark analyses, no statistically significant interaction was observed between BP during follow-up and the treatment effect of CABG over MED treatment on all-cause mortality (Figure 4).
Figure 4.
Systolic blood pressure and treatment interaction on 10-year all-cause mortality rate in landmark analyses.
Take home figure.
A landmark analysis on the relationship of systolic blood pressure and 10-year all-cause mortality rate, showing the absence of relationship between baseline BP and outcomes, and the development of a U-shaped relationship during 5 years of follow-up.
The levels of baseline BP did not associate with the mortality rate as much as the 5-year average BP. For patients who had normal baseline BP and normal 5-year average BP, their mortality rate was among the lowest. Patients who had low or high 5-year average BP had the highest mortality rate, regardless of their baseline BP levels (Supplementary material online, Table S4). Thus, the longer-term BP levels (5-year average) were more strongly associated with outcomes than one single BP measurement at baseline.
Blood pressure-lowering drugs were used in equal proportions in all groups with no significant change during the study follow-up (Supplementary material online, Table S5).
Discussion
In this study, the diagnosis of HTN and the level of BP prior to randomization were not associated with 10-year all-cause mortality among patients with ischaemic cardiomyopathy. Similarly, neither the diagnosis of HTN nor the baseline BP interacted with the treatment effect of surgical revascularization or modified the survival rates among these patients. However, a striking U-shaped relationship was found between BP during follow-up and subsequent mortality, such that the lowest mortality was observed among patients with average SBP between 120 and 130 mmHg. This U-shaped relationship became stronger as follow-up progressed, despite the smaller number of patients and the shorter duration of remaining follow-up on each subsequent landmark analysis.
A U-shaped relationship between risk factors and outcomes has been noticed previously in HF and HTN studies.14,24,25 Most recently, this was demonstrated for the relation between body weight and outcomes in HF as well as in CAD populations.26,27 The reasons for these J- or U-shaped relationships are not always easy to understand given the complexity and interplay of the multiple variables involved in the determination of BP. Mostly, such associations are not interpreted as causal relationships, and confounders are thought to be involved in the pathophysiological processes. There might also be a reverse causation as it has been shown that in older people BP tend to decrease when death is coming near.28 It is likely that the observed U-shaped relationship between follow-up BP and subsequent mortality is mediated by different mechanisms operating at either end of the BP spectrum. For example, low BP has been associated with poor outcomes in patients with HF and reduced LV systolic function.29,30 The pathophysiology underpinning this observation is different from the process that mediates the well-known adverse effect of high BP on outcomes in patients with any form of cardiovascular disease. The optimal levels of BP in HTN have been debated, and the discussion has recently been fuelled by studies implying that the lower the BP, the better the outcomes.31,32 Although the optimal BP in patients with HF has not been defined,25,32 it is generally accepted that, in patients with HF, a low BP should be tolerated in favour of the adherence to recommended treatment with beta-blockers and RAAS-blockers, which are believed to generate better organ perfusion in spite of the lower BP.33,34 In our study, the optimal SBP appeared to be in the range of 120–130 mmHg. In patients with BP below this range, risk increased linearly with decrease in BP, suggesting that this may be a consequence of HF and poor LV function.31 It is important to underscore that this was a study on HF patients following CABG surgery, and not a HTN study. Therefore, it is unlikely that over-treatment with anti-hypertensive drugs was the cause of increased mortality with low BP. Further, there were no differences in number of drugs between the treatment arms or among patients with different levels of BP. Finally, the lack of association between BP and outcomes at baseline suggests that there is a progression of the disease process during follow-up, likely as a consequence of the natural progression of HF. Keeping the poor outcome of these ischaemic HF patients with low SBP in mind, advanced therapies with LV assist device therapy or even heart transplantation may be considered in suitable patients.
Over the entire SBP spectrum, CABG treatment was more effective than MED treatment, and clearly also in the low-SBP range. This strengthens the reasoning that an improvement of LV function (with CABG) could benefit outcomes also in patients with very low BP. The potential protective effects of coronary revascularization against coronary hypoperfusion in low BP subjects appears mechanistically plausible. How a continued low BP should be targeted in the clinical situation would require a thorough evaluation of each individual patient. Although most of the patients in this study had recommended HF medication (valid for the early years post millennium shift), there are now several additional therapeutic options. The use of devices (cardiac resynchronization therapy and implantable cardioverter-defibrillator) was rather low in the study, as were the rate of mineralocorticoid receptor antagonist treatment.
Of note, the diagnosis of HTN was not associated with poor outcomes in our study, in contrast with high BP during follow-up. This suggests that it is not the diagnosis of HTN per se, but rather the BP control that is most associated with the outcomes of patients with ischaemic cardiomyopathy. Furthermore, the progressively accentuated U-shaped relationship between BP and outcomes observed in our landmark analyses reinforce that BP monitoring could be vital for best long-term outcome.
Limitations
This was a post hoc analysis and the main STICH study was not focused on BP evaluation. Further, the trial design did not include specific recommendations about BP management during follow-up. Hence, the results of this study must be interpreted with caution, as we cannot assess the potential for a causal relationship between BP management and survival in patients with ischaemic cardiomyopathy. Nevertheless, these findings provide an unbiased assessment of the relationship between BP and outcomes that—in the absence of randomized trials targeting specific BP levels—should be useful in the management of these patients. Of note, the use of BP-lowering agents may have been underestimated in this study, as information regarding the use of anti-hypertensive agents that are not used as HF medications was not prospectively collected.
Conclusion
Neither a history of HTN nor baseline BP levels influenced the survival of patients with ischaemic cardiomyopathy, nor did they affect the beneficial treatment effect of surgical revascularization. Instead, it was the level of BP during the follow-up that was strongly and progressively associated with subsequent mortality. Patients in the lower or higher BP spectrum during follow-up had increased subsequent risk of death. The U-shaped relationship observed in our study suggests that the optimal SBP for patients with ischaemic cardiomyopathy might be 120–130 mmHg.
Supplementary material
Supplementary material is available at European Heart Journal online.
Funding
This work was supported by the National Heart, Lung, and Blood Institute, National Institutes of Health Grants [U01-HL-69015, U01-HL-69013, and R01-HL-105853]. This work is solely the responsibility of the authors and does not necessarily represent the official views of the National Heart, Lung, and Blood Institute or the National Institutes of Health.
Conflict of interest: B.A.: research grants from Medtronic – significant; P.J.: clinical and public health intermediate fellowship from the Wellcome Trust-DBT India Alliance (2015–2010) – significant. R.-S.T.: honoraria for speaking and advisory board meeting from Merck – modest; honoraria for speaking from Servier – modest; E.V.: cardiovascular research grants from NIH, Alnylam Pharmaceuticals, AMGEN, Inc., General Electric, Novartis Pharmaceutical Corp., and Pfizer – all significant; consulting fees from AMGEN, Inc., Merck & Co., and Novartis Pharmaceutical Corp. – all significant; consulting fees from ABIOMED, New Century Health and Philips Ultrasound, Inc. – all modest.
Supplementary Material
References
- 1. GBD 2016 Risk Factors Collaborators. Global, regional, and national comparative risk assessment of 84 behavioural, environmental and occupational, and metabolic risks or clusters of risks, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet 2017;390:1345–1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Forouzanfar MH, Liu P, Roth GA, Ng M, Biryukov S, Marczak L, Alexander L, Estep K, Hassen Abate K, Akinyemiju TF, Ali R, Alvis-Guzman N, Azzopardi P, Banerjee A, Barnighausen T, Basu A, Bekele T, Bennett DA, Biadgilign S, Catala-Lopez F, Feigin VL, Fernandes JC, Fischer F, Gebru AA, Gona P, Gupta R, Hankey GJ, Jonas JB, Judd SE, Khang YH, Khosravi A, Kim YJ, Kimokoti RW, Kokubo Y, Kolte D, Lopez A, Lotufo PA, Malekzadeh R, Melaku YA, Mensah GA, Misganaw A, Mokdad AH, Moran AE, Nawaz H, Neal B, Ngalesoni FN, Ohkubo T, Pourmalek F, Rafay A, Rai RK, Rojas-Rueda D, Sampson UK, Santos IS, Sawhney M, Schutte AE, Sepanlou SG, Shifa GT, Shiue I, Tedla BA, Thrift AG, Tonelli M, Truelsen T, Tsilimparis N, Ukwaja KN, Uthman OA, Vasankari T, Venketasubramanian N, Vlassov VV, Vos T, Westerman R, Yan LL, Yano Y, Yonemoto N, Zaki ME, Murray CJ.. Global burden of hypertension and systolic blood pressure of at least 110 to 115 mmHg, 1990-2015. JAMA 2017;317:165–182. [DOI] [PubMed] [Google Scholar]
- 3. Mancia G, Fagard R, Narkiewicz K, Redon J, Zanchetti A, Bohm M, Christiaens T, Cifkova R, De Backer G, Dominiczak A, Galderisi M, Grobbee DE, Jaarsma T, Kirchhof P, Kjeldsen SE, Laurent S, Manolis AJ, Nilsson PM, Ruilope LM, Schmieder RE, Sirnes PA, Sleight P, Viigimaa M, Waeber B, Zannad F, Redon J, Dominiczak A, Narkiewicz K, Nilsson PM, Burnier M, Viigimaa M, Ambrosioni E, Caufield M, Coca A, Olsen MH, Schmieder RE, Tsioufis C, van de BP, Zamorano JL, Achenbach S, Baumgartner H, Bax JJ, Bueno H, Dean V, Deaton C, Erol C, Fagard R, Ferrari R, Hasdai D, Hoes AW, Kirchhof P, Knuuti J, Kolh P, Lancellotti P, Linhart A, Nihoyannopoulos P, Piepoli MF, Ponikowski P, Sirnes PA, Tamargo JL, Tendera M, Torbicki A, Wijns W, Windecker S, Clement DL, Coca A, Gillebert TC, Tendera M, Rosei EA, Ambrosioni E, Anker SD, Bauersachs J, Hitij JB, Caulfield M, De Buyzere M, De Geest S, Derumeaux GA, Erdine S, Farsang C, Funck-Brentano C, Gerc V, Germano G, Gielen S, Haller H, Hoes AW, Jordan J, Kahan T, Komajda M, Lovic D, Mahrholdt H, Olsen MH, Ostergren J, Parati G, Perk J, Polonia J, Popescu BA, Reiner Z, Ryden L, Sirenko Y, Stanton A, Struijker-Boudier H, Tsioufis C, van de Borne P, Vlachopoulos C, Volpe M, Wood DA.. 2013 ESH/ESC guidelines for the management of arterial hypertension: the Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). Eur Heart J 2013;34:2159–2219. [DOI] [PubMed] [Google Scholar]
- 4. Yusuf S, Hawken S, Ounpuu S, Dans T, Avezum A, Lanas F, McQueen M, Budaj A, Pais P, Varigos J, Lisheng L.. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): case-control study. Lancet 2004;364:937–952. [DOI] [PubMed] [Google Scholar]
- 5. Yusuf S, Rangarajan S, Teo K, Islam S, Li W, Liu L, Bo J, Lou Q, Lu F, Liu T, Yu L, Zhang S, Mony P, Swaminathan S, Mohan V, Gupta R, Kumar R, Vijayakumar K, Lear S, Anand S, Wielgosz A, Diaz R, Avezum A, Lopez-Jaramillo P, Lanas F, Yusoff K, Ismail N, Iqbal R, Rahman O, Rosengren A, Yusufali A, Kelishadi R, Kruger A, Puoane T, Szuba A, Chifamba J, Oguz A, McQueen M, McKee M, Dagenais G, Investigators P.. Cardiovascular risk and events in 17 low-, middle-, and high-income countries. N Engl J Med 2014;371:818–827. [DOI] [PubMed] [Google Scholar]
- 6. Steg PG, Goldberg RJ, Gore JM, Fox KAA, Eagle KA, Flather MD, Sadiq I, Kasper R, Rushton-Mellor SK, Anderson FA.. Baseline characteristics, management practices, and in-hospital outcomes of patients hospitalized with acute coronary syndromes in the Global Registry of Acute Coronary Events (GRACE). Am J Cardiol 2002;90:358–363. [DOI] [PubMed] [Google Scholar]
- 7. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JG, Coats AJ, Falk V, Gonzalez-Juanatey JR, Harjola VP, Jankowska EA, Jessup M, Linde C, Nihoyannopoulos P, Parissis JT, Pieske B, Riley JP, Rosano GM, Ruilope LM, Ruschitzka F, Rutten FH, van der Meer P; Authors/Task Force Members. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J 2016;37:2129–2200. [DOI] [PubMed] [Google Scholar]
- 8. Roffi M, Patrono C, Collet JP, Mueller C, Valgimigli M, Andreotti F, Bax JJ, Borger MA, Brotons C, Chew DP, Gencer B, Hasenfuss G, Kjeldsen K, Lancellotti P, Landmesser U, Mehilli J, Mukherjee D, Storey RF, Windecker S, Baumgartner H, Gaemperli O, Achenbach S, Agewall S, Badimon L, Baigent C, Bueno H, Bugiardini R, Carerj S, Casselman F, Cuisset T, Erol C, Fitzsimons D, Halle M, Hamm C, Hildick-Smith D, Huber K, Iliodromitis E, James S, Lewis BS, Lip GY, Piepoli MF, Richter D, Rosemann T, Sechtem U, Steg PG, Vrints C, Luis Zamorano J.. 2015 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: task force for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation of the European Society of Cardiology (ESC). Eur Heart J 2016;37:267–315. [DOI] [PubMed] [Google Scholar]
- 9. Windecker S, Kolh P, Alfonso F, Collet JP, Cremer J, Falk V, Filippatos G, Hamm C, Head SJ, Juni P, Kappetein AP, Kastrati A, Knuuti J, Landmesser U, Laufer G, Neumann FJ, Richter DJ, Schauerte P, Sousa Uva M, Stefanini GG, Taggart DP, Torracca L, Valgimigli M, Wijns W, Witkowski A.. 2014 ESC/EACTS guidelines on myocardial revascularization: the task force on myocardial revascularization of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS)Developed with the special contribution of the European Association of Percutaneous Cardiovascular Interventions (EAPCI). Eur Heart J 2014;35:2541–2619. [DOI] [PubMed] [Google Scholar]
- 10. Kulik A, Ruel M, Jneid H, Ferguson TB, Hiratzka LF, Ikonomidis JS, Lopez-Jimenez F, McNallan SM, Patel M, Roger VL, Sellke FW, Sica DA, Zimmerman L; American Heart Association Council on Cardiovascular Surgery and Anesthesia. Secondary prevention after coronary artery bypass graft surgery: a scientific statement from the American Heart Association. Circulation 2015;131:927–964. [DOI] [PubMed] [Google Scholar]
- 11. Saluveer O, Redfors B, Angeras O, Dworeck C, Haraldsson I, Ljungman C, Petursson P, Odenstedt J, Ioanes D, Lundgren P, Volz S, Ramunddal T, Andersson B, Omerovic E, Bergh N.. Hypertension is associated with increased mortality in patients with ischaemic heart disease after revascularization with percutaneous coronary intervention - a report from SCAAR. Blood Press 2017;26:166–173. [DOI] [PubMed] [Google Scholar]
- 12. Varnauskas E. Survival, myocardial infarction, and employment status in a prospective randomized study of coronary bypass surgery. Circulation 1985;72:V90–V101. [PubMed] [Google Scholar]
- 13. Whelton PK, Carey RM, Aronow WS, Casey DE Jr, Collins KJ, Dennison Himmelfarb C, DePalma SM, Gidding S, Jamerson KA, Jones DW, MacLaughlin EJ, Muntner P, Ovbiagele B, Smith SC Jr, Spencer CC, Stafford RS, Taler SJ, Thomas RJ, Williams KA Sr, Williamson JD, Wright JT Jr. ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: executive summary: a report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines. Hypertension 2018;71:1269–1324. [DOI] [PubMed] [Google Scholar]
- 14. Cruickshank JM. Coronary flow reserve and the J curve relation between diastolic blood pressure and myocardial infarction. BMJ 1988;297:1227–1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kannel WB, Wilson PW, Nam BH, D'Agostino RB, Li J.. A likely explanation for the J-curve of blood pressure cardiovascular risk. Am J Cardiol 2004;94:380–384. [DOI] [PubMed] [Google Scholar]
- 16. Lindholm L, Lanke J, Bengtsson B, Ejlertsson G, Thulin T, Schersten B.. Both high and low blood pressures risk indicators of death in middle-aged males. Isotonic regression of blood pressure on age applied to data from a 13-year prospective study. Acta Med Scand 1985;218:473–480. [DOI] [PubMed] [Google Scholar]
- 17. D'Agostino RB, Belanger AJ, Kannel WB, Cruickshank JM.. Relation of low diastolic blood pressure to coronary heart disease death in presence of myocardial infarction: the Framingham Study. BMJ 1991;303:385–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Badheka AO, Patel NJ, Grover PM, Shah N, Patel N, Singh V, Deshmukh AJ, Mehta K, Chothani A, Savani GT, Arora S, Rathod A, Marzouka GR, Lafferty J, Mehta JL, Mitrani RD.. Optimal blood pressure in patients with atrial fibrillation (from the AFFIRM Trial). Am J Cardiol 2014;114:727–736. [DOI] [PubMed] [Google Scholar]
- 19. Jones RH, Velazquez EJ, Michler RE, Sopko G, Oh JK, O'Connor CM, Hill JA, Menicanti L, Sadowski Z, Desvigne-Nickens P, Rouleau JL, Lee KL; STICH Hypothesis 2 Investigators. Coronary bypass surgery with or without surgical ventricular reconstruction. N Engl J Med 2009;360:1705–1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Jones RH, White H, Velazquez EJ, Shaw LK, Pietrobon R, Panza JA, Bonow RO, Sopko G, O'Connor CM, Rouleau JL.. STICH (Surgical Treatment for Ischemic Heart Failure) trial enrollment. J Am Coll Cardiol 2010;56:490–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Velazquez EJ, Lee KL, Jones RH, Al-Khalidi HR, Hill JA, Panza JA, Michler RE, Bonow RO, Doenst T, Petrie MC, Oh JK, She L, Moore VL, Desvigne-Nickens P, Sopko G, Rouleau JL; STICHES Investigators. Coronary-artery bypass surgery in patients with ischemic cardiomyopathy. N Engl J Med 2016;374:1511–1520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Cox DR. Regression methods and life-tables. J Roy Statist Soc B 1972;34:187–220. [Google Scholar]
- 23. Harrell FA., Jr Regression Modeling Strategies: With Applications to Linear Models, Logistic Regression, and Survival Analysis. New York: Springer; 2001. [Google Scholar]
- 24. Ather S, Chan W, Chillar A, Aguilar D, Pritchett AM, Ramasubbu K, Wehrens XH, Deswal A, Bozkurt B.. Association of systolic blood pressure with mortality in patients with heart failure with reduced ejection fraction: a complex relationship. Am Heart J 2011;161:567–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lee DS, Ghosh N, Floras JS, Newton GE, Austin PC, Wang X, Liu PP, Stukel TA, Tu JV.. Association of blood pressure at hospital discharge with mortality in patients diagnosed with heart failure. Circ Heart Fail 2009;2:616–623. [DOI] [PubMed] [Google Scholar]
- 26. Angeras O, Albertsson P, Karason K, Ramunddal T, Matejka G, James S, Lagerqvist B, Rosengren A, Omerovic E.. Evidence for obesity paradox in patients with acute coronary syndromes: a report from the Swedish Coronary Angiography and Angioplasty Registry. Eur Heart J 2013;34:345–353. [DOI] [PubMed] [Google Scholar]
- 27. Joyce E, Lala A, Stevens SR, Cooper LB, AbouEzzeddine OF, Groarke JD, Grodin JL, Braunwald E, Anstrom KJ, Redfield MM, Stevenson LW.. Prevalence, profile, and prognosis of severe obesity in contemporary hospitalized Heart Failure Trial populations. JACC Heart Fail 2016;4:923–931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ravindrarajah R, Hazra NC, Hamada S, Charlton J, Jackson SHD, Dregan A, Gulliford MC.. Systolic blood pressure trajectory, frailty, and all-cause mortality >80 years of age: Cohort Study using electronic health records. Circulation 2017;135:2357–2368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Chobanian AV. Hypertension in 2017-what is the right target? JAMA 2017;317:579–580. [DOI] [PubMed] [Google Scholar]
- 30. Williamson JD, Supiano MA, Applegate WB, Berlowitz DR, Campbell RC, Chertow GM, Fine LJ, Haley WE, Hawfield AT, Ix JH, Kitzman DW, Kostis JB, Krousel-Wood MA, Launer LJ, Oparil S, Rodriguez CJ, Roumie CL, Shorr RI, Sink KM, Wadley VG, Whelton PK, Whittle J, Woolard NF, Wright JT Jr, Pajewski NM; SPRINT Research Group. Intensive vs standard blood pressure control and cardiovascular disease outcomes in adults aged >/=75 years: a randomized clinical trial. JAMA 2016;315:2673–2682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Gheorghiade M, Abraham WT, Albert NM, Greenberg BH, O’Connor CM, She L, Stough WG, Yancy CW, Young JB, Fonarow GC; OPTIMIZE-HF Investigators and Coordinators. Systolic blood pressure at admission, clinical characteristics, and outcomes in patients hospitalized with acute heart failure. JAMA 2006;296:2217–2226. [DOI] [PubMed] [Google Scholar]
- 32. Raphael CE, Whinnett ZI, Davies JE, Fontana M, Ferenczi EA, Manisty CH, Mayet J, Francis DP.. Quantifying the paradoxical effect of higher systolic blood pressure on mortality in chronic heart failure. Heart 2009;95:56–62. [DOI] [PubMed] [Google Scholar]
- 33. McMurray JJ, Adamopoulos S, Anker SD, Auricchio A, Bohm M, Dickstein K, Falk V, Filippatos G, Fonseca C, Gomez-Sanchez MA, Jaarsma T, Kober L, Lip GY, Maggioni AP, Parkhomenko A, Pieske BM, Popescu BA, Ronnevik PK, Rutten FH, Schwitter J, Seferovic P, Stepinska J, Trindade PT, Voors AA, Zannad F, Zeiher A; ESC Committee for Practice Guidelines. ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: the Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC. Eur Heart J 2012;33:1787–1847. [DOI] [PubMed] [Google Scholar]
- 34. McMurray JJ, Packer M, Desai AS, Gong J, Lefkowitz MP, Rizkala AR, Rouleau JL, Shi VC, Solomon SD, Swedberg K, Zile MR; PARADIGM-HF Investigators and Committees. Angiotensin-neprilysin inhibition versus enalapril in heart failure. N Engl J Med 2014;371:993–1004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.